Abstract
Objective:
The objectives of this study were to identify functional and structural network properties that are associated with early vs. long-term seizure outcomes after mesial temporal lobe epilepsy (mTLE) surgery, and to determine how these compare to current clinically used methods for seizure outcome prediction.
Methods:
In this case-control study, 26 presurgical mTLE patients and 44 healthy controls were enrolled for 3T MRI for functional and structural connectivity mapping across an eight region network of mTLE seizure propagation including hippocampus (left and right), insula (left and right), thalamus (left and right), one midline precuneus and one midline mid cingulate. Seizure outcome was assessed annually for up to three years. Network properties and current outcome prediction methods related to early and long-term seizure outcome were investigated.
Results:
A network model was previously identified across eight seizure free mTLE patients. Results confirmed that whole-network propagation connectivity patterns inconsistent with the mTLE model predict early surgical failure. In those patients with networks consistent with the mTLE network, specific bilateral within-network hippocampal to precuneus impairment (rather than unilateral impairment ipsilateral to the seizure focus) was associated with mild seizure recurrence. No currently used clinical variables offered the same ability to predict long-term outcome.
Conclusions:
It is known that there are important clinical differences between early surgical failure leading to frequent disabling seizures, and late recurrence of less frequent mild seizures. This study demonstrated that divergent network connectivity variability, whole-network vs. within-network, were uniquely associated with these disparate outcomes.
Keywords: Functional neuroimaging, Epilepsy surgery, MRI, Connectivity
Introduction
The most common form of epilepsy, mesial temporal lobe epilepsy (mTLE), is characterized by seizures originating from a focus that is most often the hippocampus, as identified by comprehensive clinical assessments and diagnostic studies.11 While the initial course of treatment for mTLE involves the use of anti-epileptic medications, approximately 30–40% of patients fail to respond to drug treatment.12 For those patients, surgical treatment may be an option. However, only approximately 58%45 to 80%14 of these patients are seizure free one year after surgery, and fewer remain seizure free three to five years later.28
Prediction of post-surgical seizure recurrence in mTLE remains a challenge. Clinical characteristics such as duration of disease28 and presence of mesial temporal sclerosis36,42 have been proposed as potential predictors of seizure outcome. More recently, multivariate predictors (composite scores) based on several of these clinical assessments10,21,29 have also been developed. However, these clinical assessments, mostly directed at localizing the seizure focus, are not accurate predictors of long term clinical outcome.
Alternatively, magnetic resonance imaging (MRI) connectivity quantification may allow examination of seizure propagation networks beyond the seizure focus.15 We recently created a network connectivity model of mTLE by identifying the consistent connectivity properties across eight patients with seizure free (Engel Ia) one year outcome.35 We hypothesized that patients with networks similar to this model would have an Engel Ia one year outcome. We observed that similarity to the model predicted favorable (Engel I-II) seizure outcome vs. unfavorable (Engel III-IV) one year outcome with 100% sensitivity and specificity in a small, independent test cohort of surgical mTLE patients. The patients experiencing unfavorable outcome had markedly different epileptogenic network properties compared to mTLE patients who benefited from surgery, even though their baseline clinical characteristics were essentially indistinguishable. However, these overall mTLE network properties did not distinguish patients with sustained freedom from disabling seizures (Engel Ia-b) from those with some improvement (Engel Ic-II), and did not address the timing of recurrence.
Clinical evidence supports the existence of two distinct classifications of seizure recurrence.20,37 “Early failure” is generally used to describe the occurrence of multiple disabling seizures in the first two years after surgery, and is typically considered an unfavorable (Engel III-IV) surgical outcome. “Late recurrence” describes milder, less frequent seizures occurring up to several years after surgery that may be responsive to anti-epileptic medication. While not completely seizure free, these patients often do have worthwhile clinical improvement (Engel Ic-II). Exceptions to these categories, such as late severe recurrence, are much less common.
We propose that our previous results35 suggest that early failure occurs when patients whose whole eight region network (“whole-network”) connectivity is dissimilar to the mTLE model. We hypothesize that late recurrence occurs when the mTLE epileptogenic network identified above is present, but temporarily interrupted by focal resection.30 This suggests that connectivity indicators of late recurrence might be uncovered by probing specific connections within the identified network (“within-network” connectivity). Based on this premise, there are three objectives of this work. First, we develop the concepts of whole-network and within-network properties as a more comprehensive MRI network prediction method to predict early failure vs. late recurrence vs. no recurrence in mTLE. Second, we identify within-network connections that are associated with longer seizure free outcomes (late or no recurrence). Third, we compare these findings to current clinically used methods for seizure outcome prediction in mTLE.
Materials and Methods
Subject characteristics
Twenty-six unilateral mTLE patients completed the presurgical imaging procedure and had at least one year post-surgical seizure outcomes available. Study inclusion criteria included diagnosis of unilateral mTLE defined by possible mesial temporal atrophy or signal enhancement on structural imaging with MRI, lateralizing long-term ictal and interictal scalp electroencephalography (EEG), seizure semiology consistent with TLE, and mesial temporal hypometabolism on positron emission tomography (PET). Exclusion criteria included structural abnormalities on MRI outside mesial temporal lobe structures and presurgical intracranial monitoring. All patients underwent selective amygdalohippocampectomy (n=17), anterior temporal lobectomy (n=8), or hippocampal laser ablation (n=1) at Vanderbilt University Medical Center from 2012–2017. Seizure outcome was assessed by an epileptologist at each year post surgery (up to three years) using the Engel surgery outcome classification.13 Healthy controls (n=44, 21 females, age = 39.3 ± 14.3 years) with no history of head trauma or neurological or neuropsychological disease were also enrolled. This group included at least one subject that was age- (± 3 years) and gender-matched to each patient. Informed consent was obtained from each participant per Vanderbilt University Institutional Review Board guidelines (IRB #010637).
Imaging
The imaging was performed using a Philips Achieva 3T MRI scanner (Philips Healthcare, Best, Netherlands) with a 32-channel head coil. The acquisition included: 1) T1-weighted, three-dimensional scan for inter-subject normalization and tissue segmentation (1 × 1 × 1 mm3), 2) T1-weighted scan for spatial normalization acquired in the same slice orientation as the functional images (1 × 1 × 3.5 mm3 with 0.5 mm gap), 3) T2*-weighted functional MRI (fMRI) Blood Oxygenation Level Dependent scan at rest with eyes closed for functional connectivity (34 axial slices, echo time = 35 ms, repetition time = 2 sec, 3 × 3 × 3.5 mm3 with a 0.5 mm gap, 2 × 10 minutes), and 4) diffusion weighted imaging for structural connectivity (92 directions, b=1600 s/mm2, 2.5 × 2.5 x. 2.5 mm3, 50 axial slices). Physiological monitoring of cardiac and respiratory fluctuations was performed at 500 Hz using a pulse oximeter and respiratory belt.
Image processing
A potential seizure propagation network consisting of eight regions was identified on each subject’s T1-weighted image using FreeSurfer [http://freesurfer.net/].35 Regions included: left and right hippocampus, left and right insula,3,32 left and right thalamus,4,34 one midline precuneus,16,24 and one midline mid cingulate. We combined the caudal anterior cingulate and the anterior half of the posterior cingulate, in order for the mid cingulate region to be consistent with the region identified in the literature.16,27
Functional MRI images were preprocessed with SPM8 software [http://www.fil.ion.ucl.ac.uk/spm/software/spm8/] and Matlab 2013a (The MathWorks, Inc, Natick, MA). The following steps were performed: slice timing correction, motion correction, physiological noise correction using the Retrospective Correction of Physiological Motion Effects in functional MRI (RETROICOR) protocol18 using the pulse oximeter and respiratory belt time series, spatial normalization to the Montreal Neurological Institute template via the T1-weighted datasets, and spatial smoothing using a 6 × 6 × 6 mm3 full width, half maximum Gaussian kernel. Then the normalized fMRI time series were temporally band-pass filtered at 0.0067 Hz to 0.1 Hz.8
The functional connectivity between each pair of regions was computed as the partial Pearson correlation between the average preprocessed fMRI time series in each region, adjusted for the average white matter time series and the six motion parameter time series. The values were then converted to a Fisher Z-statistic17 and averaged across the two fMRI series for each subject. Next, the connectivity between each pair of regions was fit to age across all the healthy control subjects to find the linear relationship with age across each pair of regions. Then the connectivity measures in patients were normalized with respect to age-matched healthy control by using the linear fit across all healthy control subjects and dividing by the standard deviation. This resulted in functional connectivity (FC) in units of standard deviations from age-matched control, where values above and below zero reflect connectivity greater and less than the healthy control of the same age, respectively. Finally, regions were converted from left and right to ipsilateral and contralateral, referring to the side of seizure focus, to combine the right and left mTLE patients into one cohort.
The diffusion weighted images were processed using FSL [http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/]31 probabilistic fiber tracking1 as described in our previous work35. A probabilistic fiber tracking algorithm was used to investigate the same network regions utilized in the FC analyses. One region was considered the seed and the other seven regions of interest were considered targets and termination points (i.e. direct connections). Then, the fraction of the number of streamlines between the seed (A) and target (B) regions with respect to the total streamlines between seed (A) and all seven other regions was computed. This was repeated for connections from region B to A. These values were combined using a weighted average relative to the size of the regions. This is similar to the streamline density of structural connectivity6,9 and is thought to reflect the “trackability” of the connection from the seed to the target via its most likely principal diffusion direction.1,44 The connectivity values were corrected for age as described above, resulting in structural connectivity values (SC) in units of standard deviations from age-matched healthy control.
“Whole-network” connectivity analyses
First, to introduce the concept of the “whole-network” characteristics as part of our network theory of mTLE post-surgical recurrence, we briefly re-introduce our method described previously.35 In that work, we computed a weighted degree of FC and SC of each region in the network as the weighted sum of the connectivity of that region to all other seven regions in the network. The result is a vector of 16 values (8 FC and 8 SC) for each patient. Then principal component analysis was used to compute the model network using the first eight patients with Engel Ia one year outcome (Patients #1–5, 7–8, 10). Then this model was compared to completely new patients that were not used to compute the model based on two similarity measures: (1) the linear correlation to the model to reflect concordance in relative connectivity pattern across the regions, and (2) the Euclidean distance from the model to represent the magnitude of the difference. These two parameters are plotted in a two-dimensional graph, with limits of similarity defined by 99% -tile of 5000 Monte-Carlo repetitions within the mean ± 1.5 standard deviations of each pairwise connection across the eight patients used to compute the model.
“Within-network” connectivity analyses
“Within-network” connectivity in this work refers to individual pair-wise connectivity (FC or SC) across regions in the network defined above. Furthermore, the within-network analyses are focused only on those patients with whole-network properties that are similar to the mTLE model and/or have Engel I-II one year outcome. We hypothesize that these patients have some presurgical within-network connectivity that is associated with long-term seizure outcome. Long-term seizure outcome was quantified as the number of years until focal impaired awareness or focal to bilateral tonic-clonic seizure recurrence (anything other than Engel Ia or Ib outcome). Those patients with three years follow up with continued outcome of Engel Ia-b were given a score of 4, while those patients without recurrence, but without at least three years follow up were excluded from this investigation (patients #22, 24, 25).
To test our hypotheses, linear relationships between parameters of long-term seizure outcome (years until recurrence) and connectivity were investigated. In addition to the connectivity measures in each patient, two other types of clinical parameters were included in the analyses. First, the FreeSurfer derived left and right hippocampal volume for each patient was divided by the supratentorial volume measure. Then, similar to the connectivity measures, the left and right volume measures were converted to standard deviations from age-matched healthy control using the linear fitting process with the healthy control subjects and then identified as ipsilateral and contralateral to the seizure focus. Second, three published outcome prediction “scores” were computed: the modified Seizure Freedom Score,21 the Epilepsy Surgery Nomogram,29 and the Epilepsy Surgery Grading Scale.10
In order to examine multiple potential parameters related to long-term seizure outcome (FC=28, SC = 28, volumes = 2, scores = 3), we first aimed to identify the type of parameter that was associated with outcome. From those results we then identified the individual parameter within this type that was associated with outcome. In the first step, lasso regression,7 a regularized linear multiple regression model with a sparsity parameter known as the L1-norm, was used to identify the minimal number of linear predictors of years until seizure recurrence. The lasso regression was computed using 3-fold cross validation with 500 Monte-Carlo repetitions. The mean squared error of the fitted model compared to the years until seizure recurrence, as well as the standard deviation of the error across the 500 trials was computed for different combinations of predictors. Trials with lower mean squared error were used to identify the set of parameters associated with outcome (FC vs. SC vs. hippocampal volumes vs. scores). Next, the multivariate linear regression was repeated using 1000 bootstrap samples with only the type of predictors found to have the lowest mean squared error. The number of times each individual parameter was ranked as one of the top three predictors out of 1000 trials was computed. This was used to identify individual parameters most related to long-term seizure outcome.
Prediction analyses
The clinical significance of investigating associations between non-invasive imaging parameters and surgery outcome lies in the development of the parameter into a predictor of outcome. One method to quantify the prediction accuracy of continuous values such as FC, scores or volumes without arbitrarily selecting a cut-point is to use the Receiver Operating Characteristics (ROC).22 The area under the ROC curve represents a measure of the overall accuracy of the prediction and can be compared between methods.25 We used this method to quantify the accuracy of prediction of three year seizure freedom from focal impaired awareness or focal to bilateral tonic-clonic seizures of any potential connectivity predictors, and compared the prediction accuracy to that of scores and hippocampal volumes.
Results
In the present cohort of mTLE patients, 26 had one year surgical outcome data available with five having Engel III-IV rating at that time. Of the 21 remaining patients, 18 had either i) three year outcomes, or ii) focal impaired awareness or focal to bilateral tonic-clonic seizure recurrence (Engel Ic-II) before reaching three years. The clinical and demographic characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Patient # | Sex | Age (yrs) | Epilepsy Duration (yrs) | Side | MTS | Ictal/Interictal EEG | FBTC/month | Engel Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Yr1 | Yr2 | Yr3 | ||||||||
| 1 | F | 49 | 17 | R | Y | C/C | 0.0 | I-a | I-a | I-a |
| 2 | F | 18 | 18 | L | Y | C/C | 0.1 | I-a | I-a | I-a |
| 3 | F | 39 | 39 | R | Y | C/NC | 0.1 | I-a | I-a | I-a |
| 4 | M | 28 | 4 | L | Y | C/NC | 0.2 | I-a | I-a | I-d |
| 5 | F | 54 | 21 | R | N | C/NC | 0.1 | I-a | I-a | I-a |
| 6 | M | 18 | 6 | L | Y | C/NC | 0.0 | I-b | I-b | I-b |
| 7 | M | 43 | 10 | R | Y | C/C | 0.0 | I-a | I-a | III-b |
| 8 | M | 24 | 23 | R | Y | NC/NC | 0.0 | I-a | I-a | II-a |
| 9 | M | 23 | 3 | R | N | C/C | 0.0 | III-a | III-a | III-a |
| 10 | F | 41 | 18 | R | Y | C/C | 0.2 | I-a | I-a | I-a |
| 11 | F | 38 | 5 | R | Y | C/NC | 0.0 | II-c | II-c | III |
| 12 | F | 37 | 36 | R | Y | C/C | 0.1 | I-a | I-a | I-a |
| 13 | M | 68 | 50 | L | Y | C/NC | 0.0 | I-a | I-d | II-b |
| 14 | M | 21 | 20 | L | Y | C/C | 0.0 | I-a | I-a | I-a |
| 15 | F | 38 | 11 | R | N | C/C | 0.0 | IV-c | ||
| 16 | M | 38 | 10 | R | Y | C/NC | 0.4 | IV-c | IV-c | |
| 17 | F | 46 | 46 | L | N | C/NC | 4.0 | I-a | I-a | I-a |
| 18 | F | 36 | 16 | R | Y | NC/C | 0.1 | II-b | III-a | III-a |
| 19 | M | 46 | 45 | R | Y | C/C | 0.0 | III-a | III-a | |
| 20 | F | 42 | 10 | R | N | C/C | 0.0 | II-c | II-c | II-c |
| 21 | F | 62 | 31 | R | Y | C/C | 0.0 | I-a | I-a | I-a |
| 22 | F | 43 | 33 | R | Y | C/C | 0.0 | I-b | I-b | |
| 23 | M | 35 | 3 | L | Y | NC/C | 0.2 | III-a | ||
| 24 | M | 25 | 3 | R | N | C/C | 0.0 | I-a | I-a | |
| 25 | F | 42 | 22 | L | Y | C/NC | 0.0 | II-a* | ||
| 26 | M | 30 | 2 | R | N | C/C | 4.0 | II-b | ||
F = female; M = male; Side = side of surgery; MTS = mesial temporal sclerosis on MRI; EEG = electroencephalography (long-term with simultaneous video monitoring); FBTC/month = average number of focal to bilateral tonic-clonic seizures per month; L = left; R = right; Y = yes; N = no; C = fully consistent localization (ictal) or lateralization (interictal); NC = not fully consistent localization (ictal) or lateralization (interictal). * - this patient had only focal seizures with preserved awareness before and after surgery, and is classified as Engel II-a given decreased frequency of these, but no post-surgical focal impaired awareness or focal to bilateral tonic-clonic seizures.
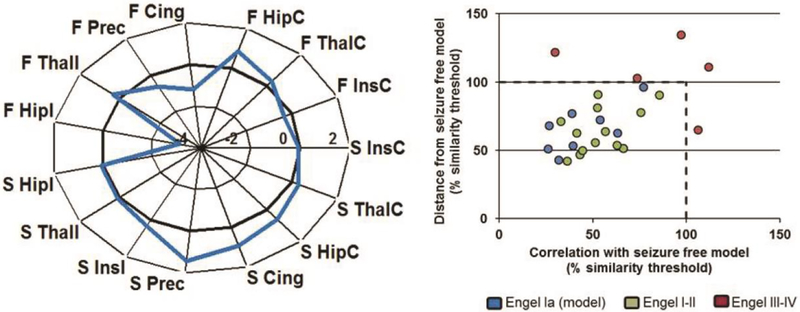
As described in our previous work,35 the whole-network similarity results are shown in Fig. 1. The connectivity model created using the first eight patients with Engel Ia one year outcome is shown in Fig. 1 (left), demonstrating the weighted sum of the connectivity of each region to all other regions in units of standard deviation from age-matched healthy control. The similarity of each of the eight patients used to compute the model are within the similarity thresholds as determined by the Monte-Carlo simulations, as expected (Fig. 1 right, blue). When tested in an independent cohort not used to compute the model, similarity to the model was successful in discriminating the early failure vs. those with favorable outcome (Fisher’s exact, p < 0.001). Specifically, all five patients with early failure and Engel III-IV outcome (red) were dissimilar to the model (outside the similarity thresholds), while the 13 patients with a more favorable Engel I-II one year outcome had similar whole-network connectivity properties (green). This work is shown to illustrate the whole-network connectivity concept. The eight patients used to compute the model described are a subset of the 26 used in the present study.
Figure 1. Whole-network dissimilarity to mTLE model identifies patients with early surgical failure (n=26).
Left) Functional and structural connectivity network model identified using eight mTLE patients with Engel Ia one year outcome. Each region value is the weighted sum of connectivity to all other regions in the network. Units are standard deviations from healthy control of the same age. Right) Similarity of each patient in the cohort to the model using two similarity metrics. Excluding those patients used to compute the model (blue), similarity to the model was successful in discriminating the early failure (red) vs. those with favorable outcome (green) (Fisher’s exact, p < 0.001). Dashed lines represent similarity limits defined by 5000 Monte-Carlo simulations using the mean +/− 1.5 standard deviations of the eight patients used to compute the model. F = functional connectivity, S = structural connectivity, I = ipsilateral to seizure focus, C = contralateral to seizure focus, Hip = hippocampus, Ins = insula, Thal = thalamus, Cing = mid cingulate, Prec = precuneus.
After confirming patients with whole-network similarity to the mTLE model (Engel I-II one year outcome), the relationship between individual within-network connectivity and long-term outcome was investigated. First, the mean squared error of 500 Monte-Carlo repetitions of the regularized multivariate regression analyses was used to determine the set of parameters that best estimated the years until seizure outcome. The results showed that the mean squared error was lower when the set of FC parameters was included (two-sample t-test, p < 0.0001, 95% confidence [−0.98,−0.56]), suggesting the association of FC parameters and long-term outcome. In contrast, the SC parameters, hippocampal volumes and clinical prediction scores provided no additional predictive information. To further investigate the individual FC parameters, the regression was repeated with 1000 bootstrap trials using only the 28 FC parameters. The FC between the contralateral hippocampus and precuneus was ranked in the top three predictors 969 times out of 1000 trials (95% confidence interval of ranking = [1,4]). The second highest occurring individual connectivity value was FC between the ipsilateral hippocampus and the precuneus (95% confidence interval of ranking = [1,17]).
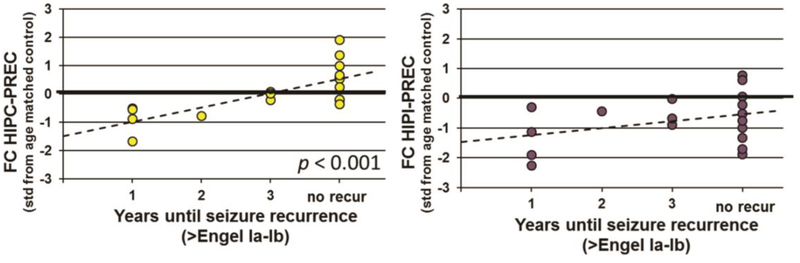
Given these findings, the relationship between these two within-network functional connectivities and long-term outcome were quantified. There is a positive linear relationship between the FC across the contralateral hippocampus and precuneus and years until post-surgical recurrence of focal impaired awareness or focal to bilateral tonic-clonic seizures (Pearson correlation, r = 0.73, p < 0.001, Fig. 2 left). Conversely, the FC between the ipsilateral hippocampus and precuneus was not linearly related to long-term seizure outcome (Pearson correlation, r = 0.34, p > 0.05, Fig. 2 right). However, this connectivity was significantly less than age-matched healthy control (i.e. the values are less than 0) across the cohort (two-sample t-test, p < 0.001, 95% confidence interval [−1.16, −0.34]).
Figure 2. Within-network functional connectivity between contralateral hippocampus and precuneus is positively correlated with long-term seizure outcome (n=18).
Left) Functional connectivity of contralateral hippocampus to precuneus (FC HIPC-PREC) is positively linearly correlated with years until post-surgical focal impaired awareness or focal to bilateral tonic-clonic seizure recurrence (Pearson correlation, r = 0.73, p < 0.001). Right) No significant linear relationship between functional connectivity of ipsilateral hippocampus to precuneus (FC HIPI-PREC) and years until seizure recurrence (Pearson correlation, r = 0.34, p > 0.05). This connectivity is less than age-matched healthy control regardless of outcome (FC HIPI-PREC < 0, two-sample t-test, p < 0.001). FC units are standard deviations from age-matched healthy control. Zero line represents FC of the estimated age-matched healthy control.
The strong linear association between FC of contralateral hippocampus to precuneus implies that this may be a predictor of three year freedom from post-surgical consciousness impairing seizures. Therefore, an ROC analysis22 was used to quantify the specificity and sensitivity of this prediction, and compare it to other potential predictors25 (Fig. 3A). The area under the ROC curve, representing a summary measure of prediction accuracy,22 was greater using the FC predictor than the modified Seizure Freedom Score21 (p < 0.01, uncorrected), the Epilepsy Surgery Grading Scale10 (p < 0.05, uncorrected) and the contralateral hippocampal volume (p < 0.05, uncorrected) (Fig. 3B). When considering the highest levels of specificity (Fig. 3A, shaded area), the functional connectivity is the only predictor with high sensitivity (≥ 0.7). To illustrate the longitudinal seizure freedom of patients in our sample using a cutoff of FC between contralateral hippocampus and precuneus greater than 0 (greater than age-matched healthy control), we used the Kaplan-Meier19 survival analysis (Fig. 3C).
Figure 3. Predictions of three year seizure freedom after mTLE surgery.
A) Receiver operating characteristics (ROC) curves to predict three year freedom from post-surgical recurrence of focal impaired awareness or focal to bilateral tonic-clonic seizures of five prediction methods: functional connectivity of contralateral hippocampus to precuneus (FC HIPC-PREC), modified Seizure Freedom Score (mSFS), the Epilepsy Surgery Grading Scale (SGS), the Epilepsy Surgery Nomogram (NOM), and the age corrected contralateral hippocampal volume (HIPC vol). Shaded region indicates area of highest specificity (> 0.7, 1-specificity < 0.3). FC HIPC-PREC is only predictor with high sensitivity (> 0.7) in this range (n=18). B) Area under the whole ROC curve (AUC) from A. Error bars are 95% confidence interval. ** - difference between AUC p < 0.01, ** - difference between AUC p < 0.05. C) Kaplan-Meier plot illustrating percent of patients with seizure freedom each year after surgery using FC HIPC-PREC > 0 (age-matched healthy control) as cutoff for good prediction (n=21, method includes censored data).
Discussion
Accurate prediction of long-term post-surgical seizure outcome in mTLE remains a challenge. MRI network analyses have shown promise over standard clinical assessments in addressing this issue,.5,26,38 However, many of these investigations consider seizure outcome as a short term binary outcome (i.e. “seizure free” vs. “seizure recurrence”) which may limit their overall clinical utility. In reality, there are important clinical differences between early surgical failure leading to frequent disabling seizures, and late recurrence of less frequent mild seizures,20,37 and thus there are likely different network properties which influence the timing and severity of seizure recurrence. In this work, MRI functional and structural connectivity was used to build on previous work to investigate the theory that differences in the underlying seizure propagation network can explain these disparate outcomes, and lead to more accurate individualized outcome prediction. Specifically, we briefly re-introduced the idea of a network model of mTLE seizure propagation required for seizure improvement from mTLE surgery,35 which we now call the whole-network properties. We used this step to identify those patients in our cohort with networks consistent with this model. Then we focused more closely on those patients to identify specific within-network variabilities associated with long-term (up to three year) seizure outcome.
Similarity of an individual patient’s seizure propagation network to the whole-network functional and structural connectivity model predicts at least some improvement from mesial temporal surgical treatment. This concept is novel in that it does not consider seizure free and seizure recurrence as two homogeneous groups of patients. Rather, the idea that the dissimilarity to the model can be very heterogeneous across the population of patients, as demonstrated by the scatter of red points in Fig. 1 right, is consistent with the idea that early failures have varied mechanisms of recurrence.30,43
While the absence of mTLE whole-network connectivity may be predictive of early surgical failure, the presence of the network connectivity alone did not distinguish patients with sustained seizure freedom (Engel Ia-b) vs. late recurrence (Engel Ic-II) over the first three years after surgery. To investigate the theory that late recurrence occurs when this mTLE epileptogenic network is only temporarily interrupted by focal resection,30 we probed within-network connections associated with long-term outcome. The association analyses revealed that the age-corrected functional connectivity between the contralateral hippocampus and the precuneus was positively correlated with number of years until seizure recurrence. Those patients with presurgical connectivity less than age-matched healthy control (FC < 0) were more likely to experience mild seizure recurrence in the first three years than those with connectivity greater than controls. Notably, hippocampal volumes and three currently used multivariate clinical prediction scores provided no additional power in predicting years to seizure recurrence.
These changes in functional connectivity may be a challenge to interpret clinically. The concept of decreased functional connectivity in epileptogenic hippocampal networks compared to controls has been reported previously.2,39,41 Similarly, we found the functional connectivity between ipsilateral hippocampus to precuneus (Fig. 2 right), ipsilateral to contralateral hippocampus, and ipsilateral insula to mid cingulate are each less than age-matched healthy control (p < 0.001), across the entire cohort of mTLE patients in this study; but there were no connections with significantly increased functional connectivity in our sample. Based on this, we conclude that ipsilateral hippocampal connectivity decreases (specifically to precuneus and contralateral hippocampus) characterize impairment that facilitates presurgical ipsilateral seizures. Furthermore, our results suggest that when this impairment includes the contralateral hippocampus to precuneus, there is a higher likelihood of mild seizure recurrence (Fig. 2 left, Fig. 4). It is not known whether this contralateral connectivity impairment represents an existing independent contralateral focus that was not detectable by other standard assessments,23 or perhaps whether this suggests increased susceptibility of the network to develop a secondary epileptogenicity once the primary seizures are stopped.40 More invasive presurgical assessments and post-surgical longitudinal monitoring may provide evidence to support one of these possibilities. However, while the mechanism is not yet understood, novel and reproducible tests for predicting bilateral network abnormalities which may influence post-surgical outcome, are critically needed. The method proposed requires about 30 minutes of additional MRI scanning generally available on most clinical scanners, while the image processing can be fully automated off-line using standard tools. An algorithm of how the whole-network and within-network analysis methods could be utilized sequentially to influence clinical decisions by identifying the optimal surgical candidates, if validated in much larger cohorts, is presented in Fig. 5.
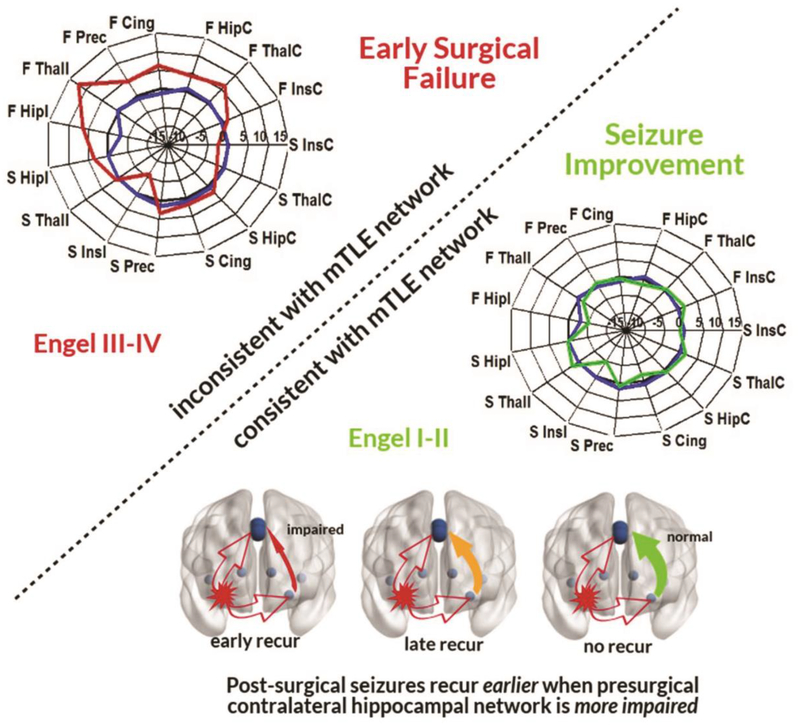
Figure 4. Seizure propagation network theory of post-surgical recurrence.
These results suggest that mTLE propagation network patterns inconsistent with the whole-network mTLE model predict early surgical failure (Top). In those patients with networks consistent with the mTLE network, specific within-network contralateral hippocampal to precuneus impairment is associated with earlier mild seizure recurrence (Bottom).
Figure 5. Identification of optimal mesial temporal surgical candidates based on network connectivity.
Possible algorithm to incorporate network models into presurgical decision making in mTLE.
There are several limitations of this study that require discussion. First, the small sample size impedes accurate estimates of generalizability. However, our sample is designed to maximize homogeneity to understand a specific population of patients. The proposed relationships with outcome, therefore, need validation in a larger, independent patient cohort. Second, these data only include three year follow up, and are measured only at yearly increments. More detailed dates of recurrence would improve future studies. Third, our cohort included patients who underwent three different types of surgical treatment. While no difference was detected in outcome between selective amygdalohippocampectomy vs. anterior lobectomy in a recent study,33 this should be considered in future validation studies. Finally, while most patients had either stable or worsening seizure outcome over time, one patient (#2) did demonstrate an improvement over time. More patients with this type of improvement are needed to understand and predict this recovery trajectory.
Conclusions
This study presents support for a theory of network connectivity related to early surgical failure vs. late recurrence in unilateral mTLE. The results suggest that there exists a functional and structural whole-network model of mTLE seizure propagation35 whose disruption via mTLE surgery reduces or eliminates consciousness impairing seizures. Within this network, more bilateral hippocampal to precuneus impairment increases susceptibility to mild seizure recurrence. If validated in a prospective cohort, this method could be readily automated for individual presurgical TLE patient evaluation and long term outcome prediction. The information provided may be valuable in determining when invasive evaluations should be performed to further localize the seizure focus prior to surgery, and when alternative treatments such as neurostimulation should be considered. And, this information may elucidate potential mechanisms of seizure recurrence after surgical treatment. Finally, while the algorithm presented is relevant only to mTLE, it provides a framework for applications to other focal epilepsies.
Disclosure
This work was supported by NIH R01 NS075270 (VLM), R01 NS110130 (VLM) and R00-NS097618 (DJE). The authors report no conflict of interest concerning the materials and methods used in this study or the findings specified in the paper.
Abbreviations:
- TLE
mesial temporal lobe epilepsy
- fMRI
functional MRI
- FC
functional connectivity
- SC
structural connectivity
- ROC
Receiver Operating Characteristics
References
- 1.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW: Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, et al. : Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. Journal of Neurology Neurosurgery and Psychiatry 81:1147–1154, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Blauwblomme T, David O, Minotti L, Job AS, Chassagnon S, Hoffman D, et al. : Prognostic value of insular lobe involvement in temporal lobe epilepsy: A stereoelectroencephalographic study. Epilepsia 54:1658–1667, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. : Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 132:999–1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilha L, Jensen JH, Baker N, Breedlove J, Nesland T, Lin JJ, et al. : The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology 84:1846–1853, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, et al. : Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 83:903–909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll MK, Cecchi GA, Rish I, Garg R, Rao AR: Prediction and interpretation of distributed neural activity with sparse models. Neuroimage 44:112–122, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. : Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22:1326–1333, 2001 [PMC free article] [PubMed] [Google Scholar]
- 9.Dinkelacker V, Valabregue R, Thivard L, Lehericy S, Baulac M, Samson S, et al. : Hippocampal-thalamic wiring in medial temporal lobe epilepsy: Enhanced connectivity per hippocampal voxel. Epilepsia 56:1217–1226, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Dugan P, Carlson C, Jette N, Wiebe S, Bunch M, Kuzniecky R, et al. : Derivation and initial validation of a surgical grading scale for the preliminary evaluation of adult patients with drug-resistant focal epilepsy. Epilepsia 58:792–800, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Engel J Jr.: Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 7:340–352,2001 [DOI] [PubMed] [Google Scholar]
- 12.Engel J Jr.: What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology 87:2483–2489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel J, Van Ness PC, Rasmussen TB, Ojemann LM: Outcome with respect to epileptic seizures, in Engel J (ed): Surgical Treatment of the Epilepsies, ed 2 New York: Raven Press, 1993, pp 609–621 [Google Scholar]
- 14.Englot DJ, Chang EF: Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurgical Review 37:389–405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englot DJ, Hinkley LB, Kort NS, Imber BS, Mizuiri D, Honma SM, et al. : Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain 138:2249–2262, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahoum F, Lopes R, Pittau F, Dubeau F, Gotman J: Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia 53:1618–1627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher RA: Frequency Distribution of the Values of the Correlation Coefficient in Samples from an Indefinitely Large Population. Biometrika 10:507–521, 1915 [Google Scholar]
- 18.Glover GH, Li TQ, Ress D: Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Goel MK, Khanna P, Kishore J: Understanding survival analysis: Kaplan-Meier estimate. International Journal of Ayurveda Research 1:274–278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goellner E, Bianchin MM, Burneo JG, Parrent AG, Steven DA: Timing of early and late seizure recurrence after temporal lobe epilepsy surgery. Epilepsia 54:1933–1941, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Gracia CG, Yardi R, Kattan MW, Nair D, Gupta A, Najm I, et al. : Seizure freedom score: A new simple method to predict success of epilepsy surgery. Epilepsia 56:359–365, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Hajian-Tilaki K: Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian Journal of Internal Medicine 4:627–635, 2013 [PMC free article] [PubMed] [Google Scholar]
- 23.Halasz P: The medial temporal lobe epilepsy is a bilateral disease - novel aspects, in Journal of Epileptology, 2016, Vol 24, p 141 [Google Scholar]
- 24.Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Stern JM: Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55:137–145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ: A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843, 1983 [DOI] [PubMed] [Google Scholar]
- 26.He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI: Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology 88:2285–2293,2017 [DOI] [PubMed] [Google Scholar]
- 27.Holmes MJ, Yang X, Landman BA, Ding Z, Kang H, Abou-Khalil B, et al. : Functional networks in temporal-lobe epilepsy: a voxel-wise study of resting-state functional connectivity and gray-matter concentration. Brain Connect 3:22–30, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, et al. : Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 128:395–404, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Jehi L, Yardi R, Chagin K, Tassi L, Lo Russo G, Worrell G, et al. : Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurology 14:283–290, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Jehi LE, Silveira DC, Bingaman W, Najm I: Temporal lobe epilepsy surgery failures: predictors of seizure recurrence, yield of reevaluation, and outcome following reoperation. J Neurosurg 113:1186–1194, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM: FSL. Neuroimage 62:782–790, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Hong SB, Seo DW: Differences in ictal hyperperfusion of limbic-related structures between mesial temporal and neocortical epilepsy. Epilepsy Res 81:167–175, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Mathon B, Bielle F, Samson S, Plaisant O, Dupont S, Bertrand A, et al. : Predictive factors of long-term outcomes of surgery for mesial temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsia 58:1473–1485, 2017 [DOI] [PubMed] [Google Scholar]
- 34.McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME: Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage 23:167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Morgan VL, Englot DJ, Rogers BP, Landman BA, Cakir A, Abou-Khalil BW, et al. : Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia 58:1251–1260, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhlhofer W, Tan YL, Mueller SG, Knowlton R: MRI-negative temporal lobe epilepsy- What do we know? Epilepsia 58:727–742, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W: Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia 54:772–782, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT: Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia 52:1733–1740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira FRS, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, et al. : Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neuroscience 11:66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitkanen A, Lukasiuk K, Dudek FE, Staley KJ: Epileptogenesis. Cold Spring Harbor Perspectives in Medicine 5:a022822, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittau F, Grova C, Moeller F, Dubeau F, Gotman J: Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53:1013–1023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radhakrishnan K, So EL, Silbert PL, Jack CR Jr., Cascino GD, Sharbrough FW, et al. : Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology 51:465–471, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Ramos E, Benbadis S, Vale FL: Failure of temporal lobe resection for epilepsy in patients with mesial temporal sclerosis: results and treatment options Clinical article. Journal of Neurosurgery 110:1127–1134, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Schumacher LV, Reisert M, Nitschke K, Egger K, Urbach H, Hennig J, et al. : Probing the reproducibility of quantitative estimates of structural connectivity derived from global tractography. NeuroImage 175:215–229, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Wiebe S, Blume WT, Girvin JP, Eliasziw M: A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318, 2001 [DOI] [PubMed] [Google Scholar]