Abstract
Introduction
A recent study demonstrated that large glucose fluctuations were associated with an increased incidence of cardiovascular disease (CVD) in patients with type 2 diabetes mellitus (T2DM) and acute myocardial infarction. However, it is unknown whether glucose fluctuations are related to the incidence of CVD or the progression of atherosclerosis in patients with T2DM with no apparent history of CVD. In this protocol, we will be investigating the relationships of glucose fluctuations evaluated by continuous glucose monitoring (CGM) to the incidence of composite cardiovascular events and the progression of atherosclerosis in patients with T2DM who had no apparent history of CVD.
Methods
This is a prospective, multicenter, 5-year follow-up observational study. Between April 2018 and October 2019, 1000 participants are expected to be recruited at 34 medical institutions. CGM using FreeStyle Libre Pro is useful for evaluating glucose fluctuations by continuously monitoring glucose levels in interstitial fluid for up to 14 days. The primary study outcome is the relationship between fluctuations in glucose levels evaluated by CGM and the incidence of composite cardiovascular events. Secondary outcomes include the relationships of fluctuations in glucose levels evaluated by CGM to changes in carotid intima media thickness evaluated by echography or grayscale median (an index of tissue characteristics of the carotid wall), brachial–ankle pulse wave velocity, development or progression of diabetic retinopathy or nephropathy, quality-of-life-related diabetes therapy, quality of sleep, development of dementia, and autonomic nerve function.
Planned Outcome
This protocol is designed to investigate the relationship between glucose fluctuations and the incidence of composite cardiovascular events. We completed the registration of 1000 participants in March 2019. Thus, results will be available in 2024. We expect that evaluating glucose fluctuations will aid the identification of patients with a high probability of developing CVD.
Trial Registration
ClinicalTrials.gov identifier, UMIN000032325.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-0665-8) contains supplementary material, which is available to authorized users.
Keywords: Cardiovascular disease, Continuous glucose monitoring, Glucose fluctuation, Progression of atherosclerosis, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is an independent risk factor for cardiovascular disease (CVD), which is one of the main causes of death in patients with type 2 diabetes mellitus (T2DM). Taking preventive measures against CVD in patients with T2DM is an urgent task that is necessary to maintain their quality of life, extend healthy lifespans, and reduce the costs incurred by the social healthcare system.
The UK Prospective Diabetes Study showed that every 1% decrease in the level of HbA1c was associated with a decrease in the incidence of CVD [1]. That study highlighted the importance of glycemic control in terms of preventing CVD in patients with T2DM. However, other large clinical trials have failed to show that intensive glycemic control has beneficial effects on the prevalence of CVD in T2DM [2–4].
On the other hand, recent clinical studies have suggested that, in patients with T2DM, glucose fluctuations are more significantly associated with atherosclerotic-related diseases than the degree of hyperglycemic exposure (as indicated by HbA1c levels) is [5, 6]. In addition, a recent study demonstrated that large glucose fluctuations were associated with higher subsequent incidence rates of myocardial infarction, acute heart failure, and cardiac death in patients with acute myocardial infarction [7] and patients with both T2DM and acute myocardial infarction [8]. Thus, evaluating glucose fluctuations may aid the identification of patients with a high probability of developing CVD. Such patients are often not identified by measurements of HbA1c alone. However, it is unknown whether glucose fluctuations are related to the incidence of CVD or to the progression of atherosclerosis, especially in patients with T2DM and no apparent history of CVD. It is important to investigate these relationships in order to identify new treatment targets for preventing CVD.
The FreeStyle Libre Pro (FLP) continuous glucose monitoring (CGM) (FLP-CGM) system is useful for evaluating glucose fluctuations by continuously monitoring glucose levels in interstitial fluid every 15 min for up to 14 days. FLP-CGM is also considerably cheaper than conventional CGM and does not require calibration by self-monitoring blood glucose levels [9]. These positive aspects of FLP-CGM may reduce patient burden. In addition, the accuracy and utility of the FreeStyle Libre system in patients with T2DM have already been demonstrated [10].
In this protocol, we will mainly be investigating the relationships of glucose fluctuations evaluated by FLP-CGM to the incidence of composite cardiovascular events and the progression of atherosclerosis, including carotid intima media thickness (IMT) progression and brachial-ankle pulse wave velocity (baPWV), in 1000 patients with T2DM who have no apparent history of CVD. In addition, we will be investigating the relationships of glucose fluctuations evaluated by FLP-CGM to the development or progression of diabetic retinopathy or nephropathy, quality-of-life-related diabetes therapy, quality of sleep, dementia development, and autonomic nerve function.
Methods
Study Design
This is a prospective, multicenter, observational study. This study has been registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), which is a nonprofit organization in Japan that meets the requirements of the International Committee of Medical Journal Editors (ICMJE) (UMIN000032325).
Study Population
The study population consists of Japanese patients with T2DM who regularly attend the outpatient diabetes clinics of 34 institutions across Japan that were asked to participate in this study (see Table S1 of the Electronic supplementary material, ESM, for a list of those institutions and the investigators involved in the study).
The inclusion criteria are as follows: (1) ≥ 30 years of age and ≤ 80 years of age (regardless of gender); (2) receiving treatment for T2DM at one of the above outpatient clinics; (3) a signed consent form for participation in the study is provided; (4) no changes (including new prescriptions) in antidiabetic medications for 6 months before written informed consent is obtained (insulin dosage changes are allowed); and no changes in antidiabetic medication are anticipated from the time of enrollment until FLP sensors are applied to the back of the upper arm (insulin dosage changes are allowed). In order to check whether the inclusion criteria are met, the investigators are advised to confirm the patient’s information, including their medication history, by checking medical records and/or questioning the patient. The following exclusion criteria are also applied: (1) type 1 or secondary diabetes; (2) presence of severe infectious disease pre- or postoperatively, or severe trauma; (3) history of myocardial infarction, angina pectoris, cerebral stroke, cerebral infarction, or arteriosclerosis obliterans; (4) current treatment with artificial dialysis; (5) moderate liver dysfunction (aspartate aminotransferase ≥ 100 IU/l); (6) moderate or severe heart failure (New York Heart Association stage III or worse); (7) pregnancy, lactation, possible pregnancy, or the patient intends to become pregnant during the study period; (8) present or past history of a malignant tumor (however, patients not currently receiving medication for a malignant tumor, with no disease recurrence to date, and with no risk of recurrence during this study are allowed to participate); (9) use of a sensor-augmented insulin pump; (10) a T2DM diagnosis within the past year; and (11) judged as ineligible by the clinical investigators.
The subjects are screened consecutively, and patients who meet the above eligibility criteria are asked to participate in the present study. All patients who agree to participate are entered into the study. The protocol was approved by the institutional review board of each participating institution in compliance with the Declaration of Helsinki and current legal regulations in Japan. The chief ethics committee among those of all the participating institutions was the hospital ethics committee of Juntendo University Hospital, as shown in Table S2 of the ESM. Written informed consent must be obtained from all participants after they have received a full explanation of the study.
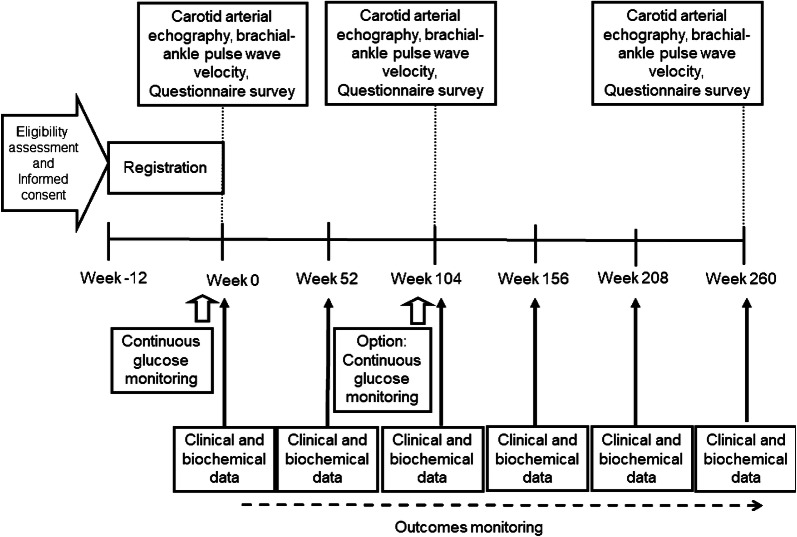
Observation Variables and Schedule
The observation parameters and schedule are shown in Table 1 and Fig. 1. The study period is 260 weeks after patient registration (registration period: April 2018 to October 2019; full study duration: April 2018 to December 2024). Patients will visit each study institution for routine visits during the study period. Each patient’s demographic data, clinical characteristics, past medical history, lifestyle habits, and treatment history will be collected at baseline using a standardized case record form after informed consent is provided. This is an observational study, and all treatment decisions will be made by the treating investigators according to usual care protocols. All patients will be followed until the end of the scheduled study period regardless of any change in treatment for diabetes or other conditions. The general health, blood pressure, and diet and exercise status of each patient will be checked at each visit. The clinical outcome of each patient will be ascertained. Clinical and biochemical data will be collected at baseline with a ± 10-week buffer period, and every 52 weeks with a ± 12-week buffer period. Blood samples will be obtained after overnight fasting at baseline and every 52 weeks thereafter. White blood cell count, red blood cell count, hemoglobin, hematocrit, platelet count, aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, creatinine, uric acid lipids, total cholesterol, low-density lipoprotein cholesterol (estimated by the Friedewald equation), high-density lipoprotein cholesterol, triglyceride, HbA1c (National Glycohemoglobin Standardization Program), and glucose will be measured with standard techniques. The estimated glomerular filtration rate (eGFR) will be calculated using the formula: eGFR (ml/min per 1.73 m2) = 194 × age−0.287 × serum creatinine−0.1094 (× 0.739 for females). Urinary albumin excretion will be measured by a latex agglutination assay using a spot urine sample at baseline and every 52 weeks thereafter. The incidence of CVD will be recorded during the 260-week follow-up period.
Table 1.
Items and schedule
| Registration | Treatment period (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 52 weeks | 104 weeks | 156 weeks | 208 weeks | 260 weeks | ||
| Informed consent | ○ | ||||||
| Patient characteristics | ○ | ||||||
| Continuous glucose monitoring | ○ | △ | |||||
| Outcomes | ○ | ○ | ○ | ○ | ○ | ○ | |
| Body weight | ○ | ○ | ○ | ○ | ○ | ○ | |
| Blood pressure | ○ | ○ | ○ | ○ | ○ | ○ | |
| Blood chemistry* | ○ | ○ | ○ | ○ | ○ | ○ | |
| Urinary albumin excretion | ○ | ○ | ○ | ○ | ○ | ○ | |
| Carotid arterial echography | △ | △ | △ | ||||
| baPWV and ABI | △ | △ | △ | ||||
| Electrocardiogram | △ | △ | △ | ||||
| Diabetes Therapy-Related Quality of Life questionnaire | △ | △ | △ | ||||
| Pittsburgh Sleep Quality Index questionnaire | △ | △ | △ | ||||
| Cardiovascular events | |||||||
baPWV brachial-ankle pulse wave velocity, ABI ankle brachial blood pressure index, △ optional examination
*Including red blood cells, white blood cells, hemoglobin, hematocrit, blood platelet count, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, creatinine, estimated glomerular filtration rate, uric acid, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, fasting plasma glucose, HbA1c
Fig. 1.
Flow chart of the study schedule
Continuous Glucose Monitoring Using the Freestyle Libre Pro
The FLP sensors are placed on the back of the upper arm of each participant by well-trained health care providers, and left in place for 14 days. There are no restrictions on participants’ daily lives. The FLP system continuously measures the glucose concentration in the interstitial fluid immediately below the skin. After the sensor is removed, it is mailed to the research support center (Soiken Inc., Tokyo, Japan). Data are downloaded from the FLP system and an ambulatory glucose profile is produced. The investigators and participants are provided with these data at the outpatient clinic appointment scheduled within 3 months after FLP-CGM. Based on the results of FLP-CGM, either the current therapy dose is increased or additional diabetes treatment is prescribed. Glucose fluctuations are assessed by measuring the mean amplitude of glycemic excursion (MAGE) [11], the standard deviation (SD), the glucose coefficient of variation (CV), the average daily risk range (ADRR) [12], and the M value, among other parameters.
MAGE is calculated as the arithmetic mean of the differences between consecutive peaks and nadirs, provided that the differences are greater than 1 SD of the mean glucose value. CV (%) is calculated by dividing the SD by the mean of the corresponding glucose readings. ADRR is computed by transforming blood glucose data to a symmetric scale, converting blood glucose measures to risk values, and eliminating values within the normal range, then averaging the daily out-of-range levels. The M value is a measure based on a weighted average of the mean and the glucose range. It is a logarithmic transformation of the deviation of blood glucose from an arbitrarily assigned ideal glucose level. Hypoglycemia is defined as the percentage of FLP-CGM values that are less than 3.9 mmol/l with or without symptoms. All patients receive FLP-CGM at baseline. Patients who undergo ultrasonographic scans of the carotid artery will be asked to receive FLP-CGM at 104 weeks as an optional examination for an exploratory analysis.
Measurement of Carotid IMT
Ultrasonographic scans of the carotid artery are performed by expert sonographers who have been specifically trained to perform the prescribed study examination, as reported previously [13, 14]. To avoid inter-sonographer variability, each participant is examined by the same sonographer at all visits, using the same equipment (high-resolution B-mode ultrasound scanner equipped with a high-frequency (> 7.5 MHz) linear transducer and with a limit of detection of < 0.1 mm). The extracranial common carotid artery, the carotid bulb, and the internal carotid artery in the neck are scanned bilaterally in transverse projections and in at least three different longitudinal projections (anterior, lateral, and posterior, which approximately correspond to 60°, 90°, and 150° for the right carotid artery and 210°, 270°, and 300° for the left carotid artery, as marked on Meijer’s arc). Additionally, the site of greatest thickness (including plaque lesions) is identified along the arterial walls. IMT represents the distance between two parallel echogenic lines corresponding to the vascular lumen and the adventitia. To avoid inter-reader variability, all scans are stored electronically and emailed to the central office (IMT Evaluation Committee, Osaka, Japan) to be read in random order by a single experienced reader blinded to the clinical characteristics of the patients, using automated digital edge-detection software (Intimascope; MediaCross, Tokyo, Japan) [13, 14]. The software system averages about 200 IMT values in the segment 2 cm proximal to the dilation of the carotid bulb (mean-IMT-CCA). In addition, the greatest IMTs, including plaque lesions in the common carotid arteries (max-IMT-CCA), are also measured separately.
The echogenicity of the arterial wall is evaluated based on the grayscale median (GSM) method with a grayscale range of 0–255 (0 as the darkest tone and 255 as the brightest), as described previously [15, 16]. Adobe Photoshop software (version 7.0; Adobe Systems, San Jose, CA, USA) is used for image standardization and the calculation of grayscale values. The standardization of the B-mode image is performed using a curve option, so that the GSM range for the blood is 0–5 and that for the adventitia is 185–195 [17]. The mean-IMT-CCA area is then delineated with a freehand tool, and the GSM of the selected area is read from the entire delineated area. Similarly, if there is an atherosclerotic plaque lesion (i.e., a focal elevated lesion with max-IMT-CCA > 1.0 mm), the GSM of the plaque lesion is also measured using the same method. If multiple plaque lesions are found in the same individual, the plaque with the greatest thickness is subjected to GSM measurement, and that GSM value is used as the subject’s representative value. To avoid inter-reader variability, all scans are stored electronically and read in random order by a single reader who is unaware of the subjects’ clinical characteristics and treatment groups.
Measurement of baPWV
In some participating centers, baPWV is measured using an automatic waveform analyzer (Colin Medical Technology, Komaki, Japan), as described previously [18]. Recording is performed with the patients in the supine position after a 5-min rest. Occlusion and monitoring cuffs are placed snugly around the appropriate areas on the upper and lower extremities. The pressure waveforms are then recorded simultaneously from the brachial arteries by the oscillometric method. All scans are automatically conducted by well-trained investigators who are blinded to the clinical information. Previous studies have confirmed the validity and reproducibility of baPWV measurements [19]. The ankle–brachial index is also measured. A resting ankle–brachial index of ≤ 0.90 is considered to indicate the presence of peripheral artery disease.
Coefficient of Variation of R–R Intervals (CVR–R) on Electrocardiogram
CVR–R at rest is measured based on R–R intervals from 100 electrocardiogram samples. CVR–R, which reflects autonomic nerve function, is calculated as (standard deviation in R–R )/(mean R–R interval) × 100%.
Diabetes Therapy-Related Quality of Life (DTR-QOL)
The DTR-QOL is a 29-item, self-administered assessment with four primary scales, including burden on social activities and daily activities (13 items), anxiety and dissatisfaction with treatment (8 items), hypoglycemia (4 items), and satisfaction with treatment (4 items) [20]. It has been demonstrated that the DTR-QOL can be used to evaluate the effect of diabetes treatment on patient QOL with high reliability and validity [20].
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is used to evaluate sleep quality in this study [18, 21]. The PSQI is a self-administered questionnaire that consists of 18 items relating to 7 components: subjective sleep quality, sleep duration, sleep onset, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Each of these is weighted equally on a 0–3 scale, and the sum yields the global PSQI score, ranging from 0 to 21, with higher scores indicating worse sleep quality. The PSQI has a high test–retest reliability and a good validity [22].
Study Outcomes
The primary study outcome is the relationship between fluctuations in glucose levels evaluated by FLP-CGM and the incidence of composite cardiovascular events, including cardiovascular death, acute myocardial infarction, unstable angina pectoris, revascularization for the treatment of coronary artery diseases, ischemic stroke, and revascularization for the treatment of arteriosclerosis obliterans.
The secondary outcomes are:
-
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and the incidence rates of the following composite cardiovascular events (a, b, or c, or each of a, b, and c):
- heart diseases (cardiovascular death, acute myocardial infarction, unstable angina pectoris, revascularization for the treatment of coronary artery diseases, or hospitalization for heart failure),
- cerebrovascular diseases (ischemic stroke, hemorrhagic stroke, or subarachnoid hemorrhage), and
- arterial diseases (arteriosclerosis obliterans, revascularization for the treatment of arteriosclerosis obliterans, or ischemia-related lower limb amputation (minor amputation and major amputation));
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and the incidence of malignant tumors;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and all-cause mortality in addition to (a) plus (b) plus (c) as described above;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and cardiovascular death, myocardial infarction, or stroke;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and cardiovascular death, myocardial infarction, unstable angina, or stroke;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and development or progression of diabetic retinopathy or nephropathy. The development of diabetic retinopathy is defined as the transition from no diabetic retinopathy at baseline to simple retinopathy or pre-proliferative retinopathy or proliferative retinopathy. The progression of diabetic retinopathy is defined as worsening of the stage of diabetic retinopathy. The development of diabetic retinopathy is defined as the transition from normoalbuminuria at baseline to micro- or macroalbuminuria. The progression of diabetic nephropathy is defined as worsening of the stage of diabetic nephropathy. Also, we will evaluate the decline in renal function (defined as a decrease in eGFR of 30% from baseline) and the eGFR slope (estimated from all available serum creatinine measurements over the study period) to investigate the relationships between fluctuations in glucose levels and those parameters;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and changes in mean-IMTCCA and max-IMT-CCA, gray-scale median, or brachial-ankle pulse wave velocity;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and changes in DTR-QOL scores or PSQI scores;
The relationship between fluctuations in glucose levels evaluated by FLP-CGM and the development of dementia;
The relationship between hypoglycemic events and cardiovascular events; and
-
The relationship between atherosclerotic risk factors, Diabetes Therapy-Related QOL scores or Pittsburgh Sleep Quality Index scores, and cardiovascular events. These secondary endpoints will be analyzed as prespecified exploratory endpoints.
The tertiary outcomes are the relationships between fluctuations in glucose levels evaluated by FLP-CGM and glucose parameters, lipid parameters, renal function, diabetic microangiopathy, mean-IMT-CCA and max-IMT CCA, gray-scale median, brachial-ankle pulse wave velocity, DTR-QOL scores or PSQI scores, and autonomic nerve function in cross-sectional analysis.
Sample Size
The sample size was not calculated based on scientific evidence because this is an explanatory study. The target number of enrolled patients was tentatively set at about 1000 subjects. We aimed to ensure that more than 35 composite cardiovascular events are included because at least 20 events are required to obtain meaningful results in multivariate Cox regression analysis [23]. According to our previous study, the event rate was estimated to be about 4% during a 5.5-year follow-up period [24]. Assuming an exponential distribution, the event rate would be about 3.64%, and 961 cases would be required to include 35 events during the 5-year follow-up. Therefore, considering participant dropout, the target number of enrolled patients was set at 1000 subjects.
Safety Evaluation
All adverse events (AEs) are to be recorded during the study for the sake of patient safety. AEs are defined as any untoward medical occurrence in a clinical trial subject who has been administered a medicinal product. In particular, the investigators are required to record AEs related to the FLP device; as soon as these are confirmed, they must immediately be reported to the principal investigator and the ethics committee within 15 days, who will judge whether the diagnosis is appropriate and then make a decision on whether the patient should withdraw from the trial. Serious AEs are defined as death or life-threatening events that necessitate inpatient hospitalization, cause prolongation of the existing hospitalization, or result in persistent or significant disability/incapacity that requires intervention to prevent permanent impairment or damage.
Statistical Analysis
Analyses of study outcomes will be performed mainly on the full analysis set. Univariate and multivariate Cox proportional hazard models will be used to determine the association of glucose fluctuations with the primary outcome. Hazard ratios (HRs) and 95% confidence intervals (CIs) will be reported. The cumulative event-free rates of the primary outcome will be estimated for different levels of glucose fluctuation using the Kaplan–Meier method, and the differences will be tested using the log-rank test.
Glucose fluctuations will be expressed as MAGE, CV, SD, ADRR, and M values. To evaluate the model fit and the ability of these parameters to predict the incidence of composite cardiovascular events, a time-dependent receiver operating characteristic (ROC) curve analysis will be performed and Akaike’s information criterion (AIC) values will be calculated.
The secondary outcomes will be analyzed in the same manner as the primary outcome. The correlations between glucose fluctuation parameters and various parameters, including mean carotid IMT, maximum carotid IMT, GSM, and baPWV, will be evaluated using Pearson’s and Spearman’s correlation coefficients.
In the exploratory study, the time trajectories of glucose fluctuation will be evaluated using the glucose fluctuation parameters at baseline and at 104 weeks. The correlation between change in glucose fluctuation parameters and changes in carotid echo parameters (mean carotid IMT, maximum carotid IMT, GSM, and baPWV) from baseline to 104 weeks will be also evaluated.
All statistical tests will be two-sided with a significance level of 5%. All analyses will be performed using SAS software (version 9.4 or above; SAS Institute, Cary, NC, USA).
Strengths and Limitations of the Study Protocol
The strength of this protocol is its large-sample, prospective, multicenter study design involving repeated clinical measurements such as risk factors for atherosclerosis and a 5-year follow-up period. On the other hand, our study protocol has certain limitations. The primary limitation of our study protocol is its explanatory nature. There are no reports to suggest that glucose fluctuations are related to the incidence of CVD or progression of atherosclerosis in patients with T2DM with no apparent history of CVD. This made it difficult to calculate the sample size besed on scientific evidence. In addition, it is still unclear which parameters of glucose fluctuations evaluated by FLP-CGM are useful in terms of predicting the incidence of CVD in patients with T2DM who have no apparent history of CVD. Thus, we will evaluate the ability of glucose fluctuation markers to predict the incidence of composite cardiovascular events. Second, glucose fluctuations will be evaluated by limited FLP-CGM measurements. One of the reasons for this is practical constraints, including study costs. However, we are focusing on investigating the ability of glucose fluctuation parameters measured by FLP-CGM at baseline to predict the incidence of composite cardiovascular events. In order to attain the best measurements of glucose fluctuations by FLP-CGM at baseline, we recruited patients whose antidiabetic medications were unchanged for 6 months before written information consent was obtained. Those subjects are expected to have stable glucose control. Third, we only recruited Japanese patients with T2DM. These constraints may limit the generalizability of our results.
Conclusions
We had completed the registration of 1000 participants in March 2019, and the results will be available in 2024. We expect that glucose fluctuations at baseline will be a risk factor for CVD and progression of atherosclerosis, and may even be an independent risk factor for these conditions after adjusting for other atherosclerotic risk factors. If our results support the hypothesis that early intervention targeting glucose fluctuations can decrease the incidence of CVD in patients with T2DM, these findings will contribute to establishing a better social healthcare system and to extending patients’ healthy lifespans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the clinical staff for their assistance with the execution of the clinical trial, and Soiken Inc. for their technical assistance in the launch and execution of this trial.
Funding
This study, including the publisher’s rapid service fee, was financially supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 19ek0210105h0002 and the Manpei Suzuki Diabetes Foundation. We confirm that all authors had access to the study data that support this publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis, and have given final approval of the version to be published.
List of Investigators
The authors wish to thank the study investigators (as shown in Table S1 of the ESM) and the participants for their contributions to this study.
Disclosures
Takashi Osonoi has received research funds from Abbott. Hirotaka Watada is a member of the advisory board of Abbott Japan and has received research funds from Abbott. None of the other authors (Tomoya Mita, Naoto Katakami, Yosuke Okada, Hidenori Yoshii, Keiko Nishida, Toshihiko Shiraiwa, Keiichi Torimoto, Akira Kurozumi, Satomi Wakasugi, Masahiko Gosho, and Iichiro Shimomura) have anything to disclose.
Compliance with Ethics Guidelines
All procedures followed are in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki, as revised in 2013. The protocol was approved by the institutional review board of each participating institution; a complete list of those review boards can be found in Table S2 of the ESM. The hospital ethics committee of Juntendo University Hospital is the chief ethics committee. Written informed consent has been or will be obtained from all patients included in the study.
Data Availability
The analyzed datasets will be available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8343701.
References
- 1.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med. 2008;358(24):2545–59. [DOI] [PMC free article] [PubMed]
- 3.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 5.Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1. doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36(4):1026–1032. doi: 10.2337/dc12-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care. 2019;42(4):674–681. doi: 10.2337/dc18-2047. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann L, Freckmann G. CGM versus FGM; or, continuous glucose monitoring is not flash glucose monitoring. J Diabetes Sci Technol. 2015;9(5):947–950. doi: 10.1177/1932296815603528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–794. doi: 10.1089/dia.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 13.Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39(3):455–64. [DOI] [PubMed]
- 14.Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care. 2016;39(1):139–48. [DOI] [PubMed]
- 15.Katakami N, Mita T, Irie Y. Effect of sitagliptin on tissue characteristics of the carotid wall in patients with type 2 diabetes: a post hoc sub-analysis of the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE) Cardiovasc Diabetol. 2018;17(1):24. doi: 10.1186/s12933-018-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irie Y, Katakami N, Mita T, Takahara M, Matsuoka TA, Gosho M, et al. Evaluation of the effect of alogliptin on tissue characteristics of the carotid wall: subanalysis of the SPEAD-A trial. Diabetes Ther. 2018;9(1):317–329. doi: 10.1007/s13300-018-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabetai MM, Tegos TJ, Nicolaides AN, Dhanjil S, Pare GJ, Stevens JM. Reproducibility of computer-quantified carotid plaque echogenicity: can we overcome the subjectivity? Stroke. 2000;31(9):2189–2196. doi: 10.1161/01.STR.31.9.2189. [DOI] [PubMed] [Google Scholar]
- 18.Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, et al. Morningness-Eveningness Questionnaire score and metabolic parameters in patients with type 2 diabetes mellitus. Chronobiol Int. 2014;31(9):1017–23. [DOI] [PubMed]
- 19.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 20.Ishii H. Development and psychometric validation of the Diabetes Therapy-Related QOL (DTR-QOL) questionnaire. J Med Econ. 2012;15(3):556–563. doi: 10.3111/13696998.2012.665111. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/S0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S, May S. Applied survival analysis. 2. New York: Wiley-Blackwell; 2011. [Google Scholar]
- 24.Yoshida M, Mita T, Yamamoto R, Shimizu T, Ikeda F, Ohmura C, et al. Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2012;35(1):178–180. doi: 10.2337/dc11-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The analyzed datasets will be available from the corresponding author on reasonable request.