Abstract
Recent large clinical trials on sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, with the aim of verifying cardiovascular safety, have revealed that these medications have a preventative advantage on adverse cardiovascular outcomes, including worsening of heart failure and deterioration of nephropathy, in patients with type 2 diabetes (T2D). These observed benefits do not seem to correlate with the glucose-lowering effect, and the underlying mechanism is being intensively investigated. Given the results from recent studies, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend that patients with T2D and clinical cardiovascular disease (CVD) with inadequate glucose control despite treatment with metformin should receive an SGLT2 inhibitor or GLP-1 receptor agonist. In this review we summarize the results of recent cardiovascular outcome trials and discuss the potential clinical advantage of SGLT2 inhibitors and GLP-1 receptor agonists. We also present practical implications of these glucose-lowering agents for reducing the risk of adverse cardiovascular events and progressive renal comorbidity in patients with T2D and CVD.
Keywords: Cardiovascular outcome, Glucagon-like peptide-1, Sodium-glucose cotransporter-2, Type 2 diabetes
Introduction
Initial concerns about the cardiovascular safety of rosiglitazone [1], which were mostly dispelled following the analysis of data on cardiovascular outcomes in the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycemia in Diabetes (RECORD) trial that assessed oral rosiglitazone combination therapy for patients with type 2 diabetes (T2D) [2], compelled the US Food and Drug Administration (FDA) to mandate that all new glucose-lowering agents must undergo post-marketing endpoint trials with the aim of verifying cardiovascular safety. In recent years, major clinical trials, including those on the cardiovascular safety of dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors, have been published.
Trials assessing the safety of DPP-4 inhibitors have reported a lack of superiority for major cardiovascular events compared with placebo [3–6] as well as an increase in the risk of hospitalization for heart failure, as seen with saxagliptin in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial [5]. To the contrary, three successive trials of SGLT2 inhibitors [7–9] showed a beneficial effect of this class of medications on cardiovascular events, accompanied by a lower risk of hospitalization for heart failure and a reno-protective effect with respect to mitigating albuminuria and deterioration of kidney function. In addition, three trials of GLP-1 receptor agonists, namely, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes 6 (SUSTAIN-6) trial, and the Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes) trial, also demonstrated risk reduction in major adverse cardiovascular events [10–12], with a consistent result recently reported on the use of dulaglutide in the Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial [13].
Given the implications of new evidence derived from these cardiovascular outcomes trials (CVOTs), the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recently recommended that patients with T2D and clinical cardiovascular disease (CVD) inadequately controlled with metformin therapy and lifestyle intervention should be treated with an SGLT2 inhibitor or GLP-1 receptor agonist and that among those in whom heart failure coexists, an SGLT2 inhibitor should be preferentially prescribed [14].
In this review we summarize the results of these CVOTs and discuss the potential clinical advantage of therapy with SGLT2 inhibitors and GLP-1 receptor agonists. We also present the practical implications of these two classes of glucose-lowering medications with respect to their potential to reduce the risk of adverse cardiovascular events and of progressive renal comorbidity in patients with T2D and CVD.
Methods
The articles cited in this review were retrieved from the literature using Google Scholar with the search terms “SGLT2 inhibitor”, “GLP-1 receptor agonist”, “cardiovascular”, and “outcome”. Related articles were also collected from a personal database.
This review article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Era Before SGLT2 inhibitors and GLP-1 Receptor Agonists
Despite the therapeutic advances in recent years driven by the emergence of novel anti-diabetic agents, CVD remains a major cause of morbidity in individuals with T2D, and CVD is still a major cause of death among people with diabetes. In the past decade, published evidence of the efficacy of intensive glycemic control on macrovascular outcome has been challenged. In the United Kingdom Prospective Diabetes Study 33 (UKPDS33) [15], which involved newly diagnosed patients with T2D in the UK between 1977 and 1991, the participants were randomly assigned to receive either intensive therapy with a sulfonylurea or insulin, or to receive conventional treatment. Although a 25% reduction in microvascular endpoints was achieved (hazard ratio [HR] 0.75, 95% confidence interval [CI] 0.60–0.93, p = 0.0099), the 11% reduction in glycated hemoglobin (HbA1c) achieved by intensive therapy was not sufficient to bring about a significant reduction in myocardial infarction (fatal or nonfatal) and sudden death (HR 0.84, 95% CI 0.71–1.00, p = 0.052). In UKPDS 34 [16], in which 1704 overweight patients were randomly assigned to conventional treatment or intensive glycemic control with a sulfonylurea, insulin, or metformin, participants allocated metformin had a significantly reduced risk of any diabetes-related endpoint (HR 0.68, 95% CI 0.53–0.87, p = 0.0023), diabetes-related death (HR 0.58, 95% CI 0.37–0.91p = 0.017), all-cause mortality (HR 0.64, 95% CI 0.45–0.91, p = 0.011), and myocardial infarction (HR 0.61, 95% CI 0.41–0.89, p = 0.01), as opposed to participants who received intensive glycemic control achieved with a sulfonylurea or insulin.
However, none of three subsequent major trials that examined intensive glycemic control, namely, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) trial, and the Veterans Affairs Diabetes Trial (VADT) [17–19], exhibited a beneficial effect of intensive control on cardiovascular events, with the exception of the ACCORD trial in which non-fatal myocardial infarction decreased in the intensive control group. The ADVANCE trial demonstrated a significant reduction in the risk of microvascular complications, consistent with the UKPDS33 results, but the favorable results were not replicated in the VADT. Furthermore, in the ACCORD trial, all-cause mortality and cardiovascular mortality were significantly increased in the intensive control group during the 3.5-year follow-up period, accompanied by severe hypoglycemia and body weight gain; this trial was therefore prematurely terminated. Given these results, intensive glycemic control is now regarded as harmful rather than beneficial, especially in elderly persons.
Since the FDA mandated postmarketing cardiovascular trials for new anti-diabetes drugs following the controversy over rosiglitazone, a number of CVOTs aimed at validating cardiovascular safety have been performed. Although a CVOT with a DPP-4 inhibitor was first reported in 2013, all four DPP-4 inhibitor trials, namely, the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial, Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), SAVOR-TIMI53, and the Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA), met the noninferiority margin in major adverse cardiac event (MACE) endpoints, but none of these trials demonstrated superiority in MACE compared with placebo [3–6].
SGLT2 Inhibitors
Mode of Action of SGLT-2 Inhibitors
Sodium-glucose cotransporter 2 is a sodium-glucose cotransporter that is highly expressed in the early part of the proximal renal tubule (S1 segment). Approximately 90% of urinary glucose is reabsorbed via SGLT2, whereas residual glucose is reabsorbed by SGLT1 situated in the more distal part of the proximal renal tubule (S3 segment). In patients with T2D, maximum reabsorption capacity is amplified, and this amplification in association with an increased concentration of systemic glucose results in persistence of hyperglycemia and glucotoxicity, leading to β-cell dysfunction [20, 21]. SGLT2 inhibitors reduce the maximum glucose reabsorption rate and lower the threshold for glycosuria, two actions which cause increased glucose excretion according to rising plasma glucose concentration [22, 23]. SGLT2 inhibitors exert their plasma glucose-lowering effect by increasing urinary glucose excretion in an insulin-independent manner, leading to reduced plasma glucose level and body weight without the risk of hypoglycemia.
Cardiovascular Outcome Trials
A summary of major CVOTs with SGLT2 inhibitors is shown in Table 1. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients Removing Excess Glucose (EMPA-REG OUTCOME) trial enrolled 7020 participants with T2D and established CVD, who were then randomly assigned to empagliflozin 10 or 25 mg or placebo. During a median follow-up of 3.1 years, empagliflozin demonstrated significant risk reduction in 3-point MACE (cardiovascular death, nonfatal myocardial infarction [MI] and nonfatal stroke) (HR 0.86, 95% CI 0.74–0.99,p = 0.04) compared to placebo [9]. The primary outcome was largely driven by a 38% reduction in cardiovascular death (HR 0.62, 95% CI 0.49–0.77, p < 0.001), whereas there was a non-significant decrease in nonfatal MI (HR 0.87, 95% CI 0.70–1.09, p = 0.22), while the occurrence of nonfatal stroke tended to be higher although the difference did not reach significance (HR 1.24, 95% CI 0.92–1.67, p = 0.16). Furthermore, there was a marked reduction in the incidence of hospitalization for heart failure in the empagliflozin arm (HR 0.65, 95% CI 0.50–0.85, p = 0.002).
Table 1.
Summary of major cardiovascular outcome trials with sodium-glucose cotransporter 2 inhibitors
| Parameters | EMPA-REG Outcome trial [9, 30] | CANVAS/CANVAS-R trial [7] | DECLARE-TIMI 58 [8, 100] | CREDENCE trial [25] |
|---|---|---|---|---|
| Drug | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin |
| Dose | 10/25 mg, once daily | 100/300 mg, once daily | 10 mg, once daily | 100 mg, once daily |
| Number of participants | 7020 | 10,142 | 17,160 | 4401 |
| Mean age (years) | 63.1 | 63.3 | 63.9 | 63.0 |
| Mean HbA1c at baseline (%) | 8.1 | 8.2 | 8.3 | 8.3 |
| Median follow-up period (years) | 3.1 | 2.4 | 4.2 | 2.6 |
| Patients with established CVD | 6964 (99%) | 6656 (65.6%) | 6974 (40.6%) | 2220 (50.4%) |
| Patients with prior history of heart failure | 706 (10.1%) | 1461 (14.4%) | 1724 (10.0%) | 652 (14.8%) |
| 3-point MACE (CV death, nonfatal MI, nonfatal stroke) | HR 0.86 (0.74–0.99) | HR 0.86 (0.75–0.97) | HR 0.93 (0.84–1.03) | HR 0.80 (0.67–0.95) |
| CV death | HR 0.62 (0.49–0.77) | HR 0.87 (0.72–1.06) | HR 0.98 (0.82–1.17) | HR 0.78 (0.61–1.00) |
| Nonfatal MI | HR 0.87 (0.70–1.09) | HR 0.85 (0.69–1.05) | HR 0.89 (0.77–1.01) | n.a. |
| Nonfatal stroke | HR 1.24 (0.92–1.67) | HR 0.90 (0.71–1.15) | HR 1.01 (0.84–1.21) | n.a. |
| Hospitalization for heart failure | HR 0.65 (0.50–0.85) | HR 0.67 (0.52–0.87) | HR 0.73 (0.61–0.88) | HR 0.61 (0.47–0.80) |
| Progression of albuminuriaa | HR 0.62 (0.54–0.72) | HR 0.73 (0.67–0.79) | HR 0.84 (0.79–0.89) | n.a. |
| Renal outcomeb | HR 0.54 (0.40–0.75) | HR 0.60 (0.47–0.77) | HR 0.53 (0.43–0.66) | HR 0.70 (0.59–0.82) |
CANVAS Canagliflozin Cardiovascular Assessment Study, CREDENCE Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation trial,CVD cardiovascular disease, CV death cardiovascular death, DECLAIR-TIMI58 Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 trial, EMPA-REG OUTCOME Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients Removing Excess Glucose Outcome, ESRD end stage of renal disease, HbA1c glycated hemoglobin, HR hazard ratio (95%CI), MACE major adverse cardiovascular events, MI myocardial infarction, n.a. not available
aDescribed as progression to macroalbuminuria in the EMPA-REG trial; as > 30% increase in albuminuria, change from either normoalbuminuria to micro-/macroalbuminuria or micro- to macroalbuminuria in the CANVAS/CANVAS-R trial; and as the composite risk of normo- to micro- or macroalbuminuria in the DECLAIR-TIMI 58 trial
bDescribed as the composite risk of doubling of serum creatinine level accompanied by an estimated glomercular filtration rate (eGFR) of ≤ 45 ml/min/1.73 m2, initiation of renal replacement therapy, or death from renal disease in the EMPA-REG trial; as the composite risk of 40% reduction in eGFR, renal replacement therapy, or renal death in the CANVAS/CANVAS-R trial; as the composite risk of > 40% decrease in eGFR to < 60 ml/min/1.73 m2, ESRD, or death from renal cause in the DECLARE-TIMI 58 trial; and as the composite outcome of end-stage kidney disease, doubling of serum creatinine level, or renal or cardiovascular death in the CREDENCE trial
In terms of renal outcome, an analysis based on prespecified renal outcomes as the secondary endpoint revealed that empagliflozin also reduced the risk for the composite renal end point (progression to macroalbuminuria, doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease) (HR 0.61, 95% CI 0.53–0.70, p < 0.001) [24]. The magnitude of reduction in the composite renal endpoint was greater in those individuals with an estimated glomerular filtration rate (eGFR) of ≥ 90 ml/min/1.73 m2 (HR 0.21, 95% CI 0.09–0.53) compared with those with an eGFR of < 60 ml/min/1.73 m2 (HR 0.66, 95% CI 0.41–1.07) or those with an eGFR of 60–90 ml/min/1.73 m2 (HR 0.61, 95% CI 0.37–1.03) [24].
The beneficial outcome for cardiovascular risk observed in the EMPA-REG OUTCOME trial was recapitulated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program [7]. The CANVAS Program enrolled 10,142 participants, 65.6% of whom had established atherosclerotic CVD when the CANVAS and CANVAS-R trials were combined. Canagliflozin demonstrated a significant reduction in the relative risk of the primary cardiovascular endpoint by 14% (HR 0.86, 95% CI 0.75–0.97, p = 0.02 for superiority) during a median follow-up period of 2.4 years [7]. This medication did not demonstrate a significant reduction in cardiovascular and all-cause mortality, in contrast to empagliflozin in the EMPA-REG OUTCOME trial.
Similar to the EMPA-REG OUTCOME trial, a significant 33% reduction in the secondary endpoint of hospitalization for heart failure (HR 0.67, 95% CI 0.52–0.87) was observed in the CANVAS Program, whereas neither the risk of MI nor that of stroke was significantly reduced. Canagliflozin also exerted a 40% reduction in the risk of the prespecified composite renal outcome which comprised a sustained 40% reduction in eGFR, the need for renal replacement therapy, or death from renal causes (HR 0.60, 95% CI 0.47–0.77).
The renal outcome with canagliflozin in patients with impaired kidney function was verified in the recently reported Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial [25]. Patients with T2D who had chronic kidney disease (CKD), defined as those with an eGFR ranging from 30 to 90 ml/min/1.73 m2 and macroalbuminuria (urine albumin [mg] to creatinine [g] ratio; UACR > 300–5000), and all 4401 participants who underwent randomization were required to have background use of an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker (ARB) for at least 4 weeks. Canagliflozin significantly reduced the relative risk of the primary composite outcome comprising end-stage renal disease (ESRD), doubling of serum creatinine, or death from renal or CVD by 30%, compared with the placebo group (HR 0.70, 95% CI 0.59–0.82, p = 0.00001). Canagliflozin reduced the slope of the change in eGFR from baseline, accompanied by an initial dip during the first 3 weeks, presumably reflecting correction of hyperfiltration, underscored by an early decrement of UACR [25].
The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial enrolled 17,160 participants including a cohort receiving primary prevention (59.4% of participants) [8]. In the DECLARE trial, dapagliflozin did not demonstrate risk reduction of MACE or cardiovascular death. Consistent with the results of the prior two trials mentioned above, however, hospitalization for heart failure was significantly reduced (HR 0.73, 95% CI 0.61–0.88), and improvement of renal outcome (composite risk of ≥ 40% decrease in eGFR to < 60 ml/min/1.73 m2, ESRD, or death from renal cause) was also observed in the DECLARE-TIMI 58 trial (HR 0.53, 95% CI 0.43–0.66) [8].
The neutral effects on MACE observed in the DECLARE trial may reflect the proportion of patients with established CVD at baseline, indicating that the impact of an SGLT2 inhibitor on primary prevention might be minimal. In contrast, an apparent beneficial effect on the reduction of cardiovascular risk in established CVD was suggested by a recent meta-analysis [24].
In the Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors (CVD-REAL 2) study, involving 235,064 propensity-matched patients across six countries, including those of the Asia Pacific, Middle East, and North American regions, eligible patients were treated with dapagliflozin (75%), empagliflozin (9%), ipragliflozin (8%), canagliflozin (4%) tofogliflozin (3%), or luseogliflozin (1%). Although only 27% of patients had established CVD, treatment with SGLT2 inhibitors was also associated with significant risk reduction in hospitalization for heart failure and all-cause death (HR 0.64, 95% CI 0.50–0.82, p = 0.001 and HR 0.51 95% CI 0.37–0.70, p < 0.001, respectively), with a directionally similar trend regardless of the existence of prior CVD [26].
Possible Mechanisms of Cardiovascular Outcome
Intriguingly, it has been observed that empagliflozin has an effect on hospitalization for heart failure and cardiovascular death during the very early phase of treatment and that cumulative event curves for cardiovascular death and for heart failure hospitalization diverge within 1–2 months of treatment initiation. This early separation suggests that the beneficial effect of this medication is not due to amelioration of atherosclerosis or glycemic control. The putative contributor is in part osmotic diuresis accompanied by glucose excretion and subsequent natriuresis and reduced intravascular volume, ameliorated arterial elasticity, and reduced cardiac preload and afterload, leading to a decrease in heart failure events and cardiovascular mortality [27, 28].
In the setting of hyperglycemia, SGLT2 is upregulated in the proximal tubule, resulting in decreased sodium delivery to the macula densa, leading to afferent vasodilation and hyperfiltration via tubuloglomerular feedback [29]. The administration of SGLT2 inhibitors restores sodium delivery to distal tubular compartments, mitigating hyperfiltration and thereby reducing intraglomerular pressure and exerting renoprotective effects. SGLT2 inhibitors comparably exert this renal hemodynamic change, as illustrated by a dose-dependent decline in eGFR during the early period after treatment initiation, in those with and without CKD [30]. Intriguingly, a previous analysis of UACR data from the EMPA-REG OUTCOME trial in accordance with the status of albuminuria at baseline [31] revealed that patients experienced an initial fall in eGFR with 4 weeks of treatment initiation with empagliflozin regardless of baseline albuminuria status, followed by a modest increase in or stabilization of eGFR. The majority of these patients had used antihypertensive drugs, including renin–angiotensin–aldosterone system (RAAS) inhibitors, and the use of these medications was mostly sustained over the course of the study. In contrast, the eGFR in the placebo arm continuously declined over time. With regards to UACR, in patients with microalbuminuria or macroalbuminuria at baseline, decreased UACR was observed as early as week 12, and significantly reduced UACR was maintained up to week 164 [31].
It should be noted that treatment with empagliflozin still brought about an advantage in renoprotection despite background RAAS blocker use in 80% of participants. An earlier study had demonstrated that initiation of antihypertensive therapy with an ARB caused an acute decrease in eGFR, which was negatively correlated with the slope of decline in renal function during the long-term follow-up period [32].
Inhibition of the RAAS by an ARB reduces intraglomerular pressure through efferent arteriolar vasodilation, resulting in improvement of intraglomerular hypertension and hyperfiltration and leading to slowing of eGFR decline [32, 33]. On the contrary, the initial dip in eGFR observed with SGLT2 inhibitors is assumed to result from afferent arteriolar vasoconstriction via the tubuloglomerular feedback system. This difference in mechanism may contribute to the additive favorable effects on renal outcome observed in patients with concomitant use of ARB.
Although the renoprotective effects of SGLT2 inhibitors seem to be a class effect, it has been suggested that SGLT2 inhibitors differentially affect the risk of acute renal failure [34]. The results of a meta-analysis of 53 trials of SGLT2 inhibitors indicated that only empagliflozin demonstrates a lower risk of acute renal impairment/failure events relative to placebo (OR 0.72; 95% CI 0.59–0.87), compared with canagliflozin (OR 1.82; 95% CI 0.28–11.77) and dapagliflozin (OR 1.93; 95% CI 0.42–8.83) [35].
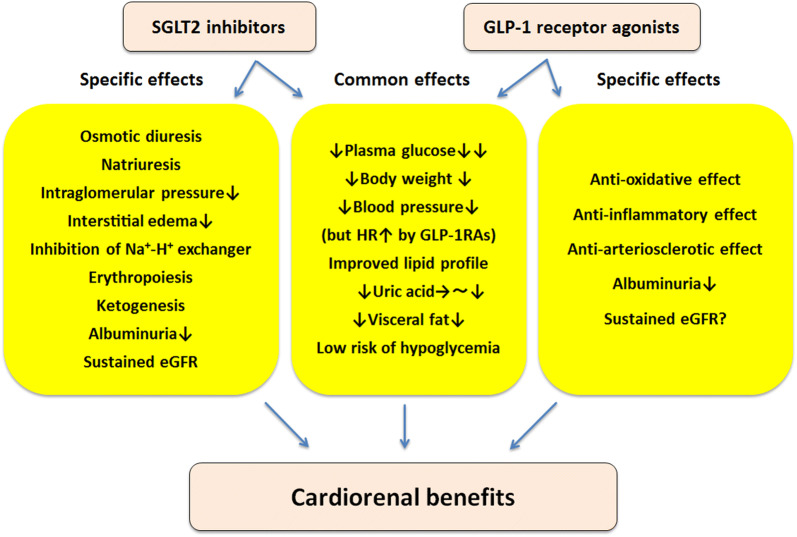
SGLT2 inhibitors promote a shift to fatty substrate utilization in response to decreased plasma glucose caused by glycosuria, leading to enhanced fatty oxidation, lipolysis, and ketogenesis. Lower plasma glucose subsequently stimulates glucagon secretion, and suppression of plasma insulin level may partially contribute to this mechanism [36], while the direct effect of SGLT2 inhibitors on alpha cells remains to be elucidated [37, 38]. Ferrannini et al. hypothesized that ketone bodies are preferentially harnessed by the heart in patients with T2D, in which myocardial insulin-mediated glucose utilization is impaired, resulting in reliance to a large degree on fatty substrates as the energy source [39]. In this setting, ketone bodies produce ATP more efficiently per molecule of oxygen than do free fatty acids [40], enabling an efficient cardiac workload in the context of triggering mitochondrial activity. In addition, it has been postulated that the increased hematocrit observed in the EMPA-REG OUTCOME trial as a consequence of stimulation of erythropoiesis, leading to increased delivery of oxygen to tissues, may contribute to the beneficial effect of SGLT2 inhibitors on cardiovascular outcomes [39]. Interestingly, in a previous study which recruited 75 participants with T2D who were assigned to placebo, dapagliflozin 10 mg/day or hydrochlorothiazide 25 mg/day, increased hematocrit occurred in the dapagliflozin group, whereas it was unchanged in the hydrochlorothiazide and placebo groups at week 12, underpinned by evidence of erythropoiesis [41]. Further investigation is needed to elucidate the underlying mechanism of the favorable effects on cardiovascular outcome. Proposed key mechanisms of SGLT2 inhibitors on cardiorenal protection are summarized in Fig. 1.
Fig. 1.
Proposed key mechanisms of cardiorenal protection by sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs) in patients with type 2 diabetes and cardiovascular disease. eGFR Estimated glomerular filtration rate, HR heart rate
Safety Concerns
SGLT2 inhibitors have been reported to increase the occurrence of mycotic genital infections, predominantly candida vaginitis in women and balanitis in men. A meta-analysis that included 77 randomized controlled trials (RCTs) involving 50,820 patients found that SGLT2 inhibitors were associated with a significantly higher risk of genital infections, whereas no significant association was seen with urinary tract infections [42]. Several case reports suggesting that SGLT2 inhibitors might predispose toward diabetic ketoacidosis (DKA) have evoked concern, although the incidence of DKA reported was relatively rare. Meta-analyses of RCTs have not shown any significant increased incidence of DKA with SGLT2 inhibitor use [43, 44].
One researcher reported 13 episodes of SGLT2 inhibitor-related euglycemic DKA, including 11 episodes in patients with type 1 diabetes (T1D) in whom several episodes were triggered by infectious disease or exercise accompanied by dose reduction of insulin prior to DKA [45]. According to post-marketing reports of Japanese drug manufacturers, one case was associated with switching from multiple therapy that included a sulfonylurea to monotherapy with a SGLT2 inhibitor during strict carbohydrate restriction [46, 47], and another case was associated with long-term starvation. Other reports indicate that increased risk was observed upon discontinuation of prior insulin therapy at the initiation of treatment with SGLT2 inhibitors in patients who had undergone distal pancreatectomy for mucinous cystadenoma [48]. Therefore, when SGLT2 inhibitors are administered to patients with impaired insulin secretion, such as those with T1D or a long duration of diabetes with insufficient β-cell function, clinicians should pay attention to the possibility of “euglycemic ketoacidosis.” The authors of one report recommended that patients with symptoms of ketoacidosis, such as dyspnea, nausea, abdominal pain, ketonuria, and/or ketonemia, should be investigated for DKA regardless of the current glucose status [49].
Canagliflozin was associated with an increased risk of lower limb amputation compared to placebo (6.3 vs. 3.4 amputations per 1000 patient-years; HR 1.97, 95% CI 1.41–2.75) in the CANVAS Program [7]. Importantly, overall amputation risk was correlated with a prior history of amputation at baseline (HR 21.31, 95% CI 15.40–29.49) [50]. In addition, the incidence of all fractures was higher with canagliflozin than with placebo (15.4 vs. 11.9 participants with fracture per 1000 patient-years; HR 1.26, 95% CI 1.04–1.52) [7]. Although a similar risk has not been observed with empagliflozin [51, 52], it remains unclear whether this risk is a class effect.
Clinical Application of SGLT2 Inhibitors
The ADA/EASD currently recommends use of SGLT-2 inhibitors as adjunct therapy to metformin in patients with T2D with established atherosclerotic CVD (ASCVD) or with heart failure or CKD, barring the existence of impaired renal function, on the basis of the results of recent large clinical trials [14, 53]. The cardiovascular benefits of SGLT2 inhibitors seem to be robust; however, beneficial effects on MACE and cardiovascular death were not apparent in those individuals without established ASCVD [24]. Thus, it would be premature to prescribe these drugs for such patients in the expectation of a benefit for primary prevention of major cardiovascular events. On the other hand, a previous meta-analysis [24] showed a comparable reduction of hospitalization for heart failure across patients regardless of history of heart failure or established ASCVD, suggesting that the effect on prevention of worsening of heart failure may be consistent independent of baseline characteristics in those patients at high risk for cardiovascular events. Interestingly, when the results of the three large trials were combined, it was revealed that the magnitude of risk reduction in hospitalization for heart failure with SGLT2 inhibitors was greatest in those with an eGFR of < 60 ml/min/1.73 m2 (40%) compared with those with an eGFR of between 60 and 90 ml/min/1.73 m2 (31%) and those with an eGFR of > 90 ml/min/1.73 m2 (12%; this change did not reach significance) (p for interaction across subgroups = 0.0073) [24]. These results indicate that because a large proportion of patients with impaired renal function were included in the high-risk group for adverse cardiovascular events, the protective effect against worsening of heart failure may be more likely in patients with reduced eGFR, even though the glucose-lowering effect decreases as eGFR decreases [54]. As monotherapy or as an adjunct to metformin, SGLT2 inhibitors do not increase the risk of hypoglycemia. However, in the setting of concomitant use of insulin or an insulin secretagogue (a sulfonylurea or glinide), the physician should consider reducing the total daily dose of insulin by ≤ 20% with careful consideration of endogenous insulin secretion and reducing the sulfonylurea or glinide dose by 50% or to half the maximum permitted dose, as well as discontinuing these agents when the patient is already on a minimal dose, in anticipation of the risk of hypoglycemia [55].
SGLT2 inhibitors increase the risk of mycotic genital infection and should be administered with caution in patients with a history of genital infection or urinary tract infection, especially in those with poor glycemic control or concomitant use of immunosuppressive agents. In patients with depleted insulin secretion, including those with T1D or with a long duration of diabetes, the risk of euglycemic ketoacidosis is raised, and thus clinicians need to be alert for symptoms associated with diabetic ketoacidosis, such as nausea, vomiting, and generalized weakness [49]. A recent experimental study has shown that dapagliflozin-induced volume depletion and simultaneous reduced insulin levels were both necessary and sufficient to generate euglycemic ketoacidosis in rats [56]. The clinical relevance of these results could be that since patients with depleted insulin secretion and with an incapability to voluntarily drink water or an impaired thirst center, especially elderly persons, may be at high risk, avoidance of dehydration and an appropriate dose of concomitant insulin therapy are important to prevent the occurrence of euglycemic ketoacidosis if a SGLT2 inhibitor is administered.
Canagliflozin was found to increase the risk of lower limb amputation in the CANVAS trial, which is inconsistent with the effects of other SGLT2 inhibitors. Moreover, the frequency of amputation and of fracture were not increased in the CREDENCE trial [25]. It is unclear whether the risk of lower limb amputation is a class effect or not, and administration of this class of medication may need to be avoided in patients with a history of prior amputation or with existing peripheral artery disease.
GLP-1 Receptor Agonists
Mode of Action of GLP-1 Receptor Agonists
Glucagon-like peptide-1 is synthesized in L-cells situated in the distal ileum, where it is secreted in response to nutrient intake [57], and it is quickly degraded within 2–3 min by DPP-4 [58]. Following administration of a GLP-1 receptor agonist, postprandial insulin secretion is stimulated and glucagon secretion is reduced in a glucose-dependent fashion, thereby delaying gastric emptying, which in turn induces satiety.
Cardiovascular Outcome Trials
A summary of major CVOTs with GLP-1 receptor agonists is shown in Table 2. The LEADER trial, in which 9340 patients, including 72.4% with established ASCVD, were randomly assigned to liraglutide or placebo, demonstrated a 13% reduction in the 3-point MACE composite (HR 0.87, 95% CI 0.78–0.97, p = 0.01) with liraglutide versus placebo during a median follow-up period of 3.8 years. Each component of the primary composite outcome showed a directionally similar trend toward a reduction in 3-point MACE, in which cardiovascular death reached statistical significance (HR 0.78, 95% CI 0.66–0.93). Patients receiving liraglutide showed a significant reduction in all-cause mortality (HR 0.85, 95% CI 0.74–0.97), predominantly driven by a reduction in cardiovascular death [11].
Table 2.
Summary of major cardiovascular outcomes trials with glucagon-like peptide-1 receptor agonists
| Parameters | LEADER trial [11, 62] | SUSTAIN-6 trial [12] | ELIXA trial [60, 103] | EXSCEL trial [59, 101] |
|---|---|---|---|---|
| Drug | Liraglutide | Semaglutide | Lixisenatide | Exenatide QW |
| Dose | 1.8 mg or tolerable maximum dose, once daily | 0.5/1 mg, once weekly | 10/20 μg, once daily | 2 mg, once weekly |
| Number of participants | 9340 | 3297 | 6068 | 14,752 |
| Mean age (years) | 64.3 | 64.6 | 60.3 | 62.0 |
| Mean HbA1c at baseline (%) | 8.7 | 8.7 | 7.7 | 8.0 |
| Median follow-up period (years) | 3.8 | 2.1 | 2.1 | 3.2 |
| Patients with established CVD | 6764 (72.4%) | 2382 (72.2%) | 6068 (100%) | 10,782 (73.1%) |
| 3-point MACE (CV death, nonfatal MI, nonfatal stroke) | HR 0.87 (0.78–0.97) | HR 0.74 (0.58–0.95) | HR 1.02 (0.89–1.17)d | HR 0.91 (0.83–1.00) |
| CV death | HR 0.78 (0.66–0.93) | HR 0.98 (0.65–1.48) | HR 0.98 (0.78–1.22) | HR 0.88 (0.76–1.02) |
| Nonfatal MI | HR 0.88 (0.75–1.03) | HR 0.74 (0.51–1.08) | HR 1.03 (0.87–1.22)e | HR 0.95 (0.84–1.09) |
| Nonfatal stroke | HR 0.89 (0.72–1.11) | HR 0.61 (0.38–0.99) | HR 1.12 (0.79–1.58)e | HR 0.86 (0.70–1.07) |
| Hospitalization for heart failurea | HR 0.87 (0.73–1.05) | HR 1.11 (0.77–1.61) | HR 0.96 (0.75–1.23) | HR 0.94 (0.78–1.13) |
| Progression of albuminuriab | HR 0.74 (0.60–0.91) | HR 0.54 (0.37–0.77) | HR 0.815 (0.665–0.999) | n.a. |
| Renal outcomec | HR 0.78 (0.67–0.92) | HR 0.64 (0.46–0.88) | HR 1.163 (0.741–1.825) | HR 0.85 (0.73–0.98) |
| Parameters | REWIND trial [13, 64] | HARMONY trial [10] | PIONEER 6 trial [102] |
|---|---|---|---|
| Drug | Dulaglutide | Albiglutide | Oral semaglutide |
| Dose | 1.5 mg once weekly | 30 mg (increased to 50 mg if inadequately controlled) once weekly | 14 mg once daily |
| Number of participants | 9901 | 9463 | 3183 |
| Mean age (years) | 66.2 | 64.1 | 66 |
| Mean HbA1c at baseline (%) | 7.3 | 8.7 | 8.2 |
| Median follow-up period (years) | 5.4 | 1.6 | 1.3 |
| Patients with established CVD | 3114 (31.5%) | 6678 (71.0%)f | 2695 (84.7%)g |
| 3-point MACE (CV death, nonfatal MI, nonfatal stroke) | HR 0.88 (0.79–0.99) | HR 0.78 (0.68–0.90) | HR 0.79 (0.57–1.11) |
| CV death | HR 0.91 (0.78–1.06) | HR 0.93 (0.73–1.19) | HR 0.49 (0.27–0.92) |
| Nonfatal MI | HR 0.96 (0.79–1.16) | HR 0.75 (0.61–0.90)e | HR 1.18 (0.73–1.90) |
| Nonfatal stroke | HR 0.76 (0.61–0.95) | HR 0.86 (0.66–1.14)e | HR 0.74 (0.35–1.57) |
| Hospitalization for heart failurea | HR 0.93 (0.77–1.12) | HR 0.85 (0.70–1.04) | HR 0.86 (0.48–1.55) |
| Progression of albuminuriab | HR 0.77 (0.68–0.87) | n.a. | n.a. |
| Renal outcomec | HR 0.85 (0.77–0.93) | n.a. | n.a. |
Exenatide QW Exenatide once weekly, ELIXA Evaluation of Lixisenatide in Acute Coronary Syndrome trial, EXSCEL Exenatide Study of Cardiovascular Event Lowering trial, HARMONY Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease trial,LEADER Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results trial, REWIND Researching Cardiovascular Events With a Weekly Incretin in Diabetes trial,PIONEER-6 Trial investigating the cardiovascular safety of oral semaglutide in subjects with type 2 diabetes, SUSTAIN-6 Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes 6 trial
aHospital admission for heart failure or urgent visit in REWIND, and composite of death from cardiovascular causes or hospital admission for heart failure in HARMONY outcomes
bDescribed as new-onset persistent macroalbuminuria in the LEADER, SUSTAIN-6, ELIXA, and REWIND trials. It was adjusted for baseline and on-trial HbA1c in an exploratory analysis of ELIXA
cRenal outcome comprised new-onset persistent macroalbuminuria, persistent doubling of serum creatinine level, an eGFR of ≤ 45 ml/min/1.73 m2 body-surface area, a need for continuous renal replacement therapy (ESRD), or death from renal disease in LEADER; as the composite of new onset of persistent macroalbuminuria, or persistent doubling of serum creatinine level and creatinine clearance of < 45 ml/min/1.73 m2 body-surface area, or the need for continuous renal replacement therapy or death from renal disease in SUSTAIN-6; as doubling of serum creatinine in ELIXA; as the composite of 40% eGFR decline, renal replacement, renal death, and new macroalbuminuria in EXSCEL; and as the composite of the first occurrence of new macroalbuminuria, a sustained decline in eGFR of ≥ 30% from baseline, or chronic renal replacement therapy in REWIND
d4-point MACE (CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina) was adopted in ELIXA
eIncluding both nonfatal and fatal MI/stroke in ELIXA and HARMONY outcomes
fNumber of patients with a history of coronary artery disease
gIncluding chronic kidney disease
The Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes 6 (SUSTAIN-6) trial, which enrolled 3297 patients who were administered semaglutide 0.5 or 1.0 mg per week or placebo, showed a significant 26% reduction in 3-point MACE (HR 0.74, 95% CI 0.58–0.95), a significant reduction in nonfatal stroke (HR 0.61, 95% CI 0.38–0.99), and a directionally concordant result in nonfatal MI (HR 0.74, 95% CI 0.51–1.08). These findings are in contrast to the results of the EMPA-REG OUTCOME trial, which revealed a 24% increase (which did not reach statistical significance) in nonfatal stroke [9, 12]. No reduction in all-cause mortality (HR 1.05, 95% CI 0.74–1.50) or cardiovascular death (HR 0.98, 95% CI 0.65–1.48) was revealed in SUSTAIN-6, unlike the effect of liraglutide in the LEADER trial. No beneficial effect on hospitalization for heart failure was observed in both trials.
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial recruited 14,752 subjects, including 73% with established ASCVD, who were randomly assigned to once-weekly exenatide versus placebo [59]. The 3-point MACE tended to be lower in the exenatide group compared with placebo, with borderline significance (HR 0.91 95% CI 0.83–1.00). Once-weekly exenatide also lowered the risk of all-cause mortality (HR 0.86, 95% CI 0.77–0.97) compared with placebo, accompanied by a directionally consistent trend in cardiovascular mortality (HR 0.88, 95% CI 0.76–1.02). The effect on hospitalization for heart failure was neutral, similarly to the effect of other GLP-1 receptor agonists. In the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial, in which 6068 patients who had experienced acute coronary syndrome within the preceding 180 days were enrolled, lixisenatide failed to demonstrate a significant reduction in a 4-point MACE composite outcome (CV death, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina), any component of MACE, or hospitalization for heart failure over a median follow-up period of 2.1 years [60]. The reasons for the conflicting results of CV outcome with GLP-1 receptor agonists are unclear. However, a partial explanation could be that the participants recruited in the ELIXA trial were at much higher risk of recurrence of adverse cardiovascular events, as reflected by the incidence of the primary outcome of 6.4/100 patient-year in ELIXA versus 3.9/100 in LEADER [61]. In addition, differences in the duration of the glucose-lowering effect between lixisenatide (short-acting GLP-1 receptor agonist) and semaglutide or liraglutide (long-acting GLP-1 receptor agonists) may have contributed to the heterogeneity among these trials.
Renal Outcome
In the LEADER trial, liraglutide was associated with a 22% reduction in nephropathy, defined as new onset of macroalbuminuria, doubling of serum creatinine level, an eGFR of < 45 ml/1.73 m2, the need for continuous renal replacement therapy, or death from renal disease (HR 0.78, 95% CI 0.67–0.92,p = 0.003). Among the components of the composite renal outcome [62], the risk of new-onset persistent macroalbuminuria significantly decreased with liraglutide (HR 0.74, 95% CI 0.60–0.91, p = 0.004), although the risks of persistent doubling of the serum creatinine level and of ESRD did not change (HR 0.89, 95% CI 0.67–1.19,p = 0.43 and HR 0.87, 95% CI 0.61–1.24,p = 0.44, respectively).
In the SUSTAIN-6 trial, semaglutide caused a 36% reduction in the risk of new or worsening nephropathy (HR 0.64, 95% CI 0.46–0.88, p = 0.005). Significant risk reduction in persistent macroalbuminuria (HR 0.54, 95% CI 0.37–0.77, p = 0.001) was demonstrated, whereas neither the risk of persistent doubling of serum creatinine level and creatinine clearance per modification of diet in renal disease of < 45 ml/min/1.73 m2 nor that of need for continuous renal replacement therapy decreased (HR 1.28, 95% CI 0.64–2.58,p = 0.48 and HR 0.91, 95% CI 0.40–2.07,p = 0.83, respectively) [12].
In AWARD-7, a multicenter, open-label, randomized study in which 576 patients with T2D and stage 3–4 CKD were randomly assigned to once-weekly dulaglutide 0.75 or 1.5 mg or daily insulin glargine with concomitant use of insulin, dulaglutide mitigated eGFR decline [63]. However, in the exploratory analysis of the REWIND trial [64], significant risk reduction was observed only in new occurrences of macroalbuminuria (HR 0.77, 95% CI 0.68–0.87, p < 0.0001) among the renal component of the composite microvascular outcome. Thus, it remains unclear whether GLP-1 receptor agonists are able to improve renal outcomes other than albuminuria, although a significant reduction in the incidence of a sustained decline in eGFR of ≥ 40% and ≥ 50%, but not ≥ 30%, , was observed in the trial [64].
Possible Mechanisms of CV Outcome
The delayed separation of the Kaplan–Meier curves seen in the trials of liraglutide and semaglutide, in contrast to the early separation of curves observed in the EMPA-REG OUTCOME trial, and the absence of the significant reduction in hospitalization for heart failure with GLP-1 receptor agonists that has been observed with SGLT2 inhibitors suggest an anti-atherothrombotic effect of GLP-1 receptor agonists rather than the hemodynamic effect related to SGLT2 inhibitors. Moreover, the 3-point MACE reduction reported in the LEADER trial, which was derived from a concordant reduction in all cardiovascular endpoints, thereby contributing to a reduction in cardiovascular death, and that in the SUSTAIN-6 trial were primarily driven by a significant reduction in nonfatal stroke, underscoring the anti-atherosclerotic mechanism of this class of medications. GLP-1 receptor agonists exert a favorable effect on cardiovascular outcome not only through glycemic control, body weight reduction, and improved blood pressure and lipid profiles, but also by amelioration of inflammatory markers, resulting in the enhanced retardation of atherosclerosis (Fig. 1) [65]. However, the precise mechanism is not yet fully elucidated.
Further analyses in LEADER were also unable to readily explain the demonstrated preventive role of GLP-1 receptor agonists in new onset of albuminuria by improvement in glycemic control, systolic blood pressure, or body weight [62]. Previous studies suggested that GLP-1 directly induces natriuresis by inhibiting sodium-hydrogen exchanger isoform-3 (NHE3) in the proximal tubule, which may contribute to reducing albuminuria through amelioration of tubuloglomerular feedback, although the hemodynamic change derived from the natriuretic effect of these drugs remains unclear [66–68].
In a study using a rodent model with progressive diabetic nephropathy, liraglutide was shown to ameliorate oxidative stress by increasing renal cAMP level and protein kinase A activation and downregulation of NOX4 and NAD(P)H oxidase activity, resulting in reduced albuminuria, mesangial expansion, and improved glomerular hyperfiltration [69]. Another study [70] showed that GLP-1 suppressed oxidative stress and inflammation and improved endothelial dysfunction in patients with T1D. Thus, GLP-1 receptor agonists may exert a renoprotective effect through these anti-oxidative and anti-inflammatory effects on the diabetic kidney. Of note, in clinical trials, GLP-1 receptor agonists did not demonstrate superiority in any hard endpoint compared to placebo, including doubling of serum creatinine level and progression to ESRD [12, 62, 64]. It has been suggested that a GLP-1 receptor-independent pathway could be involved in the development of renal interstitial fibrosis and kidney dysfunction in GLP-1 receptor knockout mice with 5/6 nephrectomy [71, 72], which may at least partially explain why GLP-1 receptor agonists seemingly do not play a major role in preventing progression of diabetic nephropathy.
Safety Concerns
The most common side effects of GLP-1 receptor agonists are nausea and vomiting, which are more likely with short-acting than with long-acting GLP-1 receptor agonists. These adverse effects are usually transient and diminish within a couple of weeks [73].
The LEADER trial showed a significant increase in acute gallstones (p < 0.001) and acute cholecystitis (p = 0.046) in patients treated with liraglutide [11]. The SUSTAIN-6 trial showed that semaglutide was associated with increased retinopathy complications (HR 1.76, 95% CI 1.11–2.78, p = 0.02), largely among those with retinopathy at baseline; these complications may be partially mediated by rapid improvement of glycemic control in patients with diabetic retinopathy and poorly controlled glycemic status [12, 74]. In contrast to the post-marketing case reports, a systematic review and meta-analysis found that GLP-1 receptor agonists do not represent an increased risk of pancreatitis or pancreatic cancer [75].
In the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) trial, which involved 300 patients with advanced heart failure, the liraglutide group showed a numerically higher (but not statistically significant) risk of rehospitalization for heart failure (HR 1.30, 95% CI 0.89–1.88, p = 0.17) compared with the placebo group [76]. Liraglutide was associated with a significantly increased rate of serious adverse cardiac events in patients with a reduced left ventricular ejection fraction of < 45% compared with placebo (12 [0%] vs. 3 [3%], respectively; p = 0.04), accompanied by increased heart rate [77]. Liraglutide has also been reported to increase mean 24-h heart rate by approximately 9 bpm following 8 weeks of treatment, versus 3 bpm with lixisenatide [78, 79]. However, the correlation between the observed negative impact on advanced heart failure and increased heart rate with liraglutide needs further investigation.
Clinical Application of GLP-1 Receptor Agonists
Glucagon-like peptide-1 receptor agonists are categorized as short-acting (exenatide, lixisenatide) or long-acting (liraglutide, exenatide once weekly, semaglutide, dulaglutide, albiglutide), and by their distinct pharmacokinetic/pharmacodynamic characteristics. Short-acting GLP-1 receptor agonists preferentially reduce postprandial plasma glucose through delayed gastric emptying and inhibition of glucagon concentration, whereas long-acting GLP-1 receptor agonists mainly exert their glucose-lowering effect on fasting plasma glucose (FPG) by stimulating insulin secretion [58].
With regard to glycemic control, exenatide 10 μg twice daily reduced HbA1c from baseline to a smaller extent compared with exenatide 2 mg once weekly (DURATION-1 and -5 trials) [80–82], liraglutide 1.8 mg once daily (LEAD-6 trial) [83], and dulaglutide 1.5 or 0.75 mg once weekly (AWARD-1 trial) (p < 0.01, respectively) [84]. Liraglutide 1.8 mg once daily showed a larger decrease in HbA1c than did lixisenatide 20 μg once daily [79, 85, 86], as did exenatide 10 μg twice daily [83] and exenatide 2 mg once weekly [87], and its glucose-lowering effect was non-inferior to that of dulaglutide 1.5 mg once weekly (AWARD-6 trial) [88], whereas semaglutide 1.6 mg once weekly showed a numerically larger decrease in HbA1c from baseline than did liraglutide 1.8 mg daily, accompanied by a comparable impact on FPG level [89]. Liraglutide resulted in a larger reduction in body weight from baseline than did duraglutide 1.5 mg once weekly [88] or exenatide 2 mg once weekly [87]. Semaglutide 1.0 mg once weekly achieved greater weight loss as well as HbA1c reduction than did exenatide 2 mg once weekly [90], and the mean change in body weight from baseline to week 12 was larger with semaglutide 0.8 mg once weekly with dose escalation than with liraglutide 1.2 mg (− 1.7 kg, 95% CI − 2.8 to − 0.7) or 1.8 mg (− 1.0 kg, 95% CI − 2.0 to − 0.0), with a marginal difference in HbA1c between each group (vs. liraglutide 1.2 mg: − 0.3%, 95% CI − 0.6 to 0.0; vs. liraglutide 1.8 mg: − 0.1%, 95% CI − 0.4 to 0.2) in accordance with the unadjusted 95% CI [89]. In previous analyses, including 11 RCTs, exenatide 2 mg and dulaglutide 0.75 mg once weekly achieved lower HbA1c compared with insulin glargine (exenatide: − 0.31%, 95% CI − 0.42 to − 0.19; dulaglutide: − 0.39%, 95% CI − 0.49 to − 0.29), whereas neither once-daily liraglutide nor twice-daily exenatide did [91].
Weight reduction has been found to be a common characteristic of all GLP-1 receptor agonists while weight gain is seen with basal insulin. In the setting of hyperglycemia despite treatment with multiple oral agents, clinicians need injectable medications with greater potency in order to overcome glucotoxicity, which is commonly experienced among such patients. These trial results suggest that a GLP-1 receptor agonist is the preferred option in these patients, especially in those with difficulty managing their body weight, given the beneficial effect of GLP-1 receptor agonists on body weight and the low risk of hypoglycemia, unless the patient develops nausea or gastroparesis or depletion of intrinsic insulin secretion.
In patients with multiple daily injections, the combination of basal insulin and a GLP-1 receptor agonist is worth considering because of less weight gain and hypoglycemia without exacerbation of glycemic control [92]. It is recommended that when a GLP-1 receptor agonist is initiated in a patient in whom HbA1c is controlled at < 8.0%, the clinician should reduce the basal insulin dose by 20% in advance to limit the risk of anticipated hypoglycemia [93].
Discussion
The FDA issued diabetes guidance in 2008 mandating that all new anti-diabetic drugs must undergo post-marketing endpoint trials for cardiovascular safety in view of the possible elevated cardiovascular risk with rosiglitazone. A series of cardiovascular outcome trials revealed an unexpected benefit on cardiovascular outcome with SGLT2 inhibitors and GLP-1 receptor agonists, highlighting the advantage of secondary prevention in patients with established CVD. A consensus report by the ADA/EASD recommends SGLT2 inhibitors for patients with ASCVD with coexistent heart failure, based on the results of recent large clinical trials. The report also recommends that SGLT2 inhibitors should be considered for patients with T2D and CKD, regardless of CVD, and if they are contraindicated or not preferred, a GLP-1 receptor agonist should be selected [14]. Both agents have demonstrated improved renal outcome, including a protective effect against albuminuria, and SGLT2 inhibitors mitigate a decline in kidney function, although the underlying mechanism needs further investigation. Both drugs are rarely associated with hypoglycemia; however, concomitant use with insulin or an insulin secretagogue could increase the risk of hypoglycemia, and thus reduction of the existing insulin dose is desirable unless there is severe hyperglycemia.
The efficacy of combination therapy with a GLP-1 receptor agonist and SGLT2 inhibitor has been elucidated [94, 95], with further improvement of glycemic control compared with monotherapy also observed, although the achieved glycemic efficacy was subadditive partially due to stimulatory effects on glucagon and hepatic glucose production correlated with glycosuria derived from SGLT2 inhibitors [96]. Three separately conducted 52-week open-label studies to evaluate the efficacy and safety of SGLT2 inhibitors, involving canagliflozin, ipragliflozin, and luseogliflozin, in combination with a GLP-1 receptor agonist showed a further decrease in HbA1c and body weight without any increase in serious adverse events including severe hypoglycemia [97–99]. This combination is therefore an attractive therapeutic option for patients with obesity refractory to treatment with an SGLT2 inhibitor or GLP-1 receptor agonist alone or in combination with insulin therapy in terms of long-term glycemic control and body weight control.
Conclusion
Recent clinical trials have revealed a novel role of SGLT2 inhibitors and GLP-1 receptor agonists beyond their glucose-lowering effect, and both agents have received more attention as a paradigm shift from “the lower glucose the better” to “how to optimize glycemic control without hypoglycemia and overweight” has been generated by current guidelines. Full advantage of the pleiotropic benefit of these agents should be taken to prevent secondary adverse cardiovascular events and to mitigate the advance of renal complications without increased risk of hypoglycemia.
Acknowledgements
The authors thank Dr. Wendy Gray, self-employed, for editing the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article. Publication fee of this article was waived by the publisher.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Yoshifumi Saisho received honoraria from Takeda Pharmaceutical Co. Ltd., Japan and Nippon Boehringer Ingelheim Co. Ltd., Japan and a grant from AstraZeneca K.K., Japan not related to the submitted work. Taichi Nagahisa has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9332171.
References
- 1.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 2.Mahaffey KW, Hafley G, Dickerson S, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166(240–249):e241. doi: 10.1016/j.ahj.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 4.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 5.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk The CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 11.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 14.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;2018(41):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed]
- 16.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352: 854–65. [PubMed]
- 17.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;24:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 20.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 21.Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–270. doi: 10.1146/annurev-med-051013-110046. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 24.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 25.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 26.Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 29.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 30.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 31.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 32.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 33.Cruzado JM, Rico J, Grinyo JM. The renin angiotensin system blockade in kidney transplantation: pros and cons. Transpl Int. 2008;21:304–313. doi: 10.1111/j.1432-2277.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 34.Chu C, Lu YP, Yin L, et al. The SGLT2 inhibitor empagliflozin might be a new approach for the prevention of acute kidney injury. Kidney Blood Press Res. 2019;44:149–157. doi: 10.1159/000498963. [DOI] [PubMed] [Google Scholar]
- 35.Tang H, Li D, Zhang J, et al. Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:1106–1115. doi: 10.1111/dom.12917. [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 37.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 38.Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. No direct effect of SGLT2 activity on glucagon secretion. Diabetologia. 2019;62:1011–1023. doi: 10.1007/s00125-019-4849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 40.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 41.Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7:2824. doi: 10.1038/s41598-017-02733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XL, Zhu QQ, Chen YH, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc. 2018;7(2):e007165. doi: 10.1161/JAHA.117.007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang H, Li D, Wang T, et al. Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2016;39:e123–e124. doi: 10.2337/dc16-0885. [DOI] [PubMed] [Google Scholar]
- 45.Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayami T, Kato Y, Kamiya H, et al. Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig. 2015;6:587–590. doi: 10.1111/jdi.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–138. doi: 10.1111/jdi.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hine J, Paterson H, Abrol E, et al. SGLT inhibition and euglycaemic diabetic ketoacidosis. Lancet Diabetes Endocrinol. 2015;3:503–504. doi: 10.1016/S2213-8587(15)00204-1. [DOI] [PubMed] [Google Scholar]
- 49.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–1642. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 50.Matthews DR, Li Q, Perkovic V, et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62:926–938. doi: 10.1007/s00125-019-4839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D, Yang JY, Wang T, et al. Risks of diabetic foot syndrome and amputation associated with sodium glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Metab. 2018;44:410–414. doi: 10.1016/j.diabet.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Kohler S, Kaspers S, Salsali A, et al. Analysis of fractures in patients with type 2 diabetes treated with empagliflozin in pooled data from placebo-controlled trials and a head-to-head study versus glimepiride. Diabetes Care. 2018;41:1809–1816. doi: 10.2337/dc17-1525. [DOI] [PubMed] [Google Scholar]
- 53.The American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care 2019;42:S90-S102. [DOI] [PubMed]
- 54.Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93:231–244. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry RJ, Rabin-Court A, Song JD, et al. Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun. 2019;10:548. doi: 10.1038/s41467-019-08466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anandhakrishnan A, Korbonits M. Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J Diabetes. 2016;7:572–598. doi: 10.4239/wjd.v7.i20.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 59.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 61.Kaul S. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 trials. Diabetes Care. 2017;40:821–831. doi: 10.2337/dc17-0291. [DOI] [PubMed] [Google Scholar]
- 62.Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 63.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 64.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 65.Bruen R, Curley S, Kajani S, et al. Liraglutide dictates macrophage phenotype in apolipoprotein E null mice during early atherosclerosis. Cardiovasc Diabetol. 2017;16:143. doi: 10.1186/s12933-017-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skov J, Pedersen M, Holst JJ, et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18:581–589. doi: 10.1111/dom.12651. [DOI] [PubMed] [Google Scholar]
- 67.Farah LX, Valentini V, Pessoa TD, et al. The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am J Physiol Renal Physiol. 2016;310:F123–F127. doi: 10.1152/ajprenal.00394.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tonneijck L, Smits MM, Muskiet MHA, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59:1412–1421. doi: 10.1007/s00125-016-3938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 70.Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasan AA, von Websky K, Reichetzeder C, et al. Mechanisms of GLP-1 receptor-independent renoprotective effects of the dipeptidyl peptidase type 4 inhibitor linagliptin in GLP-1 receptor knockout mice with 5/6 nephrectomy. Kidney Int. 2019;95:1373–1388. doi: 10.1016/j.kint.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Hasan AA, Hocher B. Role of soluble and membrane-bound dipeptidyl peptidase-4 in diabetic nephropathy. J Mol Endocrinol. 2017;59:R1–R10. doi: 10.1530/JME-17-0005. [DOI] [PubMed] [Google Scholar]
- 73.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–216. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilsboll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889–897. doi: 10.1111/dom.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 76.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 78.Owens DR, Monnier L, Barnett AH. Future challenges and therapeutic opportunities in type 2 diabetes: changing the paradigm of current therapy. Diabetes Obes Metab. 2017;19:1339–1352. doi: 10.1111/dom.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meier JJ, Rosenstock J, Hincelin-Mery A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38:1263–1273. doi: 10.2337/dc14-1984. [DOI] [PubMed] [Google Scholar]
- 80.Dalsgaard NB, Vilsboll T, Knop FK. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk factors: a narrative review of head-to-head comparisons. Diabetes Obes Metab. 2018;20:508–519. doi: 10.1111/dom.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 82.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–1310. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 83.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 84.Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37:2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 85.Nauck M, Rizzo M, Johnson A, et al. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care. 2016;39:1501–1509. doi: 10.2337/dc15-2479. [DOI] [PubMed] [Google Scholar]
- 86.Kapitza C, Forst T, Coester HV, et al. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15:642–649. doi: 10.1111/dom.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 88.Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 89.Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. doi: 10.2337/dc15-0165. [DOI] [PubMed] [Google Scholar]
- 90.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41:258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 91.Singh S, Wright EE, Jr, Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19:228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 93.Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74:2141–2152. doi: 10.1007/s40265-014-0325-2. [DOI] [PubMed] [Google Scholar]
- 94.Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 95.Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 96.DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353–1362. doi: 10.1111/dom.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harashima SI, Inagaki N, Kondo K, et al. Efficacy and safety of canagliflozin as add-on therapy to a glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes mellitus: a 52-week, open-label, phase IV study. Diabetes Obes Metab. 2018;20:1770–1775. doi: 10.1111/dom.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishihara H, Yamaguchi S, Nakao I, et al. Ipragliflozin add-on therapy to a GLP-1 receptor agonist in Japanese patients with type 2 diabetes (AGATE): a 52-week open-label study. Diabetes Ther Res Treat Educ Diabetes Rel Disorders. 2018;9:1549–1567. doi: 10.1007/s13300-018-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seino Y, Yabe D, Sasaki T, et al. Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52-week, open-label, single-arm study. J Diabetes Investig. 2018;9:332–340. doi: 10.1111/jdi.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raz I, Wiviott SD, Yanuv I, et al. 244-OR: effects of dapagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes: a predefined analysis from the DECLARE-TIMI 58 randomised, placebo-controlled trial. Diabetes. 2019;1(Suppl 1):244-OR. [Google Scholar]
- 101.Bethel MA, Mentz RJ, Merrill P, et al. Renal outcomes in the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) Diabetes. 2018;67(Suppl 1):522-P. [Google Scholar]
- 102.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019 doi: 10.1056/nejmoa1901118. [DOI] [PubMed] [Google Scholar]
- 103.Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:859–869. doi: 10.1016/S2213-8587(18)30268-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.