Abstract
Background:
Engineered cell sheet transplantation has been considered an alternative physiological therapy for endocrine disorders. In this study, we attempted to fabricate functional human thyroid cell sheets using the engineering technology by culturing primary thyrocytes in free-feeder monolayers and assessed their proliferation and function in two different media.
Methods:
The non-tumorous tissues (approximately 2 g) were dissected during surgery. Primary human thyroid cells were isolated by mechanical dispersion and treatment with isolation solution. The cells were cultured on tissue culture dishes or temperature-responsive culture dishes to induce the formation of detached cell sheets.
Results:
Primary thyroid cells isolated from nine patients were positive for thyroid transcription factor 1, thyroglobulin (TG) and cytokeratin 7. Cell sheets with follicles were fabricated by cells incubated in both Dulbecco’s Modified Eagle Medium (DMEM) and hepatocyte-defined medium (HDM) culture medium. The diameter and thickness of sheets fabricated in HDM were larger and thicker than those fabricated from DMEM. Furthermore, the cells incubated in HDM secreted higher levels of fT3 and fT4 than those incubated in DMEM. The thyroid peroxidase and TG mRNA of cells maintained in HDM were higher than those in cells maintained in DMEM.
Conclusion:
HDM appears suitable as a culture medium for maintaining primary thyrocytes and fabricating functional cell sheets. These in vitro findings may contribute to the development of appropriate culture conditions for human thyrocytes as well as engineered functional cell sheets.
Keywords: Cell sheet, Thyroid, Free-feeder monolayer, Hepatocyte-defined medium
Introduction
In cases of hormonal deficiency caused by endocrine organ diseases, continuous hormone administration is indispensable to compensate [1]. However, the maintenance of the complex homeostatic interactions of hormones via oral administration alone may be difficult. In this context, engineered cell sheet transplantation has been considered as an alternative physiological therapy for endocrine disorders [2]. Previous studies have shown that the transplantation of cell sheets is a more effective cell transfer technique than the injection of isolated cells in various cell populations [3, 4]. Furthermore, cell sheet transplantation is useful in that we can handle the cell sheets easily and control their size and number of layers [2, 5, 6].
Several reports have described the fabrication of thyroid tissues. Bell et al. [7] reported the fabrication of thyroid gland equivalents by cultivating rat thyroid cells within collagen gel. The mixture became tissue-like in vitro, and organized follicles were detected after implantation into thyroidectomized hosts. Toda et al. [8, 9] also studied the reconstruction of thyroid follicles from isolated porcine thyrocytes in three-dimensional (3D) collagen gel culture. Bechtner et al. [10] cultured isolated porcine thyroid follicles in Matrigel, thereby providing a tool for investigating the regulation of follicular growth and neoformation close to the in vivo situation. Fröhlich et al. [11] showed that porcine thyroid cells were able to form follicles with a regular basal lamina when they were cultured in a 3D environment. Takasu et al. [12] analyzed the relationships of iodine metabolism and cell polarity, using polarized monolayer porcine thyroid cells cultured on collagen-coated filters and double layered, follicle-forming cells. Arauchi et al. [2] reconstructed 3D thyroid tissues using cell sheet technology with rat thyrocytes and transplanted them into a hypothyroidism rat model; the thyroid function was restored within 1 week after cell sheet transplantation, and improvement was maintained for 4 weeks.
However, all of these previous studies were performed with non-human cells. Therefore, in the present study, we attempted to fabricate functional human thyroid cell sheets using the available engineering technology.
Materials and methods
Patients
The study was approved by the Institutional Review Board of our institution (approval number 14052642), and informed consent was obtained from all human donors. Resected human thyroid tissues were obtained during surgery (Table 1). The non-tumorous tissues (approximately 2 g) were carefully dissected by experienced operators.
Table 1.
Sources of human thyroid gland for primary thyroid cell isolation
| Case | Age (years) | Gender | Diagnosis |
|---|---|---|---|
| 1 | 32 | F | Thyroid cancer |
| 2 | 70 | F | Thyroid tumor |
| 3 | 35 | F | Graves’ disease |
| 4 | 31 | F | Thyroid tumor |
| 5 | 55 | F | Graves’ disease |
| 6 | 71 | M | Graves’ disease |
| 7 | 37 | F | Graves’ disease |
| 8 | 25 | M | Graves’ disease |
| 9 | 16 | M | Graves’ disease |
Isolation and culture of primary human thyroid cells
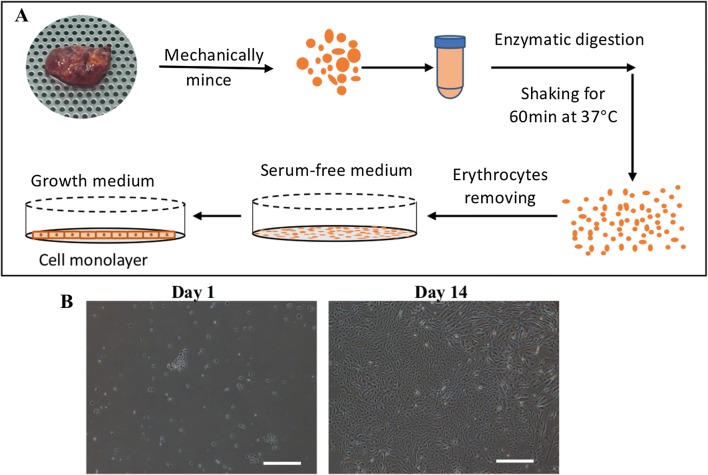
Primary human thyroid cells were isolated by mechanical dispersion and treatment with isolation solution, as previously described [13, 14] (Fig. 1). In brief, non-tumorous thyroid tissue samples were washed with phosphate-buffered saline (PBS) (Wako Pure Chemical Corp., Osaka, Japan). Part of the sample from Case 3 was used for a cytologic examination. After cutting the tissue into small pieces (< 1 mm in size), the tissue pieces were digested with 50 mL of isolation solution (HANK’s complement with 1.5 mg/mL of Collagenase I) through shaking for 60 min at 37 °C. The supernatant was then transferred into two 50-ml conical tubes, and fetal bovine serum (FBS; 5% [v/v]) was added to neutralize isolation buffer, followed by centrifugation at 1000 rpm for 5 min. The supernatant was then discarded, and the sample was resuspended and the pellet incubated with 2 mL of RBC lysis buffer (Bay bioscience Co., Ltd., Kobe, Japan) for 10 min at room temperature (RT) to remove erythrocytes. The cells were washed with PBS and centrifuged at 500 g for 5 min to collect the cell pellets. The primary thyroid cells were incubated on 100-mm tissue culture treated dishes (Nest Scientific USA Inc., Rahway, NJ, USA) in Dulbecco’s Modified Eagle’s Medium (DMEM) without FBS but supplemented with 2 mM/L of l-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin (10378-016; Thermo Fisher Scientific K.K., Tokyo, Japan) for the first 3 days to minimize the contamination of fibroblasts (Fig. 1A). Three days later, the medium was exchanged for DMEM supplemented with 10% (v/v) FBS, a process that was repeated every 2 or 3 days. The isolated cells grew as monolayers after the addition of medium with FBS (Fig. 1B). When these primary cells (passage 0, P0) reached 80–90% confluence, they were trypsinized with 2 mL of TryPLE™ Express (Thermo Fisher Scientific K.K.) and incubated on 35-mm tissue culture dishes (GC TECHNO GLASS CO.,LTD., Shizuoka, Japan) or onto temperature-responsive culture dishes (TRCDs) (CellSeed Inc., Tokyo, Japan) for different purposes. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Fig. 1.
Isolation of primary human thyroid follicular epithelial cells. A Schematic isolation protocol. Non-tumorous thyroid tissues were minced mechanically into small pieces, digested with collagenase, and plated into medium without FBS for 3 days to minimize the contamination by fibroblasts. B The cell morphologies of primary isolated cells on days 1 and 14. The primary isolated cells grew as monolayers after the addition of medium with FBS. Scale bar = 500 µm
Culture medium preparation
For the different culture medium tests, 1 × 105 thyroid cells were seeded onto 35-mm tissue culture dishes and maintained in DMEM or hepatocyte-defined medium (HDM) [15]. DMEM (Wako Pure Chemical Corp., Osaka, Japan) was supplemented with 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin. HDM (Corning Inc. NY, USA) was supplemented with 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 2 ng/L of epidermal growth factor (EGF). Medium were changed the first day after passage and then every other day subsequently.
The immunofluorescence assay for monolayer cells
The monolayer cells were fixed with 4% paraformaldehyde phosphate buffer solution (Wako Pure Chemical Corp., Osaka, Japan) for 20 min and then blocked in Tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA) and 0.1% Tween-20 for 1 h at RT. Cells were incubated overnight at 4 °C with primary antibodies as follows: rabbit anti-thyroid transcription factor 1 (TTF1) (1:500) (Abcam plc., Cambridge, UK), rabbit anti-thyroglobulin (TG) (1:100) (Abcam plc.) and rabbit anti-cytokeratin 7 (CK7) (1:500) (Abcam plc.) diluted in TBS + 5% BSA and 0.1% Tween-20. Cells then were incubated for 1 h at RT in tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG (1:400) (Sigma-Aldrich Inc., St. Louis, MO, USA) as the secondary antibody. Nuclei were stained with 4,6-diamidiono-2- phenylindole (DAPI; DOJINDO, Kumamoto, Japan). Cells were mounted with ProLong gold antifade mounting medium (Thermo Fisher Scientific K.K., Tokyo, Japan). Fluorescence images were captured using a confocal laser scanning microscope (Olympus Corp., Tokyo, Japan).
The cell proliferation assay
After subculture, 1 × 105 thyroid cells were seeded onto 35-mm tissue culture dishes and cultured in DMEM and HDM, as described above. At 1, 3 and 5 days, cells were collected after being separated by a TrypLE Express, and the total cell number was counted with a hemocytometer to calculate the cell proliferation rate.
Detection of secreted thyroid hormone
After subculture, 1 × 105 cells were incubated on 35-mm tissue dishes. The medium was changed 2 days before collecting. We usually changed the medium according when the cells reached about 90% confluence (i.e. 6 or 7 days after seeding), replacing the old medium with fresh medium. Two days later, the cells reached 100% confluence, and the medium in the culture dishes was collected and stored at − 80 °C until assayed for secreted free triiodothyronine (fT3) and free thyroxine (fT4). All samples were checked by the SRL. Inc. (Nagasaki, Japan) using routine hospital assays. The fT3 and fT4 levels in fresh medium with serum were subtracted from the rough data of culturing medium in order to remove the baseline value of the serum itself.
The quantitative real-time polymerase chain reaction (qrt-PCR) assay
Total RNA of the cell samples cultured in different media were extracted using the NucleoSpin RNA kits (MACHEREY–NAGEL GmbH & Co. KG, Duren, Germany). Complementary DNA (cDNA) was synthesized from 200 ng of total RNA using High-Capacity cDNA Reverse Transcription Kits (Thermo Fisher Scientific K.K., Tokyo, Japan). PCR was performed using the Applied Biosystems StepOnePlus Real-Time PCR System with TaqMan® Fast Universal PCR Master Mix (2x), No AmpErase® UNG (Life Technologies, Carlsbad, CA, USA). The TaqMan® Gene Expression Assay Mix (20x) of TG, TPO, interlukin-6 (IL6), transforming growth factor-beta (TGF-β) and EGF (Thermo Fisher Scientific K.K., Tokyo, Japan) used here are listed in Table 2. The expression of the analyzed genes was normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. Qrt-PCR was carried out with 1 μL of TaqMan® Gene Expression Assay Mix (20x) (Thermo Fisher Scientific K.K., Tokyo, Japan) and 10 ng of cDNA. The conditions for the reactions were set at 95 °C for 20 s and 40 cycles at 95 °C for 1 s and 60 °C for 20 s. The cycle threshold values were determined automatically by the Applied Biosystems StepOnePlus Real-Time PCR System, and the fold-change in the gene expression was calculated by the 2−ΔΔCT method.
Table 2.
List of TaqMan® gene expression assay mix (20x)/PCR primers used in RT-qPCR studies
| Gene symbol | Gene name | Assay ID | Species | Amplicon length |
|---|---|---|---|---|
| TPO | Thyroid peroxidase | Hs00892519_m1 | Human | 53 |
| TG | Thyroglobulin | Hs00174974_m1 | Human | 69 |
| TGFB | Transforming growth factor beta 1 | Hs00998133_m1 | Human | 57 |
| IL6 | Interleukin 6 | Hs00985639_m1 | Human | 66 |
| EGF | Epidermal growth factor | Hs01099999_m1 | Human | 70 |
Fabrication of cell sheets
Human primary thyroid cell sheets were constructed using TRCDs. In brief, 1 × 106 cells were incubated on 35-mm TRCDs in DMEM or HDM. The medium was changed every other day after inoculation. All cells were cultured under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. After about 1 week, once the cells reached more than 100% confluence, TRCDs were incubated at 20 °C to induce the formation of detached cell sheets. The maximal diameter of each cell sheets was measured by the relative length of the diameter of 35-mm of TRCDs.
Histology of cell sheets
Cell sheets were fixed with 4% paraformaldehyde PBS for 24-72 h. Fixed samples were then embedded in paraffin, cut into 5-mm cross-sections, mounted on MAS-coated slides (Matsunami Glass Ind., Ltd., Osaka, Japan), and deparaffinized for standard histological staining with hematoxylin and eosin (H&E) (Muto Pure Chemicals CO. LTD., Tokyo, Japan). For immunostaining, sections were heated in 10 mM Tris–HCl buffer (pH 9.0) containing 1 mM EDTA using an autoclave for antigen retrieval, incubated in 3% hydrogen peroxide solution for 10 min to quench endogenous peroxidase activity, and then blocked in TBS containing 5% BSA and 0.1% Tween-20 for 1 h at RT. Blocked sections were incubated overnight at 4 °C with rabbit anti-TTF1 (1:250), rabbit anti-TG (1:250) primary antibodies diluted in TBS + 5% BSA and 0.1% Tween-20. Sections were then incubated for 1 h at RT in horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Sigma-Aldrich Inc., St. Louis, MO, USA) secondary antibody. HRP-conjugated secondary binding was visualized using the Dako liquid DAB + substrate chromogen system (Dako, Tokyo, Japan). Nuclei were stained with hematoxylin. Slides were mounted with Permount (Fisher Scientific, Atlanta, GA, USA). Bright-field images were captured using an optical microscope (Olympus).
Measurement of thickness of cell sheet cross-sections
The thickness of the cell sheet cross-sections was measured by an optical microscope with pictures of H&E staining at the same magnification. The trisection parts of each slides were measured, the average length was used as the mean thickness of cross-sections.
Statistical analyses
The data are presented as the mean ± standard deviation (SD) of at least two time points from two independent cell preparations. Means of continuous numerical variables were compared by a two-tailed t test (GraphPad Prism version 7.0; GraphPad Software, San Diego, CA, USA). Values of ***p < 0.001, **p < 0.01 and *p < 0.05 were considered statistically significant.
Results
Characteristics and identification of primary thyroid cells
We isolated 9 thyroid tissue samples from subjects with a mean age of 41.4 ± 19.5 years. Among them, three samples were obtained from men and six from women. Three samples were diagnosed with thyroid tumor, and the other six were diagnosed with Graves’ disease (Table 1). The isolated cells grew as monolayers cultured onto tissue dishes (Figs. 1B, 2A). In order to identify those cells, immunofluorescence staining of two thyroid cell-specific markers (TTF-1 and TG) and the epithelial cell marker CK7 were analyzed (Fig. 2B–D). Data showed that the isolated cells cultured in both DMEM and HDM were thyroid epithelial cells, as demonstrated by positivity for TTF1 and CK7, and those isolated cells retained differentiated function as demonstrated by positivity for TG. The isolated cells cultured in HDM proliferated more quickly than those cultured in DMEM (Day 1: 1.65 ± 0.18 vs. 1.20 ± 0.12; Day 3: 4.82 ± 0.27 vs. 2.45 ± 0.31; Day 5: 5.38 ± 0.47 vs. 3.25 ± 0.64 × 106 cells, p < 0.001 each) (Fig. 2E).
Fig. 2.
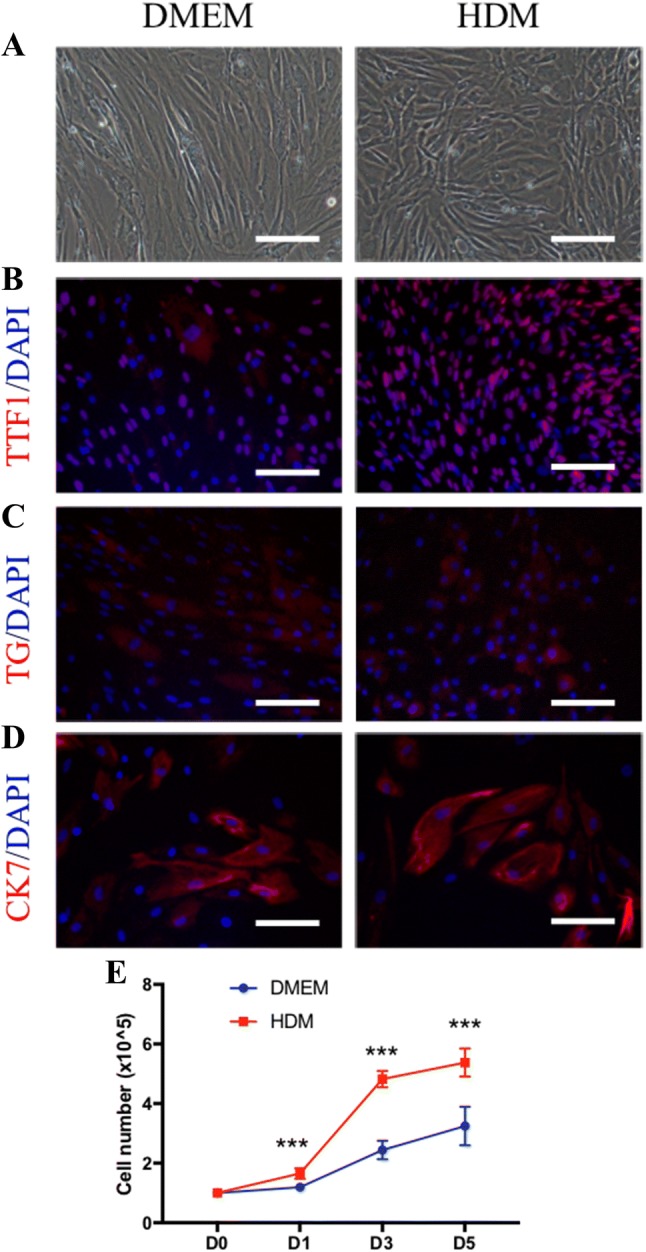
Characteristics of isolated cells after subculture. A Cells were cultured in DMEM (left panel) and HDM (right panel) after passage. Phase-contrast image of cell morphologies. B–D Immunofluorescent stained images of the thyroid cell-specific marker TTF1, TG and the epithelial cell marker CK7. Nuclei were stained with DAPI (blue). E TTF1: thyroid transcription factor-1; TG: thyroglobulin; CK7: cytokeratin 7; DAPI: 4′,6-diamidino-2-henylindole. Scale bar = 50 lm. The number of thyroid cells cultured in DMEM and HDM for 1, 3 and 5 days. ***p < 0.001 (two-way t test), (n = 5)
Fabrication of thyroid cell sheets
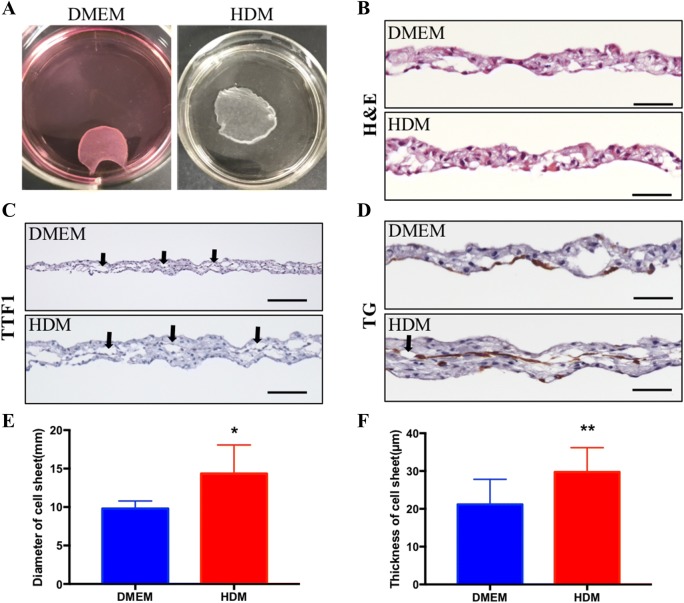
Approximately 1 × 106 primary cells were incubated on 35-mm TRCDs, and after 1-week culture, the cells reached more than 100% confluence. Dishes were stored at 20 °C for 2–4 h to allow for the detachment of thyroid cell sheets (Fig. 3A). The histological examination by H&E staining of cultured thyroid cell sheets in vitro demonstrated typical thyroid follicles with colloid stored in the center (Fig. 3B), and anti-TTF1 antibody and anti-TG antibody immunostaining revealed follicle epithelial cells lining the follicle inner surface (Fig. 3C, D). The diameters of thyroid cell sheets in DMEM and HDM were 9.8 ± 0.44 mm and 14.36 ± 1.66 mm, respectively (p = 0.0293, n = 5) (Fig. 3E), and the thickness was 21.19 ± 1.92 µm and 29.76 ± 1.78 µm in DMEM and HDM, respectively, with p = 0.0033 (n = 12) (Fig. 3F).
Fig. 3.
Fabrication of a thyroid cell sheet. A Cell sheets cultured on TRCDs in DMEM and HDM. B H&E staining of sheet cross-sections. C, D Immunostaining of TTF1 and TG of the sheet cross-sections. The black arrows show the follicle structure of the cell sheet. Scale bar = 50 lm. E Diameter of the cell sheets in DMEM and HDM (n = 5 in 3 cases). F Thickness of cell sheet cross-sections (n = 12 in 3 cases). Data are presented as the mean ± SD. *p < 0.05, **p < 0.01 (two-way t test)
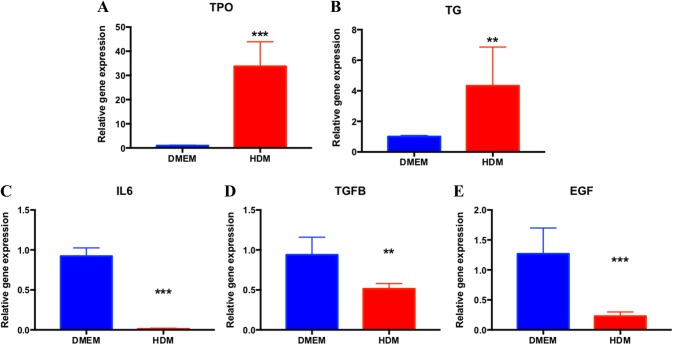
Gene analyses
Cells cultured in DMEM and HDM were used for gene analyses. Levels of TPO and TG mRNA in HDM were higher than in DMEM (Fig. 4A, B). We further analyzed the gene expression of three cytokines [IL-6, transforming growth factor-β (TGF-β) and epidermal growth factor (EGF)] related to the thyroid cell function. The gene expression of IL6, TGF-β and EGF was lower in the HDM group than in the DMEM group (Fig. 4C–E).
Fig. 4.
Changes in the gene expression of thyroid cell markers and cytokines. A-E RT-qPCR analyses of TPO, TG, IL-6, TGFB and EGF for cells cultured in DMEM and HDM. GAPDH was set as the housekeeper gene. TPO: thyroid peroxidase; TG: thyroglobulin; TGFB: transforming growth factor-β; EGF: epidermal growth factor. *p < 0.05, **p < 0.01, **p < 0.01, ***p < 0.001 (two-way ANOVA), n.s.: non significance, n = 6 in 2 cases
Levels of secreted thyroid hormones
To evaluate the function of the isolated cells, levels of fT3 and fT4 secreted into the cultured medium were measured. The medium was changed 2 days before collecting samples. The levels of fT3 in DMEM and HDM were 0.15 ± 0.02 and 0.32 ± 0.05 pg/mL (p = 0.0014), respectively (Fig. 5A). The levels of fT4 in HDM (1.22 ± 0.19 ng/dL) were much higher than in DMEM (0.04 ± 0.02 ng/dL), p = 0.0003 (Fig. 5B).
Fig. 5.
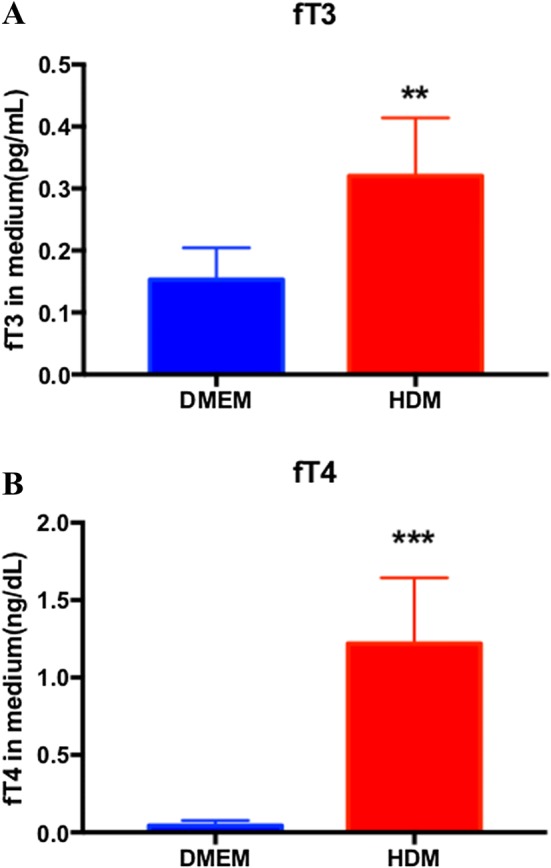
Measurement of secreted hormone in DMEM and HDM. A Levels of fT3 in DMEM and HDM (n = 3). B Levels of fT4 in DMEM and HDM (n = 3). All of the values were subtracted by the baseline of medium. **p < 0.01, ***p < 0.001 (two-way t test)
Discussion
Previous studies have described thyroid follicles crafted using non-human thyrocytes, such as porcine and rat thyrocytes, by a 3D gel-culture system [7–9, 16]. In the present study, however, we collected samples from nine human subjects with thyroid diseases and successfully isolated thyrocytes from non-tumorous thyroid glands. We also achieved our primary goal of fabricating a thyroid cell sheet using TRCDs, in which certain follicles were formed. Although thyrocytes can form stable follicles with physiological polarity in 3D collagen gel culture, our two-dimensional (2D) monolayer thyrocytes also formed some follicles that secreted fT3 and fT4.
In vivo, thyroid hormone synthesis and secretion is a complex process requiring a series of minute steps under the control of the pituitary hormone (i.e. thyroid-stimulating hormone; TSH). In in vitro culture, appropriate culture conditions are essential for maintaining the phenotype in human thyrocytes.
DMEM and HDM (described in the Materials section above) are two basic cell culture media. Many studies utilize DMEM for the culture of thyroid cell lines [17]. While HDM was originally designed for the culture of hepatocytes, it has also been reported to be suitable for the culture of epithelial cells and certain kinds of gland cells, such as hepatic epithelial cells [15], salivary epithelial cells [18] and primary human lacrimal gland cells [19]. Similarly, thyroid cells, which are follicle epithelial cells isolated from the thyroid gland, may also be well-cultured and easily proliferate in epithelial cell culture medium. In the present study, we found that thyroid cells cultured in HDM proliferated more quickly than those cultured in DMEM. While cell sheets incubated in both DMEM and HDM formed follicle structures, cells cultured in HDM secreted much higher levels of fT4 and fT3 than those cultured in DMEM. Furthermore, higher levels of TPO and TG mRNA were confirmed in HDM, suggesting that HDM may be the more appropriate culture medium for primary human thyroid cells with the up-regulation of the metabolic activity of each cell. HDM also promoted the development of functional tight junctions in salivary epithelial cells [18]. HDM contains insulin, transferrin, selenium, EGF, and 1.8 mM Ca2+. We believe that these components promoted the proliferation and function of thyroid cells, and to our knowledge, this is the first report concerning the advantages of using HDM to culture thyroid cells.
Thyroid gland homeostasis is maintained through complex interactions between TSH and other growth factors and cytokines [20]. Gene expression data obtained in the present study suggested that the downregulation of IL6, TGF-β and EGF mRNA was related to the increased proliferation rate and fT4 levels observed in cells incubated in HDM. Our finding that IL6 was capable of inhibiting the synthesis and release of fT4 and, to a greater extent, T3 from thyroid cells was consistent with those of a previous report [21]. Our data supported the roles of TGF-β and EGF in the regulation of thyroid proliferation and function at the gene level. TGF-β and EGF are physiological regulators of thyroid cell differentiation and proliferation. TGF-β is normally expressed and secreted by thyrocytes, acting as a potent inhibitor of thyroid cell growth [22]. EGF, in contrast, acts as a strong mitogen for follicular thyroid cells [23]. EGF is synthesized by the thyroid gland and is able to induce thyroid cell proliferation in several species along with the loss of thyroid-specific functions. The addition of 2 ng/L of EGF to HDM resulted in increased cell proliferation and elevated levels of fT4 but reduced levels of EGF mRNA in cells, perhaps due to negative feedback. Previous in vitro data suggest that EGF might be a modulator of the thyroid growth and function [24], and the present data support the importance of choosing the appropriate culture medium for maintaining primary thyrocytes.
Several limitations associated with the present study warrant mention. First, we only included the age and disease information of the cases and did not compare the differences in the characteristics among patients. A further study will be needed to clarify the differences in the cellular functions according to characteristics of tissues, including the age and disease. Second, we did not check the normal polarity. Polarity is important for the iodine uptake, and an appropriate follicle structure is required for thyroid hormone synthesis. Follicle-forming cells, cultured as double layers, take up iodine and organify it into thyroid hormones [12]. Third, we did not perform long-term culture. As in primary culture, human follicular cultures lose their phenotype after passage. The loss of the thyroid phenotype may be related to the culture conditions [25]. We simply compared two different culture conditions for less than five passages in the present study. Finally, although the expression of thyroid-specific transcription factors was maintained over time, it was low, and the expression of thyroid-related proteins was minimal or undetectable. To account for these shortages, a further study with long-term culture in the optimum medium will be required.
Because no chemical treatments are needed in order to harvest the cell sheets with extracellular matrix, the native cellular function is maintained [4, 6, 15]. Furthermore, cell sheets have been shown to be able to be handled easily. Previous studies have shown the effectiveness and safety of the transplantation of sheets derived from various kinds of cells, not only in animal experiments, but also in clinical situations [26, 27]. In rat experiments, the subcutaneous transplantation of a thyroid cell sheet in a hypothyroidism model by total thyroidectomy was able to restore the thyroid function with typical thyroid follicles being formed [2]. Considering the future clinical applications, we intend to transplant human thyroid cell sheets into hypothyroidism models of immunodeficient animals and evaluate their function and morphology as a preclinical study.
In conclusion, we successfully fabricated engineered human thyroid cell sheets. HDM appears suitable as a culture medium for maintaining primary thyrocytes and fabricating functional cell sheets. These in vitro findings may contribute to the development of appropriate culture conditions for human thyrocytes as well as engineered functional cell sheets.
Acknowledgement
This work was supported by Grant-in-Aid for Scientific Research (No. 26461950).
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Statement
This study was approved by the institutional review board of the Nagasaki University Hospital (Approval Number 14052642) and we obtained informed consent by each patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96:3466–3474. doi: 10.1210/jc.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arauchi A, Shimizu T, Yamato M, Obara T, Okano T. Tissue-engineered thyroid cell sheet rescued hypothyroidism in rat models after receiving total thyroidectomy comparing with nontransplantation models. Tissue Eng Part A. 2009;15:3943–3949. doi: 10.1089/ten.tea.2009.0119. [DOI] [PubMed] [Google Scholar]
- 3.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 5.Perrod G, Rahmi G, Pidial L, Camilleri S, Bellucci A, Casanova A, et al. Cell sheet transplantation for esophageal stricture prevention after endoscopic submucosal dissection in a porcine model. PLoS One. 2016;11:e0148249. doi: 10.1371/journal.pone.0148249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai Y, Yamanouchi K, Ohashi K, Koike M, Utoh R, Hasegawa H, et al. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015;65:66–75. doi: 10.1016/j.biomaterials.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 7.Bell E, Moore H, Mitchie C, Sher S, Coon H. Reconstruction of a thyroid gland equivalent from cells and matrix materials. J Exp Zool. 1984;232:277–285. doi: 10.1002/jez.1402320215. [DOI] [PubMed] [Google Scholar]
- 8.Toda S, Koike N, Sugihara H. Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: perspective for thyroid tissue engineering. Pathol Int. 2001;51:403–417. doi: 10.1046/j.1440-1827.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 9.Toda S, Sugihara H. Reconstruction of thyroid follicles from isolated porcine follicle cells in three-dimensional collagen gel culture. Endocrinology. 1990;126:2027–2034. doi: 10.1210/endo-126-4-2027. [DOI] [PubMed] [Google Scholar]
- 10.Bechtner G, Schopohl D, Rafferzeder M, Gärtner R, Welsch U. Stimulation of thyroid cell proliferation by epidermal growth factor is different from cell growth induced by thyrotropin or insulin-like growth factor I. Eur J Endocrinol. 1996;134:639–648. doi: 10.1530/eje.0.1340639. [DOI] [PubMed] [Google Scholar]
- 11.Fröhlich E, Wahl R, Reutter K. Basal lamina formation by porcine thyroid cells grown in collagen- and laminin-deficient medium. Histochem J. 1995;27:602–608. doi: 10.1007/BF02388459. [DOI] [PubMed] [Google Scholar]
- 12.Takasu N, Ohno S, Komiya I, Yamada T. Requirements of follicle structure for thyroid hormone synthesis; cytoskeletons and iodine metabolism in polarized monolayer cells on collagen gel and in double layered, follicle-forming cells. Endocrinology. 1992;131:1143–1148. doi: 10.1210/endo.131.3.1505456. [DOI] [PubMed] [Google Scholar]
- 13.Zeng L, Geng Y, Tretiakova M, Yu X, Sicinski P, Kroll TG. Peroxisome proliferator-activated receptor-delta induces cell proliferation by a cyclin E1-dependent mechanism and is up-regulated in thyroid tumors. Cancer Res. 2008;68:6578–6586. doi: 10.1158/0008-5472.CAN-08-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penna-Martinez M, Winten C, Fichtel T, Caspar-Bell G, Usadel KH, Schumm-Draeger P-M. Isolation of thyroid cells obtained by fine-needle aspiration biopsy. Thyroid. 2005;15:989–995. doi: 10.1089/thy.2005.15.989. [DOI] [PubMed] [Google Scholar]
- 15.Baimakhanov Z, Sakai Y, Yamanouchi K, Hidaka M, Soyama A, Takatsuki M, et al. Spontaneous hepatocyte migration towards an endothelial cell tube network. J Tissue Eng Regen Med. 2018;12:e1767–e1771. doi: 10.1002/term.2577. [DOI] [PubMed] [Google Scholar]
- 16.Toda S, Yonernitsu N, Hikichi Y, Sugihara H, Koike N. Differentiation of human thyroid follicle cells from normal subjects and Basedow’s disease in three-dimensional collagen gel culture. Pathol Res Pract. 1992;188:874–882. doi: 10.1016/S0344-0338(11)80247-5. [DOI] [PubMed] [Google Scholar]
- 17.Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 1990;118:48–63. doi: 10.1007/978-3-642-83816-3_6. [DOI] [PubMed] [Google Scholar]
- 18.Hegyesi O, Földes A, Bori E, Németh Z, Barabás J, Steward MC. Evidence for active electrolyte transport by two-dimensional monolayers of human salivary epithelial cells. Tissue Eng Part C Methods. 2015;21:1226–1236. doi: 10.1089/ten.tec.2014.0614. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari S, Nair RM, Vamadevan P, Ali MJ, Naik MN, Honavar SG, et al. Establishing and characterizing lacrispheres from human lacrimal gland for potential clinical application. Graefes Arch Clin Exp Ophthalmol. 2018;256:717–727. doi: 10.1007/s00417-018-3926-8. [DOI] [PubMed] [Google Scholar]
- 20.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki K, Yamada E, Kanaji Y, Shizume K, Wang DS, Maruo N, et al. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. 1996;137:4857–4863. doi: 10.1210/endo.137.11.8895357. [DOI] [PubMed] [Google Scholar]
- 22.Colletta G, Cirafici AM, Di Carlo A. Dual effect of transforming growth factor beta on rat thyroid cells: inhibition of thyrotropin-induced proliferation and reduction of thyroid-specific differentiation markers. Cancer Res. 1989;49:3457–3462. [PubMed] [Google Scholar]
- 23.Asmis LM, Gerber H, Kaempf J, Studer H. Epidermal growth factor stimulates cell proliferation and inhibits iodide uptake of FRTL-5 cells in vitro. J Endocrinol. 1995;145:513–520. doi: 10.1677/joe.0.1450513. [DOI] [PubMed] [Google Scholar]
- 24.Westermark K, Karlsson FA, Westermark B. Epidermal growth factor modulates thyroid growth and function in culture. Endocrinology. 1983;112:1680–1686. doi: 10.1210/endo-112-5-1680. [DOI] [PubMed] [Google Scholar]
- 25.Bravo SB, Garcia-Rendueles ME, Garcia-Rendueles AR, Rodrigues JS, Perez-Romero S, Garcia-Lavandeira M, et al. Humanized medium (h7H) allows long-term primary follicular thyroid cultures from human normal thyroid, benign neoplasm, and cancer. J Clin Endocrinol Metab. 2013;98:2431–2441. doi: 10.1210/jc.2012-3812. [DOI] [PubMed] [Google Scholar]
- 26.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 27.Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]