Abstract
Brain-derived neurotrophic factor (BDNF) and microRNAs (miRNAs) play a significant role in the pathogenesis of acute ischemic stroke (AIS). The present study investigates the elevated expression of BDNF and miR-124 in AIS patients. In the present study, serum samples from AIS patients and healthy controls were collected to determine the regulatory role and mechanism of operation of BDNF and to determine the regulatory miRNAs involved in AIS. Using bioinformatics analysis, we identified putative and regulatory miR-124. The effect of miR-124 on BDNF expression was examined in human neuronal cell lines. Moreover, the function of miR-124 in regulating BDNF was analyzed by assessing the serum level of BDNF in both AIS patients and healthy controls. The results indicate that the BDNF level of AIS patients is very low compared with that of controls. In contrast, real-time polymerase chain reaction (RT-PCR) data revealed a very high serum level of miR-124 in AIS patients relative to healthy individuals. The associations of the National Institutes of Health (NIH) stroke scale (NIHSS) score with BDNF and BDNF-related miR-124 serum levels were calculated using Pearson’s/Spearman’s correlation coefficient. The findings revealed a negative correlation between NIHSS score and BDNF level, whereas a positive correlation was observed between NIHSS score and miR-124. In addition, the relationship between serum BDNF and miR-124 was negative in AIS patients. In conclusion, this study provides strong evidence that serum BDNF and the BDNF-regulatory miR-124 may serve as molecular markers for AIS.
Keywords: Brain-derived neurotrophic factor, Acute ischemic stroke, miR-124, Human neuronal cell lines, NIH, NIHSS
Introduction
Stroke is an unexpected disease of brain cells caused by a block in blood flow owing to a lack of oxygen. There are two types of stroke: ischemic and hemorrhagic. A disruption in the blood supply to the brain results in an ischemic stroke, whereas a ruptured blood vessel results in a hemorrhagic stroke. Brain tissues and certain populations of neurons are highly susceptible to ischemic stroke (Mattson et al. 2001). More than 85% of strokes are ischemic, rending this condition the world’s second and foremost cause of mortality, with a high prevalence of morbidity (Jickling and Sharp 2015).
Approximately 20–40% of stroke cases show rigorous symptoms that slowly affect the quality of life of the individual and ultimately lead to disabilities (Bonita et al. 2004). Stroke is often related to certain therapeutic conditions, including inflammation, depression, and myocardial infarction (Arumugam et al. 2005; Legos and Barone 2003; Muir et al. 2007; Maddahi and Edvinsson 2010). The pathophysiology of stroke is somewhat intricate and involves acidosis, glial cell activation, disruption of the blood–brain barrier, and leukocyte infiltration (Majno and Joris 1995; Broughton et al. 2009).
At present, the cellular and molecular aspects of stroke have not been completely elucidated. However, current evidence indicates that altered gene expression may play an essential role in the pathogenesis of stroke (Tu et al. 2013). Brain-derived neurotrophic factor (BDNF) is a neurotrophin family protein that plays a vital role in regeneration, neuronal developmental, and plasticity. Interestingly, reports clearly confirm that streptozotocin induces hippocampal and hypothalamic neurons by reducing BDNF secretion (Bathina et al. 2017). In addition, BDNF protects pancreatic β cells (RIN5F) against cytotoxic effects of streptozotocin (Bathina et al. 2016). BDNF has also been considered to be a regulator of stroke (Tu et al. 2013), and a low level of BDNF protein is a major indicator of acute ischemic stroke (AIS). In addition, reports show that inflammatory (Tuttolomondo et al. 2008) as well as anti-inflammatory factors (such as cytokines) (Nicolás Vila et al. 2003) were documented during AIS.
Many studies have illustrated that polymorphism of the BDNF gene is associated with stroke-related traits. It is very difficult to directly evaluate the BDNF level in the brain; therefore, researchers are assessing the level of BDNF in the serum (Meng et al. 2017). Numerous reports have indicated that the serum BDNF level is lower in AIS patients compared with healthy individuals (Wang et al. 2017). Interestingly, the BDNF levels in serum are increased after the intravenous (Jiang et al. 2017; Cooper-Kuhn 2007), nanoparticle (Harris et al. 2016) or hydrogel (Cook et al. 2017) delivery of BDNF to stroke patients, suggesting that serum BDNF level can be used as a biomarker to analyze AIS and the efficiency of treating stroke.
Hence, it is necessary to identify the factor that alters the BDNF level in the serum of AIS patients. In recent years, a surprising discovery related to microRNAs (miRNAs) was made. miRNAs are non-coding, highly conserved RNAs approximately 18–25 nucleotides in length that silence their mRNA targets by monitoring post-transcriptional gene expression (Bartel 2004). MiRNAs are further processed to mature miRNAs, where they partially and specifically bind to the 3’ untranslated region (UTR) of the target mRNA (Lee et al. 2003; Yi et al. 2003) and thereby repress translation (Bartel 2004) or degrade the mRNA (Ambros 2004; Yekta et al. 2004).
Evidence related to miRNAs indicates that abnormal expression of miRNAs plays a vital role in the pathogenesis of AIS. miR-9 and miR-124a are highly expressed and associated with AIS (Lagos-Quintana et al. 2002). The upregulation of miR-147 and miR-34a has also been noted in human modulate inflammation after stroke (Witwer et al. 2010). Together, these findings suggest that miRNAs play a crucial role in the pathogenesis of AIS. In this context, the present study was designed to identify the miRNA that controls the serum BDNF level and to identify associations between miRNAs and AIS.
Materials and methods
Study population
This research received approval from the Internal Institutional Ethical Review Board, Ethical Committee, and Research Advisory Committees of the affiliated hospital and research institute. All the applicable guidelines and regulations of each institution were followed. Proper written consent was acquired from both the patients and the spectators before sample collection. The study protocols were explained thoroughly. Forty patients aged 50–75 years were diagnosed with AIS based on magnetic resonance imaging and were enrolled in the current study. Patients were diagnosed by a neurologist using the National Institutes of Health (NIH) stroke scale (NIHSS) within 24 h after the onset of stroke. Patients suffering from a malignant tumor, an autoimmune disorder, or another unidentified disease as well as those with a short survival time were excluded. Forty normal controls that underwent yearly medical checkups at the affiliated institute were also enrolled. The clinical characteristics of controls and AIS patients are presented in Table 1.
Table 1.
Clinical characteristics of the AIS and normal samples
| Parameter | AIS patients (n = 40) | Normal (n = 40) |
|---|---|---|
| Age (median) | 63 (50–75) | 63 (50–75) |
| Sex (M/F) | 22/18 | 21/19 |
| BDNF (ng/ml) | 19.14 ± 4.87 | 27.34 ± 4.28 |
| miR-124 (103 copies/ml) | 0.56 | 0.11 |
| NIHSS | 10 (5–15) | 0 |
Detection of BDNF in serum
Blood samples were collected from both AIS patients and healthy individuals and subjected to centrifugation. The serum was collected and stored at 80 °C. The serum BDNF level was analyzed using an enzyme-linked immunosorbent assay (ELISA). Serum samples were incubated in a 96-well plate, and a rabbit anti-human antibody against BDNF (AV41970; Sigma-Aldrich, St. Louis, MO, USA) was added. The binding efficiency was detected using a horseradish peroxidase (HRP) labeled secondary antibody. The color developed was read spectrophotometrically at 450 nm using an ELISA reader (Bio-Tek Instruments Inc., Winooski, VT, USA) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (RT-PCR)
MiRNA was isolated from serum samples using a mirVana™ miRNA Kit (Ambion, Inc., Foster City, CA, USA) according to protocol guidelines. From RNA samples, cDNA was transcribed at 37 °C for 30 min followed by inactivating the reverse transcriptase at 95 °C for 10 min. The primers used for miR-124 were (forward) 5′-CATACCTAAGGCACGCGG-3′ and (reverse) 5′-GTCGGTGGGCATTCACC-3′. Reverse transcription (RT)-PCR was performed by initial denaturation (95 °C) followed by annealing (52 °C) and extension (72 °C). The expression of miR-124 was detected by means of the Quantitect SYBRGreen Kit (Qiagen, Hilden, Germany), and fluorescence was read at 585 nm. The experiments were performed in triplicate to obtain accurate data.
Cell culture and transfection
Human neuroblastoma cell lines were purchased and cultured in DMEM/F12 medium supplemented with 10% fetal calf serum and 100 µl/ml penicillin–streptomycin. miR-124 was chemically synthesized by means of small interfering RNA. Cells were transfected with 0.5 mg miRNA according to the manufacturer’s protocol.
Western blot analysis
Protein lysates were prepared from transfected cell lines and resolved by 10% SDS-PAGE. The resolved protein samples were transferred to a PVDF membrane and blocked with 5% skim milk for 2 h. The membrane was washed three times with Tris-buffered saline containing Tween 20 (TBST) and incubated with the anti-human BDNF primary antibody (AV41970; Sigma-Aldrich) overnight at 4 °C. The membrane was washed again with TBST and incubated with the HRP-labeled secondary antibody for 1 h, and signals were visualized using DAB (3,3′-diaminobenzidine; Sigma-Aldrich). Actin served as a loading control.
Statistical analysis
The results were analyzed using the F test and are presented as mean ± standard deviation (SD). Student’s t test, Pearson’s correlation coefficient, and Spearman’s rank test were used to evaluate the normality of the distributions of BDNF levels, NIHSS scores, and miR-124 values. A P value less than 0.05 was considered statistically significant in all analysis.
Results
Clinical analysis of AIS patients and controls
Eighty individuals were enrolled in the current study: 40 were AIS patients, and the remaining 40 were healthy controls. In total, 53% were male, and 46% were female. Subjects ranged in age from 50 to 75 years. The NIHSS assigns scores to stroke patients based on seriousness: 0 indicates stroke symptoms, 1–4 indicates minor stroke, 5–15 indicates moderate stroke, 16–20 indicates moderate to severe stroke, and 21–42 indicates severe stroke. This study included individual with moderate stroke (5–15), with a median NIHSS score of 10. The clinical parameters were tabulated and are presented in Table 1.
Serum BDNF levels in AIS patients and controls
The serum BDNF levels in AIS patients and controls were determined by ELISA. The mean serum BDNF level of AIS patients was significantly higher (19.14 ± 4.87 ng/ml) than that of healthy controls (27.34 ± 4.28 ng/ml) (Fig. 1). These results suggest that BDNF can be used as a molecular marker for AIS.
Fig. 1.
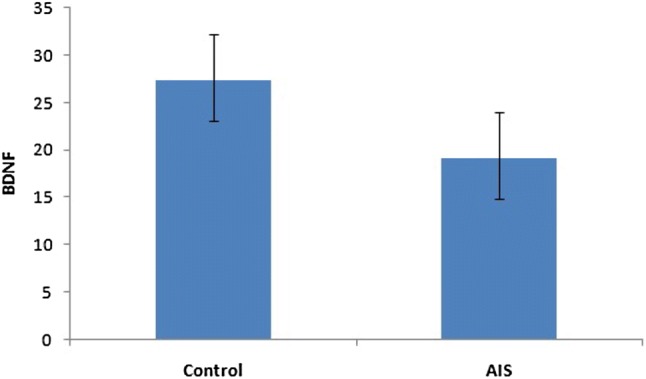
Serum BDNF concentration. BDNF levels in patients and control individuals. The BDNF levels in the serum of AIS patients were significantly decreased when compared with controls
BDNF-related miRNAs in AIS patients and controls
To clarify the relationship between BDNF and miRNA, it is essential to characterize the specific miRNA that standardizes the level of BDNF. Using miRNA analysis software, we performed a comprehensive analysis (http://www.microrna.org/microrna/getMirnaForm.do) to identify the specific miRNAs that target specific genes. Based on this computational analysis, we determined that miR-124 was targeted by the 3′ UTR of BDNF mRNA.
The functional characteristic of miR-124 in association with BDNF was examined using cell lines. Cells were transfected with miR-124, and the expression of BDNF was analyzed by Western blot. The BDNF level was lower in miR-124-treated cells compared to controls (Fig. 2). These findings suggest that miR-124 plays a crucial role in regulating BDNF expression.
Fig. 2.
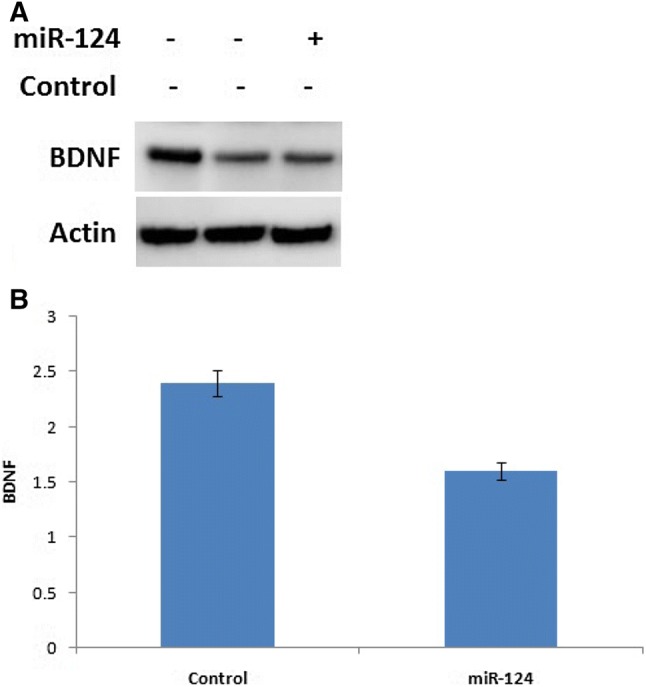
Regulation of miR-124 in expressing BDNF using western blot. a Expression of BDNF in miR-124 transfected human neuronal cell lines. b Relative intensity of the blot in graphical form
To confirm these findings, real-time PCR was carried out in human trials by analyzing the serum level of BDNF-regulating miR-124. AIS patients had a higher serum level of miR-124 (0.56 × 103 copies/ml; n = 40) than healthy controls (0.11 × 103 copies/ml; n = 40; P < 0.01) (Fig. 3). These results confirm that miR-124 is the putative target that regulates the expression of BDNF, which may be causative of AIS.
Fig. 3.
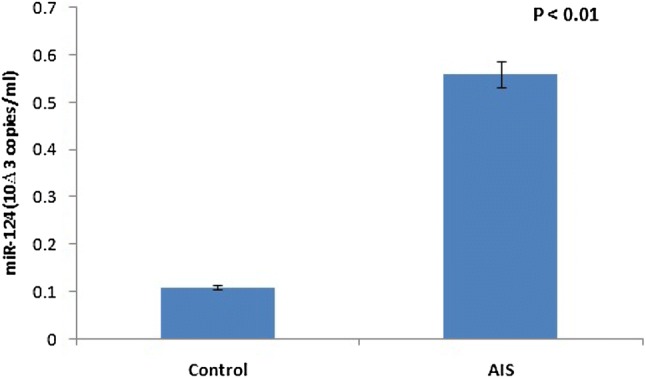
Detection of serum miR-124 by real time PCR. Serum miR-124 in AIS and healthy control. RT-PCR analysis illustrated that the level of serum miR-124 in AIS patients was higher than the normal. P value < 0.01
Serum BDNF level and its regulatory miRNA, miR-124
The serum BDNF level was lower and the BDNF-related miR-124 level was higher in AIS patients than in healthy controls. The correlation between BDNF and miR-124 was analyzed by Spearman’s coefficient. The data revealed a negative correlation between serum BDNF level and miR-124 (Spearman rs = − 0.418; P = 0.007) in AIS patients and healthy controls (Fig. 4).
Fig. 4.
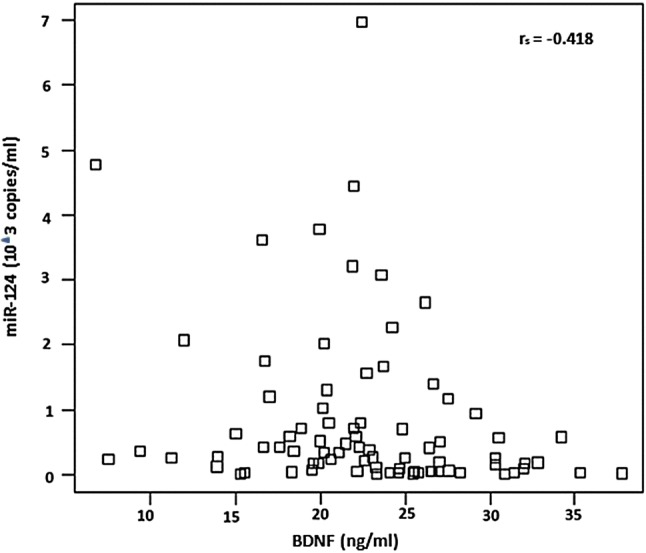
Correlation among BDNF in serum and its related miR-124. Spearman coefficient analysis showed a negative correlation among serum BDNF and miR-124 in AIS and controls
Serum level of BDNF-related miR-124 and NIHSS score
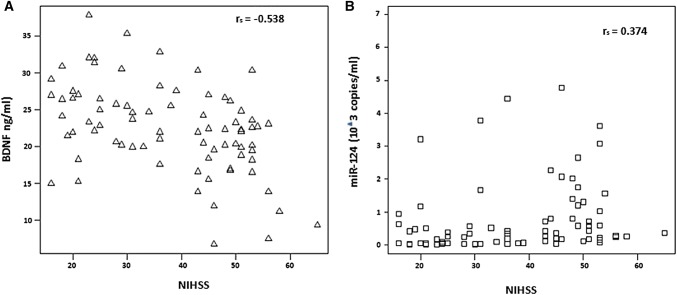
To determine whether this miRNA can be used as a diagnostic tool in AIS, an association between the serum level of miRNA and the NIHSS score was examined by Pearson’s or Spearman’s coefficient. The results indicated a negative correlation (Pearson r = − 0.538; P = 8.75E-05) between serum BDNF and NIHSS score in both AIS patients (n = 40) and healthy controls (n = 40) (Fig. 5a). In contrast, a positive correlation (Spearman rs = 0.374; P = 0.002) between serum miR-124 and NIHSS score was noted in all 80 individuals (Fig. 5b). This finding suggests that the serum level of miRNA is associated with the NIHSS score, which may aid in AIS diagnosis.
Fig. 5.
Correlation among serum BDNF-related miR-124 and NIHSS score. a Pearson coefficient analysis showed a negative correlation among serum BDNF and NIHSS score in AIS patients along with control. b Spearman coefficient analysis showed a positive correlation between serum miR-124 and NIHSS score in AIS patients and control
Discussion
The present study characterized the regulation of BDNF and its associated miRNA and evaluated their functions related to the pathogenesis of AIS. BDNF, a member of the neurotrophin family, plays a vital role in cell differentiation, proliferation, and synapsis regulation (Tu et al. 2013). Using a bioinformatics approach, we identified the putative BDNF-regulatory miR-124. Using human neuronal cell lines, we validated the negative regulation of BDNF. AIS patients exhibited a marked decrease in serum BDNF level compared with healthy controls. Interestingly, the serum miR-124 level was higher in AIS patients than in controls. The statistical analysis revealed a negative correlation between BDNF/miR-124 and BDNF level/NIHSS score in both AIS patients and controls. Furthermore, a positive correlation between miR-124/NIHSS score was noted in AIS patients and healthy controls.
As AIS is such a serious condition, it is essential to identify a molecular marker for its early diagnosis. Several reports have indicated that the serum BDNF level is low in stroke patients (Wang et al. 2017). These findings are consistent with ours, which revealed decreased serum BDNF levels in AIS patients relative to healthy controls. Moreover, we identified a negative correlation between BDNF and NIHSS score in both AIS patients and healthy controls. Therefore, we conclude that serum BDNF can be used as a molecular marker for AIS.
Previous studies have reported decreased BDNF levels in neurodegenerative disorders, such as schizophrenia (Ikeda et al. 2008) and Alzheimer’s disease (Yasutake et al. 2006). However, these results have not aided in diagnosis. Hence, in the current study, we investigated BDNF-related miRNA and serum BDNF level to increase the possibility of diagnosing AIS through the BDNF-regulated miRNA.
Numerous miRNAs are expressed in both neuronal and non-neuronal cells; however, their expression may vary under different conditions (Anacker et al. 2011). miR-124 and miR-9 are expressed in neurons, whereas miR-26 and miR-23 are more strongly expressed in astrocytes (Smirnova et al. 2005). Several studies have determined that miRNAs, such as miR-14, miR-107, and miR-21 (from plasma and peripheral blood samples), are highly expressed in stroke patients (Long et al. 2013; Peng et al. 2015); however, only a limited number of miRNAs are brain-specific. miR-124 expression is abundant in the brain (Laterza et al. 2009). miR-9 is also expressed in the developing as well as the adult brain. Moreover, previous reports have noted miR-9 expression in stroke patients (Coolen et al. 2013). A recent study demonstrated an elevated level of serum exosomal miR-124 and miR-9 in AIS patients. A positive correlation between the levels of miRNAs and the NIHSS scores was also observed (Ji et al. 2016). Similarly, our findings confirm that miR-124 is elevated in AIS patients, whereas transfection of miR-124 in human neuronal cells decreases BDNF expression.
Conclusion
From this study, we conclude that, miR-124 is the putative miRNA target that plays a crucial role in the regulation and expression of BDNF in serum. Although the miR-124 level of AIS patients was very high compared to that of healthy controls, we observed marked decrease in the serum BDNF level in AIS patients. These findings strongly suggest that BDNF and its associated miRNA (miR-124) play a vital role in the pathogenesis of AIS. We also recommend that BDNF be used as a molecular biomarker for AIS diagnosis. Additional studies are necessary to obtain more conclusive results.
Acknowledgements
The authors acknowledge the Institution, Institutional Review Board and Ethics Committee for providing support and funding to carrying out this research.
Authors contribution
XPW is the corresponding of the manuscript, who planned and designed the experiments for the project. In addition, first draft of the manuscript was written by corresponding author along with JD Research work was carried out and executed equally by JW and QH In addition, part of the research experiments such as statistical analysis was carried out by JD.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Human and animal rights
This article includes human study and it was approved by the Institutional Ethical Review Board, Ethical Committee, and Research Advisory Committees of the affiliated hospital and research institute. Also, proper written consent was acquired from both the patients and the spectators before collecting samples.
Footnotes
Jie Wang and Qiong Huang contributed equally.
References
- Ambros V. The functions of animal microRNAs Nature 431: 350–355. Proc Natl Acad Sci USA. 2004;103:3687–3692. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bathina S, Srinivas N, Das UN. BDNF protects pancreatic β cells (RIN5F) against cytotoxic action of alloxan, streptozotocin, doxorubicin and benzo(a)pyrene in vitro. Metabolism. 2016;65:667–684. doi: 10.1016/j.metabol.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Bathina S, Srinivas N, Das UN. Streptozotocin produces oxidative stress, inflammation and decreases BDNF concentrations to induce apoptosis of RIN5F cells and type 2 diabetes mellitus in Wistar rats. Biochem Biophys Res Commun. 2017;486:406–413. doi: 10.1016/j.bbrc.2017.03.054. [DOI] [PubMed] [Google Scholar]
- Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Nguyen C, Chun HN. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37:1030–1045. doi: 10.1177/0271678X16649964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M, Katz S, Bally-Cuif L. miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci. 2013;7:220. doi: 10.3389/fncel.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Harris NM, Ritzel R, Mancini NS. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav. 2016;150:48–56. doi: 10.1016/j.pbb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Yahata N, Ito I. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008;101:58–66. doi: 10.1016/j.schres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Ji Q, Ji Y, Peng J. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11:e0163645. doi: 10.1371/journal.pone.0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Zhu J. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm. 2017 doi: 10.1155/2010/372423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015;46:915–920. doi: 10.1161/STROKEAHA.114.005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Laterza OF, Lim L, Garrett-Engele PW. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Legos JJ, Barone FC. Update on pharmacological strategies for stroke: prevention, acute intervention and regeneration. Curr Opin Investig Drugs. 2003;4:847–858. [PubMed] [Google Scholar]
- Long G, Wang F, Li H. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13:178. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation. 2010;7:14. doi: 10.1186/1742-2094-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3. [PMC free article] [PubMed] [Google Scholar]
- Mattson M, Duan W, Pedersen W, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/A:1009676112184. [DOI] [PubMed] [Google Scholar]
- Meng WD, Sun SJ, Yang J, Chu RX, Tu W, Liu Q. Elevated serum brain-derived neurotrophic factor (BDNF) but not BDNF gene Val66Met polymorphism is associated with autism spectrum disorders. Mol Neurobiol. 2017;54:1167–1172. doi: 10.1007/s12035-016-9721-9. [DOI] [PubMed] [Google Scholar]
- Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- Peng G, Yuan Y, Wu S, He F, Hu Y, Luo B. MicroRNA let-7e is a potential circulating biomarker of acute stage ischemic stroke. Transl Stroke Res. 2015;6:437–445. doi: 10.1007/s12975-015-0422-x. [DOI] [PubMed] [Google Scholar]
- Smirnova L, Gräfe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- Tu WJ, Dong X, Zhao SJ, Yang DG, Chen H. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol. 2013;25:771–778. doi: 10.1111/jne.12052. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Davalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- Wang J, Gao L, Yang YL. Low serum levels of brain-derived neurotrophic factor were associated with poor short-term functional outcome and mortality in acute ischemic stroke. Mol Neurobiol. 2017;54:7335–7342. doi: 10.1007/s12035-016-0236-1. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Sisk JM, Gama L, Clements JE. MicroRNA regulation of IFN-β protein expression: rapid and sensitive modulation of the innate immune response. J Immunol. 2010;184:2369–2376. doi: 10.4049/jimmunol.0902712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-α and IL-1β levels in dementia patients. Eur Arch Psychiatry Clin Neurosci. 2006;256:402–406. doi: 10.1007/s00406-006-0652-8. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]