Abstract
Purpose:
Recent advances in retinal imaging allow visualization of structural abnormalities in retinal disease at the cellular level. Here we use adaptive optics (AO) microperimetry to assess visual sensitivity with high spatial precision and examine how function varies across two phenotypic features observed in choroideremia (CHM) – atrophic lesion borders and outer retinal tubulations (ORTs).
Design:
Cross-sectional study.
Subjects:
12 CHM patients.
Methods:
A custom adaptive optics scanning light ophthalmoscope (AOSLO) equipped with both confocal and non-confocal split-detection imaging modalities was used to image the photoreceptor inner and outer segment mosaics. For AO microperimetry, circular 550-nm stimuli were presented through the AOSLO system; stimuli were either 9.6 or 38.3 arcmin^2 (~60 or 15 times smaller than a Goldman III stimulus). Test locations were identified in structural images and stimuli were targeted to these locations using real-time retinal tracking combined with measurements of transverse chromatic aberration. Psychophysical detection thresholds were measured at the atrophic border in 12 patients. Additionally, visual sensitivity was probed along ORTs in four patients.
Main Outcome Measure:
Visual sensitivity thresholds measured with AO microperimetry at retinal locations corresponding to structural phenotypes observed in AOSLO retinal images.
Results:
In CHM, sharp borders between intact central islands of the photoreceptor mosaic and complete atrophy of the outer retina and retinal pigment epithelium were observed in both split detection and confocal structural images. AO microperimetry at locations spanning these borders show a commensurately sharp decrease in function, with readily measurable visual sensitivity on one side and dense scotoma on the other. These functional transitions often occurred over a distance smaller than the diameter of the Goldman III stimulus. Thresholds measured along ORTs showed dense scotoma over the tubule in all four subjects, despite the visibility of remnant cone inner segments in the AO images.
Conclusions:
CHM patients exhibit sharp functional transitions that collocate with structural transitions from intact to severely degenerated retina. We found no evidence of visual sensitivity over ORTs. Measuring cone function with high resolution offers insight to disease mechanisms and may enable precise assessment of whether experimental therapies, such as gene therapy, provide a functional benefit.
Précis:
Adaptive optics microperimetry revealed sharp functional transitions that co-located between relatively intact and severely-degenerated retina in choroideremia. Outer retinal tubulations containing cone inner segments did not exhibit measurable visual sensitivity when probed with cellular-scale stimuli.
Introduction
Choroideremia (CHM) is an X-linked inherited retinal degeneration with a prevalence of approximately 1 in 50,000.1 CHM is caused by mutations in the CHM gene, which interrupt the encoding of rab-escort protein 1 (REP1).2 REP1 is involved in the prenylation of Rab proteins which are necessary for intercellular trafficking and phagocytosis.3 Lack of functional REP1 results in progressive degeneration of the photoreceptors, retinal pigment epithelium (RPE), and choroid.4, 5 In CHM patients, with established disease and central retinal involvement, fundus appearance is characterized by a central island of relatively preserved retina and RPE with characteristic scalloped edges, surrounded by atrophic, depigmented retina (Figure 1A).5 Structurally, a sharp transition between atrophic and relatively preserved, ostensibly viable, retina is suggested by retained central fundus autofluorescence (FAF) (Figure 1B).6 Optical coherence tomography (OCT) has shown initial layer disruption occurring in the interdigitation zone between the photoreceptor tips and the RPE,6 and inner retinal thickening within the central island.4 Interlaminar bridges mark the transition from the central island to atrophic areas showing loss of outer retinal layers and loss of RPE pigmentation.7 OCT imaging has also shown that a high proportion of CHM patients exhibit outer retinal tubulations (ORTs) peripheral to the central island (Figure 1C, arrowheads).4, 8
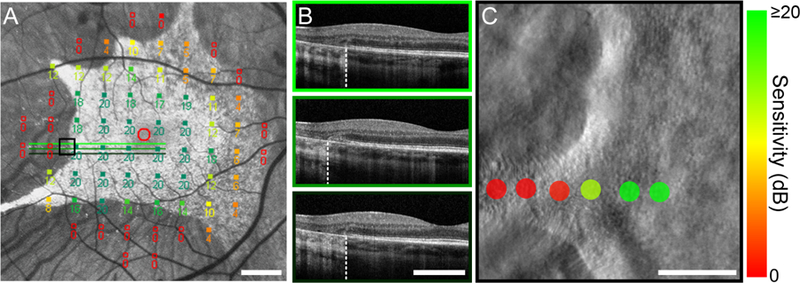
Figure 1. Clinical assessment of structure and function in choroideremia.
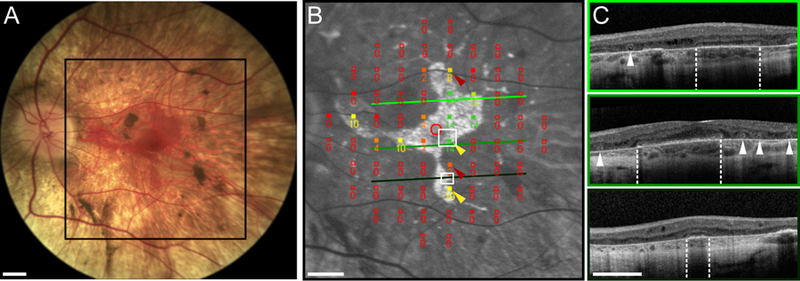
(A) Color fundus photograph from the left eye of subject 13125 showing a central island of preserved retina bordered by scalloped edges demarcating the sharp transition to depigmentation of the RPE and peripheral atrophy. (B) FAF image with 10–2 MP1 sensitivity values superimposed. The region shown here is denoted by the black box in (A). The hyper-fluorescent region corresponds to the autofluorescence signal and presumably delineates the boundaries of the viable retina. The red circle indicates the subject’s fovea. MP1 sensitivity values are shown numerically in decibels (dB); a sensitivity value of 0 dB indicates a dense scotoma at that location on the retina. Yellow arrowheads indicate locations where residual sensitivity appears to be preserved near the atrophic border, whereas red arrows highlight locations in the atrophic retina with reduced but still measurable sensitivity. Green scan lines indicate locations of the OCT B-scans shown in (C). White boxes outline regions where AO microperimetry was conducted (see Figure 2). (C) OCT B-scans corresponding to the lines in (B). Retinal lamination is mostly preserved in regions co-localized with a measurable autofluorescence signature; the OCT layer corresponding to the photoreceptor-RPE interdigitation zone is absent. Outer retinal structure changes abruptly at the atrophic border (white dashed lines) with loss of the OCT layers corresponding to the outer nuclear layer, the external limiting membrane, the ellipsoid zone (or inner segment/outer segment junction) and the RPE. ORTs, visible as hyperreflective bands encircling hyporeflective cores, are indicated by white arrowheads. Scale bars in all panels represent 3 degrees of visual angle.
At a broad level, loss of visual function in CHM progresses essentially in lockstep with the pattern of structural degeneration.4, 8 Initial symptoms include nyctalopia and peripheral visual field loss.5 Progressive functional loss proceeds centripetally and centrifugally from the midperiphery degeneration leading to tunnel vision and ultimately blindness. High visual acuity is commonly maintained and only becomes compromised in later stages of the disease, as degeneration encroaches on the central fovea.4, 9 Cone and rod sensitivity losses in CHM are detectable within the midperipheral and/or central retinal from the earliest disease stages.4 Clinical microperimetry shows that CHM patients have an absolute scotoma in the atrophic area corresponding to loss of the outer retina and RPE, but can have either normal or reduced sensitivities within the centrally retained retinal area.10, 11
Currently there are no approved treatments for CHM, although multiple gene therapies are currently being tested in clinical trials with some indications of success.12, 13 Gene therapeutic approaches aim to restore function to surviving cells. Thus, there is a critical need to understand both the extent to which cellular structure remains intact in CHM and how cellular structure correlates with visual function. Studies addressing these issues are critical for guiding the targeting and assessment of gene therapies (as well as other therapeutic approaches), and for maximizing the sensitivity of outcome measures for assessing therapeutic safety and efficacy.
Adaptive optics (AO) ophthalmoscopy has enabled the acquisition of retinal images with cellular resolution.14 AO studies in CHM have shown that the cone photoreceptor mosaic remains present and contiguous up to the atrophic border between the central island of preserved retina and the peripheral atrophy.6, 15, 16 Using adaptive optics scanning laser ophthalmoscopy (AOSLO), our group previously showed that some local regions within the retained central island exhibit normal cone density while others showed reduced cone density.6 While these studies provide important descriptions of the cellular structural phenotype of CHM, they have not related retained cellular structure to visual function at the same high spatial resolution.
Here, we measure both retinal structure and visual function in CHM with high spatial resolution. We combine AO with microperimetry to examine CHM patients’ visual sensitivity to very small stimuli targeted to retinal locations just inside the atrophic border (regions showing preserved photoreceptor structure) in comparison to retinal locations just outside the atrophic border (regions showing loss of the photoreceptor mosaic). Our results show a sharp transition in retinal function that closely follows the co-localized changes in photoreceptor structure. We further targeted small visual stimuli along ORTs and at retinal locations adjacent to ORTs but just inside the atrophic border. Our results again show a sharp transition in retinal function, with loss of sensitivity at retinal locations corresponding to ORTs.
Our results provide a better understanding of disease mechanisms at play in CHM, and have implications for applying both structural and functional outcome measures to assess CHM disease progression and treatment efficacy.
Methods
Subjects
12 male subjects with CHM (Table 1) participated in this study. Eight of these were part of an ongoing longitudinal study evaluating the efficacy of a gene therapy intervention. All data presented in this study from patients in the intervention group were collected from the untreated eye but were collected after the gene therapy had been delivered to the contralateral eye. This study was approved by the Institutional Review Board the University of Pennsylvania. All study procedures adhered to the principles enumerated in the Declaration of Helsinki. Prior to enrollment, the nature of the study and its associated risks were explained, and informed consent was obtained from each subject.
Table 1|.
Subject information
| Patient ID | Genetic Mutation | Age | Eye Tested | Axial Length (mm) | Visual Acuity | Test Eccentricity* (°) | Stimulus Diameter (arcmin) | Visit† |
|---|---|---|---|---|---|---|---|---|
| 13004 | CHM Deletion, Exon 1-hemizygous-EPP=3 | 53 | OS | 26.86 | 20/20–2 | 4 N | 3.5 | Visit 1 |
| 13035 | CHM Hemizygous c.315_318delTCAG | 21 | OD | 24.51 | 20/32–4 | 2 N | 7.0 | Visit 1 |
| 13048 | CHM Hemizygous c.700A>T/p.Lys234Term | 27 | OD | 24.39 | 20/20–2 | 6.2 T | 7.0 | Visit 1 |
| 13057 | CHM Hemizygous for the Novel c.745delT mutation | 36 | OS | 27.04 26.85 |
20/40 20/32–3 |
2.8 S-T 1.5 I |
3.5 3.5 |
Visit 1 +5 mos. |
| 13071 | CHM-Glu382Stop GAA>TAA hemizygous-Epp=3 | 35 | OD | 23.88 23.91 23.91 |
20/25 20/25–4 20/20–4 |
10.5 T 10.5 T 14 I-T |
3.5 3.5 7.0 |
Visit 1 +6 mos. +12 mos. |
| 13125 | CHM Hemizygous c.1437dupA/p.Glu480ArgfsX12 | 34 | OS | 23.42 23.47 |
20/20–2 20/25–4 |
1.25 I-T 4.2 I 6.5 S-T |
3.5 7.0 3.5 |
Visit 1 Visit 1 +6 mos. |
| 13131 | CHM-Met443 del2aacAT hemizygous-EPP=3 | 39 | OD | 24.99 24.97 24.97 |
20/20–2 20/25 20/25 |
4 S-N 4 S-N 0.8 T |
3.5 7.0 7.0 |
Visit 1 +6 mos. +6 mos. |
| 13159 | CHM-Glu382Stop GAA>TAA hemizygous-EPP=3 | 45 | OS | 25.25 25.25 |
20/25 20/25 |
3 N 1.1 S-N |
7.0 7.0 |
Visit 1 Visit 1 |
| 13195 | CHM-IVS6–2 A>G hemizygous-EPP=3 | 38 | OD | 23.44 | 20/20 | 6 T | 7.0 | Visit 1 |
| 13224 | CHM-Hemizygous c.41dupT/p.Gly15Argf sX6 | 58 | OS | 24.30 24.27 24.31 |
20/20 20/20 20/20 |
8 T 8 T 8 T |
7.0 7.0 7.0 |
Visit 1 +6 mos. +12 mos. |
| 13226 | CHM-IVS7–2 A>T hemizygous-EPP=3 | 41 | OS | 23.87 | 20/20–1 | 3.8 T | 3.5 | Visit 1 |
| 13249 | CHM-Phe282 del2gtcTT hemizygous – EPP=3 | 20 | OS | 24.14 | 20/20 | 5 N | 3.5 | Visit 1 |
eccentricity relative to fovea; S = superior; I = inferior; N = nasal; T = temporal
relative to Visit 1
Clinical Measurements of Retinal Structure and Function
Subjects underwent a comprehensive ophthalmic exam, including axial length measurement, fundus photography, spectral domain OCT cross-sectional imaging, short wavelength FAF imaging (Spectralis, Heidelberg Engineering, Carlsbad, CA), visual sensitivities with a commercially available instrument (Nidek, MP-1, Fremont, CA) using a conventional 10–2 protocol grid and an achromatic Goldman size III stimuli presented to the dark-adapted eye (30 min adaptation period) on a 1.27 cd/m2 background. Some subjects were able to return at a different date for additional testing, at which point, all procedures were repeated. Molecular testing by a CLIA laboratory was carried out to confirm the diagnosis of CHM. Subject information can be found in Table 1.
Imaging the Retina with Adaptive Optics Scanning Light Ophthalmoscopy
Prior to AOSLO imaging, subjects’ pupils were dilated using 1% tropicamide and 2.5% phenylephrine ophthalmic solutions. Once mydriasis was achieved, subjects were aligned to the AOSLO imaging beam using a bite bar fitted with a custom dental impression. Subject fixation was guided by a secondary light source viewed through a pellicle beam splitter.
The details of imaging the retina with a multimodal AOSLO have been described elsewhere.17, 18 The AOSLO system used in this study featured three input wavelengths: 848 nm (for wavefront sensing), 795 nm (for retinal imaging), and 550 nm (for visual stimulation; see below). To ensure each input channel would be well focused on the retina, the relative vergences of the three beams were adjusted to compensate for longitudinal chromatic aberration of the human eye according to normative data.19 The input beams were co-aligned prior to entry into the main optical path, where they were scanned across the retina in a raster pattern by vertical and horizontal scanning mirrors positioned in optical planes conjugate to the pupil. The light returning from the eye was descanned and entered the collection arm of the system. For 795 nm light, three photo-multiplier tubes (PMTs) were arrayed to detect incoming photons. One PMT was positioned behind a pinhole (1.13 Airy Disk Diameters [ADD]) to provide a confocal image of the cone mosaic, whereas the remaining PMTs were arranged in a non-confocal split-detector configuration to sense multiply-scattered light.18 The 550 nm collection channel also featured a confocal PMT (1.40 ADD pinhole) for concurrent imaging at the stimulus wavelength. The 848nm light was directed towards a Shack-Hartmann wavefront sensor to monitor ocular aberrations, which were corrected in closed-loop using a 97-actuator deformable mirror (DM97–15, ALPAO, Montbonnot-Saint-Martin, France).
The angular size of the imaging raster was determined by analyzing images of a Ronchi ruling acquired with either a horizontal or vertical orientation; the former was also used to measure and correct for the sinusoidal image distortion introduced by the horizontal resonant scanner. Visual angle units may be converted to microns on the retina using axial length measurements obtained with a Zeiss IOLMaster (Carl Zeiss Meditec, Jena, Germany) and provided in Table 1, according to previously published methods.20 For structural AO imaging, a 1-by-1 degree imaging raster was used; for AO microperimetry, the imaging field was expanded to subtend roughly 1.5-by-1.5 degrees (see Adaptive Optics Microperimetry below).
To assess cone mosaic structure, spatially overlapping AOSLO videos were acquired at and surrounding each retinal location tested with AO microperimetry. Each AOSLO video comprised 150 frames acquired at 16.7 Hz. Video frames were aggregated offline using custom strip-registration software.21 The near-infrared detector configuration enabled the concurrent acquisition of confocal (outer segments) and split-detector (inner segments) retinal images with cellular resolution.18
Adaptive Optics Microperimetry
The AOSLO used to image the retina in this study was also equipped with microperimetry capabilities—i.e., the ability to track the moving retina and deliver visual stimuli to targeted loci.22, 23 To achieve tracking, retinal videos were digitized by a field-programmable gate array (FPGA) board and relayed strip-by-strip to a graphics processing unit (GPU) for real-time stabilization and retinal position estimation via strip-based registration to a reference image 24. The stabilized videos were displayed in a custom video rendering interface, and retinal locations targeted for perimetric testing were selected manually in stabilized image coordinates by the examiner.25 Stimuli were delivered pixel-by-pixel by modulating the intensity of the scanned stimulus beam with an acousto-optic modulator (AOM; Brimrose Corp., Sparks, MD, USA). The timing of stimulus delivery was guided by the GPU, which used retinal position information to generate a prediction of when the scanned beam would encounter the retinal target and, thus, when the AOM should be triggered by the FPGA board. Delivery predictions were generated independently for each frame of stimulus delivery. Delivery locations were encoded directly into each delivery frame by inserting a digital marker at the image pixel corresponding to the center of the rendered stimulus.
For AO microperimetry, the imaging field size was set to 1.5-by-1.5 degrees. The rationale behind this choice is that the image-based eye tracking algorithm requires some overlap between the reference image and subsequent video frames to achieve a successful registration. The image shift, in pixels, associated with a fixational eye movement of fixed magnitude will increase as the imaging field size decreases (assuming the pixel sampling rate remains constant). By contrast, imaging over larger fields can also hamper image-based retinal tracking due to degradations in image quality associated with undersampling and the difficulty of correcting high-order ocular aberrations across non-isoplanatic fields. The 1.5° imaging field size used in this study thus represents a practical middle ground, providing sufficient resolution for the image-based retinal tracking algorithm while still remaining tolerant of ordinary fixational eye motion, ultimately producing fewer tracking failures and more successful stimulus deliveries.
Microperimetry stimuli were 550 ± 15 nm (chosen to equate the sensitivity of the long-and middle-wavelength-sensitive cone photoreceptors) and circular in shape, with diameters of either 3.5 or 7.0 arcmin. For comparison, the standard Goldmann III stimulus used routinely in clinical microperimetry subtends approximately 26 arcmin (0.43°). Note also that because our stimuli are delivered through the AO system, their retinal size is not increased because of blurring by the eye’s normal optical point spread function. Stimulus size was chosen by the examiner based on the test eccentricity and a preliminary assessment of the local visual sensitivity. Specifically, at the beginning of each session, one or two 20-trial practice runs were conducted to familiarize the subject with the response paradigm (see below). For these trials, retinal tracking was disabled to ensure the stimulus was delivered on every trial (albeit at slightly different retinal locations from one trial to the next, depending on fixational eye movement). Stimuli in the practice runs were centered away from the atrophic border so that most trials landed in the contiguous cone mosaic. Stimulus size was deemed appropriate if the threshold algorithm (see below) converged on a value near the lower-half of the dynamic range of the system; this allowed enough headroom for higher thresholds to be measured as the lesion border was approached. If the preliminary threshold estimate was near the upper end of the system’s dynamic range, we opted for the larger stimulus size to bring the thresholds down. Note that for a given subject and eccentricity, stimulus size was kept constant for all test locations.
For each test location, increment threshold was determined using a 20-trial adaptive staircase26 guided by a yes-no response paradigm and set to converge on the test intensity producing a 78% frequency-of-seeing. Subject responses were recorded using a programmable game controller. The subject initiated each trial by button press, which triggered the recording of a one-second retinal video during which the stimulus was delivered for three frames (187.5 ms) to the targeted location. Stimulus delivery was accompanied by an audible cue, and delivery locations were encoded into the retinal videos via a digital marker corresponding to the center pixel of the AOM-rendered stimulus. Subjects indicated whether the stimulus was “seen” or “not seen” by pressing buttons corresponding to those responses on the game controller. A “repeat trial” button was also available, although its use was reserved for instances where the examiner observed failed deliveries during the experiment (e.g., due to blinks, tear film evaporation, drifts in fixation, or retinal tracking software failures). Subjects were encouraged not to repeat trials unless instructed to do so by the examiner. At each test location, at least three 20-trial staircases were obtained.
After testing, the delivery location of each stimulus was identified by locating the digital marker inserted into the video on the stimulus frames. For a trial to be included in subsequent analyses, the delivery location for each stimulus frame had to fall within a 15-by-15 arcmin inclusion window centered on the median delivery coordinates for all deliveries at that test location. Psychometric functions were fit to the remaining data using the Palamedes toolbox27; threshold was defined as the test intensity that yielded 78% frequency-of-seeing. In cases where 8 or more trials at the maximum deliverable stimulus intensity were not detected by the subject, a sensitivity value of 0 dB was assigned (i.e. a dense scotoma at the modulation limits of the system). For all other test locations, measurements where fewer than half of the total trials (i.e., 30 out of 60, typically) were delivered accurately were excluded altogether. Threshold intensities (It) were converted to sensitivity in decibels (SdB) using the following formula: SdB = 10 * log10(Imax/It), where Imax represents the maximum stimulus intensity (~580 cd/m2; Weber contrast = 42.9) the system is capable of presenting. For comparison, the Zeiss Humphrey Field Analyzers HFA3 and the Nidek MP1 microperimeter have maximal stimulus luminances of ~3000 cd/m2 and ~125 cd/m2, respectively.28 The background on which the incremental stimuli were presented was a combination of baseline light leak through the 550 nm AOM, the infrared imaging light, and the dim wavefront sensing light; its cumulative luminance was approximately 13.5 cd/m2, the majority of which was caused by the infrared imaging light. The dynamic range of stimulus modulation was 24 dB; the dimmest non-zero stimulus corresponded to a Weber contrast of 0.16.
Transverse chromatic aberration (TCA) causes a displacement between the imaging (848 nm) and stimulus (550 nm) beams at the retinal plane. Because AO microperimetry test locations were selected in the coordinates of the infrared image used for retinal tracking, TCA must be accounted for to enable a precise correlation of visual sensitivity to the underlying mosaic structure. Before and after each measurement session, TCA was estimated using a variant of a previously-published approach.29 For each measurement, four 40-frame AOSLO videos were acquired concurrently at the imaging and stimulus wavelengths. The spatial offsets between corresponding infrared and visible-light video frames were estimated using full-frame image registration.30 The resultant shifts, in the x and y image dimensions, are a byproduct of the laterally-displaced imaging and stimulation beams on the retina. TCA was defined as the median x-and y-shift between each frame pair measured across the quartet of videos (160 samples total). The TCA shift information was used in offline analyses to refine the estimate of stimulus delivery locations relative to the structures observed the infrared retinal images. Note that imaging at the stimulus wavelength requires using light intensities that cause photopigment bleaching. To allow the subjects to readapt, the eye selected for testing was patched for 10 minutes prior to beginning data collection.
In all 12 subjects, visual sensitivity was assessed at test locations spanning a lesion boundary. To quantify the gradient of sensitivity near the atrophic border, the distance from each test location to the nearest lesion border was estimated. This was done by placing a measurement circle centered on each test location in the confocal image and expanding it concentrically until the examiner judged that it encroached on the lesion; the distance to the atrophic border was taken as the radius of this circle. We note that while the atrophic retina in CHM is readily distinguishable from regions containing clear evidence of cone photoreceptors (i.e. hexagonal packing), structural heterogeneity in the narrow transition zone makes it difficult to apply an objective, image-based criterion for what constitutes the exact location of the atrophic border in CHM.
In four subjects with whom we had additional time for testing, we also examined visual sensitivity along ORTs protruding from the atrophic border. There were no systematic differences between the subset selected for ORT testing and the larger cohort with respect to age, central visual acuity, or extent of visual field (atrophic border locations can be inferred from the test eccentricity column in Table 1).
Results
An example of the typical clinical assessment of retinal structure and function in a patient with CHM is shown in Figure 1. Color fundus photography (Figure 1A) revealed a characteristic posterior pole appearance, with a central island of preserved retina featuring scalloped edges. In the circumjacent region, the retina and RPE were degenerated and deeper choroidal structures and white sclera were visible. FAF imaging (Figure 1B) more clearly delineated these features, with a central region of preserved FAF signal surrounded by hypo-FAF and background autofluorescence areas. Microperimetry (Nidek MP1, 10–2 test pattern) sensitivity values were generally well-correlated with the underlying, relatively preserved retina and RPE, with some exceptions near the atrophic border (Figure 1B, red arrowheads). OCT imaging (Figure 1C) showed relatively preserved retinal lamination in the intact retina, with an abrupt transition in thickness occurring at the atrophic borders (Figure 1C, white dashed lines). ORTs were evident in regions with severely thinned but detectable outer nuclear layer (ONL overlaying severely depigmented RPE, appearing in the OCT images as hyperreflective rings encircling hyporeflective cores (Figure 1C, white arrowheads).
Multi-modal adaptive optics ophthalmoscopy demonstrated that contiguous mosaics of cone photoreceptors were maintained up to the edge of the atrophic border. Example images from two retinal eccentricities acquired from subject 13125 are shown in Figure 2. In the confocal images (left column), the tightly-packed bright spots occupying the majority of the images represent light reflected from individual cone photoreceptor outer segments. The co-localized features observed in the split-detector images acquired simultaneously (right column) are presumed to correspond to multiply-scattered light arising from the cone inner segment.18 In the confocal images, the transition to the atrophic retina was often marked by a hyporeflective band in which cones appeared dim or not visible, followed by a hyperreflective region with a speckled appearance. By contrast, split-detector imaging revealed more phenotypic heterogeneity in outer retinal structure at the margin. In some cases, there appeared to be cones with a disrupted orientation along the border, resembling felled trees (Figure 2, top-right panel). The furrowed appearance of the circumlesional region has been termed “variegated structure” by previous investigators.31 Elsewhere, the transition from relatively normal inner segment mosaic structure to the atrophic retina was more abrupt (Figure 2, bottom-right panel).
Figure 2. Cellular-scale retinal structure and function at the atrophic border measured with AOSLO.
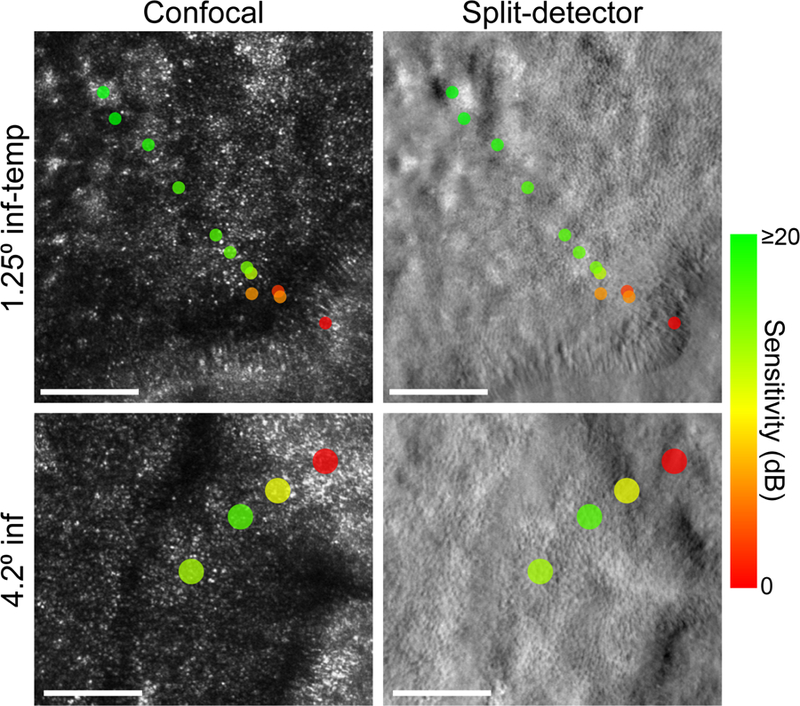
Spatially-registered confocal (left column) and split-detector (right column) AOSLO images from the left eye of subject 13125. The top row images are centered roughly 1.25 degrees inferior-temporal from the fovea. The bottom row images were acquired 4.2 degrees from the fovea in the inferior retina. These retinal regions are outlined in white on the FAF image in Figure 1B. Colored markers indicate the visual sensitivity at each AO microperimetry test location. Markers are color-coded to reflect the measured sensitivity (in dB) and scaled to represent the size of the microperimetry stimulus on the retina (upper row: 3.5 arcmin diameter; bottom row: 7.0 arcmin diameter). Stimulus markers were placed after accounting for transverse chromatic aberration between the imaging and stimulus wavelengths. The scale bar represents the retinal diameter of the Goldmann III perimetry stimulus (0.43 degrees) routinely used in clinical microperimetry.
AO microperimetry offers the stimulation resolution to probe cone-mediated function across these cellular-scale phenotypic variations. Color-coded markers representing the measured sensitivity of the underlying retina are shown in Figure 2. On this color scale, greenish markers indicate areas where sensitivity was preserved, while red markers represent locations where the subject was unable to detect the highest-intensity stimulus (i.e. scotomas). At both eccentricities shown in Figure 2, sensitivity was maintained in regions where the cone mosaic structure was relatively intact. As the atrophic border was approached, sensitivity dropped, and dense scotomas were typically observed at and beyond the structural margin. Additional examples of how sensitivity changes across the atrophic border in CHM are shown in Figure 3. Note that the drop in sensitivity with change in test location is very rapid, and can occur across a retinal region that is commensurate with the size of the Goldman III stimulus routinely used in clinical microperimetry. Across all included trials, the precision of stimulus delivery (defined as the standard deviation of the Euclidean distance from each delivery location to the center of the inclusion window) was 1.25 arcmin, or approximately 6.1 µm.
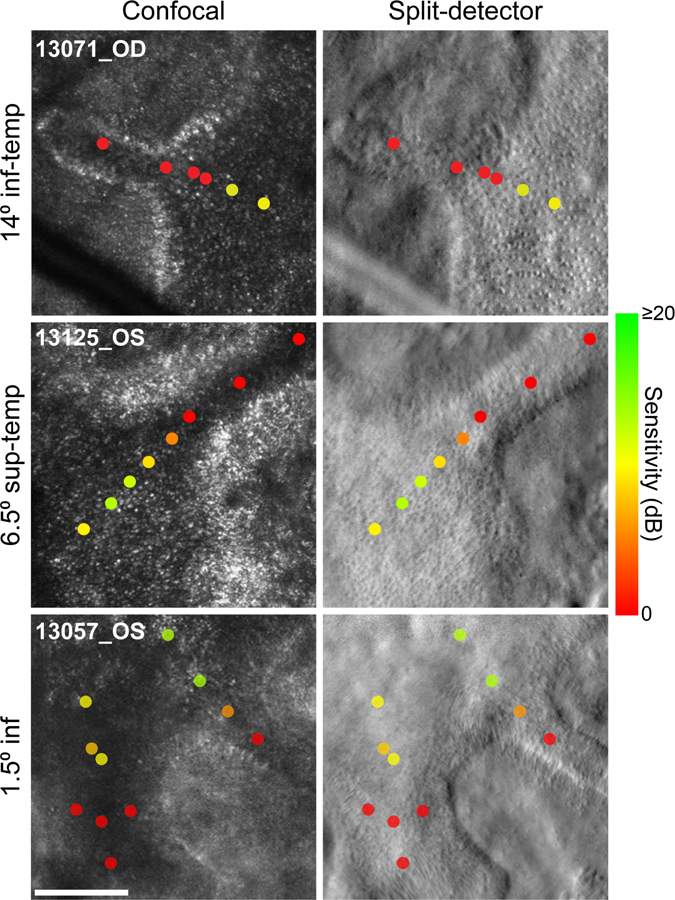
Figure 3. Additional examples of the sensitivity gradient at the atrophic border in CHM.
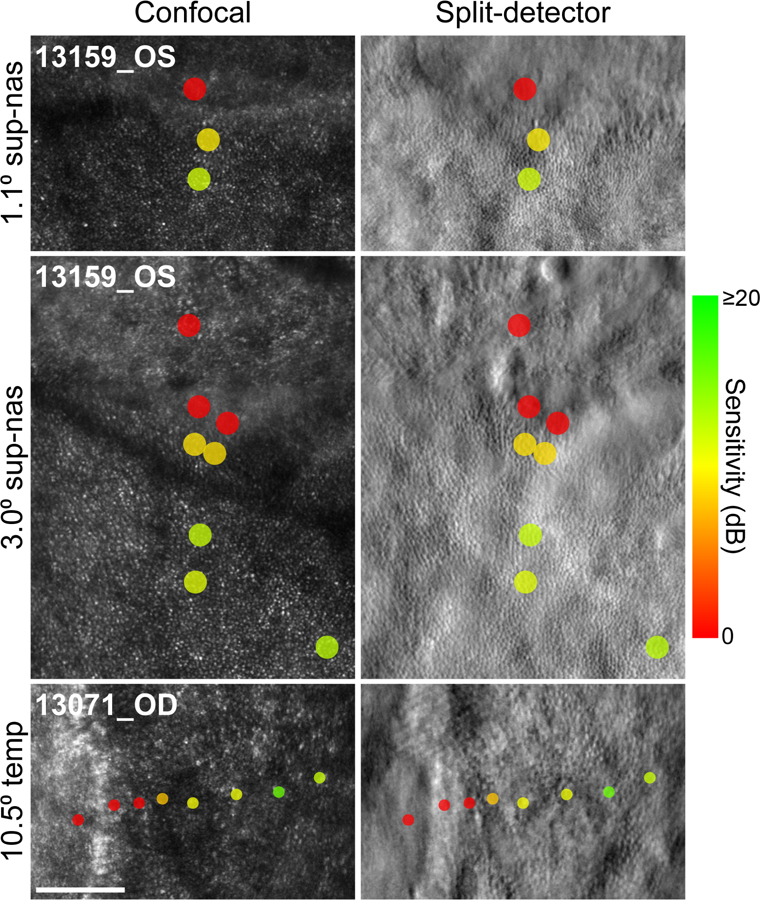
As in Figure 2, the left column shows confocal images and the right column contains the corresponding split-detector images. The test eccentricity, relative to the center of each image, is indicated by the text to the left of each row. Sensitivity markers are scaled and color coded as described in Figure 2. In the top and middle panels, the atrophic border runs across the upper section of the image; in the bottom panels, the atrophic border is oriented vertically along the left side of the image. In all three cases, visual sensitivity is maintained in areas with clearly-resolved photoreceptors and drops off steeply as the atrophic border is approached. Dense scotomas were measured at all test points located entirely within the severely degenerated retina. Scale bars in all panels depict the size of the Goldmann III stimulus (0.43°).
Together, these results suggest that the gradient of sensitivity at the outer retinal transition zone in CHM is steep. To examine this relationship more quantitatively, we plotted visual sensitivity as a function of the distance to the atrophic border for each test point from our 12 subjects (Figure 4), excepting the measurements that were obtained across ORTs (see Figures 5 and 6). One striking feature of these data was that essentially every test site located within the atrophic retina (negative distances in Figure 4) exhibited a dense scotoma (i.e., 0 dB sensitivity), including those situated directly adjacent to the atrophic border (red line). Moving into the preserved retina, sensitivity recovered sharply with increasing distance from the lesion margin, often improving by 10 dB (i.e. 1 log unit) or more across the distance subtended by the Goldmann III stimulus used in clinical perimetry (gray shaded area, ~130 µm). It is worth noting that we made no attempt in this summary analysis to account for inter-subject differences in test eccentricity, stimulus size, or stage of degeneration, all of which impact visual sensitivity and could introduce additional variability from subject to subject. Despite this, our aggregate results demonstrate clearly that visual sensitivity falls precipitously at the atrophic border in CHM.
Figure 4. Visual sensitivity gradient at the atrophic border in CHM.
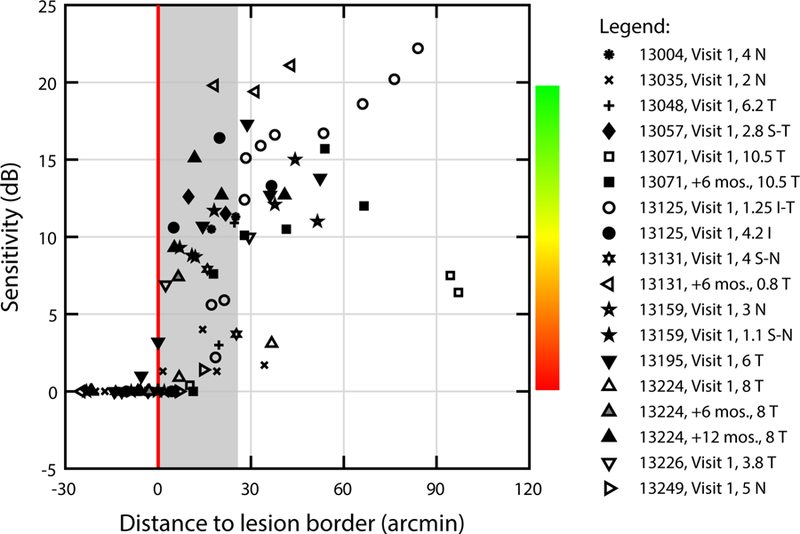
Visual sensitivity is plotted as a function of distance to the lesion border (red line) for all subjects. Sensitivity is shown in dB; the color scale used elsewhere in this report is included for reference. Points to the left of the red line are located within the lesion. The gray shaded area represents the diameter of the Goldmann III stimulus (0.43°).
Figure 5. Reduced visual sensitivity along an ORT.
(A) MP1 10–2 microperimetry map registered to the FAF image from the right eye of subject 13195. Green scan lines show the location of the cross-sectional OCT images in (B) obtained near an ORT protruding from the atrophic border in the temporal retina (~6°). The black box outlines the region shown at higher resolution in (C). (B) OCT B-scans acquired along the atrophic border, the location of which is indicated by the dashed white line. The middle OCT scan passes through the longitudinal axis of the ORT. (C) Adaptive optics microperimetry visual sensitivity markers shown on the split-detector AOSLO image. Sensitivity markers are scaled and color coded as described in Figure 3. Photoreceptor inner segments appear to be preserved well into the ORT, but sensitivity falls off steeply with increasing distance from the base of the ORT. Scale bars in (A) and (B) are 3°; in (C), scale bar represents the Goldmann III stimulus (0.43°).
Figure 6. Additional examples of dense scotomas along ORTs.
Adaptive optics microperimetry results for test stimuli delivered along ORTs in three subjects. Cones and variegated structures are observed in the split-detection AO images. In our apparatus, confocal AO images do not show clearly waveguiding cones within the ORTs. All conventions as in Figure 3.
ORTs are a second phenotypic hallmark of outer retinal degenerations with prominent associated RPE degeneration, including CHM. All 12 subjects in the present study exhibited ORTs visible on OCT. Although histological32 and high-resolution retinal imaging16, 31 evidence has accumulated to demonstrate that photoreceptor structure is partially preserved in ORTs, the degree to which these remnant cones might support visual function is not clear. Figure 5 shows imaging and adaptive optics microperimetry data obtained along an ORT in subject 13195. Consistent with other subjects with CHM, FAF imaging and MP1 sensitivities suggested a sharp structural and functional transition at the atrophic border (Figure 5A). In the temporal retina, OCT images (Figure 5B) revealed the cross-sectional structure at the atrophic border. In the middle OCT image, the ELM appears to descend towards Bruch’s membrane, suggesting the scan passed through the longitudinal axis of an ORT protruding temporally. A split-detector image obtained from this region confirmed the presence of an ORT in which remnant photoreceptor inner segments appeared to be present (Figure 5C). AO microperimetry data collected along the ORT demonstrated a steep decline in sensitivity with increasing distance from the preserved retina, with dense scotomas (red markers) measured at the most distal loci. A similar pattern of sensitivity along ORTs was observed in three additional subjects (Figure 6).
Discussion
The centripetal pattern of central photoreceptor loss in CHM implies that cones situated near the atrophic border may be next to succumb to degeneration. Previous structural studies using adaptive optics ophthalmoscopy have shown that contiguous cone mosaics are preserved close to the atrophic border6, 15, 16, 31 but the degree to which these cones on the healthy side of the border support visual function has been a critical and heretofore unanswered question. Using adaptive optics microperimetry to assess visual function with cellular precision, we found a tight relation between structure and function at the atrophic border in CHM: photopic visual sensitivity was preserved in regions with a contiguous cone mosaic while dense scotomas were invariably observed in atrophic areas (Figure 4). Unlike steep transitions in visual sensitivity documented in conventional clinical microperimetry, AO microperimetry demonstrated a gradient of sensitivity loss across the transition zones that co-localized with a progressively disorganized cone mosaic. This finding suggests the structural disorganization of the cone photoreceptor mosaic with photoreceptor loss and cone outer segment shortening and/or loss underlies the cone sensitivity losses in CHM.
This finding not only has implications for our understanding of how CHM progresses naturally, but also illustrates how adaptive optics microperimetry may fit into the complement of tests used to evaluate the efficacy of novel treatments intended to slow down or reverse its course. A number of clinical trials investigating gene therapies for CHM are currently ongoing, with some promising early results.12, 13 The prospects for preserving or expanding the area of visual function with these treatment approaches hinge on whether dysfunctional border cones retain a capacity for driving downstream circuitry. Our finding of reduced-but-measurable sensitivities within 30 arcmin (~150 µm) of the atrophic retina suggests that improving function at the margin of the intact retina with therapeutic interventions may indeed be possible. Further, our finding of dense scotomas at and beyond the atrophic border suggests that structural measurements, such as the width of intact outer retinal layers on OCT or the area of a continuous photoreceptor mosaic on AO imaging, may be appropriate for use as outcome measures for assessing therapeutic safety and efficacy. The fine-grained functional testing offered by the AO microperimetry approach comes at the cost of limited retinal coverage and high technical complexity, making it difficult to envision its translation into routine clinical use. Nevertheless, our results highlight how clarifying structure-function relationships at the cellular scale in a small experimental cohort may help validate the inferences made using ophthalmic devices (e.g. OCT) better suited for large-scale studies.
The sensitivity with which any measurement can ascribe a gain (or loss) of function to a treatment is critically limited by the measurement’s test-retest variability. In the macula of CHM patients, the repeatability of conventional microperimetry measurements obtained within the preserved retina is roughly ±5 dB.10, 33 However, near the atrophic border, test-retest variability increases twofold, to ±10 dB.10 This outcome could reflect genuine functional variability along the atrophic border, or it could be due to limitations in the precision with which border regions are stimulated. In normal subjects, test-retest variability is substantially higher for test points near the optic nerve head compared to data obtained from the macular and peripapillary regions,34 suggesting that the poor repeatability stems in part from some stimuli inadvertently landing in scotomatous locations. Likewise, in our own clinical microperimetry data in CHM, we occasionally observed test loci within the atrophic zone that appeared to retain residual sensitivity (Figure 1, red arrows). This could have been due to errant stimulus deliveries. The cellular-scale delivery precision of adaptive optics microperimetry 22, 24 helps resolve this ambiguity: nearly every atrophic location we tested with this technique exhibited a dense scotoma, even those positioned directly adjacent to contiguous cone mosaics (Figure 4). Although practical considerations precluded us from conducting a formal analysis of test-retest variability in the current study, our data suggest that the repeatability of adaptive optics microperimetry measurements near degenerated retina will be less influenced by delivery uncertainty, compared to data acquired with conventional perimetry devices.
Our results also provide new insights about the functional integrity of remnant cones located within ORTs,31 a phenotypic signature encountered in many outer retinal degenerations with potential RPE involvement, including CHM.8, 35 Evidence from histology 32, 36 and longitudinal OCT imaging 37 has led to a mechanistic model of ORT development in which RPE degeneration in regions with photoreceptor degeneration triggers Müller glia cells to guide a descent and eventual subduction of the ELM, ultimately forming a tubular structure containing photoreceptors oriented radially towards the lumen. Abnormal cones with preserved inner and outer segments, presumably in a nascent stage of degeneration, have been observed in ORTs in histological preparations; as the degeneration progresses to end-stage, both inner and outer segments are eventually lost.32
Despite the findings demonstrating that the cellular morphology of cones situated in ORTs can, in some cases, remain grossly intact, our measurements provided no evidence of visual sensitivity for stimuli delivered along ORTs (Figures 5 and 6). While we cannot exclude the possibility that larger or more intense stimuli may have evoked a residual response from these cones, our results indicate that visual sensitivity within ORTs is, at minimum, severely impoverished. This is perhaps not surprising, considering that the functional viability of these photoreceptors would depend on additional factors, the most obvious being the existence of healthy synaptic contacts with synaptic partners in the inner nuclear layer. Whether functional synapses between affected cones and downstream bipolar cells are preserved after ORT formation is not well-established. In addition, healthy cones act as optical waveguides, funneling incident light into the outer segment for phototransduction. Similar to previous results,16, 31 we found evidence of cone inner segments in ORTs using the split-detector modality (Figures 5 and 6). In the corresponding confocal images, however, cone mosaics were not well-resolved. While this finding is consistent with the idea that the infolded architecture of ORTs disrupts normal photoreceptor waveguiding,38 we cannot exclude the possibility that adjustments to the confocal imaging parameters (e.g. changes to the detection aperture size or imaging focal depth) might reveal waveguiding cones not visible in the current images.31 Finally, normal photoreceptors depend on the RPE for sustenance and regeneration of the visual pigment. Longitudinal imaging studies have demonstrated that ORTs can remain stable over time,16, 31 however, leading to the hypothesis that fluid exchange with nearby intact retina might provide some replacement trophic support in the absence of underlying RPE.36 It is not clear if this nourishment would be sufficient to maintain an operational visual cycle. For example, previous work suggests that cone photoreceptors exhibit delayed dark adaptation in choroideremia39; exposure to higher light levels (such as those used to measure TCA prior to testing) might differentially affect the sensitivity of any remnant cones within the ORT.
The present results add to an emerging body of work in which adaptive optics has been leveraged to examine, at the cellular scale, the relationship between structure and function in retinal disease. In the first clinical application of high-resolution perimetry with adaptive optics, Makous et al.40 analyzed psychometric functions for detecting cone-sized stimuli delivered without retinal tracking to infer the presence of microscotomas in a dichromatic patient whose retina was missing roughly 30% of its cones. The incorporation of retinally-contingent stimulus delivery methods into the AOSLO imaging modality 22 has enabled more direct comparisons of structure and function, including a few examples where the link between the two was counterintuitive. In one patient with type 2 macular telangiectasia, cone-mediated sensitivity was measured in locations where the confocal AO images suggested cone photoreceptors were absent.41 Longitudinal AOSLO imaging in the same patient demonstrated how cone reflectivity in the confocal modality can be capricious, with a contiguous mosaic of cones appearing in a region near the temporal lesion border that previous images had suggested was devoid of cones. More recently, further evidence for abnormally-reflecting cones with residual function (termed “dysflective” cones) was reported in a patient with acute bilateral foveolitis.42 In the current results, the link between cone mosaic structure and function was more straightforward, with no evidence of preserved visual sensitivity in regions where cones were not resolved. Collectively, however, these diverse findings underscore how incorporating functional testing at a cellular scale helps refine and guide our interpretation of the ambiguous structures often observed in high-resolution images of the diseased retina.
In summary, we used AO microperimetry to reveal a steep gradient in cone-mediated visual sensitivity across the atrophic border in choroideremia. We also found precipitous drops in sensitivity when stimuli were delivered along ORTs that appeared to contain cone inner segments. In the future, cone-targeted microperimetry may be combined with other recently-developed AOSLO techniques for probing photoreceptor function, including cellular-scale measurements of rod sensitivity43 and optophysiological assays of cone function,44 to provide a more complete assessment of outer retinal integrity in retinal dystrophy.
Acknowledgements:
The authors thank Robert F. Cooper for technical assistance, Alfredo Dubra for sharing the AOSLO optical design, as well as AO control, image acquisition and image registration software, and Austin Roorda for sharing the AO microperimetry design and software.
Financial Support:
This manuscript was supported by NIH R01EY028601, NIH U01EY025477, NIH P30 EY001583, Research to Prevent Blindness Stein Innovation Award, Foundation Fighting Blindness, the F. M. Kirby Foundation, the Paul and Evanina Mackall Foundation Trust, Pennsylvania Lions Sight Conservation, Eye Research Foundation, Brenda and Matthew Shapiro Stewardship, and the Robert and Susan Heidenberg Investigative Research Fund for Ocular Gene Therapy. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest:
JIWM is an inventor on US Patent 8,226,236 and receives funding from AGTC. WST is an inventor on US Patent 10,130,253. JIWM, WST, and DHB have filed a provisional patent. JB and AMM have a patent licensed by Spark Therapeutics, but waived potential financial gain. JB serves as an advisor for Gensight Biologics, Perch Therapeutics and Akouos, and receives funding from Biogen and Limelight. AMM receives funding from Spark Therapeutics. All other authors report no conflict of interest.
Abbreviations:
- ADD
Airy disk diameters
- AO
adaptive optics
- AOM
acousto-optic modulator
- AOSLO
adaptive optics scanning laser ophthalmoscopy
- CHM
Choroideremia
- dB
decibels
- ELM
external limiting membrane
- FAF
fundus autofluorescence
- OCT
optical coherence tomography
- ORT
outer retinal tubulation
- PMT
photo-multiplier tube
- REP1
rab escort protein 1
- RPE
retinal pigment epithelium
- TCA
transverse chromatic aberration
Footnotes
Meeting presentation:
Results from this study were presented in part at the Association for Research in Vision and Ophthalmology 2018 annual meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDonald I, Hume S, Chan S, et al. GeneReviews® [Internet] In: Adam MPAH, Pagon RA, et al. , editors., ed. Seattle (WA): University of Washington, Seattle, 2003. February 21 [Updated 2015 Feb 26]; v. 1993–2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1337/. [Google Scholar]
- 2.McTaggart KE, Tran M, Mah DY, et al. Mutational analysis of patients with the diagnosis of choroideremia. Human mutation 2002;20(3):189–96. [DOI] [PubMed] [Google Scholar]
- 3.Preising M, Ayuso C. Rab escort protein 1 (REP1) in intracellular traffic: a functional and pathophysiological overview. Ophthalmic genetics 2004;25(2):101–10. [DOI] [PubMed] [Google Scholar]
- 4.Aleman TS, Han G, Serrano LW, et al. Natural History of the Central Structural Abnormalities in Choroideremia: A Prospective Cross-Sectional Study. Ophthalmology 2017;124(3):359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic genetics 2012;33(2):57–65. [DOI] [PubMed] [Google Scholar]
- 6.Morgan JI, Han G, Klinman E, et al. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Investigative ophthalmology & visual science 2014;55(10):6381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson SG, Cideciyan AV, Sumaroka A, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Investigative ophthalmology & visual science 2006;47(9):4113–20. [DOI] [PubMed] [Google Scholar]
- 8.Heon E, Alabduljalil T, McGuigan ID, et al. Visual Function and Central Retinal Structure in Choroideremia. Investigative ophthalmology & visual science 2016;57(9):OCT377–87. [DOI] [PubMed] [Google Scholar]
- 9.Coussa RG, Kim J, Traboulsi EI. Choroideremia: effect of age on visual acuity in patients and female carriers. Ophthalmic genetics 2012;33(2):66–73. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos IS, Tseng C, MacDonald IM. Microperimetry as an Outcome Measure in Choroideremia Trials: Reproducibility and Beyond. Investigative ophthalmology & visual science 2016;57(10):4151–61. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos IS, Freund PR, Knowles JA, MacDonald IM. The Natural History of Full-Field Stimulus Threshold Decline in Choroideremia. Retina 2018;38(9):1731–42. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren REG,M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FPM, Black GCM, Lotery AJ, Downes SM, Webster AR, Seabra MC Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. The Lancet 2014. [DOI] [PMC free article] [PubMed]
- 13.Edwards TL, Jolly JK, Groppe M, et al. Visual Acuity after Retinal Gene Therapy for Choroideremia. The New England journal of medicine 2016;374(20):1996–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roorda A, Romero-Borja F, Donnelly WJ III, et al. Adaptive optics scanning laser ophthalmoscopy. Optics Express 2002;10(9):405–12. [DOI] [PubMed] [Google Scholar]
- 15.Syed R, Sundquist SM, Ratnam K, et al. High-resolution images of retinal structure in patients with choroideremia. Investigative ophthalmology & visual science 2013;54(2):950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun LW, Johnson RD, Williams V, et al. Multimodal Imaging of Photoreceptor Structure in Choroideremia. PloS one 2016;11(12):e0167526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubra A, Sulai Y. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomedical optics express 2011;2(6):1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scoles D, Sulai YN, Langlo CS, et al. In vivo imaging of human cone photoreceptor inner segments. Investigative ophthalmology & visual science 2014;55(7):4244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atchison DA, Smith G. Chromatic dispersions of the ocular media of human eyes. Journal of the Optical Society of America A, Optics, image science, and vision 2005;22(1):29–37. [DOI] [PubMed] [Google Scholar]
- 20.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 1994;232(6):361–7. [DOI] [PubMed] [Google Scholar]
- 21.Dubra A, Harvey Z. Registration of 2D Images from Fast Scanning Ophthalmic Instruments. Lecture Notes in Computer Science 2010;6204:60–71. [Google Scholar]
- 22.Tuten WS, Tiruveedhula P, Roorda A. Adaptive optics scanning laser ophthalmoscope-based microperimetry. Optometry and vision science : official publication of the American Academy of Optometry 2012;89(5):563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuten WS, Cooper RF, Tiruveedhula P, et al. Spatial summation in the human fovea: Do normal optical aberrations and fixational eye movements have an effect? Journal of vision 2018;18(8):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arathorn DW, Yang Q, Vogel CR, et al. Retinally stabilized cone-targeted stimulus delivery. Optics express 2007;15(21):13731–44. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, Arathorn DW, Tiruveedhula P, et al. Design of an integrated hardware interface for AOSLO image capture and cone-targeted stimulus delivery. Optics express 2010;18(17):17841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King-Smith PE, Grigsby SS, Vingrys AJ, et al. Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision research 1994;34(7):885–912. [DOI] [PubMed] [Google Scholar]
- 27.Prins N, Kingdom FAA. Applying the Model-Comparison Approach to Test Specific Research Hypotheses in Psychophysical Research Using the Palamedes Toolbox. Frontiers in psychology 2018;9:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acton JH, Bartlett NS, Greenstein VC. Comparing the Nidek MP-1 and Humphrey field analyzer in normal subjects. Optometry and vision science : official publication of the American Academy of Optometry 2011;88(11):1288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmening WM, Tiruveedhula P, Roorda A, Sincich LC. Measurement and correction of transverse chromatic offsets for multi-wavelength retinal microscopy in the living eye. Biomedical optics express 2012;3(9):2066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guizar-Sicairos M, Thurman ST, Fienup JR. Efficient subpixel image registration algorithms. Optics letters 2008;33(2):156–8. [DOI] [PubMed] [Google Scholar]
- 31.King BJ, Sapoznik KA, Elsner AE, et al. SD-OCT and Adaptive Optics Imaging of Outer Retinal Tubulation. Optometry and vision science : official publication of the American Academy of Optometry 2017;94(3):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaal KB, Freund KB, Litts KM, et al. OUTER RETINAL TUBULATION IN ADVANCED AGE-RELATED MACULAR DEGENERATION: Optical Coherence Tomographic Findings Correspond to Histology. Retina 2015;35(7):1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolly JK, Xue K, Edwards TL, et al. Characterizing the Natural History of Visual Function in Choroideremia Using Microperimetry and Multimodal Retinal Imaging. Investigative ophthalmology & visual science 2017;58(12):5575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Jung CJ, Ayton LN, et al. Test-Retest Repeatability of Microperimetry at the Border of Deep Scotomas. Investigative ophthalmology & visual science 2015;56(4):2606–11. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg NR, Greenberg JP, Laud K, et al. Outer Retinal Tubulation in Degenerative Retinal Disorders. Retina 2013. [DOI] [PMC free article] [PubMed]
- 36.Litts KM, Messinger JD, Dellatorre K, et al. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA ophthalmology 2015;133(5):609–12. [DOI] [PubMed] [Google Scholar]
- 37.Dolz-Marco R, Litts KM, Tan ACS, et al. The Evolution of Outer Retinal Tubulation, a Neurodegeneration and Gliosis Prominent in Macular Diseases. Ophthalmology 2017;124(9):1353–67. [DOI] [PubMed] [Google Scholar]
- 38.Litts KM, Wang X, Clark ME, et al. Exploring Photoreceptor Reflectivity through Multimodal Imaging of Outer Retinal Tubulation in Advanced Age-Related Macular Degeneration. Retina 2017;37(5):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mura M, Sereda C, Jablonski MM, et al. Clinical and functional findings in Choroideremia due to complete deletion of the CHM gene. Arch Ophthalmol 2007; 125(8):1107–1113. [DOI] [PubMed] [Google Scholar]
- 40.Makous W, Carroll J, Wolfing JI, et al. Retinal microscotomas revealed with adaptive-optics microflashes. Investigative Ophthalmology & Visual Science 2006;47(9):4160–7. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Tuten WS, Lujan BJ, et al. Adaptive optics microperimetry and OCT images show preserved function and recovery of cone visibility in macular telangiectasia type 2 retinal lesions. Investigative ophthalmology & visual science 2015;56(2):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu JH, Foote KG, Lujan BJ, et al. Dysflective cones: Visual function and cone reflectivity in long-term follow-up of acute bilateral foveolitis. American journal of ophthalmology case reports 2017;7:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domdei N, Domdei L, Reiniger JL, et al. Ultra-high contrast retinal display system for single photoreceptor psychophysics. Biomedical optics express 2018;9(1):157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper RF, Tuten WS, Dubra A, et al. Non-invasive assessment of human cone photoreceptor function. Biomedical optics express 2017;8(11):5098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]


