Mounting evidence shows that several classes of bioactive and emerging environmental contaminants, including endocrine-active compounds, metals, and nanomaterials, adversely affect fetal development and birth outcomes1,2. Because the prenatal period is believed to be one of the most vulnerable periods of human development, understanding the link between maternal and fetal exposure to environmental contaminants is critical to anticipating factors that may predispose a woman to adverse birth outcomes3. For example, inorganic arsenic, which is ubiquitous in the earth’s crust and rock and found in water, has been associated with spontaneous abortion3, stillbirth3, preterm birth3 (less that 37 weeks gestational age), and hypertension4. Our group previously showed that in utero arsenic exposure increased fetal gene expression in genes that play roles in birth-related and reproductive effects3,5,6. However, there is a gap in our knowledge regarding perinatal outcomes of chronic arsenic exposure in pregnancy.
Prior studies of amniotic fluid to identify indicators of adverse fetal and neonatal outcomes such as altered fetal gene expression7 and cytokines8 have been reported. Amniotic fluid can also be used to determine fetal exposure to environmental contaminants9. Using previously collected amniotic fluid supernatant samples, our objective was to measure second trimester arsenic levels using state of the art inductively coupled plasma mass spectrometry (ICP-MS). Essential metals (selenium, zinc, manganese) and other toxic metals (mercury, lead) were also measured in second trimester group and previously reported by our group3 in the context of fetal gene expression alterations related to pathways specific to preterm birth. In this study, we hypothesized that second trimester amniotic fluid inorganic arsenic levels would be higher in pregnancies resulting in preterm birth (<37 weeks) compared to those resulting in term birth.
This was a retrospective case-control study utilizing a pre-existing database of previously collected amniotic fluid samples from women who underwent clinically indicated second trimester (14–24 weeks) amniocentesis for diagnostic genetic testing at the University of North Carolina – Chapel Hill. Since 2003, amniotic fluid supernatant has been banked for research purposes and stored at −80°C for research purposes. We queried our database from 2003–2015 to identify the 42 samples in this study. The UNC School of Medicine Institutional Review Board approved this study. Women with known fetal aneuploidy, structural abnormalities, or cervical shortening (≤20 mm) at the time of genetic amniocentesis were excluded. A total of 42 subjects were identified in the cohort.
Cases were defined as women who had spontaneous preterm birth, defined as labor without a precipitating factor (i.e. women with preterm premature rupture of membranes or abruption were excluded) at <37 weeks); n=21] and controls were women who had a term birth [(>37 weeks); n=21], matched by fetal sex, maternal age (within 2 years), gestational age at amniocentesis (within 1 week), maternal race, year of amniocentesis (within one year), and psychoactive medication exposure (Table 1). This fluid was obtained as part of a parent study to evaluate fetal gene expression in sPTB and TB6. Because several factors could affect fetal gene expression such as fetal sex and maternal medication exposure, we matched base on these factors.
Table 1.
Demographic characteristics
| Spontaneous Preterm Birth (<37 weeks) |
Term Birth (≥37 weeks) |
|
|---|---|---|
|
Total Number of Women |
21 | 21 |
|
Maternal Age (years), mean (SD)[range] |
34.6 (5.04) [22–40] |
35.8 (6.98) [21–44] |
| Ethnicity n(%) | ||
| Caucasian | 12 | 12 |
| African American | 5 | 5 |
| Hispanic | 1 | 1 |
| Asian | 2 | 2 |
| Unknown | 1 | 1 |
|
Fetal Sex (number of males/number of females) |
14/7 | 14/7 |
|
Gestational age at time of amniocentesis (weeks), mean (SD)frangel |
17.6 (15.2) [16.0–23.3] |
17.5 (14.3) [15.1–20.1] |
| Psychiatry Medications | 6 | 6 |
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) quantified amniotic fluid inorganic arsenic levels. The measurement of total inorganic arsenic content was performed using the Agilent Technologies 7500cx inductively coupled plasma mass spectrometer (Santa Clara, CA. USA) [22]. External calibration and quality control standards were prepared from NIST (National Institutes of Standards and Technology) traceable solutions (High Purity Standards, Charleston, SC. USA). There were measurable amounts of inorganic arsenic levels in all specimens. Mean, median, and range of inorganic arsenic levels were calculated. Wilcoxon rank sum tests compared levels between cases and controls; Spearman correlation coefficients were used in the analysis of arsenic levels and gestational age at delivery. Statistical significance was defined at level P<0.05. We conducted all analyses using STATA 14.1 (StataCorp, Inc., College Station, TX).
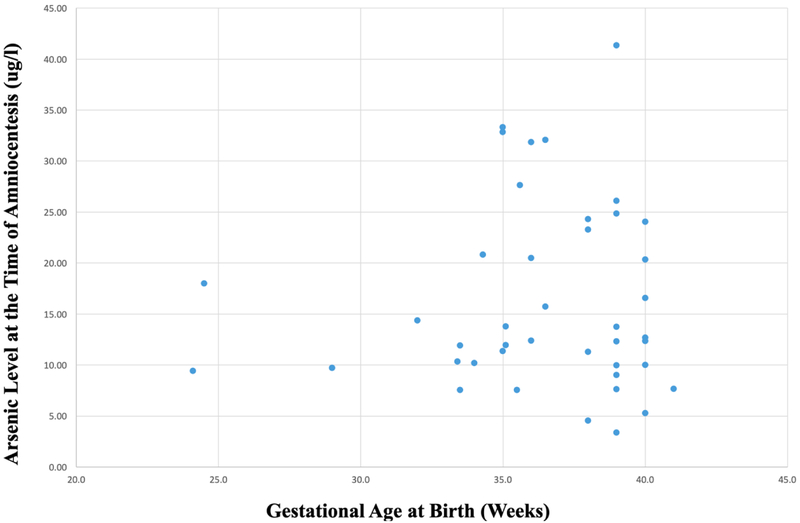
The term birth and spontaneous preterm birth groups did not differ significantly in maternal age or gestational age at the time of amniocentesis and cases and controls were matched as discussed previously. The mean gestational age at birth within the spontaneous preterm birth cases was 33.6 weeks and in the term controls was 39.2 weeks. The mean arsenic level was 16.3 ug/L, median was 12.5 ug/L, and range 3.4–41.3 ug/L. Arsenic was detected in all amniotic fluid samples at the time of second trimester amniocentesis (Table 2), there was no statistical difference in second trimester arsenic levels (17.29 ug/l (8.95) vs. 15.26 ug/l (9.35), p=0.24) between cases and controls respectively. There was also no correlation between second trimester amniotic fluid arsenic level and gestational age at delivery (p=0.79; R=0.042). There was also no significant difference in amniotic fluid arsenic levels between male and female fetuses in this cohort.
Although inorganic arsenic exposure is ubiquitous and certain areas in the United States have higher exposures through drinking water, it was unexpected to identify measurable levels of arsenic in all the amniotic fluid in this study. Little is known about the reproductive implications of arsenic exposure. International studies have investigated birth outcomes in areas such as Bangladesh and Chile where chronic inorganic arsenic exposure is known from contaminated drinking water. In Bangladesh, it was found that in the women whose drinking water had more than 100 ug/L of arsenic detected, there was a statistically higher rate of spontaneous abortion, stillbirth, and preterm birth3. In Chile, arsenic exposure measured in the water supply from contaminated drinking water correlated with higher rates of late term still birth and neonatal death in exposed versus unexposed controls.10 Neither study measured inorganic arsenic levels in maternal serum or amniotic fluid samples.
It is difficult to determine “normal” serum or amniotic fluid inorganic arsenic levels. The Environmental Protection Agency set the inorganic arsenic standard for drinking water to 10ppb, or 10 micrograms per liter. About three-fourths of amniotic fluid samples exceeded this amount at the time of amniocentesis. A study of North Carolina drinking well-water detected inorganic arsenic concentrations above the EPA drinking water standards in 2.5% of the wells tested11. The clinical significance of our findings is that, to our knowledge, this is the first study to use state of the art technology (ICP-MS) to measure inorganic arsenic levels in amniotic fluid, which was ubiquitously identified in all amniotic fluid specimens suggesting maternal and thus fetal exposure. As such, it is important to increase our understanding of the effects of inorganic arsenic on fetal and maternal health. It is not altogether surprising that arsenic was found in all amniotic fluid specimens given that certain foods, especially rice, is a major determinant of exposure when concentrations in drinking water is low12. There is currently no known reason why arsenic levels would be different based on fetal gender or maternal medication exposures. In one study, arsenic metabolism differed by gender as measured by arsenic levels in urine with women having better methylation efficiency than men.13 However, it is currently unknown whether this translates to a difference in arsenic levels in utero.
Our study is not without limitations. While the sample size is small, the cases and controls were carefully matched. Because this was a pilot study and our sample size was a convenience sample, we were not powered to detect a difference in arsenic levels between fluid from women who had sPTB compared to TB or between fetal genders. Obtaining second trimester amniotic fluid is difficult because many women choose not to have an amniocentesis for fetal genetic diagnosis, thus, we were able to query a database of amniotic fluid supernatant and identified these cases and controls. It is possible that the women choosing amniocentesis were different in some way compared to women who did not choose amniocentesis and more predisposed to having arsenic in amniotic fluid. However, this seems unlikely because all the amniocenteses were done for routine indications (advanced maternal age, ultrasonographic soft markers of aneuploidy, abnormal serum screening results, maternal request) and we excluded any fetus with chromosome abnormalities and structural abnormalities.
In summary, this pilot study shows proof of principal that arsenic can be measured using ICP-MS in amniotic fluid and that arsenic is present in all of the samples tested. This study expands our current scope of measurement of arsenic in amniotic fluid. Future studies including a larger sample size with power to detect a difference between arsenic levels in fetuses destined to deliver preterm compared to term as well as further speciation of arsenic components could facilitate future studies of monitoring environmental exposures throughout pregnancy, also known as the exposome, and enable studies on the association specific amniotic fluid contaminants may have on maternal and fetal outcomes.
Figure 1.
Gestational age of samples at time of birth and measured arsenic levels during second trimester amniocentesis
Highlights.
Inorganic arsenic was measureable in all amniotic fluid specimens
This suggest ubiquitous maternal, and thus fetal, exposure
This expands our scope of knowledge in environmental contaminants in amniotic fluid
Funding Information:
Cefalo-Bowes Research Award from UNC-Chapel Hill Department of Obstetrics and Gynecology; NICHD: RO3 HD080788; Building Interdisciplinary Research Careers in Women’s Health K12 HD001441–16; K23 HD088742; NIEHS: R01 ES019315, P42 ES005948, T32ES007018; This work was supported in part by Grant 2015213 from the Doris Duke Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have no conflicts of interest.
This research was presented at 64th annual meeting of the Society of Reproductive Investigation, Orlando, Florida.
References
- 1.Boekelheide K, Blumberg B, Chapin RE, et al. Predicting later-life outcomes of early-life exposures. Environ Health Perspect. 2012;120(10):1353–1361. Doi: 10.1289/ehp.1204934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey KA, Laine J, Rager JE, et al. Prenatal arsenic exposure and shifts in the newborn proteome: Interindividual differences in tumor necrosis factor (TNF)-responsive signaling. Toxicol Sci. 2014;139(2):328–337. Doi: 10.1093/toxsci/kfu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad SA, Sayed MH, Barua S, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–631. doi: 10.1289/ehp.01109629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farzan SF, Howe CG, Zens MS, Palys T, Channon JY, Li Z, Chen Y, Karagas MR. Environmental Health Perspectives. 2017. December 15; 125(12): 127002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smeester L, Martin EM, Cable P, et al. Toxic metals in amniotic fluid and altered gene expression in cell-free fetal RNA. Prenat Diagn. 2017;37(13):1364–1366. doi: 10.1002/pd.5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rager JE, Bailey KA, Smeester L, et al. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014. doi: 10.1002/em.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vora NL, Smeester L, Boggess K, et al. Investigating the Role of Fetal Gene Expression in Preterm Birth. Reprod Sci. 2017;24(6):824–828. doi: 10.1177/1933719116670038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa R, Garry D, Elimian A, et al. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Neonatal Med. 2005;18(4):241–247. doi: 10.1080/13506120500223241 [DOI] [PubMed] [Google Scholar]
- 9.Windham G, Fenster L. Environmental contaminants and pregnancy outcomes. Fertil Steril. 2008;89 (2 SUPPL.):e111–e116. doi: 10.1016/j.fertnstert.2007.12.041 [DOI] [PubMed] [Google Scholar]
- 10.Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, et al. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 2000;108(7):667–673. doi: 10.1289/ehp.00108667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders AP, Messier KP, Shehee M, et al. Arsenic in North Carolina: public health implications. Environ Int. 2012;38(1):10–16. doi: 10.1016/j.envint.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson D Arsenic consumption in the united states. J Environ Health. 2015;78(3):8–14. [PubMed] [Google Scholar]
- 13.Lindberg A-L, Ekström E-C, Nermell B, Rahman M, Lönnerdald B, Persson A-A, Vahter M Gender and Age Differences in the Metabolism of Arsenic in a Highly Exposed Population in Bangladesh. Environ. Res 2008;106:110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]