Abstract
The pharmaceutical and chemical industries depend on additives to protect enzymes and other proteins against stresses that accompany their manufacture, transport, and storage. Common stresses include vacuum-drying, freeze-thawing, and freeze-drying. The additives include sugars, compatible osmolytes, amino acids, synthetic polymers, and both globular and disordered proteins. Scores of studies have been published on protection, but the data have never been analyzed systematically. To spur efforts to understand the sources of protection and ultimately develop more effective formulations, we review ideas about the mechanisms of protection, survey the literature searching for patterns of protection, and then compare the ideas to the data.
Graphical Abstract

Biologics, such as protein-based drugs, are some of the most effective therapeutic treatments on the market.1 However, these medications are inherently unstable and can easily degrade with time or changes to their environment.2 The World Health Organization even varies its stability guidelines for biologic drugs to accommodate the temperature and humidity ranges of different regions.2 Furthermore, biologics usually require transport and storage in refrigerated conditions, also called the cold chain, which adds additional challenges and expense to maintaining drug integrity.3 Enzymes are similarly poised to revolutionize the industry of chemical synthesis; however, optimizing these reactions requires the stabilization of enzymes in nonphysiological, and often non-aqueous, environments.4
We summarize the ability of a variety of additives to protect enzymes and other proteins against common stresses of biologic and industrial enzyme manufacture, transport, and storage, specifically, vacuum-drying (desiccation), freeze-thawing, and freeze-drying. Many of the additives are already used as, or have the potential to serve as, excipients, non-active ingredients formulated with biologics to stabilize and protect the active ingredient.5 More specifically, we define excipients as compounds appearing as such in the Appendix of the book chapter by Gokam et al. and mostly focus on protection data published after the book by Franks and Auffret.6
STRESSES
Vacuum-Drying (desiccation) Stress.
Removing water from biologic drugs and industrial enzymes drastically reduces the weight of these products, which decreases shipping costs. Water can be removed from liquid formulations in a vacuum chamber, which is often combined with a centrifuge (e.g., a Speedvac).
Freeze-Thawing and Freeze-Drying Stress.
The chemistry of protein degradation usually requires the inherent motion in liquid formulations. Therefore, it can be advantageous to freeze enzymes, but freeze-thawing is often damaging.7 In addition to vacuum-drying, water can be removed from frozen samples via lyophilization, resulting in both freeze-thawing and vacuum-drying stress.
MECHANISMS OF PROTECTION
Several mechanisms for protection from the stresses mentioned above have been considered. Some groups suggest that vitrification, when additives form an amorphous glass rather than ordered crystals as they dry, protects the enzymes by encasing them. This idea is often investigated using scanning calorimetry to observe the glass transition temperature.8-15 Additional support for this idea comes from the vitrification observed in desiccation-tolerant organisms.14,16,17 However, like all of the mechanisms, this idea is not universally accepted.18,19
Crowe et al. suggest that preferential hydration protects proteins against freezing-induced inactivation.7 The idea behind this exclusion is that in aqueous solution, cosolutes such as simple sugars and compatible osmolytes are repelled by the protein backbone, favoring the more compact folded form of globular proteins, which stabilizes the protein by increasing its standard-state free energy of unfolding.20,21 These repulsive cosolute–protein interactions have been especially well quantified for sugars.21 Stability could also be increased via hard-core repulsions caused by high concentrations of inert cosolutes, which favor the compact native state of globular proteins.22,23 It is unclear, however, if the effects of preferential hydration or macromolecular crowding extend to the dry state.
Some investigators suggest that inhibiting aggregation during vacuum drying,24-28 freeze-thawing,29 and freeze-drying is protective.30 Chakrabortee et al. propose the molecular shield hypothesis, in which shield molecules use physical interference to reduce the frequency of cohesive interactions between potentially aggregating species.26 This protection is conferred through nonspecific interactions. From efforts using solvatochromic dyes, Ferreira et al. suggest that the shield effect of protein additives called dehydrins arises from changes in water structure.31 Several studies consider this hypothesis directly24,26,29 or describe a similar phenomenon.32,33
Other groups report damage to enzyme structure from these stresses. Circular dichroism spectropolarimetry, Fourier transform infrared spectroscopy, and nuclear magnetic resonance-detected amide-proton exchange have been used to observe damage to secondary structure.28,33-37 Others report inhibition of enzyme-subunit dissociation19,33,38,39 and suggest that maintenance of quaternary structure can compensate for a lack of water replacement during drying.19,39 Several studies attribute dissociation to the precipitation of sodium phosphate buffer during freezing and the concomitant acidification of the sample.39-42 However, this explanation fails to account for dissociation under other buffer conditions or other stresses.
Seguro et al. suggest that cryo-denaturation is caused by ice crystal formation.41 In addition to freeze–thaw protection, a few groups report inhibition or slowing of ice crystal formation by additives that may, in turn, prevent enzyme damage.11,33,43 Hillgren et al. postulate that surfactants may protect enzymes by covering ice crystals as they form,11 which could explain why 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), polidocanol, polyethylene glycol (PEG) dodecyl ether, polysorbate, and sucrose fatty acid monoester protect some enzymes against freeze-thawing- and freeze-drying-induced inactivation.11,19,30,44,45 Nevertheless, this explanation fails to explain protection from vacuum-drying damage.
ADDITIVES AND PROTECTION FROM VACUUM-DRYING STRESS
We compiled reports of enzyme protection during vacuum drying (Table S1). The highest degree of protection for each additive is further summarized in Figure 1. Focusing on the strongest protection tends to increase false positives. This approach, however, helps decrease the number of false negatives, which are likely because many studies test only a single concentration or mole ratio.
Figure 1.
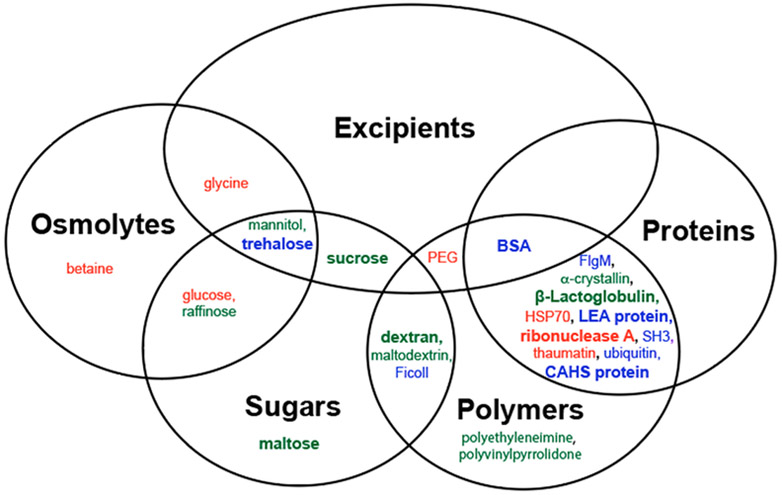
Venn diagram for protection from vacuum-drying stress. Additives in blue have at least one report of full protection. Additives in green have no reports of full protection but at least one report of partial protection. Additives in red have no reports of protection. A bold font represents two or more reports.
The stress of vacuum-drying resembles anhydrobiosis. Therefore, interrogating desiccation-tolerant organisms could result in the discovery of new excipients. Many such creatures, including Caenorhabditis elegans, Saccharomyces cerevisiae, and Anopheles gambiae, use the nonreducing disaccharide trehalose to combat desiccation stress.27,46,47 As might be expected, heterologous expression of a trehalose transporter improves the desiccation tolerance of Chinese hamster ovary cells,48 and trehalose biosynthetic genes improve the drought resistance of rice.49 In vitro studies show that high concentrations of trehalose protect lactate dehydrogenase (LDH) against vacuum-drying-induced inactivation.9,14,24,50,51 Trehalose also protects the activity of the restriction enzymes BamHI, EcoRI, and HindIII.8,52
Most sugars tested have some ability to protect enzymes against desiccation. The sucrose polymer Ficoll 70 fully protects LDH, although a higher grams per liter concentration is required than for trehalose.51 The glucose polymer dextran 20, the disaccharide sucrose, and the sugar alcohol mannitol partially protect LDH in a concentration-dependent manner.51 Additionally, Ficoll and dextran reduce the level of desiccation-induced aggregation of the water soluble T-REx293 proteome.26 Maltodextrin and raffinose protect the restriction enzyme EcoRI against desiccation-induced inactivation, although protection was not quantified.8 The disaccharide maltose partially protects LDH activity at intermediate concentrations, but protection decreases at both low and high concentrations.51 Of the sugars tested, only glucose has no protective ability.51 The protective ability of sugars may depend on the absence of reactive carbonyl groups.51 Finally, it is important to bear in mind that there are closely related studies that we have not included because the identical drying method was not used. For instance, Colaco et al.53 and Carpenter and Crowe54 studied air-dried, rather than freeze-dried, samples. Although we do not discuss these results or include them in Figure 1, they are generally consistent with the statements above and are included in Table S1.
The sugars trehalose, raffinose, mannitol, and glucose are also compatible osmolytes, a class of molecules that restore homeostasis in organisms under osmotic stress.55,56 However, only sugar-based compatible osmolytes protect enzymes against desiccation. The compatible osmolyte and sugar alcohol mannitol has some ability to protect LDH, but even at high concentrations, it protects only a fraction of the enzyme.51 The compatible osmolyte and amino acid glycine, along with its methylated form, betaine, do not protect LDH.51
Although the sugar polymers dextran and Ficoll are protective, the synthetic polymer polyethylene glycol has no ability to protect LDH against vacuum-drying regardless of concentration.51 Polyethylenimine provides partial protection to LDH, and polyvinylpyrrolidone protects EcoRI during vacuum-drying, although protection of the latter was not quantified.8,57 These mixed results suggest that hard-core excluded volume from macromolecular crowding23 in solution does not confer desiccation tolerance.
Organisms across all kingdoms of life also synthesize intrinsically disordered proteins58 in response to stress.59 These proteins are often classified as hydrophilins, defined by Garay-Arroyo et al.60 as having both a high glycine content (>6%) and a hydrophilicity index61 of >1.0. Late embryogenesis abundant (LEA) proteins are the best known of this family. Their heterologous expression increases the desiccation tolerance of yeast and the hyperosmotic tolerance of human cells.62,63 During vacuum-drying, LEA proteins reduce the level of desiccation-induced aggregation of the water soluble T-REx293 and Aphelenchus avenae proteomes and partially protect ADP-glucose-pyrophosphorylase and glucose-6-phosphate dehydrogenase activity in the soluble leaf proteome of Arabidopsis thaliana.25,26,28 In vitro, LEA proteins protect LDH, fumarase, and citrate synthase activity and inhibit the latter’s aggregation.24,26,28,50,62,64,65 Additionally, formulation with LEA proteins reduces the loss of fluorescence of the red fluorescent protein mCherry upon vacuum-drying and rehydration.26
Another family of intrinsically disordered proteins, cytosolic abundant heat soluble (CAHS) proteins, is required by tardigrades to survive desiccation.14 Heterologous expression of these proteins increases the desiccation tolerance of both Escherichia coli and Saccharomyces cerevisiae, and in vitro, CAHS proteins protect LDH against desiccation.14,51 Mitochondrially abundant heat soluble proteins from tardigrades increase the hyperosmotic tolerance of human cells, but these proteins have not yet been studied as protectants of purified proteins.63
Despite having no link to desiccation tolerance, many other proteins protect enzymes against vacuum-drying in vitro. The most common globular protein control for vacuum-drying experiments, bovine serum albumin (BSA), protects the activity of LDH and citrate synthase and also prevents aggregation of the latter, although it does not protect the activity of fumarase or the fluorescence of mCherry 14,24,26,50,51,62,64. The disordered bacterial signaling protein flgM protects LDH against vacuum-drying-induced inactivation more effectively than CAHS proteins.51 Ubiquitin and an SH3 domain are similarly effective.51 α-Crystallin and β-lactoglobulin have some protective ability.28,50 Ribonuclease A, thaumatin from pathogenesis-related protein family 5,66 and the chaperone HSP70 have no protective ability,26,28,62 but this result could arise from an insufficient amount of additive.
The fact that so many proteins with well-studied functions and no known link to desiccation tolerance protect enzymes against vacuum drying-induced inactivation suggests that protection is not conferred by a particular amino acid sequence.51 Rather, protection may be a general property of proteins.51 Along these lines, it has been suggested that the ability to survive repeated cycles of hydration and dehydration is a prerequisite for the emergence of life.67
ADDITIVES AND PROTECTION FROM FREEZE–THAW STRESS
We summarized the data using the fewest freeze–thaw cycles reported in each paper (Table S2). The highest degree of protection reported for each additive is summarized in Figure 2. However, it is important to use caution when comparing these studies because many parameters, including cooling rates,11,12,44 the enzyme batch, and the sample volume, can affect the results.
Figure 2.
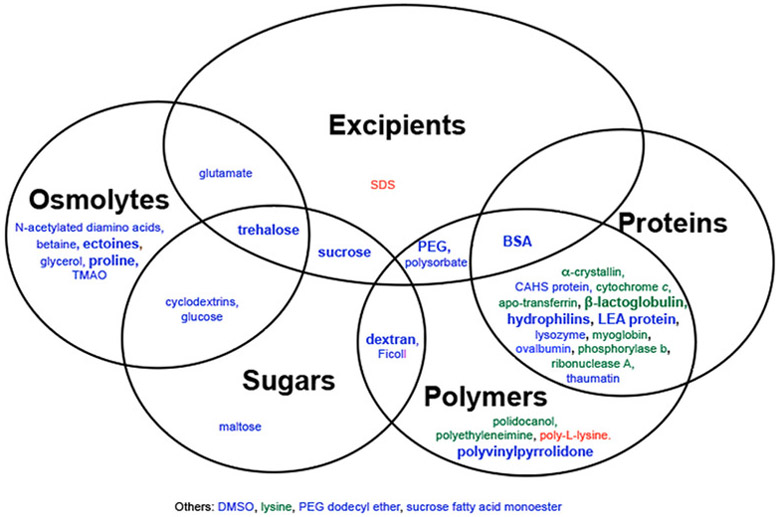
Venn diagram for protection from freeze–thaw stress. Additives in blue have at least one report of full protection. Additives in green have no reports of full protection but at least one report of partial protection. Additives in red have no reports of protection. A bold font represents two or more reports. DMSO, dimethyl sulfoxide; SDS, sodium dodecyl sulfate.
In cells, trehalose protects both membranes and proteins against freeze–thaw damage.68 Exogenous trehalose increases the tolerance of a variety of cells and organelles, including Lactobacillus bulgaricus, S. cerevisiae, isolated thylakoids, and ram spermatozoa.69-72 Heterologous expression of the Staphylococcus aureus hemolysin transmembrane pore allows trehalose uptake and improved cryopreservation of 3T3 fibroblasts and human keratinocytes.73 Reports of in vitro enzyme protection, however, are inconsistent. There are conflicting reports concerning the cryoprotection of LDH,9,19,45,74-77 and trehalose fully protects phosphofructokinase74 but fails to protect lipoprotein lipase.51
Inconsistent results are reported for many sugars. One study reports full protection of LDH by glucose, but another reports no cryoprotection.19,45 The same studies test three cyclodextrin variants, β-cyclodextrin, 2,6-di-O-methyl-β-cyclodextrin, and hydroxypropyl-β-cyclodextrin, and report no protection to full protection of the enzyme.19,45 Most studies report full protection of LDH by sucrose,19,74-76,78 but other experiments report incomplete protection of LDH and phosphofructokinase.76,79 Similarly, maltose fully protects LDH and partially protects phosphofructokinase against freeze–thaw stress.74 Dextran fully protects both catalase and LDH,19,80 and Anchordoquy et al. report full protection of LDH by Ficoll.19 Despite this variability, there is at least one report of full protection for every sugar molecule tested. It is likely that reports of partial or no protection result from using an insufficient amount of sugar, inhospitable buffer conditions, or other easily altered conditions.
The sugars trehalose, glucose, and cyclodextrin are also compatible osmolytes, and like sugars, reports of compatible osmolyte protection are inconsistent. Two reports observe partial protection of LDH activity by betaine, one of which also reports protection of LDH structural integrity via tryptophan fluorescence.62,75 However, other experiments report no protection of LDH activity but full protection of phosphofructokinase activity by betaine.75,76 Similarly, ectoine and its derivatives demethylectoine, homoectoine, and hydroxyectoine provide full or partial protection to LDH and full protection to phosphofructokinase.74-76 Glutamate partially protects alcohol dehydrogenase, malate dehydrogenase, and pyruvate kinase.40,76 Other studies, however, report full, partial, and no protection of LDH,40,41,45 and one study reports no protection of glucose-6-phosphate dehydrogenase.40 Glycerol partially protects catalase activity and fully protects LDH activity.75,80 The N-acetylated diamino acids Nα-acetyllsyine, Nε-acetyllsyine, Nα-acetylornithine, and Nδ-acetylornithine fully protect the structure of LDH as assessed by tryptophan fluorescence but only partially protect its activity.75,76 The opposite is true of proline, which fully protects LDH activity but only partially inhibits its structural perturbation.35,75 Trimethylamine N-oxide (TMAO) fully protects LDH activity and structure, but there is only one report.76 Like sugars, there is at least one report of full protection for every compatible osmolyte tested. Again, it is likely that reports of partial or no protection result from insufficient compatible osmolyte concentration, inhospitable buffer conditions, or other easily modified conditions.
Similar to their behavior in desiccation tolerance, intrinsically disordered LEA proteins fully protect LDH and citrate synthase in vitro and partially protect ADP-glucose-pyrophosphorylase and glucose-6-phosphate dehydrogenase in the soluble leaf proteome of A. thaliana.24,26,28,29,43,62,77,79,81,82 Two hydrophilins outside the LEA protein family also protect LDH.77,83 Futhermore, an intrinsically disordered CAHS protein protects lipoprotein lipase against freeze–thaw inactivation.51 On a molar basis, all of these intrinsically disordered proteins are more effective protectants than the globular protein BSA. Nevertheless, BSA protects the enzymes malate dehydrogenase and pyruvate kinase in addition to LDH, citrate synthase, glucose-6-phosphate dehydrogenase, and lipoprotein lipase.24,29,39,40,43,51,62,78,79,81,82
Among globular proteins, partial and full LDH protection is reported for lysozyme and ovalbumin.29,78,79,81 Partial cryoprotection of LDH is reported for α-crystallin, apo-transferrin, cytochrome c, myoglobin, and phosphorylase b.77,81 Ribonuclease A has no cryoprotective effect on ADP-glucose-pyrophosphorylase and glucose-6-phosphate dehydrogenase in the soluble leaf proteome of A. thaliana,28 yet full, partial, and no protection are reported for LDH.28,77,79,81,82 Both full cryoprotection and no cryoprotection are reported for proteins from pathogenesis-related protein family 5.62,78 Like ribonuclease A, β-lactoglobulin has no cryoprotective effect on ADP-glucose-pyrophosphorylase and glucose-6-phosphate dehydrogenase in the soluble leaf proteome of A. thaliana,28 but multiple groups report partial protection of LDH activity.28,79,81,82
Although full protection is reported for only a subset of these globular proteins, at least one study reports partial cryoprotection by each protein. It is likely that reports of no protection result from insufficient concentration, incompatible buffer conditions, or other facile variables.
In addition to proteins, several synthetic polymers confer cryoprotection. Polyethylene glycol fully protects both LDH and phosphofructokinase.11,18,19,33,45 Full cryoprotection and partial cryoprotection of LDH are reported for the related polymer, polyethylene glycol dodecyl ether.11,19 Polidocanol, a short polyethylene glycol attached to a hydrocarbon chain, also partially protects LDH.45 Polyvinylpyrrolidone fully protects LDH and catalase,39,80 and polyethylenimine partially protects LDH against freezing and thawing.57 The emulsifier polysorbate partially protects LDH.19 Poly-L-lysine has no ability to protect LDH, but only one concentration was tested.77
Some studies report an increased level of enzyme protection with an increasing molecular weight of the synthetic polymer, sugar polymer, or LEA protein.19,33,57,84 Given the ability of numerous monosaccharides, disaccharides, and other compatible osmolytes to protect enzymes against freeze–thaw stress, additional studies are needed to determine the mechanism of protection.
ADDITIVES AND PROTECTION FROM FREEZE-DRYING STRESS
In addition to vacuum drying, water can be removed from frozen samples via lyophilization, resulting in both freeze-thawing and vacuum drying stress. We have compiled reports of enzyme protection during freeze-drying stress (Table S3). The highest degree of protection for each additive is summarized in Figure 3.
Figure 3.
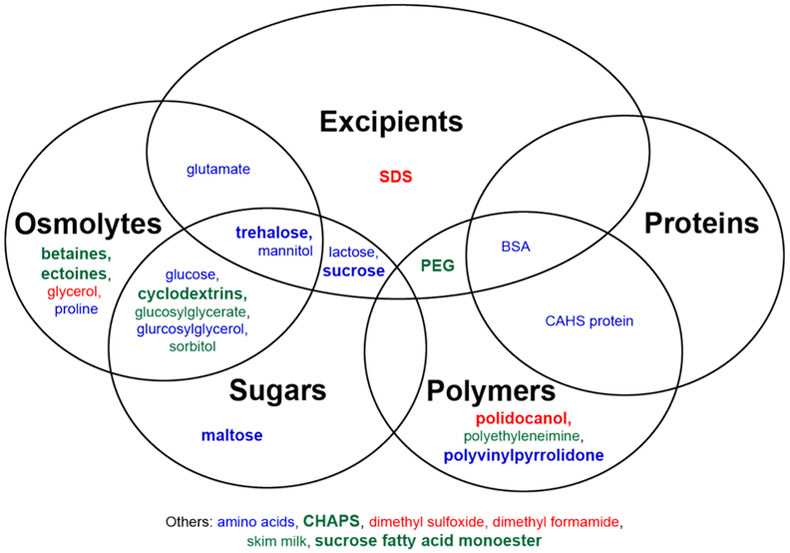
Venn diagram for protection from freeze-drying stress. Additives in blue have at least one report of full protection. Additives in green have no reports of full protection but at least one report of partial protection. Additives in red have no reports of protection. A bold font represents two or more reports. CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate; SDS, sodium dodecyl sulfate.
Trehalose exhibits mixed results. Formulating trehalose with Lactobacillus reuteri CICC6266 cells protects native LDH and ATPase,85 and trehalose provides full protection to β-galactosidase and L-asparaginase.30,32 Alkaline phosphatase lyophilized with trehalose even retains activity for up to 84 days.86 However, trehalose confers only partial protection to lipase and mannitol dehydrogenase.87,88 Trehalose was most frequently studied with LDH, but at best, only partial protection is reported.18,30,45,74,75,87 Furthermore, trehalose did not protect the enzymes phosphofructokinase and lipoprotein lipase against lyophilization-induced inactivation.18,51
A variety of results are also reported for other sugars. Sucrose fully protects LDH activity in L. reuteri CICC6266 cells and L-asparaginase activity in lyophilized formulations and inhibits aggregation of monoclonal antibodies.32,85,89 However, others report that sucrose partially protects LDH and phosphofructokinase and does not protect ATPase in L. reuteri CICC6266 cells.13,37,74,75,85 Maltose fully protects L-asparaginase but only partial protects LDH and phosphofructokinase.13,32,74 Glucose fully protects β-galactosidase and L-asparaginase but does not protect LDH or phosphofructokinase activity.18,30,32,45 Similarly, lactose fully protects alkaline phosphatase and L-asparaginase, but no or only partial protection is reported for lactate dehydrogenase.13,18,32,86 Two studies examine five cyclodextrin variants (α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, 2,6-di-O-methyl-β-cyclodextrin, and hydroxypropyl-β-cyclodextrin), which confer, at best, only partial protection to β-galactosidase and LDH.30,45 Additional reports examine the sugar alcohols mannitol and sorbitol. Mannitol fully protects L-asparaginase; mixed results are reported for LDH, and no protection is observed for alkaline phosphatase and phosphofructokinase.18,32,37,86 Sorbitol partially protects LDH, but no protection is observed when formulated with lipase.87 Like these sugar alcohols, glucosylglycerol fully protects and glucosylglycerate partially protects mannitol dehydrogenase.88 If we consider the greatest degree of protection, all of these sugars confer at least partial protection.
The sugars trehalose, glucose, cyclodextrin, mannitol, sorbitol, glucosylglycerol, and glucosylglycerate are also classified as compatible osmolytes. Reports of protection are less frequent among non-sugar compatible osmolytes. Glutamate and proline fully protect β-galactosidase against freeze drying, although glutamate only partially protects LDH and proline confers no protection to phosphofructokinase.30,45,75 Betaine partially protects phosphofructokinase but does not protect LDH or lipase.74,75,87 However, derivatives of betaine, dimethylthetin and homodeanol betaine, have a limited ability to protect LDH and lipase.87 Similarly, ectoine provides no protection to LDH but partially protects phosphofructokinase. Its derivative hydroxyectoine partially protects both enzymes.74,75 Glycerol is the only compatible osmolyte with no documented protective ability, at least in limited studies with LDH and phosphofructokinase.75
Few proteins have been studied as protectants during freeze-drying and subsequent storage. Anchordoquy et al. report that BSA fully protects LDH.39 Piszkiewicz et al. report that BSA partially protects, and a CAHS protein fully protects, lipoprotein lipase.51 Furthermore, several enzymes protect themselves against freeze-drying with increasing concentrations.30,39,45 Additional work is needed to determine if other proteins that protect enzymes against vacuum-drying and freeze-thawing stress are as protective against freeze-drying-induced inactivation.
Slightly more data are available for protection by synthetic polymers. Polyvinylpyrrolidone fully protects both catalase and LDH.39,90 Polyethylene glycol confers partial protection of β-galactosidase, partial or no protection of LDH, and no protection of phosphofructokinase.18,30,45 Polyethylenimine partially protects LDH activity.57 Polidocanol is ineffective against freeze-drying.30,45 Nevertheless, these studies are too limited to draw robust conclusions.
PROTECTION BY MIXTURES
Several groups report levels of protection from mixtures that are greater than those of the sum of the individual additives. Goyal et al. report protection of LDH and citrate synthase against desiccation-induced inactivation by a mixture of LEA proteins and trehalose.24 Tamiya et al. report synergistic protection of LDH, malate dehydrogenase, glucose-6-phosphate dehydrogenase, and pyruvate kinase against freeze–thaw stress by BSA combined with sodium glutamate.40 Mannitol and glycine formulated in 1:1 5% (w/w) ratios protect LDH from freeze-drying inactivation.12 This study, however, does not investigate the protective ability of these compatible osmolytes individually and shows that the concentration of buffer in the original solution has a strong effect on protection. Mattern et al.10 sought to assess the protective ability of vitrification using glasses made from amino acids, but most amino acids form crystals. These authors found that arginine forms a glass with a low water content when mixed with phenylalanine. The low water content is important because the authors posit that residual water adversely affects protection. Phenylalanine and arginine together protect LDH against vacuum drying inactivation and inhibit granulocyte colony-stimulating factor aggregation, but these amino acids are not tested individually.10 The authors made similar observations about mixtures of maltose or sucrose and phenylalanine.91 Carpenter et al. observed that polyethylene glycol, which protects against freeze–thaw damage, can be combined with trehalose, glucose, or lactose, each of which protects against water removal, to protect LDH and phosphofructokinase against freeze-drying.18 Miller et al. report that combining borate with trehalose synergistically improves the long-term storage of LDH after vacuum-drying.9
Each report investigates a limited set of potential synergistic interactions. More thorough studies are needed to confirm the conclusions and understand the mechanism(s) leading to synergism.
ANALYSIS AND CONCLUSIONS
Dozens of studies report on the ability of additives to protect enzymes against vacuum-drying, freeze–thaw, and freeze-drying stress, but there remains much we do not understand. For instance, although several mechanisms have been advanced, few studies have been designed to tell one mechanism from another. Even so, uncovering the mechanism(s) will be difficult because some molecules have no protective ability in one report and full protective ability in another. Some of these results may be false negatives because an insufficient quantity of additive is used. Given the absence of obvious patterns, it is likely that different test proteins are protected via different mechanisms. For instance, some test proteins are more sensitive to stress-induced changes in tertiary or quaternary structure while others are more prone to stress-induced aggregation. Additionally, most papers study only a small subset of additives. Another caveat is that additives with the largest number of reported protective effects may reflect the number of studies that used them rather than better protection.
Looking across Figures 1-3 shows that representatives from all classes of additives can have a protective effect. Given the wide range of chemical properties, this generalization provides support for the molecular shield mechanism. The most striking observation is that trehalose is generally protective against all three types of stress. Enhanced hydrogen bonding between trehalose and test proteins is a partial, but not full, explanation.92
Another general observation comes from comparing the results from vacuum-drying (Figure 1) to the those from freeze-drying (Figure 3) and freeze-thaw (Figure 2) experiments. Except for trehalose, compatible osmolytes, which stabilize proteins in solution56 and in cells,93 protect poorly against vacuum-drying (Table S1), suggesting that simple ideas about protein stability in solution cannot necessarily be related to this type of protection and perhaps that inactivation arises from protein–protein interactions.
We performed a separate analysis on LDH, the enzyme with the most protection data. We focused on additives reported to protect >90% of the activity. Where multiple concentrations were tested, we focused on the lowest concentration affording full protection. The data are summarized in Table S4. There are too few data to analyze freeze-dry inactivation.
Data on LDH protection were divided into effects of small (<1 kDa) and large additives (>1 kDa). The median ratios are reported in Table 1 with one exception. The exception is that of trehalose, because it is the only small molecule that fully protects against desiccation stress.
Table 1.
Analysis of Additives That Protect LDHa
| additive:LDH | |||
|---|---|---|---|
| stress | additive | mole:mole | gram:gram |
| desiccation | large | 4 × 102 | 80 |
| small (trehalose) | 1 × 105 | 250 | |
| freeze-thaw | large | 25 | 4 |
| small | 2 × 106 | 3 × 103 | |
See the text for details.
Analysis of the data in Table 1 shows that large molecules are generally more effective than small molecules at protecting against both desiccation and freeze–thaw inactivation, which suggests a macromolecular effect. For freeze–thaw stress, however, protection cannot be attributed to hard-core repulsions because the concentrations of the protein additives studied [always ≤1 g/L (Table S4)] reflect minute fractions of the total volume. Freeze-thaw protection by large molecules may arise from nonspecific chemical interactions. Such interactions would appear to be concentration-dependent because LDH, malate dehydrogenase, alcohol dehydrogenase, pyruvate kinase, phosphofructokinase, and β-galactosidase all retain more activity when frozen at higher concentrations in the absence of additives.30,39,40,42,45,74
Upon comparison of desiccation data and freeze–thaw data, it is important to bear in mind that the concentration of the additive increases massively during desiccation but changes little during the freeze–thaw process. Trehalose, BSA, and Ficoll protect against both desiccation and freeze–thaw stress. However, more macromolecular additive is required to protect against desiccation-induced inactivation (Table1), suggesting that desiccation is more damaging. This idea is bolstered by examining the additives that protect against freeze-dry stress but not desiccation stress: the reducing sugars, dextran, maltose, and glucose. This lack of protection may arise from covalent modification by these sugars (glycation, a known problem in formulation)94 as their concentration increases during desiccation. Further support for the suggestion that different mechanisms operate for freeze-thaw- and desiccation-induced inactivation comes from the observation that lysozyme denatures LDH in concentrated solutions, but dilute lysozyme solutions can fully protect against freeze–thaw inactivation.51
Nevertheless, the simple idea that polymers protect better against desiccation-induced inactivation is not valid because neither dextran nor PEG protects against desiccation. For Ficoll, the difference could arise from the reactivity of reducing linkages as suggested above, but it is unclear why PEG offers no protection against desiccation-induced inactivation but full protection against freeze–thaw stress.51
As stated at the beginning of this Perspective, cosolute-induced preferential hydration and/or hard-core crowding effects along with the molecular shield hypothesis involving hydrogen bonding between the protectants and the test proteins are the most popular proposed mechanisms. With respect to the ideas behind hydrogen bonding and the molecular shield hypothesis, it must be remembered that attractive interactions between cosolutes and the backbone of globular proteins are destabilizing because unfolding exposes more backbone. These attractive interactions are what make urea a protein denaturant.21 In terms of ideas about hydration and crowding, we need to know if these concepts are operational in the solid state. Techniques that can interrogate protein structure and stability in both solutions and solids include Fourier transform infrared spectroscopy,16 solid-state NMR spectroscopy,95 and synchrotron circular dichroism spectropolarimetry.96 Although challenging, more such studies will be required to understand protection.
Most importantly, a direct comparison of a larger number of these additives under the same conditions using a variety of test proteins is needed. These suggestions should also be applied to the investigation of synergistic interactions to enhance protection. Systematic, hypothesis-driven studies utilizing many additives and several test proteins will determine which, if any, of the mechanisms are operative and ultimately lead to more effective formulations.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Elizabeth Pielak for comments on the manuscript and Jhoan Aguilar for designing and preparing the Table of Contents graphic.
Funding
Our research is supported by the National Science Foundation (Grants MCB 1410854 and CHE 1607359 to G.J.P.) and the National Institutes of Health (R01GM127291). G.J.P. thanks the K. C. Wong Education Foundation for travel support. S.P. thanks the UNC Graduate School for a dissertation completion fellowship.
ABBREVIATIONS
- BSA
bovine serum albumin
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CAHS
cytosolic abundant heat soluble
- LDH
lactate dehydrogenase
- LEA
late embryogenesis abundant
- mCherry
red fluorescent protein
- PEG
polyethylene glycol
- SDS
sodium dodecyl sulfate
- TMAO
trimethylamine N-oxide
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.bio-chem.9b00675.
Abilities of additives to protect proteins against vacuum drying (Table S1), abilities of additives to protect proteins against freeze–thaw stress (Table S2), abilities of additives to protect against freeze-drying (Table S3), and comparison of additives that protect LDH (Table S4) (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Morrow T, and Felcone LH (2004) Defining the difference: What makes biologics unique. Biotechnology Healthcare 1, 24–29. [PMC free article] [PubMed] [Google Scholar]
- (2).Huynh-Ba K (2009) Handbook of stability testing in pharmaceutical development: Regulations, methodologies, and best practices, Springer, New York. [Google Scholar]
- (3).Higgins A, Mangan A, Kerrigan A, Laffan S, and Klein S (2009) Activity, ICT, and material infrastructure in complex multi-organizational settings: An assessment of innovation potential for pharmaceutical cold chain transport and handling BLED 2009 Proceedings, Vol. 28, Association for Information Systems. [Google Scholar]
- (4).Lee MY, and Dordick JS (2002) Enzyme activation for nonaqueous media. Curr. Opin. Biotechnol. 13, 376–384. [DOI] [PubMed] [Google Scholar]
- (5).Gokarn YR, Kosky A, Kras E, McAuley A, and Remmele RL Jr. (2006) Excipients for protein drugs In Excipient development for pharmaceutical, biotechnological, and drug delivery sytstems (Katdare A, and Chaubal MV, Eds.) pp 291–331, Informa Healthcare, New York. [Google Scholar]
- (6).Franks F, and Auffret T (2007) Freeze-drying of pharmaceuticals and biopharmaceuticals: Principles and practice, Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- (7).Crowe JH, Carpenter JF, Crowe LM, and Anchordoguy TJ (1990) Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 27, 219–231. [Google Scholar]
- (8).Rossi S, Buera MP, Moreno S, and Chirife J (1997) Stabilization of the restriction enzyme ecori dried with trehalose and other selected glass-forming solutes. Biotechnol. Prog. 13, 609–616. [DOI] [PubMed] [Google Scholar]
- (9).Miller DP, Anderson RE, and de Pablo JJ (1998) Stabilization of lactate dehydrogenase following freeze-thawing and vacuum-drying in the presence of trehalose and borate. Pharm. Res 15, 1215–1221. [DOI] [PubMed] [Google Scholar]
- (10).Mattern M, Winter G, Kohnert U, and Lee G (1999) Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems. Pharm. Dev. Technol 4, 199–208. [DOI] [PubMed] [Google Scholar]
- (11).Hillgren A, and Alden M (2002) A comparison between the protection of LDH during freeze-thawing by PEG 6000 and Brij 35 at low concentrations. Int. J. Pharm 244, 137–149. [DOI] [PubMed] [Google Scholar]
- (12).Pyne A, Chatterjee K, and Suryanarayanan R (2003) Solute crystallization in mannitol-glycine systems-implications on protein stabilization in freeze-dried formulations. J. Pharm. Sci 92, 2272–2283. [DOI] [PubMed] [Google Scholar]
- (13).Kawai K, and Suzuki T (2007) Stabilizing effect of four types of disaccharide on the enzymatic activity of freeze-dried lactate dehydrogenase: Step by step evaluation from freezing to storage. Pharm. Res 24, 1883–1890. [DOI] [PubMed] [Google Scholar]
- (14).Boothby TC, Tapia H, Brozena AH, Piszkiewicz S, Smith AE, Giovannini I, Rebecchi L, Pielak GJ, Koshland D, and Goldstein B (2017) Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 65, 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Startzel P (2018) Arginine as an excipient for protein freeze-drying: A mini review. J. Pharm. Sci 107, 960–967. [DOI] [PubMed] [Google Scholar]
- (16).Sakurai M, Furuki T, Akao K, Tanaka D, Nakahara Y, Kikawada T, Watanabe M, and Okuda T (2008) Vitrification is essential for anhydrobiosis in an african chironomid. Proc. Natl. Acad. Sci. U. S. A 105, 5093–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hengherr S, Worland MR, Reuner A, Brummer F, and Schill RO (2009) High-temperature tolerance in anhydrobiotic tardigrades is limited by glass transition. Physiol. Biochem. Zool 82, 749–755. [DOI] [PubMed] [Google Scholar]
- (18).Carpenter JF, Prestrelski SJ, and Arakawa T (1993) Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. I. Enzyme activity and calorimetric studies. Arch. Biochem. Biophys 303, 456–464. [DOI] [PubMed] [Google Scholar]
- (19).Anchordoquy TJ, Izutsu KI, Randolph TW, and Carpenter JF (2001) Maintenance of quaternary structure in the frozen state stabilizes lactate dehydrogenase during freeze-drying. Arch. Biochem. Biophys 390, 35–41. [DOI] [PubMed] [Google Scholar]
- (20).Rose GD, Fleming PJ, Banavar JR, and Maritan A (2006) A backbone-based theory of protein folding. Proc. Natl. Acad. Sci. U. S. A 103, 16623–16633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Auton M, Rosgen J, Sinev M, Holthauzen LM, and Bolen DW (2011) Osmolyte effects on protein stability and solubility: A balancing act between backbone and side-chains. Biophys. Chem 159, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Davis-Searles PR, Saunders AJ, Erie DA, Winzor DJ, and Pielak GJ (2001) Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu. Rev. Biophys. Biomol. Struct 30, 271–306. [DOI] [PubMed] [Google Scholar]
- (23).Sarkar M, Li C, and Pielak GJ (2013) Soft interactions and crowding. Biophys. Rev 5, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Goyal K, Walton LJ, and Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem. J 388, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chakrabortee S, Boschetti C, Walton LJ, Sarkar S, Rubinsztein DC, and Tunnacliffe A (2007) Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. U. S. A 104, 18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chakrabortee S, Tripathi R, Watson M, Kaminski Schierle GS, Kurniawan DP, Kaminski CF, Wise MJ, and Tunnacliffe A (2012) Intrinsically disordered proteins as molecular shields. Mol. BioSyst 8, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Tapia H, and Koshland DE (2014) Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol 24, 2758–2766. [DOI] [PubMed] [Google Scholar]
- (28).Popova AV, Rausch S, Hundertmark M, Gibon Y, and Hincha DK (2015) The intrinsically disordered protein lea7 from Arabidopsis thaliana protects the isolated enzyme lactate dehydrogenase and enzymes in a soluble leaf proteome during freezing and drying. Biochim. Biophys. Acta, Proteins Proteomics 1854, 1517–1525. [DOI] [PubMed] [Google Scholar]
- (29).Hughes S, and Graether SP (2011) Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Sci. 20, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ken-ichi I, Sumie Y, and Tadao T (1993) Stabilization of β-galactosidase by amphiphilic additives during freeze-drying. Int. J. Pharm 90, 187–194. [Google Scholar]
- (31).Ferreira LA, Walczyk Mooradally A, Zaslavsky B, Uversky VN, and Graether SP (2018) Effect of an intrinsically disordered plant stress protein on the properties of water. Biophys. J 115, 1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ward KR, Adams GDJ, Alpar HO, and Irwin WJ (1999) Protection of the enzyme L-asparaginase during lyophilisation - a molecular modelling approach to predict required level of lyoprotectant. Int. J. Pharm 187, 153–162. [DOI] [PubMed] [Google Scholar]
- (33).Mi YL, Wood G, and Thoma L (2004) Cryoprotection mechanisms of polyethylene glycols on lactate dehydrogenase during freeze-thawing. AAPS J. 6, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Desai UR, Osterhout J, and Klibanov AM (1994) Protein structure in the lyophilized state: A hydrogen isotope exchange NMR study with BPTI. J. Am. Chem. Soc 116, 9420–9422. [Google Scholar]
- (35).Rajendrakumar CSV, Reddy BVB, and Reddy AR (1994) Proline-protein interactions - protection of structural and functional integrity of M4 lactate-dehydrogenase. Biochem. Biophys. Res. Commun 201, 957–963. [DOI] [PubMed] [Google Scholar]
- (36).Allison SD, Chang B, Randolph TW, and Carpenter JF (1999) Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys 365, 289–298. [DOI] [PubMed] [Google Scholar]
- (37).Izutsu K, and Kojima S (2002) Excipient crystallinity and its protein-structure-stabilizing effect during freeze-drying. J. Pharm. Pharmacol 54, 1033–1039. [DOI] [PubMed] [Google Scholar]
- (38).Chilson OP, Costello LA, and Kaplan NO (1965) Studies on mechanism of hybridization of lactic dehydrogenases in vitro. Biochemistry 4, 271–281. [Google Scholar]
- (39).Anchordoquy TJ, and Carpenter JF (1996) Polymers protect lactate dehydrogenase during freeze-drying by inhibiting dissociation in the frozen state. Arch. Biochem. Biophys 332, 231–238. [DOI] [PubMed] [Google Scholar]
- (40).Tamiya T, Okahashi N, Sakuma R, Aoyama T, Akahane T, and Matsumoto JJ (1985) Freeze denaturation of enzymes and its prevention with additives. Cryobiology 22, 446–456. [DOI] [PubMed] [Google Scholar]
- (41).Seguro K, Tamiya T, Tsuchiya T, and Matsumoto JJ (1990) Cryoprotective effect of sodium glutamate and lysine-HCl on freeze denaturation of lactate-dehydrogenase. Cryobiology 27, 70–79. [DOI] [PubMed] [Google Scholar]
- (42).Soliman FS, and Van den Berg L (1971) Factors affecting freezing damage of lactic dehydrogenase. Cryobiology 8, 73–78. [DOI] [PubMed] [Google Scholar]
- (43).Wisniewski M, Webb R, Balsamo R, Close TJ, Yu XM, and Griffith M (1999) Purification, immunolocalization, cryoprotective, and antifreeze activity of pca60: A dehydrin from peach (Prunus persica). Physiol. Plant 105, 600–608. [Google Scholar]
- (44).Hillgren A, Lindgren J, and Alden M (2002) Protection mechanism of Tween 80 during freeze-thawing of a model protein, LDH. Int. J. Pharm 237, 57–69. [DOI] [PubMed] [Google Scholar]
- (45).Izutsu K, Yoshioka S, and Terao T (1994) Stabilizing effect of amphiphilic excipients on the freeze-thawing and freeze-drying of lactate dehydrogenase. Biotechnol Bioeng. 43, 1102–1107. [DOI] [PubMed] [Google Scholar]
- (46).Erkut C, Penkov S, Khesbak H, Vorkel D, Verbavatz JM, Fahmy K, and Kurzchalia TV (2011) Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol 21, 1331–1336. [DOI] [PubMed] [Google Scholar]
- (47).Liu K, Dong Y, Huang Y, Rasgon JL, and Agre P (2013) Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc. Natl. Acad. Sci. U. S. A 110, 17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Chakraborty N, Menze MA, Elmoazzen H, Vu H, Yarmush ML, Hand SC, and Toner M (2012) Trehalose transporter from african chironomid larvae improves desiccation tolerance of chinese hamster ovary cells. Cryobiology 64, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, and Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. U. S. A 99, 15898–15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Reyes JL, Rodrigo MJ, Colmenero-Flores JM, Gil JV, Garay-Arroyo A, Campos F, Salamini F, Bartels D, and Covarrubias AA (2005) Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant, Cell Environ. 28, 709–718. [Google Scholar]
- (51).Piszkiewicz S, Gunn KH, Warmuth O, Propst A, Mehta A, Nguyen KH, Kuhlman E, Guseman AJ, Stadmiller SS, Boothby TC, Neher SB, and Pielak GJ (2019) Protecting activity of desiccated enzymes. Protein Sci. 28, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Uritani M, Takai M, and Yoshinaga K (1995) Protective effect of disaccharides on restriction endonucleases during drying under vacuum. J. Biochem 117, 774–779. [DOI] [PubMed] [Google Scholar]
- (53).Colaco C, Sen S, Thangavelu M, Pinder S, and Roser B (1992) Extraordinary stability of enzymes dried in trehalose: Simplified molecular biology. Nat. Biotechnol 10, 1007–1011. [DOI] [PubMed] [Google Scholar]
- (54).Carpenter JF, and Crowe JH (1988) Modes of stabilization of a protein by organic solutes during desiccation. Cryobiology 25, 459–470. [Google Scholar]
- (55).Yancey PH, Clark ME, Hand SC, Bowlus RD, and Somero GN (1982) Living with water stress: Evolution of osmolyte systems. Science 217, 1214–1222. [DOI] [PubMed] [Google Scholar]
- (56).Rydeen AE, Brustad EM, and Pielak GJ (2018) Osmolytes and protein–protein interactions. J. Am. Chem. Soc 140, 7441–7444. [DOI] [PubMed] [Google Scholar]
- (57).Andersson MA, and Hatti-Kaul R (1999) Protein stabilising effect of polyethyleneimine. J. Biotechnol 72, 21–31. [Google Scholar]
- (58).Theillet F-X, Binolfi A, Frembgen-Kesner T, Hingorani K, Sarkar M, Kyne C, Li C, Crowley P, Gierasch L, Pielak GJ, Elcock A, Gershenson A, and Selenko P (2014) Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs). Chem. Rev 114, 6661–6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Boothby TC, and Pielak GJ (2017) Intrinsically disordered proteins and desiccation tolerance: Elucidating functional and mechanistic underpinnings of anhydrobiosis. BioEssays 39, 1700119. [DOI] [PubMed] [Google Scholar]
- (60).Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, and Covarrubias AA (2000) Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem 275, 5668–5674. [DOI] [PubMed] [Google Scholar]
- (61).Kyte J, and Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol 157, 105–132. [DOI] [PubMed] [Google Scholar]
- (62).Dang NX, Popova AV, Hundertmark M, and Hincha DK (2014) Functional characterization of selected LEA proteins from Arabidopsis thaliana in yeast and in vitro. Planta 240, 325–336. [DOI] [PubMed] [Google Scholar]
- (63).Tanaka S, Tanaka J, Miwa Y, Horikawa DD, Katayama T, Arakawa K, Toyoda A, Kubo T, and Kunieda T (2015) Novel mitochondria-targeted heat-soluble proteins identified in the anhydrobiotic tardigrade improve osmotic tolerance of human cells. PLoS One 10, No. e0118272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Grelet J, Benamar A, Teyssier E, Avelange-Macherel MH, Grunwald D, and Macherel D (2005) Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 137, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Palmer SR, De Villa R, and Graether SP (2019) Sequence composition versus sequence order in the cryoprotective function of an intrinsically disordered stress-response protein. Protein Sci., DOI: 10.1002/pro.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Liu JJ, Sturrock R, and Ekramoddoullah AKM (2010) The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 29, 419–436. [DOI] [PubMed] [Google Scholar]
- (67).Spitzer J, Pielak GJ, and Poolman B (2015) Emergence of life: Physical chemistry changes the paradigm. Biol. Direct 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Leslie SB, Israeli E, Lighthart B, Crowe JH, and Crowe LM, (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol 61, 3592–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Hincha DK (1989) Low concentrations of trehalose protect isolated thylakoids against mechanical freeze-thaw damage. Biochim. Biophys. Acta, Biomembr 987, 231–234. [Google Scholar]
- (70).Deantoni GL, Perez P, Abraham A, and Anon MC (1989) Trehalose, a cryoprotectant for Lactobacillus bulgaricus. Cryobiology 26, 149–153. [Google Scholar]
- (71).Diniz-Mendes L, Bernardes E, de Araujo PS, Panek AD, and Paschoalin VM (1999) Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng 65, 572–578. [DOI] [PubMed] [Google Scholar]
- (72).Aisen EG, Medina VH, and Venturino A (2002) Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology 57, 1801–1808. [DOI] [PubMed] [Google Scholar]
- (73).Eroglu A, Russo MJ, Bieganski R, Fowler A, Cheley S, Bayley H, and Toner M (2000) Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol 18, 163–167. [DOI] [PubMed] [Google Scholar]
- (74).Lippert K, and Galinski EA (1992) Enzyme stabilization by ectoine-type compatible solutes - protection against heating, freezing and drying. Appl. Microbiol. Biotechnol 37, 61–65. [Google Scholar]
- (75).Galinski EA (1993) Compatible solutes of halophilic eubacteria - molecular principles, water-solute interaction, stress protection. Experientia 49, 487–496. [Google Scholar]
- (76).Goller K, and Galinski EA (1999) Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J. Mol. Catal B: Enzym 7, 37–45. [Google Scholar]
- (77).Reyes JL, Campos F, Wei H, Arora R, Yang YI, Karlson DT, and Covarrubias AA (2008) Functional dissection of hydrophilins during in vitro freeze protection. Plant, Cell Environ. 31, 1781–1790. [DOI] [PubMed] [Google Scholar]
- (78).Dave RS, and Mitra RK (1998) A low temperature induced apoplastic protein isolated from Arachis hypogaea. Phytochemistry 49, 2207–2213. [DOI] [PubMed] [Google Scholar]
- (79).Lin CT, and Thomashow MF (1992) A cold-regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem. Biophys. Res. Commun 183, 1103–1108. [DOI] [PubMed] [Google Scholar]
- (80).Ashwood-Smith MJ, and Warby C (1972) Protective effect of low and high molecular weight compounds on the stability of catalase subjected to freezing and thawing. Cryobiology 9, 137–140. [DOI] [PubMed] [Google Scholar]
- (81).Kazuoka T, and Oeda K (1994) Purification and characterization of COR85-oligomeric complex from cold-acclimated spinach. Plant Cell Physiol. 35, 601–611. [Google Scholar]
- (82).Thalhammer A, Bryant G, Sulpice R, and Hincha DK (2014) Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 166, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Konrad Z, and Bar-Zvi D (2008) Synergism between the chaperone-like activity of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta 227, 1213–1219. [DOI] [PubMed] [Google Scholar]
- (84).Hughes SL, Schart V, Malcolmson J, Hogarth KA, Martynowicz DM, Tralman-Baker E, Patel SN, and Graether SP (2013) The importance of size and disorder in the cryoprotective effects of dehydrins. Plant Physiol. 163, 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Li B, Tian F, Liu X, Zhao J, Zhang H, and Chen W (2011) Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl. Microbiol. Biotechnol 92, 609–616. [DOI] [PubMed] [Google Scholar]
- (86).Ford AW, and Dawson PJ (1993) The effect of carbohydrate additives in the freeze-drying of alkaline phosphatase. J. Pharm. Pharmacol 45, 86–93. [DOI] [PubMed] [Google Scholar]
- (87).Vasudevamurthy MK, Weatherley LR, and Lever M (2005) Enzyme stabilization using synthetic compensatory solutes. Biocatal Biotransform. 23, 285–291. [Google Scholar]
- (88).Sawangwan T, Goedl C, and Nidetzky B (2010) Glucosylglycerol and glucosylglycerate as enzyme stabilizers. Biotechnol J. 5, 187–191. [DOI] [PubMed] [Google Scholar]
- (89).Shire SJ (2009) Formulation and manufacturability of biologics. Curr. Opin. Biotechnol 20, 708–714. [DOI] [PubMed] [Google Scholar]
- (90).Darbyshire B (1974) The influence of dehydration on catalase stability. A comparison with freezing effects. Cryobiology 11, 148–151. [DOI] [PubMed] [Google Scholar]
- (91).Mattern M, Winter G, Rudolph R, and Lee G (1997) Formulation of proteins in vacuum-dried glasses. I: Improved vacuum-drying of sugars using crystallising amino acids. Eur. J. Pharm. Biopharm 44, 177–185. [Google Scholar]
- (92).Allison SD, Chang B, Randolph TW, and Carpenter JF (1999) Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys 365, 289–298. [DOI] [PubMed] [Google Scholar]
- (93).Stadmiller SS, Gorensek-Benitez AH, Guseman AJ, and Pielak GJ (2017) Osmotic-shock induced protein destabilization in living cells and its reversal by glycine betaine. J. Mol. Biol 429, 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Chi EY (2017) Excipients used in biotechnology products In Pharmaceutical excipients: Properties, functionality, and applications in research and industry (Koo O, Ed.) pp 145–198, Wiley, Hoboken, NJ. [Google Scholar]
- (95).Yoshioka S, Forney KM, Aso Y, and Pikal MJ (2011) Effect of sugars on the molecular motion of freeze-dried protein formulations reflected by NMR relaxation times. Pharm. Res 28, 3237–3247. [DOI] [PubMed] [Google Scholar]
- (96).Yoneda JS, Miles AJ, Araujo APU, and Wallace BA (2017) Differential dehydration effects on globular proteins and intrinsically disordered proteins during film formation. Protein Sci. 26, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


