Abstract
Objective:
To elucidate the relationship between vessel density (VD) measurements and signal strength in optical coherence tomography angiography (OCTA).
Design:
Cross-sectional study.
Subjects:
Healthy volunteers.
Methods:
OCTA images obtained from healthy volunteers were analyzed to demonstrate the relationship between signal strength index (SSI) and VD. Experiments were performed to determine the effects of signal strength reduction on VD measurements on the Optovue/AngioVue and Cirrus/AngioPlex OCTA systems. Signal strength reduction was generated by either neutral density filters (NDF) or defocus.
Main Outcome Measures:
Regression analysis of signal strength effects on VD.
Results:
VD decreased linearly with signal strength with high statistical significance on both OCTA systems tested and for all analyzed sources of variation in signal strength. The slope of VD v. SSI was greatest when signal strength was adjusted by NDF, followed by defocus, inter-scan difference, inter-individual variation, and left-right eye difference. Multivariate analysis revealed that both SSI and age had a significant effect on the inter-individual variation in VD.
Conclusions:
VD measurements using OCTA are significantly affected by OCT signal strengths on two OCTA platforms. Investigators should exercise caution when interpreting VD data from OCTA scans. Quantification algorithms for OCTA should ideally remove the signal strength bias.
Vessel density decreases linearly with signal strength in optical coherence tomography angiography. Investigators should exercise caution when interpreting vessel density data from optical coherence tomography angiography scans.
Introduction
Optical coherence tomographic angiography (OCTA) is a new imaging modality1–7 that can visualize the 3 dimensional pattern of retinal and choroidal circulation down to the capillary level.8,9 One of the advantages of OCTA is that it allows for quantitative perfusion analyses that are relevant to the investigation of physiology, diagnosis of disease, and assessment of treatment response. For example, the flow index has been used to investigate the retinal vascular autoregulation in response to breathing oxygen enriched air,10 the vessel density (VD) has been used to detect diabetic retinopathy3,11,12 and diagnose glaucoma,13–15 and the vessel area has been used to assess the response of choroidal neovascularization to anti-angiogenic treatment.16,17
OCTA perfusion quantification algorithms distinguish vascular from nonvascular pixels on en face OCTA based on the value of the flow signal compared to a threshold value. Some investigators have pointed out that the flow signal is correlated with the amplitude of the reflectance signal and that VD measurements could be affected by the OCT signal strength of the image.18–21 Quantification algorithms that use adaptive thresholding to compensate for signal strength variation have been developed.22 However, to our knowledge, these advanced algorithms have not been adapted by commercial OCTA platforms. Some clinical investigators are not aware that signal strength could bias VD measurements. This could lead to under-estimation or over-estimation on the effects of disease or aging on VD. One publication concluded that cataract surgery increases VD because the authors assumed that the increased signal strength after cataract extraction should theoretically have no effect on VD measurement.23 This misconception is understandable as the dependence of VD on signal strength has not been described in a clinical journal using a commercial OCTA quantification algorithm, and the mechanisms by which signal strength could affect VD measurement have not been convincingly demonstrated and explained.
In this study, we aim to clearly demonstrate that VD, as measured by commercial OCTA platforms, is affected by OCT signal strength variation. Two mechanisms of signal strength variation – beam attenuation and defocus – were investigated experimentally to determine how they might affect signal strength and VD measurements differently, and whether they might explain the variability of VD measurements in a population with healthy eyes.
Methods
Human Subjects
This study adhered to the tenets of the Declaration of Helsinki and received Institutional Review Board approval from the Oregon Health & Science University. Written informed consent was obtained from all subjects. Participants with healthy eyes were recruited. Inclusion criteria were: no evidence of retinal pathology or glaucoma, intraocular pressure lower than 21 mm Hg, no chronic or systemic corticosteroid use, and corrected distance visual acuity better than 20/40.
Optical Coherence Tomography Angiography
OCTA scans were performed on both eyes of each participant on a 70-kHz spectral-domain OCT system (RTVue-XR Avanti) with the AngioVue OCTA software (version 2016.2.0.35, Optovue, Inc., Fremont, CA, USA). The standard definition macular angiography OCTA scan pattern covering a 3×3-mm area was used. The SSADA algorithm was applied to detect flow as previously described.5 The scanning software computed a signal strength index (SSI) value based on the volumetric OCT reflectance signal. The SSI is an integer score that ranged from 0 to 100. Every scan comprised two consecutive raster scans performed in orthogonal directions (x-priority and y-priority) that were registered and merged into a single volume by OptoVue’s proprietary Motion Correction Technology (MCT).24,25 In this manner, the prevalence of motion artifacts was reduced. In addition to MCT registration, the tracking system was turned on to reduce motion artifacts caused by blinks and microsaccades.26
VD of the superficial vascular complex (SVC) slab (between inner limiting membrane and 80% of the ganglion cell complex) was calculated by the AngioVue AngioAnalytics software (version 2018.0.0.8). The segmentation of tissue layers was performed automatically by the AngioAnalytics software. No manual corrections of the segmentation were needed. En face projections of the SVC were generated by the AngioAnalytics software and the whole macula vessel density was calculated as the percentage of pixels with suprathreshold flow signal. Two repeat scans were taken of both eyes of each participant. This allows us to determine the difference in signal strength and VD between the left and right eyes of the same individual and between repeat scans of the same eyes.
In an additional population of healthy eyes, OCTA scans were obtained in the right eyes with experimental signal strength reduction by two mechanisms. In the beam attenuation experiment, serial scans were performed with neutral density filters (NDF; NEK01, Thorlab, Newton, NJ, USA) placed in front of the eye. Filters with optical densities ranging from 0.1 to 0.6 were used. In the defocus experiment, scans were obtained with varying levels of defocus, ranging from −0.5 to −4.5 diopters relative to the baseline. The baseline measurement for each subject was obtained without NDF and with optimal focus. The AngioVue has automated software for optimizing focus along with polarization matching. The software also allowed manual focus setting, which we used to dial in the defocus.
We repeated the NDF experiment in the same subjects using the Cirrus/AngioPlex OCT/OCTA system (version 9.5, Carl Zeiss Meditec, Inc., Dublin, CA, USA). Again, the 3×3-mm OCTA scan pattern was used. The AngioPlex OCTA utilizes horizontal-priority raster scanning and uses active tracking to reduce microsaccadic motion artifacts. The optical microangiography (OMAG) algorithm was used to generate OCTA flow signal.27 Since VD quantification was not available on the AngioPlex software at the time of the experiment, we calculated VD by applying a fixed threshold on flow signal such that the mean vessel density of unattenuated scans was the same for healthy subjects imaged by both instruments. The AngioPlex metric for signal strength is different from the AngioVue SSI. SSI is an integer value ranging from 0–100, whereas the AngioPlex system uses a ten-point scale to measure signal strength. In order to enable comparison between the two OCTA systems, SSI values from the AngioPlex scans were calculated with a custom algorithm to obtain a signal strength scale similar to the AngioVue SSI. The algorithm averaged the logarithmic reflectance (i.e. OCT signal) of the retinal voxels and multiplied the average with a normalization factor. The normalization factor equalized the SSI values of AngioPlex and AngioVue scans obtained in the sets of healthy eyes scanned in the beam attenuation experiments.
Statistical Analysis
Linear and non-linear regressions were performed using Prism 7 software (GraphPad, San Diego, CA, USA). The slope coefficients from linear regression analysis were compared with the null hypothesis that the slope is zero. The threshold for statistical significance was set at a p-value of 0.05. In the NDF and defocus experiments, normalized SSI and VD values were calculated by dividing the SSI or VD values by the baseline measurements for each eye. The baseline measurements were obtained with neither beam attenuation nor defocus.
Results
SSI partially explains VD variation in the normal population
We first determined the relationship between VD and SSI in a population of individual with healthy eyes on the AngioVue OCTA system (Figure 1A–B). Forty one participants (23 female, 18 male) were included in this portion of the study. The age of the participants was 57.2 ± 20.7 (mean ± standard deviation; range 26–89). Measurements from the two scans from the two eyes were averaged for this analysis. On univariate regression analyses, VD measurements increased linearly with SSI and decreased linearly with age with a slope of −0.128% per year. SSI also decreased linearly with age with a slope of −.352 units SSI per year (R2=0.638, p<0.0001, plot not shown). Multivariate regression showed that both SSI (p=0.005) and age (p=0.002) had a significant effect on VD. After accounting for the effect of SSI, the slope of the age effect on VD was reduced to 0.075% per year. The slope of VD v. SSI was 0.152% per unit SSI in the multivariate analysis.
Figure 1.
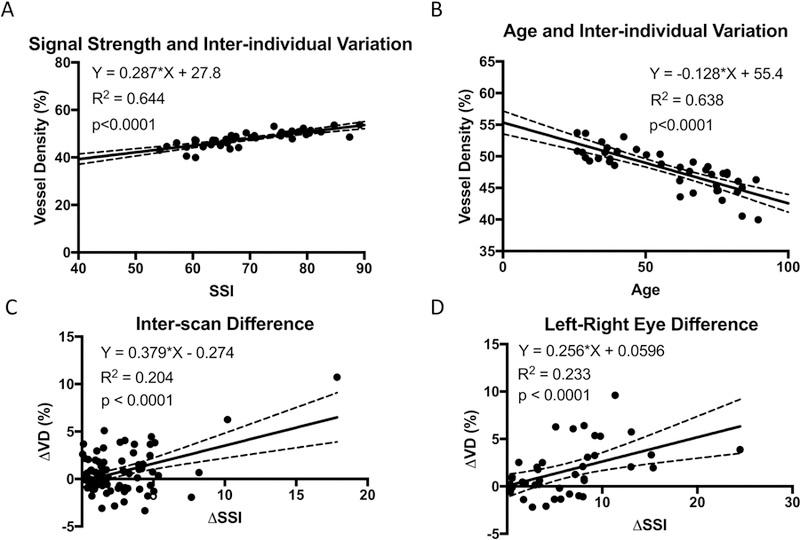
Macular retinal vessel density results obtained using the AngioVue optical coherence tomographic angiography (OCTA) system on human individuals with healthy eyes. (A) The vessel density, averaged between left and right eyes of each individual, was significantly correlated with Signal Strength Index (SSI). (B) The vessel density, averaged between left and right eyes of each individual, was significantly correlated with age. (C) The vessel density difference (∆VD) between repeat scans in the same eyes was correlated with the difference in SSI (∆SSI). (D) ∆VD between left and right eyes in the same individual was correlated with ∆SSI.
SSI affects VD measurement repeatability
We next looked at the effect of SSI on VD differences between repeat scans of the same eyes (Figure 1C). The scans were ordered so that the measurements from the scan with weaker signal strength were subtracted from the scan with the stronger signal strength to obtain ∆SSI and ∆VD. The slope of ∆VD v. ∆SSI was 0.378% per unit SSI. The regression analysis explained 20.4% of the variance (R2=0.204).
SSI partially explains inter-eye difference
Regression analysis of the difference between left and right eyes of the same individual showed that SSI was a significant factor (Figure 1D). The measurements from repeat scans in each eye were averaged for this analysis. The eyes were ordered so that the measurements from the eye with weaker signal strength were subtracted from the eye with the stronger signal strength to obtain ∆SSI and ∆VD. The slope of ∆VD v. ∆SSI was 0.256% per unit SSI. The regression analysis explained 23.3% of the variance (R2=0.233).
The effects of beam attenuation and beam defocus
The beam attenuation and defocus experiments were conducted on the right eyes of eight healthy male participants. The age of the participants was 32.8 ± 7.3 (mean ± standard deviation; range 27–48).
The effects of beam attenuation by neutral density filter (NDF) and beam defocus were qualitatively different (Figure 2). Beam attenuation decreased the flow signal (brightness on en face OCTA images), but the capillaries remained thin and sharply defined. Beam defocus blurred the edges of the blood vessels and made the capillaries appear wider, as well as decreased the brightness of the OCTA images.
Figure 2.
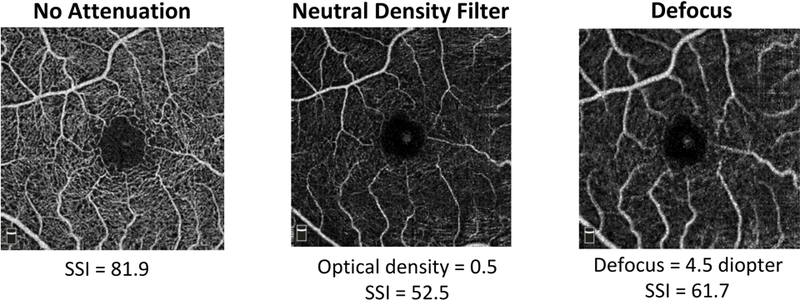
Effects of experimental signal attenuation on macular retinal OCTA by the AngioVue OCTA system on a healthy eye.
Neutral density filters (NDF) placed in the beam path attenuated signal strength in the expected fashion – the SSI decreased linearly with the optical density of the NDF (Figure 3A). We expected SSI to decrease nonlinearly with defocus – the quadratic fit showed this nonlinearity to be small (Figure 3B). The normalized VD decreased linearly with increasing NDF optical density (Figure 3C). The normalized VD decreased nonlinearly with defocus (Figure 3D); there was almost no change in VD with 1D of defocus and steep drop in VD beyond 2D of defocus.
Figure 3.
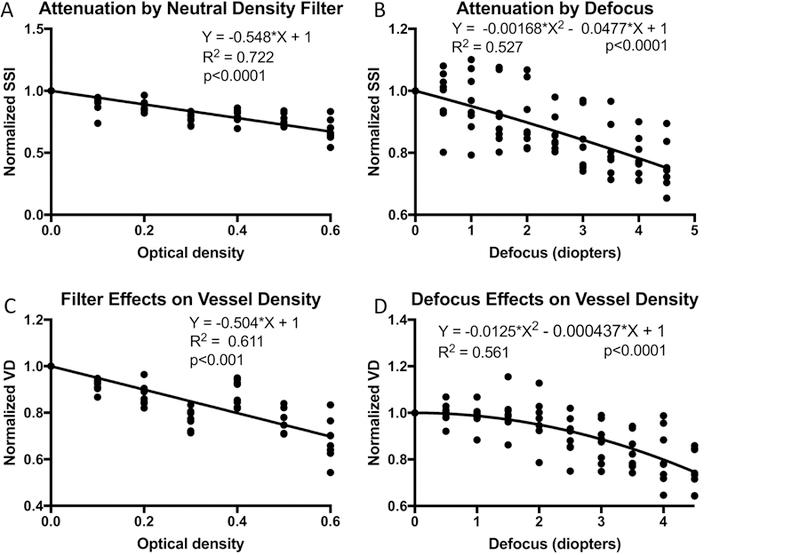
Effects of experimental signal attenuation on macular retinal vessel density measured by the AngioVue OCTA system on human participants with healthy eyes. (A) The SSI, normalized by the unattenuated value, is plotted against the optical density of neutral density filters (NDF). (B) Normalized SSI is plotted against diopters of defocus. C) Normalized vessel density (VD) plotted against NDF optical density. D) Normalized VD plotted against diopters of defocus. Linear regression models were applied to the NDF plots while quadratic models were applied to the defocus plots. The y-intercepts of for the regression models have been set to 1.
* Scans obtained on Optovue AngioVue OCTA system
VD measurements decreased linearly with decreasing SSI in both the beam attenuation and defocus experiments (Figure 4). The slope of VD v. SSI was greater for beam attenuation (0.509) compare to beam defocus (0.442), but the difference was not statistically significant (p=0.65). The slopes obtained by experimentally reducing signal strength with NDFs and defocus were significantly steeper than that obtained from inter-individual variation (0.15% per unit SSI after accounting for age-related VD change) and left-right eye difference (0.26% per unit SSI), but not for inter-scan difference (0.38% per unit SSI, Figure 1).
Figure 4.
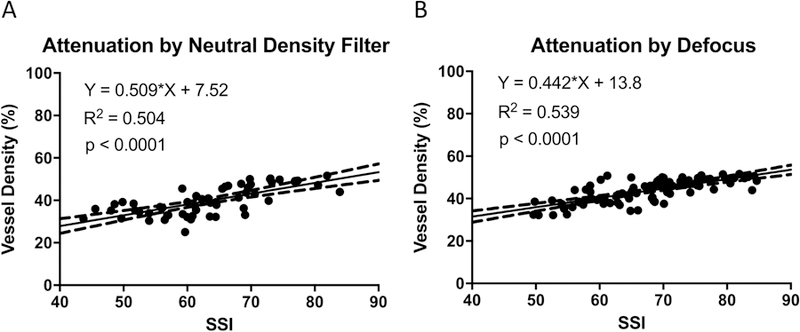
Effects of experimental signal attenuation on macular retinal vessel density measured by the AngioVue OCTA system on human participants with healthy eyes. (A) Vessel density had significant linear correlation with SSI in the experiment with NDF. (B) Vessel density had significant linear correlation with SSI in the defocus experiment.
In order to ensure that the relationship between signal strength and VD measurements was not specific to a single OCTA system, we also conducted a beam attenuation experiment using the AngioPlex system on random eyes of three healthy subjects. These subjects were separate from the eight healthy male subjects described above. We again found that VD decreased linearly with decreasing SSI (Figure 5). The slope obtained from the AngioPlex data was significantly steeper than that obtained from the AngioVue data (p<0.0001).
Figure 5:
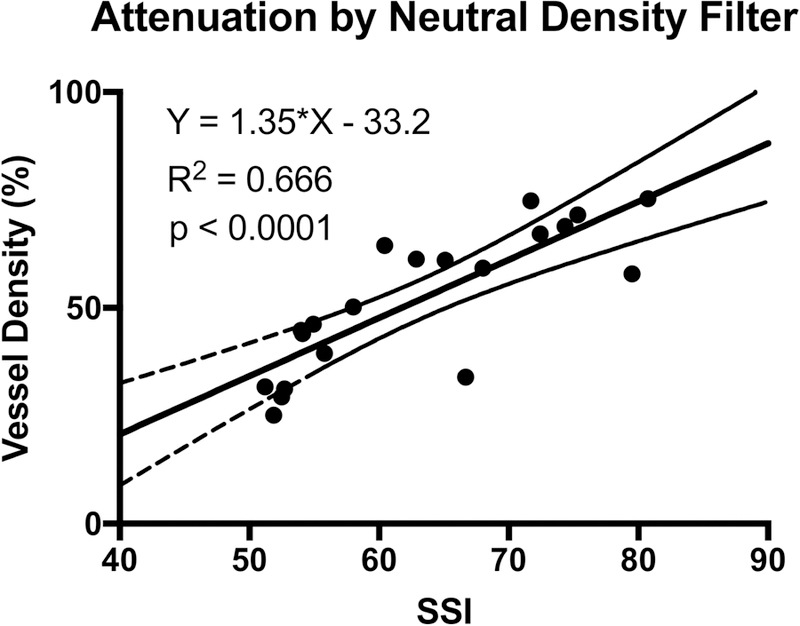
Effect of experimental signal attenuation with NDF on macular retinal vessel density measured by the AngioPlex OCTA system on human participants with healthy eyes.
Discussion
Understanding the effects of signal strength on VD measurements requires a theoretical framework that considers the various mechanisms behind observed variation in OCT signal strength and how they affect the calculation of flow signal in OCTA. These mechanisms fall into three categories: beam coupling, beam aberration, and tissue reflectivity. We will discuss these in turn.
The coupling of the OCT beam with sample tissue is affected by media opacity, which attenuates the probe beam by absorption or scattering, and media birefringence, which cause polarization mismatch between the sample and reference beams in the interferometer. These mechanisms attenuate the reflectance signal, making OCT images dimmer. However, they do not affect the beam focal spot size in the retinal plane, thus preserving transverse spatial resolution and the sharpness of the images. In this paper, we used absorptive attenuation by neutral density filter to study the effects of beam coupling. The signal strength was measured by the SSI, which is based on the average tissue reflectance amplitude on the OCT image on a logarithmic scale. The measured VD on both the AngioVue and AngioPlex systems were linearly related to SSI, thus the experiment showed beam attenuation decreases the flow signal in a log linear fashion. The flow signal in OCTA is based on voxel-wise analysis of OCT signal variation between successive B-scans at the same location. The SSADA algorithm5 measures signal variation using the decorrelation function, which is mathematically equivalent to the variance divided by the square of the average amplitude. The division operation should remove the effect of the average amplitude (signal strength) on flow signal. Thus some authors have stated that it is mathematically impossible for flow signal to be affected by signal strength.23 However, the SSADA algorithm also suppresses noise by setting a constant floor value on OCT signal amplitude, thereby reducing the measured flow in low signal voxels. This reduces the flow signal (decorrelation) in voxels with low OCT (reflectance) signal strength. All OCTA algorithms, including SSADA and OMAG, must employ a noise-suppression subroutine to avoid artifactual flow in low-signal voxels that are dominated by noise, as noise has random (maximally decorrelated) amplitude and phase. Thus the dependence of measured VD on SSI is expected, unless a special compensatory algorithm is used to remove this dependence. It should be noted that the slope relating VD and SSI was significantly steeper for the AngioPlex compared to the AngioVue. The primary reason is that AngioPlex’s OMAG algorithm is based on variance (not normalized to average amplitude) and AngioVue’s SSADA algorithm is based on decorrelation (mathematically equivalent to the variance divided by the square of the average amplitude). Thus, an extra normalization step during post-processing is required by OMAG to quantify vessels in deeper layers such as choroidal neovascularization28 and choriocapillaris,29 where signal strength is more variable. The two systems may also differ in the proprietary algorithms used to identifying vascular pixels on en face OCTA images, which could affect how much measured VD depends on the signal strength.
Beam aberration is a separate category that includes defocus, astigmatism, and higher order aberrations. These mechanisms not only reduce the OCT signal, but also increase the focal spot size of the OCT beam, making the en face OCT and OCTA images blurry. The blurring effect increases the apparent width of blood vessels. One would expect that this broadening might increase the measured VD, partially counteracting the effect of signal strength reduction. Indeed, we found that the VD v. SSI slope was slightly smaller in the beam defocus experiment, compared to the beam attenuation experiment. The effect of beam defocus should be negligible below the Rayleigh range, which is approximately 1D in the AngioVue system. This was observed in the experiment, which showed drop in VD only beyond 1D of defocus. The effect of astigmatism on beam spot area is approximately half of defocus on a per diopter basis. Thus we would expect less than 2D of astigmatism to have negligible effect on SSI and VD for the AngioVue. The effect of defocus and other aberration would be greater for OCT systems with wider beams (at the corneal plane).
Tissue reflectivity is a third mechanism by which the SSI of an OCT or OCTA image might vary. Some retinal layers, such as the nerve fiber layer (NFL) and retinal pigment epithelium (RPE), are more reflective than others. Thus if the scanned region has thicker NFL or more highly pigmented RPE, the SSI would be higher, given the same beam coupling and aberration. Since stronger reflectivity of nonvascular tissue could cast stronger shadows and weaken the reflectance from blood cells below, this type of SSI variation should be inversely related with measured VD (e.g. thicker NFL would overshadow and reduce flow signal from deeper retinal vessels), opposite of the effect of SSI reduction due to beam defocus or attenuation.
The slope dependence of VD was 0.15% per unit SSI in multivariate regression of inter-individual variance, approximately one third of that found in the attenuation and defocus experiments (0.51% and 0.44% per unit SSI, respectively). The positive slope indicates that SSI variation was mostly due to beam attenuation and defocus, which overwhelmed the counteracting effect of tissue reflectivity variation. The reduction in SSI with increasing age could be explained by an increase in media opacity (e.g. cataract, vitreous floaters), and increase in media aberrations (e.g. cataract, poor tear film quality), as well as reduced tissue reflectivity (e.g. NFL thinning). The VD v. SSI slope of inter-individual VD variance was approximately 1/3 of the slope for experiment beam attenuation and defocus, indicating that most of the inter-individual VD variation was real. Aging caused both a real decline in VD as well as an artefactual decline associated with SSI reduction. It is important to note that after adjusting for SSI effect, the rate of VD decline with age dropped from −0.128% to −0.07 per year in our study. Several papers studied aging changes in VD without accounting for signal strength effects; these may have overestimated the rate of VD decline with aging.30,31 Our results show that it is important to take signal strength into account when interpreting physiological or pathological changes in VD.
In contrast to inter-individual variation, the difference in VD measurements from repeat scans could not be explained by difference in tissue reflectivity or VD and must be related to the effects of beam coupling or aberration. Indeed, we found that the VD v. SSI slope for inter-scan difference to be statistically equivalent to those due to beam defocus or attenuation. This slope is large, thus studies that monitor VD change over time need to closely watch for artefactual change due to signal strength variation. The use of VD measurement algorithms that compensate for signal strength variation could improve the reliability of change analysis.
Our investigation showed that OCT signal strength could have a significant impact on retinal VD measurements with OCTA. This is of growing clinical significance as the use of OCTA is increasing. Retinal VD quantification in OCTA has a wide range of diagnostic and prognostic applications in glaucoma and retinal diseases.3,11,13,14,16 Thus it is important to find effective methods to reduce the biases on OCTA measurements caused by signal strength variation.
There are several approaches to reduce the effects of signal strength variation: quality improvement, quality control, and algorithmic compensation. Operators of OCTA systems should be trained to optimize scan quality by minimizing defocus, polarization mismatch, tear film disturbance, and pupil vignetting. But because media opacity, astigmatism, and other ocular aberrations are always present in the human population, methods to control and compensate for signal strength variation are also important.
The quality control solution involves excluding OCTA scans with signal strength below a certain threshold. A wide range of signal strength cutoff values has been used as a quality control measure in OCTA studies.32–34 The manufacturer’s guidelines for the AngioVue system specify a minimum SSI of 45 (out of 100) for macular OCTA. The AngioPlex system uses a ten-point scale to measure signal strength and recommends an inclusion criterion of 6 or greater. A limitation of the quality control solution is that the acceptable scans still range widely in signal strength. Between the SSI scores of 45 and 80, there would be a 5% artifactual variation of VD based on our multivariate population analysis of AngioVue results (0.15% per unit SSI), and ~15% VD variation if the SSI change was caused by beam attenuation or defocus. Lim et al. reported a significant relationship between VD and signal strength up to an signal strength value of 9 on the AngioPlex system by directly comparing the differences in VD quantification between different signal strength values.18 It is not practical to further reduce variation through stricter quality control, as this would exclude too many patients who needs OCTA evaluation. Thus although it is important to have quality control criteria, methods to compensate for signal strength variation in are needed in OCTA quantification algorithms.
Gao et al. developed a retinal VD quantification algorithm that compensates for signal strength variation by slab-based local reflectance analysis.22 The inner retinal slab was used, but the highly variable NFL was excluded to minimize the effect of tissue reflectivity variation. The algorithm was effective in minimizing the VD dependence on SSI, improve repeatability, and narrow the reference range from a normal population.22 This was a significant improvement for the purpose of glaucoma diagnosis and monitoring.13 However, there remains a concern that this algorithm could be biased by retinal tissue reflectivity variation when applied to patients with retinal diseases. The ideal compensation algorithm would need to completely separate beam coupling and aberration effects from tissue reflectivity effects. Camino et al. developed a regression-based bulk-motion subtraction algorithm that iteratively optimized the separation of vascular-flow and nonvascular voxels and analyzed the reflectance in these two types of voxels separately.35,36 A side benefit of this algorithm is that VD measurement was adjusted based on the reflectance of vascular-flow voxels. Since the reflectance of blood cells should not vary much between subjects, this approach could theoretically better separate the effects of beam coupling and aberration from tissue reflectivity variation. This algorithm was effective in reducing the VD dependence on SSI in 24 scans from a population of eight healthy subjects.
Given the results of our study, we urge investigators to exercise caution when interpreting OCTA vessel density measurements using current commercial OCTA platforms. Operators should be trained to minimize defocus and optimize signal strength. A minimum signal strength threshold should be included as a quality-control measure. A signal strength index should be used as a covariate in statistical analysis. Measurements made in eyes with dense cataract, high astigmatism, or high aberration should be interpreted cautiously. We urge manufacturers of OCTA systems to implement quantification algorithms that compensate for signal strength variation and provide quality assessment output that should include a signal strength index. A defocus index would also be useful as an additional covariate for analysis as we have shown that signal strength variation due to beam aberration (e.g. defocus), beam decoupling (e.g. media opacity), and tissue reflectivity changes could have different effects on OCTA measurements.
Acknowledgments
Financial Support: NIH grants R01 EY024544, R01 EY027833, R01 EY023285, P30 EY010572, and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflict of interest: OHSU, Yali Jia, and David Huang have financial interest in Optovue, Inc., a company that may have a commercial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Simon S. Gao is an employee of Genentech, Inc. Other authors do not have financial interest in the participant of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fingler J, Zawadzki RJ, Werner JS, Schwartz D, Fraser SE. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Opt Express 2009;17(24):22190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express 2008;16(15):11438–52. [DOI] [PubMed] [Google Scholar]
- 3.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci 2015;112(18):E2395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariampillai A, Leung MKK, Jarvi M, et al. Optimized speckle variance OCT imaging of microvasculature. Opt Lett 2010;35(8):1257–9. [DOI] [PubMed] [Google Scholar]
- 5.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao SS, Liu G, Huang D, Jia Y. Optimization of the split-spectrum amplitude-decorrelation angiography algorithm on a spectral optical coherence tomography system. Opt Lett 2015;40(10):2305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao SS, Jia Y, Zhang M, et al. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016;57(9):OCT27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang TS, Zhang M, Bhavsar K, et al. Visualization of 3 Distinct Retinal Plexuses by Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol 2016;134(12):1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagag AM, Pechauer AD, Liu L, et al. OCT Angiography Changes in the 3 Parafoveal Retinal Plexuses in Response to Hyperoxia. Ophthalmol Retina 2018;2(4):329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y. Automated Quantification of Nonperfusion in Three Retinal Plexuses Using Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 2016;57(13):5101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang TS, Hagag AM, Wang J, et al. Automated Quantification of Nonperfusion Areas in 3 Vascular Plexuses With Optical Coherence Tomography Angiography in Eyes of Patients With Diabetes. JAMA Ophthalmol 2018;136(8):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 2017;124(11):1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133(9):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121(7):1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. OCT Angiography of Time Course of Choroidal Neovascularization in Response to Anti-angiogenic Treatment. Retina Phila Pa 2015;35(11):2260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClintic SM, Gao S, Wang J, et al. Quantitative Evaluation of Choroidal Neovascularization under Pro Re Nata Anti-Vascular Endothelial Growth Factor Therapy with OCT Angiography. Ophthalmol Retina 2018;2(9):931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim HB, Kim YW, Kim JM, Jo YJ, Kim JY. The Importance of Signal Strength in Quantitative Assessment of Retinal Vessel Density Using Optical Coherence Tomography Angiography. Sci Rep 2018;8(1):12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Huang X, Meng X, et al. In vivo assessment of macula in eyes of healthy children 8 to 16 years old using optical coherence tomography angiography. Sci Rep [Internet] 2017. [cited 2018 Jul 19];7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5567180/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br J Ophthalmol 2018;102(3):352–7. [DOI] [PubMed] [Google Scholar]
- 21.Rao HL, Pradhan ZS, Weinreb RN, et al. Determinants of Peripapillary and Macular Vessel Densities Measured by Optical Coherence Tomography Angiography in Normal Eyes. J Glaucoma 2017;26(5):491–7. [DOI] [PubMed] [Google Scholar]
- 22.Gao SS, Jia Y, Liu L, et al. Compensation for Reflectance Variation in Vessel Density Quantification by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016;57(10):4485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Wen W, Jiang C, Lu Y. Changes in macular vasculature after uncomplicated phacoemulsification surgery: Optical coherence tomography angiography study. J Cataract Refract Surg 2018;44(4):453–8. [DOI] [PubMed] [Google Scholar]
- 24.Kraus MF, Potsaid B, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express 2012;3(6):1182–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus MF, Liu JJ, Schottenhamml J, et al. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed Opt Express 2014;5(8):2591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camino A, Zhang M, Gao SS, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express 2016;7(10):3905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express 2007;15(7):4083–97. [DOI] [PubMed] [Google Scholar]
- 28.Zhang A, Zhang Q, Wang RK. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed Opt Express 2015;6(10):4130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu Z, Chen C-L, Zhang Q, Pepple K, Durbin M, Gregori G. Complex signal-based optical coherence tomography angiography enables in vivo visualization of choriocapillaris in human choroid. J Biomed Opt 2017;22(12):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2016;57(13):5780–7. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci 2015;56(5):3212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S, Tanabe T, Hangai M, Morishita S, Kurimoto Y, Yoshimura N. Scanning Laser Polarimetry With Variable Corneal Compensation and Optical Coherence Tomography in Tilted Disk. Am J Ophthalmol 2006;142(3):475–82. [DOI] [PubMed] [Google Scholar]
- 33.Kim T-W, Zangwill LM, Bowd C, Sample PA, Shah N, Weinreb RN. Retinal Nerve Fiber Layer Damage as Assessed by Optical Coherence Tomography in Eyes with a Visual Field Defect Detected by Frequency Doubling Technology Perimetry but Not by Standard Automated Perimetry. Ophthalmology 2007;114(6):1053–7. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Wang J, Jiang H, et al. Retinal Microvasculature Alteration in High Myopia. Invest Ophthalmol Vis Sci 2016;57(14):6020–30. [DOI] [PubMed] [Google Scholar]
- 35.Camino A, Jia Y, Liu G, Wang J, Huang D. Regression-based algorithm for bulk motion subtraction in optical coherence tomography angiography. Biomed Opt Express 2017;8(6):3053–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camino A, Zhang M, Liu L, Wang J, Jia Y, Huang D. Enhanced quantification of retinal perfusion by improved discrimination of blood flow from bulk motion signal in OCTA. Trans. Vis. Sci. Tech 2018;7(6):20. [DOI] [PMC free article] [PubMed] [Google Scholar]


