Abstract
Mental- and physical-health conditions co-occur at a rate much higher than chance. Of patients who have a mental-health condition, more than half also have a physical disease, and these cases are associated with increased human suffering and societal cost. Comorbidity research to date has focused on co-occurring mental- and physical-health disorders separately, and relatively little research has examined the co-occurrence of mental- and physical-health dysfunction. In addition, even less is known about why mental- and physical-health dysfunction co-occurs or how to treat these cases. Thus, the aims of this article are to highlight the need for research at the intersection of physical- and mental-health dysfunction and to provide guidance on how to research cases of comorbidity. Toward these ends, we begin by presenting a selective overview of the possible role of biological processes in the co-occurrence of physical- and mental-health dysfunction using specific illustrative examples. Specifically, we outline how biological processes within the immune system and gastrointestinal system could underlie depression, irritable bowel syndrome, and their co-occurrence. We then advance and discuss a proposed research framework, including methodological and analytic guidance, that researchers could use when studying the phenomenon of co-occurring physical- and mental-health dysfunction.
Keywords: comorbidity, inflammation, gut microbiome, depression, irritable bowel syndrome (IBS), mental health, physical health
Mental- and physical-health conditions commonly co-occur and pose substantial personal- and public-health costs, and these co-occurrences are poorly understood (Fulop, Strain, Vita, Lyons, & Hammer, 1987; Kroenke et al., 2010; Sareen et al., 2006). According to epidemiological data, 68% of adults with a mental-health disorder also have one or more physical-health conditions, and 29% of adults with a physical-health condition have a co-occurring mental-health disorder (Goodell, Druss, Walker, & MAT, 2011). Whereas psychological science has closely examined comorbidity between mental-health disorders (Kessler, Chiu, Demler, & Walters, 2005; Klein & Riso, 1993; Neale & Kendler, 1995), co-occurring mental- and physical-health conditions have received less empirical attention. Research that elucidates the causes of these comorbidities is a public health imperative that stands to inform empirical models and clinical treatments (Dickey, Normand, Weiss, Drake, & Azeni, 2002).
The disability experienced by those with mental- and physical-health comorbidities highlights the need for research in this area (Scott et al., 2009). For example, individuals with both a chronic pain condition and mental-health disorder are unable to do usual activities for twice as many days as those with chronic pain alone (Bair, Wu, Damush, Sutherland, & Kroenke, 2008). Psychiatric conditions among asthma patients are associated with a four-fold increase in hospitalizations and a five-fold increase in emergency-room visits (Feldman et al., 2005; Richardson et al., 2006). Likewise, physical-health conditions likely augment the already high costs of mental-health conditions, including increased economic hardships and mortality rates (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011; Mokdad, Marks, Stroup, & Gerberding, 2004). Despite its high prevalence and public-health costs, relatively little is known about why mental- and physical-health dysfunction co-occurs or how it should be treated.
Overview and Goals of Article
The purpose of this article is to highlight the need for and advance a research framework that would allow researchers to test how physical health, mental health, and biological processes are related. First, we present evidence that biological processes are related to mental- and physical-health dysfunction and may underlie co-occurring dysfunction across mental- and physical-health domains. For illustrative purposes, we discuss depression as the exemplar mental-health condition and irritable bowel syndrome (IBS) as the exemplar physical-health condition. To contextualize our research framework, we present a selective overview of studies linking depression, IBS, and processes within the immune and gastrointestinal (GI) systems. The suggested framework is intended to be relevant for a wide range of co-occurring mental- and physical-health conditions and the biological systems and processes that may underlie their co-occurrence.
In the second part of the article, we present a research framework, with corresponding methodological suggestions, to guide studies in testing how mental health (e.g., cognitive functioning, mood, discrete psychiatric conditions), physical health (e.g., health behaviors, discrete medical conditions), and biological processes (e.g., in the immune and GI systems) are related. A more comprehensive theoretical understanding of co-occurring mental- and physical-health dysfunction would, in turn, support the development and refinement of effective treatments for these complex but common cases. For example, some mental- and physical-health conditions may be treated simultaneously by targeting a single biological process, but this type of clinical application relies on a basic understanding of the causal relationships between the disorders and biological processes. Likewise, shared biological risk factors may underlie the eventual manifestation of mental- and physical-health conditions, thus warranting preventative strategies that target the shared risk factor. Thus, the proposed framework would help elucidate the shared mechanisms of mental and physical functioning, thereby advancing basic and clinical science in this area.
Since the biological mechanisms of mental health have been elucidated (e.g., Research Domain Criteria; Insel et al., 2010), the distinction between mental health and physical health has become less clear. Despite the substantial overlap between mental health and physical health, this distinction remains relevant to how we diagnose and treat mental-versus physical-health conditions. Therefore, we refer to categories of co-occurring dysfunction as mental health and physical health.
Biological Processes
The immune system, GI system, and signaling between these two systems are broadly implicated in well-being across several physical- and mental-health conditions, but we chose to limit our discussion here to these systems’ role in depression and IBS. We chose to highlight depression and IBS as exemplar mental- and physical-health conditions because of robust evidence linking depression to immune-system processes (Dantzer, 2012; Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Smith, 1991) and to GI processes (Foster & McVey Neufeld, 2013). Likewise, IBS has been linked to both immune-system functioning (Öhman & Simrén, 2010) and functioning within the gut (Collins, 2014). In addition, these examples were selected on the basis of the high rate of co-occurrence between depression and IBS (Fond et al., 2014). We provide a brief introduction to the immune system and GI system in the sections that follow to contextualize the examples provided through-out the article.
Immune system
Aberrant immune functioning, such as when a hyperactive immune response adversely affects host health, healing, homeostasis, or normal development, has been linked to mental- and physical-health dysfunction. Thus, studying immunological processes may aid in our understanding of co-occurring physical- and mental-health dysfunction. There is an important, ongoing debate about how the immune system relates to well-being (e.g., Hoebe, Janssen, & Beutler, 2004; Medzhitov, 2001), and the summary below is based on current hypotheses rather than a comprehensive review of the literature.
The immune system is a complex set of interrelated mechanisms and feedback loops that coordinates the inflammatory response, among other functions, to protect the body. One set of working hypotheses suggests that the immune system relies on germline-encoded pattern-recognition machinery such as Toll-like receptors (TLRs) to identify invading pathogens and activate the appropriate immune cells. In the periphery, signaling cascades trigger the production and circulation of pro- inflammatory cytokines, which promote and sustain inflammation. These proinflammatory-signaling molecules communicate with the brain (Quan & Banks, 2007), where they induce additional neuromodulators of inflammation (Fernandez-Lizarbe, Pascual, & Guerri, 2009) and trigger the activation of microglia (i.e., the immune cells of the brain), resulting in neuroinflammation (Mayfield, Ferguson, & Harris, 2013). It seems that neuroinflammation can also occur directly, whereby internal and/or external triggers start a proinflammatory cascade in the brain.
The inflammatory response is adaptive and necessary under certain conditions (e.g., disease, infection, acute stressors), and immune signaling and immune-brain communication play an important role in healthy development and homeostatic processes (Merrill & Jonakait, 1995). Overactive and chronic inflammation, however, has been linked to various mental- and physical-health problems.
GI system
Studies on the relation between the GI system and well-being have largely focused on bacteria residing in the GI tract. Approximately 100 trillion bacteria inhabit the normal human GI tract, and these bacteria belong to more than 400 species that may produce effects that are adverse, benign, or beneficial to the host organism, sometimes by interacting with each other (Collins, Surette, & Bercik, 2012). The microbial species that inhabit the GI tract are collectively known as gut microbiota, whereas the genomes of the microbiota are known as the gut microbiome. A standard, measurable index of GI health is the diversity of microbiota in the gut, defined as the number, abundance, and distribution of organism types (Gevers et al., 2012). The abundance of certain microbial species is associated with health outcomes, and greater microbial diversity is thought to be a marker of gut health. Therefore, the microbiome can characterize the abundance and diversity of species of gut microbiota (Amato, 2017).
A variety of factors influence gut microbiota composition, including age, environment, and host genetics (Lozupone, Stombaugh, Gordon, Jansson, & Knight, 2012). Some evidence suggests that each microbiota environment is fairly stable and distinct over time (Caporaso et al., 2011; Turnbaugh et al., 2009), but findings also suggest that human gut microbiota can change rapidly in response to environmental and life-style factors, including diet, pregnancy, and antibiotics (David et al., 2014). Exposure to acute stress, for example, is associated with gut microbiota composition, such that greater stress is associated with reduced diversity (Bailey et al., 2011). Finally, the degree to which the host and microbes cooperate when extracting energy from resources can also contribute to the host’s micro-biome environment. That is, host-microbiome competition may lead to an inflamed and irritated environment, whereas host-microbiome cooperation could encourage host health (Wasielewski, Alcock, & Aktipis, 2016). Thus, GI health, as measured by gut microbiota composition, varies among individuals, is susceptible to change, and may have important effects on well-being.
Consummatory behaviors (e.g., diet, alcohol use) seem to affect the composition of gut microbiota. Consummatory behaviors also seem to affect intestinal permeability, which permits some contents of the gut to translocate into the bloodstream. Once in the blood-stream, bacteria can be the source of additional health problems (Cani et al., 2009). It is noteworthy that the adverse effects of intestinal permeability are exacerbated by a greater abundance of proinflammatory bacteria (Mutlu et al., 2009).
The microbiome may influence physical health, mental health, and related constructs via multiple pathways. In some cases, the microbiome may directly influence host health (e.g., projections from smooth muscle in the intestinal wall to the brain). In other cases, the microbiome may influence health via pathways that involve signaling between the immune system and GI system (see below).
Interactions between the immune and GI systems
The mechanisms and processes within the immune and GI systems discussed above are likely to play a role in physical- and mental-health dysfunction. In addition, signaling between the immune and GI systems also likely plays a role in the manifestation of physical- and mental-health phenotypes. Recent research has emphasized the effects of communication between the immune and GI systems on health and behavior. For example, the gut-brain axis comprises bidirectional influences between the GI tract and the brain while also involving hormonal, immunological, and neural communication by way of the endocrine, immune, and autonomic nervous systems (Grenham, Clarke, Cryan, & Dinan, 2011). Signaling among and between these systems, including the GI and immune systems, has downstream implications for neural and peripheral functioning as well as behavior. Thus, signaling between the immune and GI systems could underlie manifestations of physical- and mental-health dysfunction.
Immune and GI systems: mental and physical health
Immune and GI system functioning, including biological processes within the immune and GI systems, are associated with physical- and mental-health outcomes. For exemplary purposes, we discuss the associations between immune and GI system functioning and depression and IBS.
Immune system and depression.
In this section, for illustrative purposes, we discuss documented associations between depression as an exemplar mental-health problem and processes within the immune system. A growing body of literature suggests that the activation of innate immune genes and inflammatory mediators might facilitate or exacerbate the expression of depressive symptoms (e.g., negative affect) via reduced neurogenesis and neurodegeneration. For example, innate immune gene induction (including TLRs) in the brain has been linked to negative mood and depressive behavior (Kelley & Dantzer, 2011; Raison, Capuron, & Miller, 2006). Further, inflammatory processes have been associated with stress-induced inhibition of neurogenesis (i.e., the production of new cells in the brain) and depressive-behaviors via extracellular glutamate and excitotoxicity (i.e., cell death in the brain; Zou & Crews, 2006). These processes may lead to the inactivation of the frontal lobes, which may underlie some symptoms associated with depression (e.g., inattentiveness, reduced cognitive flexibility, difficulty concentrating; Crews et al., 2006).
Environmental factors, such as chronic psychological stress, could trigger or perpetuate depression and a pro(neuro)inflammatory state. Laboratory studies using repeated stress exposure suggest that innate immune-inducing molecules (e.g., tumor necrosis factor a, a TLR4 agonist; lipopolysaccharides) act as a substitute for an episode of stress (Breese et al., 2008) and can increase depressed mood (Eisenberger et al., 2010). Note that lipopolysaccharides are also found on the surface of pathogenic bacteria, providing an important link between microbes and the immune response (Lebeer, Vanderleyden, & Keersmaecker, 2010). A growing body of literature supports the role of elevated stress hormones (e.g., corticosterone) in priming microglia, thereby sensitizing microglial responses to a secondary immune challenge (Frank, Watkins, & Maier, 2013; Weber, Frank, Sobesky, Watkins, & Maier, 2013). Increased stress hormones are notably associated with depression-like behaviors and reduced neurogenesis in rats (Johnson, Fournier, & Kalynchuk, 2006).
Immunological processes are also associated with cognitive dysfunction, which characterizes the symptomatology of depression in many cases; thus, immune-mediated cognitive dysfunction could partly account for the role of immunological functioning in depression. For example, in a study of cancer patients, plasma interleukin 6 (a proinflammatory cytokine) was negatively associated with measures of executive functioning (Meyers, Albitar, & Estey, 2005). Studies also suggest a link between proinflammatory cytokines (e.g., interferon a and interleukin 2) and cognitive dysfunction following immunotherapies (Capuron et al., 2002). These results suggest a connection between treatments that cause inflammation and measures of psychological and cognitive sequelae, including complaints of moderate-to-severe cognitive symptoms, including loss of concentration (30% of patients), psychomotor retardation (40% of patients), word-finding deficits (15% of patients), and memory impairment (15% of patients).
GI system and depression.
GI-system characteristics and processes, including the composition of gut microbiota and intestinal permeability, have also been implicated in depression and related cognitive constructs (Mayer, Tillisch, & Gupta, 2015). Preclinical animal studies and some human studies have implicated perturbations to gut microbiota in acute stress and depression (Bailey et al., 2011; Collins & Bercik, 2009; Rogers et al., 2016). The diversity of gut microbiota species is associated with attentional control and mood (Foster & McVey Neufeld, 2013; Leclercq et al., 2012, 2014), and intestinal permeability has been linked to changes in attentional control and depression during inpatient treatment for alcohol-use disorder (Leclercq et al., 2014). Furthermore, animal and human studies find that the administration of probiotics, which are known to adaptively modulate the gut environment, reduces anxiety and depression-like behavior and symptoms (Benton, Williams, & Brown, 2007; Messaoudi et al., 2011).
The mechanisms by which gut-related processes might contribute to depression are not entirely clear. Some evidence suggests that there are direct pathways between the gut microbiome and psychological and behavioral outcomes related to mental health and cognitive functioning. For example, administering a probiotic formulation (live lactobacilli and bifidobacteria mixture) to rats has been associated with cognitive improvements (long- and short-term memory). Consumption of the probiotic formulation was associated with changes in brain metabolites, suggesting that live microorganisms can alter neural signaling (O’Hagan et al., 2017). Other research suggests that administering a probiotic formulation to rodents may mitigate adverse effects of a Western/cafeteria diet, including reduced microbial diversity and impaired memory (Beilharz, Kaakoush, Maniam, & Morris, 2018). This study also found that probiotic administration was associated with changes in gene expression in the brain, suggesting another mechanism by which the gut could affect the brain. In addition to the potential direct pathways between the gut and behavioral/cognitive phenotypes, some research suggests that signaling between the immune and GI systems contribute to the gut-depression link (see below).
Immune- and GI-system signaling and depression.
The evidence discussed above suggests that processes within the immune and GI systems are associated with depression and related constructs. Although the pathophysiological mechanisms underlying the manifestation of depression are not entirely clear, some evidence suggests that signaling between the immune and GI systems contributes to depression and other mental-health conditions. For example, animal models of increased inflammation in the GI tract and periphery show increased anxiety- and depression-like behavior, and probiotics reverse or moderate inflammation-related increases in these behaviors (Bercik et al., 2010, 2011), suggesting a possible immune-mediated gut effect. However, the immune-mediated relationship between the GI system and behavior could be due to immune pathways that both involve and bypass a peripheral immune response. For example, rodents injected with a common gram-negative pathogen (Campylobacter jejuni) display increased anxiety- and depression-like behavior in the absence of an overt peripheral immune response (i.e., proinflammatory circulating cytokines and blood leukocyte populations; Lyte, Varcoe, & Bailey, 1998). These results are consistent with findings that immunological triggers within the GI system (i.e., infection) are associated with depression-like behavior in the absence of systemic inflammation, suggesting that some immune insults might affect depression without evoking a peripheral immune response (Lyte, Li, Opitz, Gaykema, & Goehler, 2006).
Immune system and IBS.
Aberrant functioning within the immune system is thought to influence the development and progression of health problems related to GI health, including IBS, Crohn’s disease, and diabetes (Duboc et al., 2012; Sato et al., 2014). IBS is the most common GI disorder. It accounts for more than 50% of visits to primary-care doctors for GI complaints (Wilson et al., 2004) and is discussed here as an example of a physical-health problem related to processes within the immune system.
Several lines of research suggest a link between IBS and immune-system functioning, including research linking inflammation and infection to the onset and progression of IBS. For example, chronic inflammation is associated with IBS symptoms (Collins, 2014). Along these lines, it has been suggested that a proinflammatory microenvironment in the gut may also promote an aberrant inflammatory response and exacerbate IBS symptoms (Jeffery, Quigley, Öhman, Simrén, & O’Toole, 2012). Moreover, proinflammatory cytokines are elevated in patients with IBS, and IBS symptoms are associated with increased inflammatory cytokine levels (Dinan et al., 2006). Finally, intestinal permeability could be both a cause and a consequence of aberrant immune functioning, as evidence suggests an association between immune-system activation, intestinal permeability, and IBS (Matricon et al., 2012).
Moreover, intestinal infections, and the resulting immune response, have been shown to precede the onset of some cases of IBS (Spiller & Lam, 2012). In fact, postinfectious IBS has been coined as a subset of IBS that is characterized by the onset of IBS after an infection. Estimates suggest that 6% to 17% of patients with IBS report that the onset of their IBS symptoms followed a digestive infection (Matricon et al., 2012). The high incidence of infection preceding IBS has caused renewed interest in whether inflammation plays a role in the development of IBS. Thus, a growing body of evidence suggests that processes within the immune system are associated with the pathophysiology of IBS.
GI system and IBS.
The pathophysiological mechanisms responsible for the etiology and progression of IBS are not yet entirely clear. However, GI-system processes and characteristics, such as the composition of gut microbiota (Ohman & Simrén, 2013; Rajilić-Stojanović et al., 2015) and intestinal permeability, have been proposed as potentially important factors (Camilleri & Gorman, 2007).
Gut microbiota-composition profiles, derived from fecal samples, can differentiate healthy control subjects from patients with IBS. Specifically, one study found that the composition of gut microbiota differed between patients with IBS and those without, and this difference remained present across several microbiota-composition quantitative analytic techniques (Kassinen et al., 2007). Specific bacterial species are also linked to IBS, including probiotic bacteria (bifidobacteria), which have approximately half the relative abundance in IBS cases compared with control subjects (Kerckhoffs et al., 2009). Patients with IBS also have more variable gut microbiota composition over time (Mättö et al., 2005) as well as less overall diversity (Noor et al., 2010). To counter this imbalance, there is some support for prescribed probiotics to reduce IBS symptoms (Clarke, Cryan, Dinan, & Quigley, 2012). Microbiota dysregulation, marked by intestinal infections, may also precede the onset of some cases of IBS, suggesting a complex bidirectional relationship (Spiller & Lam, 2012).
In addition to gut microbiota composition, intestinal permeability is also associated with IBS. Intestinal permeability is the degree to which the intestinal membrane enables the passage of a solute by unmediated diffusion (Bjarnason, Macpherson, & Hollander, 1995). Although the pathobiological role of intestinal permeability in IBS etiology and progression is not comprehensively understood, research suggests that the degree of intestinal permeability is associated with a diagnosis of IBS (Camilleri & Gorman, 2007). Furthermore, intestinal permeability is associated with specific symptoms and IBS subtypes. For example, IBS patients characterized by diarrhea-predominant IBS demonstrate increased intestinal permeability (Dunlop et al., 2006; Spiller et al., 2000; Zhou, Zhang, & Nicholas Verne, 2009).
Immune- and GI-system signaling and IBS.
As discussed above, processes within the immune and GI systems are associated with IBS. In addition, signaling between the immune and GI systems might underlie the manifestation of IBS. The current evidence base suggests at least two ways in which the immune and GI system interact to potentially underlie IBS: translocation of pro-inflammatory, pathogenic bacteria due to intestinal permeability and greater abundance of proinflammatory species of microbiota. For example, intestinal permeability allows some contents of the gut, including pathogenic bacteria, to translocate into the bloodstream. Once in the bloodstream, bacteria can be the source of additional health problems (Cani et al., 2009). It is noteworthy that the adverse effects of intestinal permeability are exacerbated by a greater abundance of proinflammatory bacteria (Mutlu et al., 2009). Finally, the degree to which the host and microbes cooperate when extracting energy from resources can also contribute to the host’s microbiome environment. That is, host-microbiome competition may lead to an inflamed and irritated environment, whereas host-microbiome cooperation could encourage host health (Wasielewski et al., 2016).
There is also some indirect evidence that immune- and GI-system signaling contributes to the manifestation of IBS. For example, patients with IBS who were randomly assigned to consume a probiotic (Bifidobacterium infantis 35624) realized fewer IBS symptoms and lower levels of proinflammatory-circulating cytokines compared with patients who consumed a placebo (O’Mahony et al., 2005). This suggests a relationship between gut microbiota composition and inflammation in the context of IBS.
Immune and GI systems: co-occurring physical- and mental-health dysfunction
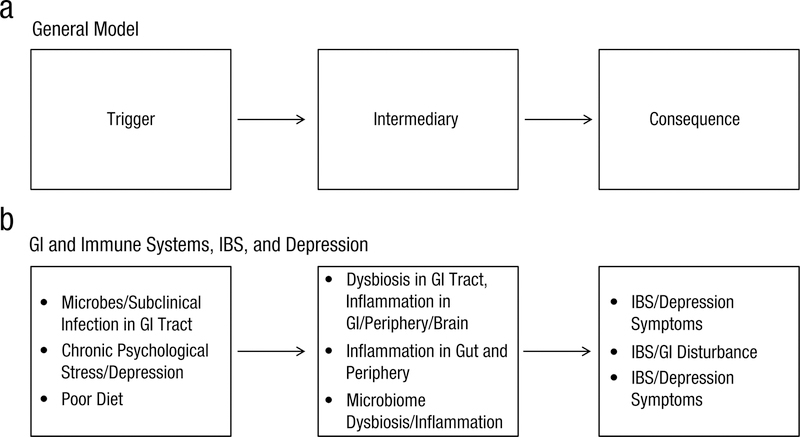
The evidence discussed above suggests that processes within the immune and GI systems and signaling between the two systems might contribute to manifestations of depression and IBS. Epidemiological data suggest that depression and IBS co-occur at a rate much higher than chance (Fond et al., 2014). Therefore, it seems logically and theoretically plausible that processes/functioning within either the immune and/or GI systems are associated with—or even play a causal role in—the co-occurrence of depression and IBS. With this reasoning and the empirical base overviewed above, consider depression and IBS in the three plausible scenarios (Scenarios 1–3) outlined in Figure 1b. In Scenario 1, the presence of certain microbes could cause a sub-clinical infection in the GI tract (trigger) and, in turn, inflammation and dysbiosis within the GI tract, periphery, and brain (intermediary), resulting in both IBS symptoms and depression symptoms (consequence). Alternatively, as shown in Scenario 2, chronic psychological stress and depression (trigger) could cause a stress-induced proinflammatory environment in the periphery and gut (intermediary), resulting in IBS and other GI disturbance (consequence). Finally, in Scenario 3, poor diet (trigger) could lead to gut microbiome dysbiosis and inflammation in the periphery and brain (intermediary), resulting in GI disturbance (e.g., IBS) and disrupted cognitive and affective processes (consequence; e.g., depression, anxiety, cognitive disturbance).
Fig. 1.
Plausible relationships between physical-health conditions, mental-health conditions, and biological processes within the gastrointestinal and immune systems. GI = gastrointestinal; IBS = irritable bowel syndrome.
These scenarios represent important, testable hypotheses largely based on piecing together evidence from the animal, preclinical, and clinical studies discussed in the previous sections. Some of the available research supports the plausibility of certain links in the proposed pathways, but the proposed scenarios themselves are based on theoretical interpretations of the available literature rather than empirical data. Empirical studies that are specifically designed to test mechanistic pathways are needed to test the proposed relationships between biological processes within the immune and GI systems (e.g., microbiota dysbiosis, inflammation, intestinal permeability), physical-health conditions (e.g., IBS), and mental-health conditions (e.g., depression, cognitive dysfunction). Therefore, in the next section, we advance a research framework that provides guidance on how to conduct the type of research that would address the questions about causal scenarios and mechanisms. Although we chose to discuss depression and IBS as exemplar mental- and physical-health conditions for the sake of brevity, the immune, GI, and other biological systems are implicated in a host of other mental-health (e.g., anxiety, substance-use disorders) and physical-health (e.g., cancer, dementia, diabetes) conditions. Thus, this research framework was developed to be applied broadly to studying cases of co-occurring mental- and physical-health dysfunction.
Research Framework
As discussed previously, comorbidity is common and problematic, and it has primarily been studied within the domains of mental- and physical-health conditions separately. Much less work has examined the confluence of physical- and mental-health conditions, particularly from a biological-systems perspective. Research initiatives such as the Research Domain Criteria project have laid the foundation for pushing the field of psychology toward a transdiagnostic model, specifically by understanding the role of biological mechanisms and correlates that cut across conditions and broadly underlie mental-health functioning. Here, we discuss evidence that supports the adoption of a broader research framework that seeks to understand the relationships between processes within biological systems, physical conditions, and mental-health disorders. We argue that understanding these relationships is key from a basic science perspective (i.e., clarifying theoretical models of comorbidities) and from a clinical perspective (i.e., determining empirically driven treatment plans on the basis of the circumstances of each case in question). Below we present a proposed research framework and discuss the research methods that one could use to investigate comorbidities depending on the specific research question(s) of interest.
Main components
The research framework presented below addresses how to conduct comorbidity research with human participants exclusively, although it should be noted that animal studies would complement this work. We have also focused in this article on the immune and GI systems, although this framework could be applied to studying biological processes relevant to mental and physical health across other biological systems, including the cardiovascular system, nervous system, and endocrine system.
Once comorbid disorders of interest have been established and relevant biological processes are identified, longitudinal studies can help to inform the relationship between the biological processes and emergence of the comorbid conditions. The utility of this framework is thus derived not only from identifying relevant biological processes and correlates but also from characterizing their relationships with comorbid conditions (i.e., temporal, etiological, causal). This second step of clarifying the temporal relationships between conditions and biological processes/correlates could prove to be critical with regard to intervention. Understanding these relationships will help to determine which biological processes serve as effective treatment targets given the causal relationships at play. From a basic science perspective, understanding the causal relationships between these conditions and biological processes could aid in developing and refining a diagnostic system for mental-health disorders that is based on etiology rather than syndrome-like categories. Taken together, we propose the following main components of our suggested mental-and physical-health comorbidity research framework: (a) determining co-occurring physical- and mental-health conditions of interest; (b) identifying biological processes/correlates across broad biological systems that demonstrate relevance to the co-occurring physical- and mental-health dysfunction in question; and (c) determining the temporal relationships between the relevant biological processes/correlates, physical health, and mental health.
The proposed research framework seeks to address these overall objectives in five steps. Table 1 outlines the five-step approach and provides guidance on analytic approaches and suggested samples for each step.
Table 1.
Proposed Research Framework
| Framework component |
Framework objective | Ideal Sample(s) | Proposed analytic approaches |
|---|---|---|---|
| 1 | 1. Determine comorbid mental- and physical-health conditions/dysfunction of interest |
• Large, representative epidemiological samples | • Test concurrence of specific physical- and mental-health conditions (correlations) |
| 2 | 2. Determine biological correlates shared across comorbid conditions | • Large, representative samples with available biological samples (e.g., blood/fecal samples for immune/gut microbiome analysis) | • Examine correlations between biological processes and each of the comorbid conditions of interest |
| 3. Determine whether biological correlate(s) could plausibly mediate the concordance between the comorbibd disorders | • Large samples of clinical populations with available biological samples corresponding to biological processes of interest | • Test cross-sectional mediation models | |
| 3 | 4. Determining temporal relationships among and between biological correlate(s) and comorbid conditions | • Large longitudinal samples of clinical populations with relevant biological samples • Large familial samples with relevant biological samples |
• Autoregressive cross-lag mediation models (longitudinal samples) • Longitudinal latent profile analysis (longitudinal samples) |
| 5. Test proposed causal pathways to refine comorbidity models | Selective clinical samples meeting narrow inclusion and exclusion criteria | • Quasi-experimental and experimental investigations of hypothesized pathways |
Note. The proposed research framework includes three main components and five objectives. For each research objective, samples and analytic approaches are suggested as proposed methods. The suggested methods outlined in the research framework are proposed best practices when investigating cases of co-occurring physical health and mental health conditions.
A user’s guide
The research framework outlined in Table 1 addresses the key components of comorbidity research. According to the proposed framework, the first step is to determine comorbid conditions of interest by testing the concurrence rate of disorders across large, epidemiological data sets. This technique has been used previously to assess overall prevalence rates of comorbid psychiatric conditions using large representative samples (Kessler et al., 2005) and prevalence rates of comorbidity between specific disorders and disorder categories (Regier et al., 1990). Surprisingly few studies to date have examined the concordance rate between mental- and physical-health conditions in large, epidemiological samples (e.g., Rehm, Gmel, Sempos, & Trevisan, 2003), further prompting the need for this type of research.
Next, the proposed framework suggests identifying biological processes that demonstrate shared relevance to the comorbid conditions of interest. Toward this end, the framework suggests that researchers should procure large representative data sets complete with biological samples (e.g., blood samples, fecal samples). Given these data, researchers could then examine the association between the biological processes (including interactions between biological processes) and each of the comorbid disorders in question. In addition to identifying potential relevant biological processes shared between comorbid disorders by examining associations in separate studies, the proposed framework suggests testing cross-sectional mediation models to determine whether there is support (i.e., significant partial or full mediation) for a biological process accounting for the association between the medical and psychiatric conditions of interest (Baron & Kenny, 1986; Hayes, 2009).
The last component of the proposed framework is to determine the temporal/causal relationships between the comorbid conditions of interest and the shared biological processes. First, the proposed framework suggests leveraging large, longitudinal samples of clinical populations that include biological samples. Given these samples, researchers could test autoregressive cross-lag mediation models (Selig & Little, 2012) and longitudinal latent-profile analysis models (Muthén & Muthén, 2000). Applied to longitudinal data, these models could be used to characterize the heterogeneity of developmental trajectories and temporal relationships between the emergence of aberrant biological processes and the onset of comorbid conditions. Finally, once researchers have a preliminary understanding of the temporal relationships on the basis of the longitudinal designs, the proposed framework suggests that researchers consider testing specific pathways with quasi-experimental designs. For example, large longitudinal familial samples with relevant biological data allow a platform to test the causal and temporal relationships between biological processes and comorbid conditions, with rigorous methodologies that control for important confounding factors and shared biological and environmental influences.
Conducting research based on this approach requires collaboration across fields and disciplines, including neuroscience, clinical psychology, biology, epidemiology, integrated physiology, biostatistics, and others. For example, studies related to identifying biological processes/correlates across broad biological systems would require expertise in collecting and managing large human-sample databases, preparing and analyzing biological samples, familiarity with transforming biological data into usable formats, and statistical expertise. The breadth of expertise required to conduct research at this single stage of the proposed research framework exemplifies the heavy reliance of this research framework on interdisciplinary collaborations. Although research based on this proposed framework could advance clinical science, adopting these approaches will likely come with notable challenges and growing pains, including the challenges associated with collaboration (Golde & Gallagher, 1999; Suls & Rothman, 2004).
Treatment implications
Understanding the common mechanisms that underlie the co-occurrence between physical- and mental-health dysfunction could strengthen our theoretical models and inform optimally effective treatments. Thus, behavioral-health interventions may be part of an effective treatment plan when addressing co-occurring physical- and mental-health concerns, especially in the context of primary care. This is of timely interest (Silberner, 2017), especially given that the top five conditions driving overall health-care costs include mood disorders, diabetes, heart disease, asthma, and hypertension (Druss et al., 2001), all of which are treated in primary-care settings and could be targeted, in part, by psychological/behavioral-health interventions.
Some behavioral-health interventions may be effective because of their effects on immune mechanisms. For example, evidence suggests that treatments such as mindfulness-based stress reduction and cognitive behavioral therapy are effective at reducing psychological distress in cancer patients while also increasing the adaptive lymphocyte proliferative response and normalizing cortisol secretion (Antoni et al., 2006; Carlson, Speca, Patel, & Goodey, 2004; McGregor et al., 2004). These treatments might work to limit the impact of stress on the immune response, which could help ameliorate or slow down aberrant immune responses that underlie the development and progression of depression and cancer.
Some behavioral-health interventions may work to improve GI health and downstream mental and physical functioning. For example, behavioral therapies and cognitive training may mitigate the effects of psychological stressors on GI health by reducing physiological stress, thereby improving downstream mood disorders (e.g., mindfulness-based interventions; Zernicke et al., 2013). Further, motivational interviewing may help change behavior (e.g., reducing alcohol consumption; Vasilaki, Hosier, & Cox, 2006), thereby reducing psychiatric and GI symptoms indirectly via reduced alcohol consumption and improved gut health. Overall, mechanistic research could inform translational treatment targets for co-occurring physical- and mental-health concerns.
Concluding Remarks
First we reviewed evidence suggesting that the cooccurrence of mental- and physical-health conditions is a common phenomenon. In addition, using illustrative examples, we provided evidence that mechanisms within broad biological systems are shared among co-occurring mental- and physical-health conditions. We then outlined a research framework for identifying co-occurring mental-and physical-health conditions of interest and investigating shared biological mechanisms and the nature of their relationships (i.e., causal, correlational, bidirectional) to the comorbid conditions.
Developing a research framework for studying cases of co-occurring mental- and physical-health conditions is important from both basic science and clinical perspectives. Mental- and physical-health disorders co-occur at a rate much higher than chance. Results consistently suggest that nearly half of patients who suffer from a mental-health condition have a physical disease, and of these patients who have comorbid conditions across mental- and physical-health domains, roughly half suffer from an additional unrecognized/undiagnosed physical illness that caused or exacerbates their mental-health diagnosis (Lambert, Velakoulis, & Pantelis, 2003). Research that seeks to understand the etiology and optimal treatment of co-occurring physical- and mental-health conditions should be prioritized.
Acknowledgments
I thank Jarrod Ellingson for his contribution to the research framework.
Funding
This material is based on work supported by National Science Foundation Graduate Research Fellowship Program Grant DGE 1144083 (to S. L. Hagerty) and National Institute on Alcohol Abuse and Alcoholism Grant K23-AA026635 (to J. M. Ellingson).
Footnotes
Action Editor
Timothy McNamara served as action editor for this article.
Declaration of Conflicting Interests
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
References
- Amato KR (2017). An introduction to microbiome analysis for human biology applications. American Journal of Human Biology, 29(1), Article e22931. doi: 10.1002/ajhb.22931 [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, … Carver CS. (2006). How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology, 74, 1143–1152. doi: 10.1037/0022-006X.74.6.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, & Lyte M (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain, Behavior, and Immunity, 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Wu J, Damush TM, Sutherland JM, & Kroenke K (2008). Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosomatic Medicine, 70, 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Kaakoush NO, Maniam J, & Morris MJ (2018). Cafeteria diet and probiotic therapy: Cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Molecular Psychiatry, 23, 351–361. doi: 10.1038/mp.2017.38 [DOI] [PubMed] [Google Scholar]
- Benton D, Williams C, & Brown A (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition, 61, 355–361. doi: 10.1038/sj.ejcn.1602546 [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, … Verdu EF (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology & Motility, 23, 1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, … Collins SM (2010). Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology, 139, 2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063 [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Macpherson A, & Hollander D (1995). Intestinal permeability: An overview. Gastroenterology, 108, 1566–1581. doi: 10.1016/0016-5085(95)90708-4 [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, & Brewer RD (2011). Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine, 41, 516–524. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, & Angel RA (2008). Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology, 33, 867–876. doi: 10.1038/sj.npp.1301468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, & Gorman H (2007). Intestinal permeability and irritable bowel syndrome. Neurogastroenterology & Motility, 19, 545–552. doi: 10.1111/j.1365-2982.2007.00925.x [DOI] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, … Lambert DM (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut, 58, 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, … Knight R (2011). Moving pictures of the human microbiome. Genome Biology, 12(5), Article R50. doi: 10.1186/gb-2011-12-5-r50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, & Dantzer R (2002). Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular Psychiatry, 7, 468–473. doi: 10.1038/sj.mp.4000995 [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, & Goodey E (2004). Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology, 29, 448–474. doi: 10.1016/S0306-4530(03)00054-4 [DOI] [PubMed] [Google Scholar]
- Clarke G, Cryan JF, Dinan TG, & Quigley EM (2012). Review article: Probiotics for the treatment of irritable bowel syndrome—Focus on lactic acid bacteria. Alimentary Pharmacology & Therapeutics, 35, 403–413. doi: 10.1111/j.1365-2036.2011.04965.x [DOI] [PubMed] [Google Scholar]
- Collins SM (2014). A role for the gut microbiota in IBS. Nature Reviews Gastroenterology & Hepatology, 11, 497–505. doi: 10.1038/nrgastro.2014.40 [DOI] [PubMed] [Google Scholar]
- Collins SM, & Bercik P (2009). The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology, 136, 2003–2014. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, & Bercik P (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology, 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, … Zou J (2006). Cytokines and alcohol. Alcoholism: Clinical & Experimental Research, 30, 720–730. doi: 10.1111/j.1530-0277.2006.00084.x [DOI] [PubMed] [Google Scholar]
- Dantzer R (2012). Depression and inflammation: An intricate relationship. Biological Psychiatry, 71, 4–5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, … Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut micro-biome. Nature, 505, 559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B, Normand S-LT, Weiss RD, Drake RE, & Azeni H (2002). Medical morbidity, mental illness, and substance use disorders. Psychiatric Services, 53, 861–867. doi: 10.1176/appi.ps.53.7.861 [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EMM, Ahmed SMM, Scully P, O’Brien S, O’Mahony L, … Keeling PWN (2006). Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology, 130, 304–311. doi: 10.1053/j.gastro.2005.11.033 [DOI] [PubMed] [Google Scholar]
- Druss BG, Marcus SC, Olfson M, Tanielian T, Elinson L, & Pincus HA (2001). Comparing the national economic burden of five chronic conditions. Health Affairs, 20, 233–241. doi: 10.1377/hlthaff.20.6.233 [DOI] [PubMed] [Google Scholar]
- Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, … Seksik P (2012). Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology & Motility, 24, 513–520. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, & Spiller RC (2006). Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. The American Journal of Gastroenterology, 101, 1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation- induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 68, 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JM, Siddique MI, Morales E, Kaminski B, Lu S-E, & Lehrer PM (2005). Psychiatric disorders and asthma outcomes among high-risk inner-city patients. Psychosomatic Medicine, 67, 989–996. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, & Guerri C (2009). Critical role of TLR4 response in the activation of microglia induced by ethanol. Journal of Immunology, 183, 4733–4744. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, … Boyer L (2014). Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. European Archives of Psychiatry & Clinical Neuroscience, 264, 651–660. [DOI] [PubMed] [Google Scholar]
- Foster JA, & McVey Neufeld K-A (2013). Gut-brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences, 36, 305–312. doi: 10.1016/j.tins.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, & Maier SF (2013). Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain, Behavior, and Immunity, 33(Suppl. C), 1–6. doi: 10.1016/j.bbi.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop G, Strain JJ, Vita J, Lyons JS, & Hammer JS (1987). Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: A preliminary report. American Journal of Psychiatry, 144, 878–882. [DOI] [PubMed] [Google Scholar]
- Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, … Huttenhower C (2012). The human microbiome project: A community resource for the healthy human microbiome. PLOS Biology, 10(8), Article e1001377. doi: 10.1371/journal.pbio.1001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde CM, & Gallagher HA (1999). The challenges of conducting interdisciplinary research in traditional doctoral programs. Ecosystems, 2, 281–285. [Google Scholar]
- Goodell S, Druss BG, Walker ER, & MAT M (2011). Mental disorders and medical comorbidity (Research Synthesis Report No. 21). Retrieved from the Robert Wood Johnson Foundation website: https://www.rwjf.org/en/library/research/2011/02/mental-disorders-and-medical-comorbidity.html [PubMed]
- Grenham S, Clarke G, Cryan JF, & Dinan TG (2011). Brain-gut-microbe communication in health and disease. Frontiers in Physiology, 2, Article 94. doi: 10.3389/fphys.2011.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. doi: 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hoebe K, Janssen E, & Beutler B (2004). The interface between innate and adaptive immunity. Nature Immunology, 5, 971–974. doi: 10.1038/ni1004-971 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Jeffery IB, Quigley EMM, Öhman L, Simrén M, & O’Toole PW (2012). The microbiota link to irritable bowel syndrome: An emerging story. Gut Microbes, 3, 572–576. doi: 10.4l6l/gmic.21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, & Kalynchuk LE (2006). Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behavioural Brain Research, 168, 280–288. doi: 10.1016/j.bbr.2005.11.019 [DOI] [PubMed] [Google Scholar]
- Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, … Palva A (2007). The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology, 133, 24–33. doi: 10.1053/j.gastro.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Kelley KW, & Dantzer R (2011). Alcoholism and inflammation: Neuroimmunology of behavioral and mood disorders. Brain, Behavior, and Immunity, 25(Suppl. 1), S13–S20. doi: 10.1016/j.bbi.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs APM, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, & Akkermans LMA (2009). Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World Journal of Gastroenterology, 15, 2887–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, & Riso LP (1993). Psychiatric disorders: Problems of boundaries and comorbidity In Costello CG (Ed.), Basic issues in psychopathology (pp. 19–66). New York, NY: Guilford Press. [Google Scholar]
- Kroenke K, Theobald D, Wu J, Loza JK, Carpenter JS, & Tu W (2010). The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. Journal of Pain and Symptom Management, 40, 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert TJR, Velakoulis D, & Pantelis C (2003). Medical comorbidity in schizophrenia. Medical Journal of Australia, 178(Suppl.), S67–S70. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, & Keersmaecker SCJD (2010). Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nature Reviews Microbiology, 8, 171–184. doi: 10.1038/nrmicro2297 [DOI] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, … de Timary P (2012). Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain, Behavior, and Immunity, 26, 911–918. doi: 10.1016/j.bbi.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, … Delzenne NM (2014). Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proceedings of the National Academy of Sciences, USA, 111, E4485–E4493. doi: 10.1073/pnas.1415174111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, & Knight R (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Li W, Opitz N, Gaykema RP, & Goehler LE (2006). Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiology & Behavior, 89, 350–357. [DOI] [PubMed] [Google Scholar]
- Lyte M, Varcoe JJ, & Bailey MT (1998). Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiology & Behavior, 65, 63–68. doi: 10.1016/S0031-9384(98)00145-0 [DOI] [PubMed] [Google Scholar]
- Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, & Ardid D (2012). Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 36, 1009–1031. doi: 10.1111/apt.12080 [DOI] [PubMed] [Google Scholar]
- Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, & Saarela M (2005). Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome—A longitudinal study in IBS and control subjects. FEMS Immunology & Medical Microbiology, 43, 213–222. doi: 10.1016/j.femsim.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, & Gupta A (2015). Gut/brain axis and the microbiota. Journal of Clinical Investigation, 125, 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, & Harris RA (2013). Neuroimmune signaling: A key component of alcohol abuse. Current Opinion in Neurobiology, 23, 513–520. doi: 10.1016/j.conb.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, & Carver CS (2004). Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. Journal of Psychosomatic Research, 56, 1–8. doi: 10.1016/S0022-3999(03)00036-9 [DOI] [PubMed] [Google Scholar]
- Medzhitov R (2001). Toll-like receptors and innate immunity. Nature Reviews Immunology, 1, 135–145. doi: 10.1038/35100529 [DOI] [PubMed] [Google Scholar]
- Merrill JE, & Jonakait GM (1995). Interactions of the nervous and immune systems in development, normal brain homeostasis, and disease. TheFASEBJournal, 9, 611–618. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, … Cazaubiel J-M (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition, 105, 755–764. doi: 10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, & Estey E (2005). Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer, 104, 788–793. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. Journal of the American Medical Association, 291, 1238–1245. [DOI] [PubMed] [Google Scholar]
- Muthén B, & Muthen LK (2000). Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical & Experimental Research, 24, 882–891. doi: 10.1111/j.1530-0277.2000.tb02070.x [DOI] [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, & Gillevet P (2009). Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcoholism: Clinical & Experimental Research, 33, 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, & Kendler KS (1995). Models of comorbidity for multifactorial disorders. American Journal of Human Genetics, 57, 935–953. [PMC free article] [PubMed] [Google Scholar]
- Noor SO, Ridgway K, Scovell L, Kemsley EK, Lund EK, Jamieson C, … Narbad A (2010). Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterology, 10, Article 134. doi: 10.1186/1471-230X-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan C, Li JV, Marchesi JR, Plummer S, Garaiova I, & Good MA (2017). Long-term multi-species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiology of Learning and Memory, 144, 36–47. doi: 10.1016/j.nlm.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Öhman L, & Simrén M (2010). Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nature Reviews Gastroenterology & Hepatology, 7, 163–173. [DOI] [PubMed] [Google Scholar]
- Ohman L, & Simrén M (2013). Intestinal microbiota and its role in irritable bowel syndrome (IBS). Current Gastroenterology Reports, 15(5), Article 323. doi: 10.1007/s11894-013-0323-7 [DOI] [PubMed] [Google Scholar]
- O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, … Quigley EMM (2005). Lactobacillus and bifido-bacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology, 128, 541–551. doi: 10.1053/j.gastro.2004.11.050 [DOI] [PubMed] [Google Scholar]
- Quan N, & Banks WA (2007). Brain-immune communication pathways. Brain, Behavior, and Immunity, 21, 727–735. doi: 10.1016/j.bbi.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, & Miller AH (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. doi: 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilić-Stojanovic M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, … Penders J (2015). Intestinal microbiota and diet in IBS: Causes, consequences, or epiphenomena? The American Journal of Gastroenterology, 110, 278–287. doi: 10.1038/ajg.2014.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, & Goodwin FK (1990). Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) Study. Journal of the American Medical Association, 264, 2511–2518. doi: 10.1001/jama.1990.03450190043026 [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel G, Sempos CT, & Trevisan M (2003). Alcohol-related morbidity and mortality. Alcohol Research & Health, 27, 39–51. [PMC free article] [PubMed] [Google Scholar]
- Richardson LP, Lozano P, Russo J, McCauley E, Bush T, & Katon W (2006). Asthma symptom burden: Relationship to asthma severity and anxiety and depression symptoms. Pediatrics, 118, 1042–1051. [DOI] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, & Wesselingh S (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J, Jacobi F, Cox BJ, Belik S-L, Clara I, & Stein MB (2006). Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Archives of Internal Medicine, 166, 2109–2116. [DOI] [PubMed] [Google Scholar]
- Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, … Ogihara T (2014). Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care, 37, 2343–2350. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Alonso J, Angermeyer MC, Bromet E, Fayyad J, … Gureje O (2009). Mental-physical co-morbidity and its relationship with disability: Results from the World Mental Health Surveys. Psychological Medicine, 39, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP, & Little TD (2012). Autoregressive and cross-lagged panel analysis for longitudinal data In Laursen B, Little TD, & Card NA (Eds.), Handbook of developmental research methods (pp. 265–278). New York, NY: Guilford Press. [Google Scholar]
- Silberner J (2017, August 9). The doctor will analyze you now. Politico. Retrieved from https://www.politico.com/agenda/story/2017/08/09/mental-illness-primary-care-000486 [Google Scholar]
- Smith RS (1991). The macrophage theory of depression. Medical Hypotheses, 35, 298–306. doi: 10.1016/0306-9877(91)90272-Z [DOI] [PubMed] [Google Scholar]
- Spiller R, & Lam C (2012). An update on post-infectious irritable bowel syndrome: Role of genetics, immune activation, serotonin and altered microbiome. Journal of Neurogastroenterology and Motility, 18, 258–268. doi: 10.5056/jnm.2012.18.3.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, & Neal KR (2000). Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut, 47, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, & Rothman A (2004). Evolution of the biopsychosocial model: Prospects and challenges for health psychology. Health Psychology, 23, 119–125. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, … Gordon JI. (2009). A core gut microbiome in obese and lean twins. Nature, 457, 480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki EI, Hosier SG, & Cox WM (2006). The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol and Alcoholism, 41, 328–335. doi: 10.1093/alcalc/agl016 [DOI] [PubMed] [Google Scholar]
- Wasielewski H, Alcock J, & Aktipis A (2016). Resource conflict and cooperation between human host and gut microbiota: Implications for nutrition and health. Annals of the New York Academy of Sciences, 1372(1), 20–28. doi: 10.1111/nyas.13118 [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, Watkins LR, & Maier SF (2013). Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain, Behavior, and Immunity, 32(Suppl. C), 112–121. doi: 10.1016/j.bbi.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Longstreth GF, Knight K, Wong J, Wade S, Chiou CF, … Ofman JJ (2004). Quality of life in managed care patients with irritable bowel syndrome. Managed Care Interface, 17, 24–28, 34. [PubMed] [Google Scholar]
- Zernicke KA, Campbell TS, Blustein PK, Fung TS, Johnson JA, Bacon SL, & Carlson LE (2013). Mindfulness-based stress reduction for the treatment of irritable bowel syndrome symptoms: A randomized wait-list controlled trial. International Journal of Behavioral Medicine, 20, 385–396. doi: 10.1007/s12529-012-9241-6 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhang B, & Nicholas Verne G (2009). Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain, 146, 41–46. doi: 10.1016/j.pain.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, & Crews F (2006). CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and Molecular Neurobiology, 26, 385–405. doi: 10.1007/s10571-006-9045-9 [DOI] [PubMed] [Google Scholar]