Abstract
Introduction
The aim of this Delphi study is to unveil the management of patients with type 2 diabetes (T2D) and different levels of complexity in the clinical practice in Spain.
Methods
Based on the common management practices of T2D profiles reported by Spanish endocrinologists, a Delphi questionnaire of 55 statements was developed and responded to by a national panel (n = 101).
Results
A consensus was reached for 30 of the 55 statements. Regarding overweight patients inadequately controlled with metformin, treatment with a sodium-glucose transport protein 2 inhibitor (SGLT2-I) is preferred over treatment with a dipeptidyl peptidase-4 inhibitor (DPP4-I). If the patient is already being treated with a DPP4-I, an SGLT2-I is added on to the treatment regimen rather than replacing the DPP4-I. Conversely, if the treatment regimen includes a sulfonylurea, it is usually replaced by other antihyperglycemic agents. Current treatment trends in uncontrolled obese patients include the addition of an SGLT2-I or a glucagon-like peptide-1 receptor agonist (GLP1-RA) to background therapy. When the glycated hemoglobin target is not reached, triple therapy with metformin + GLP1-RA + SGLT2-I is initiated. Although SGLT2-Is are the treatment of choice in patients with T2D and heart failure or uncontrolled hypertension, no consensus was reached regarding the preferential use of SGLT2-Is or GLP1-RAs in patients with established cardiovascular disease.
Conclusion
Consensus has been reached for a variety of statements regarding the management of several T2D profiles. Achieving a more homogeneous management of complex patients with T2D may require further evidence and a better understanding of the key drivers for treatment choice.
Funding
Logistic support was provided by ESTEVE Pharmaceuticals S.A Spain.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-0671-x) contains supplementary material, which is available to authorized users.
Keywords: Clinical practice, Complex patient, Delphi questionnaire, Endocrinology, Type 2 diabetes
Introduction
Current estimates show that there are 425 million adults worldwide with type 2 diabetes (T2D), of whom 3.6 million are in Spain [1].
Although T2D has a genetic component, multiple risk factors can contribute to the development and progression of the disease, including lifestyle factors (e.g., an unhealthy diet or decreased physical activity) related to obesity [2]. Developing T2D is associated with a significant increase in the risk of cardiovascular (CV) and microvascular disease, but reducing levels of glycated hemoglobin (HbA1c) can significantly decrease the risk of these complications [3]. To achieve and maintain long-term metabolic control, it is necessary to combine a number of changes in the patient’s lifestyle with different pharmacological drugs [4]. However, only about 50% of people with diabetes in Spain achieve their HbA1c target [5–8].
A healthy lifestyle and reduction in body weight are the first therapeutic measures to be considered in a patient newly diagnosed with T2D [9]. Primary pharmacologic therapy usually begins with metformin, unless otherwise contraindicated or not tolerated [4, 10, 11]. Once metformin fails to maintain a satisfactory metabolic control, initiation of a combination therapy should be considered [10]. However, the key drivers influencing the endocrinologist’s choice of a second antihyperglycemic agent to be added to background therapy are not well known, and the management of T2D patients in clinical practice may vary significantly. While recent position statements and guidelines consider obesity, risk of hypoglycemia, and CV disease to be critical factors for the selection of a second agent [4, 9, 10], T2D heterogeneity, complex patient profiles with overlapping comorbidities and circumstances, and the availability of a wide range of antihyperglycemic agents with varying efficacy and side effects make the choice of the appropriate therapeutic program a difficult issue.
The Delphi study reported here was designed to gather together the opinions of a representative panel of Spanish endocrinologists specialized in diabetes management on the available treatment options and possible therapy combinations for different T2D patient profiles with different levels of complexity and to identify common practices that may be used as guidance for other physicians.
Methods
Delphi Methodology
A scientific committee of six experts defined five different T2D patient profiles in ascending order of complexity (T2D patient not controlled on: metformin; metformin + dipeptidyl peptidase-4 inhibitor [DPP4-I]; metformin + DPP4-I + sulfonylureas [SU]; metformin + DPP4-I + basal insulin; metformin + basal-bolus insulin [> 1 IU/kg/day]) and an array of variables that could condition treatment choice in each profile (including body mass index [BMI], HbA1c levels, CV risk, heart failure, hypertension, albuminuria, estimated glomerular filtration rate [eGFR], patient frailty, and non-alcoholic fatty liver disease [NAFLD]).
In the first phase of the Think Twice Program, an online patient management survey of 145 questions matching the five patient profiles and conditioning variables was developed. Sixty-nine Spanish endocrinologists with experience in the treatment of T2D (≥ 5 years treating T2D, > 50 T2D patient visits/month, and members of the Spanish Society of Diabetes [SED] and/or the Spanish Society of Endocrinology and Nutrition [SEEN]) were invited to participate; these specialists answered the survey according to their clinical practice and gathered in local meetings to discuss the results.
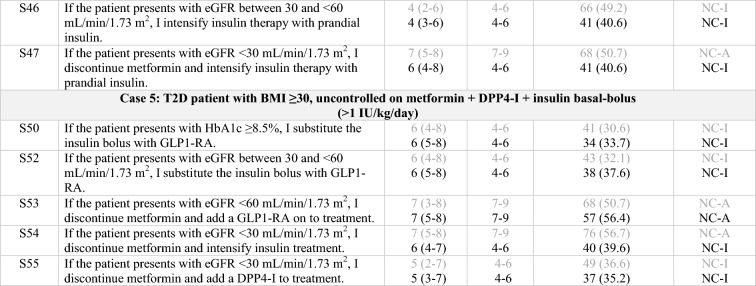
In a second phase, the scientific committee developed 55 statements presenting treatment options for complex T2D profiles that were based on the main treatment patterns and controversies identified in the local meetings (Tables 1, 2). Statements were divided into six blocks, with a block representing each of the five previously defined T2D profiles and an additional block of common practices identified across all patient profiles. To be established as recommendations, consensus needed to be reached on the statements in accordance with the Delphi methodology [12]. Each statement was rated on a Likert-like scale from 1 (“completely disagree”) to 9 (“completely agree”). Responses were grouped by tertiles in which 1–3 indicated disagreement, 4–6 indicated indeterminate and 7–9 indicated agreement. Consensus on a statement was reached when the responses of two thirds or more participants (≥ 66.6%) were located in the same tertile as the median value of all the reported responses for that statement. Statements were rated on an online questionnaire by an extended panel of endocrinologists (each original panelist could invite up to two additional endocrinologists with proven experience in the treatment of T2D [same criteria as those described above]). All endocrinologists were assigned an alphanumeric access code to the questionnaire website to maintain their anonymity.
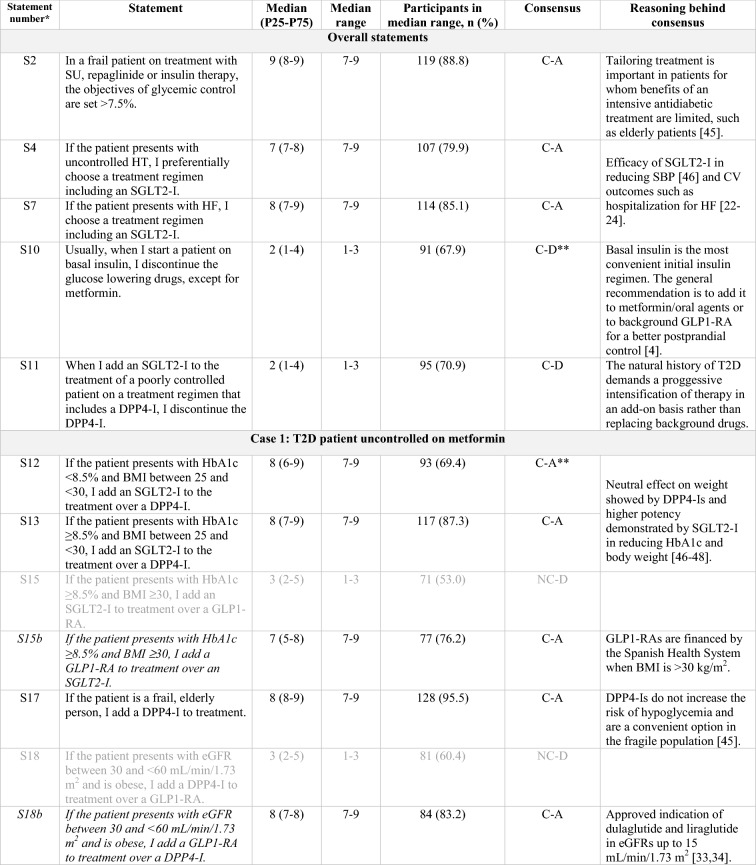
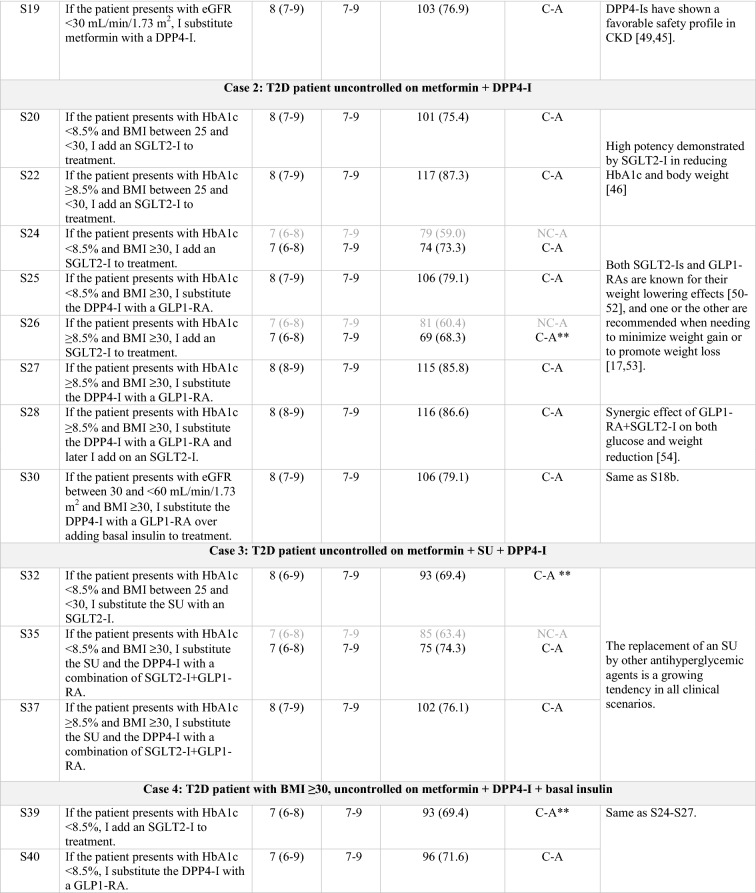
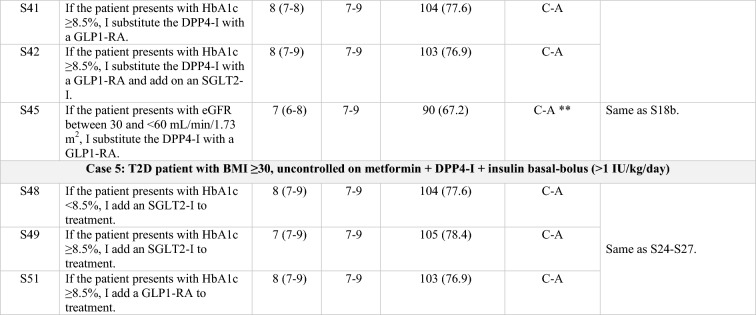
Table 1.
Statements that achieved consensus in the Delphi study
S statement, SU sulfonylurea, HT hypertension, SGLT2-I sodium/glucose cotransporter-2 inhibitor, SBP systolic blood pressure, CV cardiovascular, HF heart failure, GLP1-RA glucagon-like peptide-1 receptor agonist, DPP4-I dipeptidyl peptidase-4 inhibitor, T2D type 2 diabetes, HbA1c glycated hemoglobin, BMI body mass index, eGFR estimated glomerular filtration rate, CKD chronic kidney disease, C consensus, NC non-consensus, A agreement, D disagreement
Clear grey font: First round results for those statements that achieved consensus on the second round
Black font: Consensus, either on first or second round
Italics: Statements reworded for the second round
*According to the order in which the statements were presented in the Delphi questionnaire
**Consensus close to the limit (66.6%)
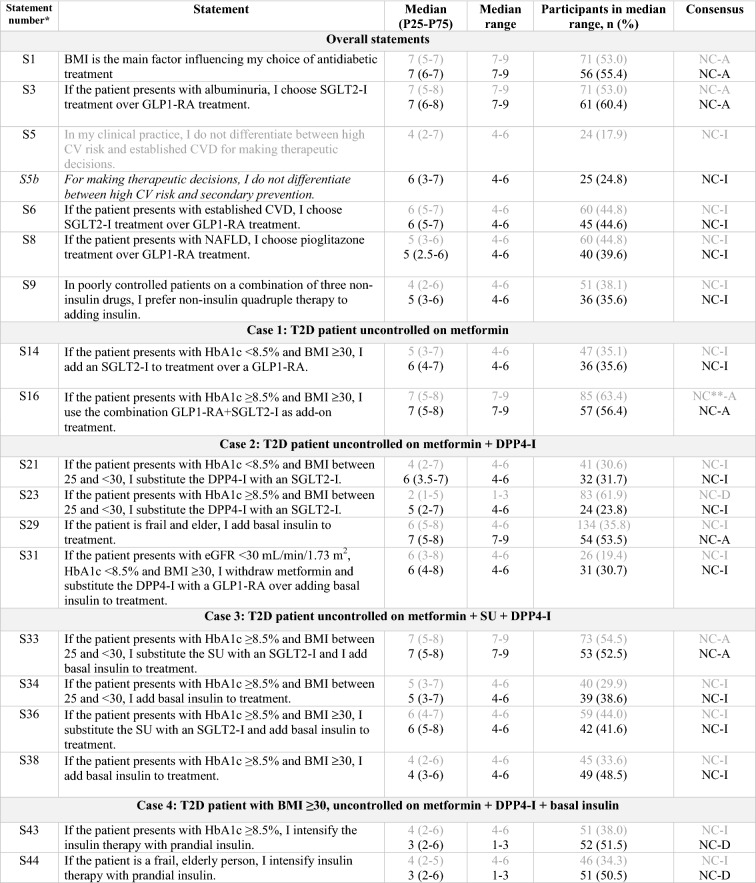
Table 2.
Statements that did not achieve consensus in the Delphi study
S statement, BMI body mass index, SGLT2-I sodium/glucose cotransporter-2 inhibitor, GLP1-RA glucagon-like peptide-1 receptor agonist, CV cardiovascular, CVD cardiovascular disease, NAFLD non-alcoholic fatty liver disease, T2D type 2 diabetes, HBA1c glycated hemoglobin, DPP4-I dipeptidyl peptidase-4 inhibitor, eGFR estimated glomerular filtration rate, SU sulfonylurea, C consensus, NC non-consensus, A agreement, D disagreement, I indeterminate
Clear grey font: First round results
Black font: Second round results
Italics: Statements reworded for the second round
*According to the order in which the statements were presented in the Delphi questionnaire
**Close to the limit of achieving consensus (66.6%)
Statements which did not achieve consensus in the first round were submitted to a subsequent round.
Statistical Analysis
A descriptive analysis of all items using the mean ± standard deviation, median (p25–p75), and minimum and maximum values was performed. The Kolmogorov–Smirnov test was used to check for “goodness” of fit of the data to a normal distribution.
The internal consistency of the questionnaire was measured by the Cronbach’s alpha (Cα), which can range between 0 and 1, from lower to higher reliability (acceptable values: > 0.7; high reliability: 0.7–0.9; very high reliability > 0.9) [13]. In addition, inter-rater reliability was assessed by the intra-class correlation coefficient (ri) (poor: ri < 0.40; fair: ri = 0.40–0.59; good: ri = 0.60–0.74; excellent: ri = 0.75–1.0) [14]. Correlation between the two rounds of the questionnaire was measured by the Spearman coefficient (rs) (none or poor: rs = 0–0.25; weak: rs = 0.26–0.50; moderate to strong: rs = 0.51–0.75; and strong to very strong: rs = 0.76–1) [15]. The Kappa index (k) was calculated to estimate the qualitative agreement between rounds of the questionnaire, taking into account the three response groups (1–3, 4–6 and 7–9), with k < 0.20 indicating no or poor qualitative agreement, k = 0.21–0.40 indicating weak agreement, k = 0.41–0.60 indicating moderate agreement, k = 0.61–0.80 indicating good agreement, and k = 0.81–1 indicating very good agreement [16]. Statistics were calculated for the overall survey and for each of the six blocks. Statistical significance was considered when p < 0.05.
The coefficient of variation (COV) of the questionnaire was calculated for every round, along with the delta or relative change in the second round above the first (COVsecond – COVfirst/COVfirst). When the absolute value of delta is ≤ 10%, there is no large variability between the rounds and, thus, there is no need for another round, since no relevant changes are expected.
Compliance with Ethics Guidelines
This study is based on a clinical practice questionnaire that does not involve the participation of human subjects or patient data management and does not aim to modify the current clinical practice of participants. As such, this study was deemed exempt from requiring ethical approval. Consent for publication of survey results was granted from all the experts participating in the program and undertaking the survey.
Results
Of the 192 endocrinologists from all over Spain who were invited to participate, 134 (70%) answered the first round of the Delphi questionnaire. The respondents were representative of the diverse local realities and administrations of the autonomous communities; their mean age was 45.2 ± 10.3 years, 57.5% were women, and the median number of years of experience in treating patients with T2D was 17 (interquartile range [IQR] 10–27 years). Consensus was reached for 25 of the 55 statements (agreement on 23 statements; disagreement on two statements).
A second round of the Delphi questionnaire was then performed with the 30 statements for which consensus had not been reached. Statements number S5, S15, and S18 were rephrased for this second round for clarity. A total of 101 endocrinologists (75.4% of the extended panel) answered the second round (mean age of respondents was 45.0 ± 10.0 years; 55.4% were women; median years of experience in treating patients with T2D was 19 [IQR 11–26.5 years]), with consensus being reached for 5 additional sentences (agreement).
The overall questionnaire showed high internal consistency (Cα) (Electronic Supplementary Material [ESM] Table S1). Spearman correlation values (rs) were moderate to very strong, indicating an acceptable quantitative correlation between rounds. Kappa index (k) values showed a moderate to very good qualitative agreement between rounds in all blocks (ESM Table S2).
The COV of the questionnaire in the first and second rounds was 40 and 36%, respectively, achieving a relative increase of 10%, which is within the limits for third round consideration. However, since the absolute difference between rounds was < 5% (i.e., 4%), further rounds were not undertaken. Thus, at the end of the Delphi study, consensus was reached for a total of 30 statements (agreement on 28 statements; disagreement on two statements), while consensus was not reached on 25 statements (Table 2). Approved sentences and reasoning underlying each consensus are summarized in Table 1.
Overall, regardless of patient profile, a sodium-glucose transport protein 2 inhibitor (SGLT2-I) is added to the treatment of patients with T2D and uncontrolled hypertension (S4) or heart failure (S7).
Regarding overweight patients inadequately controlled with metformin, treatment with an SLGT2-I is preferred over treatment with a DPP4-I (S12, S13). If the patient is already being treated with a DPP4-I, an SGLT2-I is added onto the treatment regimen (S11, S20, S22) rather than it replacing the DPP4-I [21, 23]. Conversely, if the treatment regimen includes a SU, the SU is usually replaced by an SGLT2-I (S32).
Current treatment trends in uncontrolled obese patients include the addition of a glucagon-like peptide-1 receptor agonist (GLP1-RA; S15) or, alternatively, an SGLT2-I (S24, S26, S39) to background therapy. If the treatment regimen already includes a DPP4-I, it is replaced by a GLP1-RA (S25, S27, S40, S41). When the HbA1c target is not reached, there was consensus to progress to triple therapy with metformin and a combination of GLP1-RA and SGLT2-I (S28, S42). Finally, in uncontrolled complicated obese patients already on insulin basal-bolus therapy, either an SGLT2-I or a GLP1-RA are added to treatment (S48, S49, S51) rather than substituting the insulin bolus with a GLP1-RA (S50).
The panel sets the objectives of glycemic control to a target of > 7.5% in frail patients on treatment with SU, repaglinide, or insulin therapy (S2). In elderly patients uncontrolled on metformin, DPP4-Is remain the preferred treatment option (S17, S19). However, in complex patients with an eGFR < 60 mL/min/1.73 m2, the use of some GLP1-RAs, such as liraglutide and dulaglutide, is displacing the use of DPP4-Is (S18, S45) and even of insulin (S30).
A variety of statements did not reach consensus in our study, and so the situations that they present remain controversial in clinical practice.
Discussion
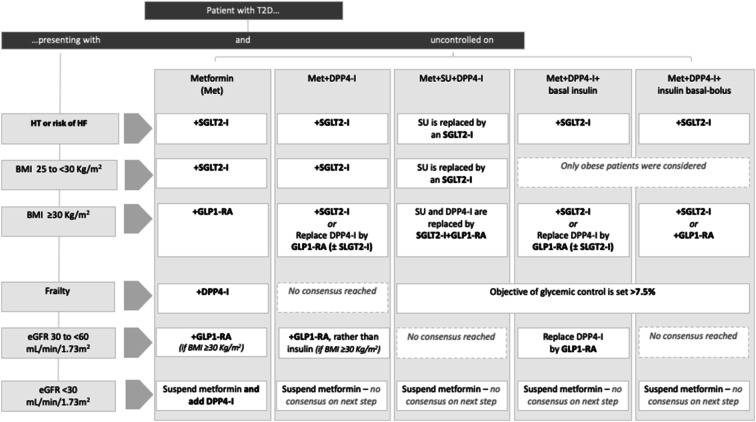
In this Delphi study, consensus was reached for a variety of statements regarding the treatment of patients with T2D and different levels of complexity, reflecting that the clinical practice in Spain generally follows the recommendations of the main clinical guidelines. Main findings on statements for each patient profile are depicted in the treatment algorithm shown in Fig. 1.
Fig. 1.
Treatment algorithm based on the main findings on statements for each patient profile. T2D Type 2 diabetes, DPP4-I dipeptidyl peptidase-4 inhibitor, SU sulfonylurea, HT hypertension, HF heart failure, SGLT2-I sodium/glucose cotransporter-2 inhibitor, BMI body mass index, GLP1-RA glucagon-like peptide-1 receptor agonist, eGFR estimated glomerular filtration rate
Consensus was not reached on a number of statements, mainly because of the array of treatment options available in clinical practice.
Firstly, it remains unclear whether BMI is the major factor influencing treatment choice (S1). A plausible explanation is that current clinical practice involves a patient-centered approach, with a tailored selection of medications based on a global assessment of comorbidities, costs, and patient preferences [4, 17]. Additionally, the key drivers influencing treatment choice have changed over time, moving from a glycocentric or adipocentric view [18] to a CV-based model where the CV safety of T2D treatments prevails [19].
Management of patients with T2D in primary or secondary CV prevention is highly heterogeneous in clinical practice (S5). Until recently, the main body of evidence regarding the use of GLP1-RAs and SGLT2-Is came from trials mainly involving secondary prevention in patients (> 60%). In these trials, treatment with both liraglutide and semaglutide showed lower rates of CV death, of nonfatal myocardial infarction, or of nonfatal stroke versus placebo, along with a beneficial effect on a composite outcome of prespecified renal events [20, 21]. Similar results were observed with empagliflozin and canagliflozin (although it should be pointed out that while GLP1-RAs mainly help by reducing albuminuria, SGLT2-Is also help by preventing a decrease in the GFR), along with a reduction in the rates of hospitalization for heart failure [22, 23]. Recently, the results of the DECLARE trial assessing the CV safety of dapagliflozin have been published, broadening the evidence base regarding the use of SGLT2-Is in primary prevention in patients. This latter study included > 17,000 patients with T2D with either atherosclerotic CV disease (ASCVD; secondary prevention [40%]) or multiple risk factors for CV disease (CVD; primary prevention [60%]) [24]. A significant reduction in the co-primary composite endpoint of CV death or hospitalization for heart failure was observed, mainly due to a lower rate of hospitalization for heart failure [24]. In addition, preliminary results from the REWIND trial have pointed out a significant reduction of major adverse CV events in the subpopulation without established CVD [25].
No consensus was reached regarding the use of SGLT2-Is over GLP1-RAs in patients with T2D and established CVD (S6). This finding may be related to logistic issues, as GLP1-RAs are not financed by the Spanish Health System in people with a BMI of < 30 kg/m2. In addition, the current trend of splitting the definition of “established CVD” into ASCVD and heart failure, given the differing benefits shown by SGLT2-Is and GLP1-RAs on the prevention of specific CV outcomes [20–24], may have had a strong influence on treatment choice. In fact, the most recently updated guidelines recommend an SGLT2-I or a GLP1-RA for the management of patients with T2D and established ASCVD, while an SGLT2-I is preferred for patients at risk of heart failure. A GLP1-RA is only recommended for this latter group of patients when an SGLT2-I is not tolerated or contraindicated, or the eGFR is less than adequate [10, 17]. Head-to-head studies regarding CV outcomes comparing SGLT2-Is and GLP1-RAs could add supporting evidence on this issue.
As previously mentioned, most of the SGLT2-I CV outcome trials have reported consistent benefits in renal endpoints, including albuminuria [23, 24, 26, 27]. In fact, both the American Diabetes Association (ADA) and the European. Association for the Study of Diabetes (EASD) recommend an SGLT2-I in patients with T2D and chronic kidney disease, with or without CVD, or the use of a GLP1-RA if the SGLT2-I is contraindicated or not preferred [17]. In our study, no final consensus was reached regarding the addition of an SGLT2-I over a GLP1-RA to the treatment of uncontrolled patients with T2D and albuminuria. Nevertheless, 60% of the endocrinologists supported this option (S3), probably due to the evidence supporting a greater role for SGLT2-Is rather than for GLP1-RAs in renoprotection [28].
Regarding the use of pioglitazone in patients with NAFLD (S8), despite evidence of its favorable effect in this context and its CV benefits [29, 30], no consensus was reached, possibly due to concerns regarding the adverse effects of pioglitazone in patients with comorbidities [29, 31].
In general, when adding more than one antihyperglycemic agent to monotherapy, or replacing two drugs from a triple therapy, endocrinologists follow a stepwise approach. This may be the explanation for the lack of consensus regarding the simultaneous addition of SGLT2-I + GLP1-AR to metformin (S16), or the replacement of SU + DPP4-I in a patient also receiving basal insulin with this combination (S35, S37). Nevertheless, more studies on the simultaneous versus sequential addition of antidiabetic agents would be needed to broaden the evidence available on this issue.
Regarding the clinical practice in more complex patients uncontrolled on a combination of three non-insulin drugs, no consensus was reached on what to do next (S9). Interestingly, when given the option to add insulin to the therapeutic program of these patients, 24% of the endocrinologists preferred a non-insulin quadruple therapy despite the absence of specific recommendations in the main guidelines, reflecting that insulin is the last resort for some physicians [32]. Similar results were observed for uncontrolled patients on a therapeutic program of metformin + DPP4-I + basal insulin and HbA1c levels of ≥ 8.5%, where the addition of an SGLT2-I or the replacement of the DPP4-I by a GLP1-RA (± SGLT2-I) prevailed over intensifying the insulin therapy with prandial insulin (S41–S43), confirming the apparent preference of Spanish endocrinologists for non-insulin therapies.
No consensus was reached regarding the use or intensification of insulin in frail patients (S29, S44). Likewise, there was no agreement on intensifying therapy with prandial insulin in patients non-controlled with metformin + basal insulin + DPP4-I and with an eGFR between 30 and < 60 mL/min/1.73 m2 (S46), or in more complex patients with an eGFR of < 30 mL/min/1.73 m2 (S47), possibly because the panel preferred the option of replacing the DPP4-I with a GLP1-RA (S45). This preference may be partly explained by the regulatory approval of dulaglutide and liraglutide in eGFRs up to 15 mL/min/1.73 m2 [33, 34], together with the greater effectiveness demonstrated by GLP1-RAs in reducing weight and hypoglycemic episodes in comparison to prandial rapid-acting insulins [35–38]. Nevertheless, these studies may not reflect the complexity of patients in clinical practice, as they included patients initially treated only with metformin and/or SU [37–43] who therefore had a potentially better beta-cell function, which is associated with better glycemic responses to GLP1-RA therapy [44].
Conclusions
In this Delphi study, consensus was reached on a number of statements regarding the management of patients with T2D and different levels of complexity. These statements reflect the most frequent behavior in current clinical practice of Spanish endocrinologists in the treatment of T2D, and may be used as guidance for other physicians, especially in those scenarios where the evidence available is scarce. Achieving a more homogeneous management of complex T2D profiles may require further evidence on the strengths and weaknesses of therapies currently available and a better understanding of the key drivers influencing treatment choice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the panel of endocrinologists for their participation in the Delphi study. The authors would also like to extend their special thanks to the participants in the local meetings of the Think Twice program, a full list of whom are listed in ESM 3.
Funding
Logistic support, including the journal’s Rapid Service fee, was provided by ESTEVE Pharmaceuticals S.A Spain. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Eva Pineda at ESTEVE Medical Affairs provided scientific support. Almudena Pardos and Anna Nualart at Ogilvy Health provided medical writing services. This assistance was funded by ESTEVE Pharmaceuticals S.A Spain.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Carlos Morillas has participated in advisory boards organized by Sanofi, Novo Nordisk, Lilly, Boehringer, Janssen, Esteve, and Astra-Zeneca; and has acted as a speaker for MSD, Astra-Zeneca, Novartis, Sanofi, Novo Nordisk, Boehringer, Esteve, Janssen, and Lilly. Javier Escalada has participated in advisory boards organized by Lilly, MSD, Novo Nordisk, Sanofi, and Esteve, and has presented data in events organized by Almirall, Astra-Zeneca, Boehringer, Janssen, Lilly, MSD, Novo Nordisk, and Sanofi. Rafael Palomares has taken part in advisory boards for Boehringer, Novartis, Sanofi-Aventis, Novo Nordisk, Abbot, and AstraZeneca Pharmaceuticals LP; and has acted as a speaker for Astra-Zeneca, Abbott, Almirall, Bayer, Boehringer, Bristol-Myers, Esteve, Faes Farma, Ferrer, GSK, Janssen, Lacer, Lifescan, Lilly, Menarini, Merck, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi-Aventis, Servier and Takeda. Diego Bellido has taken part in advisory boards for Sanofi, Boehringer Lilly, and Novo Nordisk; and has acted as a speaker for MSD, Lilly, Novo Nordisk, AstraZeneca, Esteve, and Sanofi. Fernando Gómez-Peralta has taken part in advisory panels for Sanofi and Novo Nordisk; has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, and Lilly; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, and Lilly. Antonio Pérez has served as a consultant for or received lecture fees or travel reimbursement from Sanofi Aventis, Almirall, Novo Nordisk, Eli Lilly, MSD, Boehringer Ingelheim, Esteve, Bristol-Myers Squibb, Novartis, Amgen, Menarini, and Astra Zeneca.
Compliance with Ethics Guidelines
This study is based on a clinical practice questionnaire that does not involve the participation of human subjects nor patient data management and does not aim to modify the current clinical practice of participants. Consequently, as per ethical approval procedures in Spain and the Council of Europe’s classification of this article as a scientific audit, the questionnaires compiled in this study did not require ethical approval. Consent for publication of survey results was granted from all the experts participating in the program and undertaking the survey.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8869955.
References
- 1.International Diabetes Federation. IDF diabetes atlas. http://www.diabetesatlas.org (2017). Accessed 5 Oct 2018.
- 2.Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13(3):e0194127. doi: 10.1371/journal.pone.0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association of American Diabetes 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-s009. [DOI] [PubMed] [Google Scholar]
- 5.Vinagre I, Mata-Cases M, Hermosilla E. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain) Diabetes Care. 2012;35(4):774–779. doi: 10.2337/dc11-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez A, Franch J, Cases A. Relationship between the degree of glycemic control and diabetes characteristics and hyperglycemia treatment in type 2 diabetes. DIABES Study. Med Clin (Barc) 2012;138(12):505–511. doi: 10.1016/j.medcli.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Perez A, Mediavilla JJ, Minambres I, Gonzalez-Segura D. Glycemic control in patients with type 2 diabetes mellitus in Spain. Rev Clin Esp. 2014;214(8):429–436. doi: 10.1016/j.rce.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Minambres I, Mediavilla JJ, Sarroca J, Perez A. Meeting individualized glycemic targets in primary care patients with type 2 diabetes in Spain. BMC Endocr Disord. 2016;16:10. doi: 10.1186/s12902-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber AJ, Abrahamson MJ, Barzilay JI. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25(1):69–100. doi: 10.4158/CS-2018-0535. [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Garcia R, Moreno-Pérez O, Tejera-Pérez C, et al. Documento de Abordaje Integral de la Diabetes Tipo 2. http://www.seen.es/docs/apartados/791/abordaje%20integral%20dm2019.pdf. (2019). Accessed 28 Feb 2019. [DOI] [PubMed]
- 11.Gunton JE, Cheung NW, Davis TM, Zoungas S, Colagiuri S, Australian Diabetes Society A new blood glucose management algorithm for type 2 diabetes: a position statement of the Australian Diabetes Society. Med J Aust. 2014;201(11):650–653. doi: 10.5694/mja14.01187. [DOI] [PubMed] [Google Scholar]
- 12.New Jersey Institute of Technology. The Delphi method. Techniques and applications. Newark: New Jersey Institute of Technology; 2002.
- 13.Schmitt N. Uses and abuses of coefficient alpha. Psychol Assess. 1996;8(4):350–353. doi: 10.1037/1040-3590.8.4.350. [DOI] [Google Scholar]
- 14.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instrument in psychology. Psychol Assess. 1994;6(4):284–290. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 15.Dawson B, Trapp RG. Basic and clinical biostatistics (LANGE Basic Science) 4. New York: McGraw-Hill; 2004. [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ, D’Alessio DA, Fradkin J. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorgojo Martinez JJ. Glucocentricity or adipocentricity: a critical view of consensus and clinical guidelines for the treatment of type 2 diabetes mellitus. Endocrinol Nutr. 2011;58(10):541–549. doi: 10.1016/j.endonu.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano D, Maiorino MI, Bellastella G, Esposito K. Diabetes is a cardiovascular disease, isn’t it? Diabetes Res Clin Pract. 2018;135:229–231. doi: 10.1016/j.diabres.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Daniels GH, Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marso SP, Bain SC, Consoli A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/nejmoa1607141. [DOI] [PubMed] [Google Scholar]
- 22.Zinman B, Wanner C, Lachin JM. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 23.Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 24.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–57. 10.1056/nejmoa1812389. [DOI] [PubMed]
- 25.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. 10.1016/s0140-6736(19)31149-3. [DOI] [PubMed]
- 26.Wanner C, Inzucchi SE, Lachin JM. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 27.Jardine MJ, Mahaffey KW, Neal B. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462–472. doi: 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muskiet MH, Tonneijck L, van Bommel EJ, Smits MM, van Raalte DH. Renoprotection in LEADER and EMPA-REG OUTCOME. Lancet Diabetes Endocrinol. 2016;4(10):812–814. doi: 10.1016/s2213-8587(16)30214-5. [DOI] [PubMed] [Google Scholar]
- 29.Kernan WN, Viscoli CM, Furie KL. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bril F, Kalavalapalli S, Clark VC. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol. 2018;16(4):558–566. doi: 10.1016/j.cgh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, Habel LA, Quesenberry CP. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314(3):265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 32.Peyrot M, Rubin RR, Lauritzen T. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 33.European Medicines Agency. Dulaglutide. SPC. https://www.ema.europa.eu/documents/product-information/trulicity-epar-product-information_en.pdf (2018). Accessed 3 July 2019.
- 34.European Medicines Agency. Liraglutide. SPC. https://www.ema.europa.eu/documents/product-information/victoza-epar-product-information_en.pdf (2018). Accessed 3 July 2019.
- 35.Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19(2):216–227. doi: 10.1111/dom.12804. [DOI] [PubMed] [Google Scholar]
- 36.Singh S, Wright EE, Jr, Kwan AY. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017;40(4):614–624. doi: 10.2337/dc16-1957. [DOI] [PubMed] [Google Scholar]
- 38.Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35(1):e3082. doi: 10.1002/dmrr.3082. [DOI] [PubMed] [Google Scholar]
- 39.Bunck MC, Diamant M, Corner A. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell-Jones D, Vaag A, Schmitz O. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamant M, Van Gaal L, Guerci B. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464–473. doi: 10.1016/S2213-8587(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 42.Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract. 2014;106(3):567–575. doi: 10.1016/j.diabres.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 43.D’Alessio D, Haring HU, Charbonnel B. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–178. doi: 10.1111/dom.12406. [DOI] [PubMed] [Google Scholar]
- 44.Jones AG, McDonald TJ, Shields BM. Markers of beta-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39(2):250–257. doi: 10.2337/dc15-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Huelgas R, Gomez Peralta F, Rodriguez Manas L. Treatment of type 2 diabetes mellitus in elderly patients. Rev Clin Esp. 2018;218(2):74–88. doi: 10.1016/j.rce.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Storgaard H, Gluud LL, Bennett C. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166125. doi: 10.1371/journal.pone.0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenstock J, Bailey CJ, Mathieu C, Chen H, Garcia-Sanchez R, Saraiva GL. Composite endpoint analysis of dapagliflozin versus saxagliptin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin. Endocr Pract. 2016;23(1):38A–39A. doi: 10.20945/2359-3997000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13(1):7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 49.Coppolino G, Leporini C, Rivoli L. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol Res. 2018;129:274–294. doi: 10.1016/j.phrs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Manigault KR, Thurston MM. Liraglutide: a glucagon-like peptide-1 agonist for chronic weight management. Consult Pharm. 2016;31(12):685–697. doi: 10.4140/TCP.n.2016.685. [DOI] [PubMed] [Google Scholar]
- 51.Goring S, Hawkins N, Wygant G. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab. 2014;16(5):433–442. doi: 10.1111/dom.12239. [DOI] [PubMed] [Google Scholar]
- 52.Zhang YJ, Han SL, Sun XF. Efficacy and safety of empagliflozin for type 2 diabetes mellitus: meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97(43):e12843. doi: 10.1097/MD.0000000000012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez-Peralta F, Escalada San Martin FJ, Menendez Torre E. Spanish Diabetes Society (SED) recommendations for the pharmacologic treatment of hyperglycemia in type 2 diabetes: 2018 update. Endocrinol Diabetes Nutr. 2018;65(10):611–624. doi: 10.1016/j.endinu.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Frias JP, Guja C, Hardy E. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.