Abstract
There is evidence that mGlu2/3 receptors regulate 5-HT2A signaling, interactions that have been theorized to play a role in the antipsychotic-like effects of mGlu2/3 agonists as well as the hallucinogenic effects of 5-HT2A agonists. One approach to unraveling this interaction is through chronic administration of agonists at the two receptors, which should influence the functional properties of the targeted receptor due to receptor downregulation or desensitization and thereby alter cross-talk between the two receptors. In this study, we investigated whether chronic treatment with the mGlu2/3 agonist LY379268 would alter the behavioral response to a phenethylamine hallucinogen, 25CN-NBOH, which acts as a selective 5-HT2A agonist. We first confirmed that 25CN-NBOH dose-dependently increased the head twitch response (HTR), a 5-HT2A-mediated behavior, in mice. The selective 5-HT2A antagonist M100907 completely blocked the HTR induced by 25CN-NBOH, whereas the selective 5-HT2C antagonist SB242084 had no effect on the HTR. Administration of LY379268 (10 mg/kg SC) attenuated the HTR induced by 1 mg/kg 25CN-NBOH by ~50%. Chronic treatment (21 days) with LY379268 also attenuated the HTR response to 25CN-NBOH when tested 48 hours after the last dose of LY379268. In locomotor tests, acute LY379268 significantly attenuated PCP-induced locomotor activity in the chronic vehicle treatment group; by contrast, there was only a trend for an overall interaction in the chronic LY379268 group, with LY379268 blocking the locomotor-stimulating effects of PCP only during the last 20 min. These data are consistent with a functional interaction between mGlu2/3 and 5-HT2A receptors, although the specific mechanism for the interaction is not known. These data support the hypothesis that mGlu2/3 receptors play a prominent role in modulating the behavioral response to 5-HT2A receptor activation.
Keywords: serotonin, 5-HT2A, metabotropic glutamate receptor, mGlu2/3, hallucinogen, head twitch
INTRODUCTION
Interactions between glutamate (Glu) and serotonin (5-hydroxytryptamine, 5-HT) are hypothesized to play a role in the pathophysiology of psychosis and the mechanism of action of hallucinogenic drugs. There is interest in metabotropic glutamate 2/3 (mGlu2/3) receptors as a potential therapeutic target for schizophrenia based on evidence that the illness is associated with excessive glutamatergic neurotransmission and the fact that mGlu2/3 agonists attenuate Glu release (Lorrain et al. 2003; Moghaddam and Adams 1998). mGlu2/3 agonists show efficacy in several different rodent models predictive of antipsychotic drug effects (Aghajanian and Marek 1999; Benneyworth et al. 2007; Conn and Jones 2009; Marek et al. 2006). One clinical trial found that pomaglumetad methionil (LY2140023), a prodrug for a selective mGlu2/3 agonist, possesses significant antipsychotic efficacy (Patil et al. 2007). Although the clinical effectiveness of LY2140023 was not confirmed by follow-up studies (Downing et al. 2014; Kinon et al. 2011), the response to this drug may be influenced by single nucleotide polymorphisms in the 5-HT2A gene (Liu et al. 2012; Nisenbaum et al. 2016).
The 5-HT2A receptor is responsible for mediating the characteristic effects of serotonergic hallucinogens such as (+)-lysergic acid diethylamide (LSD), psilocybin, and mescaline (Halberstadt 2015; Nichols 2004). There is evidence that mGlu2/3 receptors regulate 5-HT2A signaling, interactions that have been theorized to may play a role in the antipsychotic-like effects of mGlu2/3 agonists (Fribourg et al. 2011). Many of the behavioral, neurochemical, and electrophysiological effects of 5-HT2A activation are attenuated by mGlu2/3 agonists and facilitated by mGlu2/3 antagonists (Benneyworth et al. 2007; Carbonaro et al. 2015; Gewirtz and Marek 2000; Klodzinska et al. 2002; Marek et al. 2000; Molinaro et al. 2009; Riga et al. 2014; Wieronska et al. 2013; Winter et al. 2004; Wischhof and Koch 2012; Zhai et al. 2003). For example, the ability of the hallucinogen 2,5-dimethoxy-4-iodoamphetamine (DOI) to elicit the head twitch response (HTR), a rapid reciprocal side-to-side head movement induced by 5-HT2A activation in rodents (Canal and Morgan 2012; Halberstadt 2015), is suppressed by the mGlu2/3 agonists LY354740 and LY379268 and enhanced by the mGlu2/3 antagonist LY341495 (Gewirtz and Marek 2000; Klodzinska et al. 2002; Wieronska et al. 2013). Likewise, according to (Benneyworth et al. 2007), the mGlu2 positive allosteric modulator biphenyl-indanone A inhibits the HTR induced by R-(–)-2,5-dimethoxy-4-bromoamphetamine (R-(–)-DOB), a 5-HT2 agonist with hallucinogenic effects. The discriminative stimulus effects of hallucinogens in rats are potentiated by LY341495 and partially antagonized by LY379268 (Carbonaro et al. 2015; Winter et al. 2004). It has also been reported that mice lacking mGlu2 receptors are insensitive to the HTR and other effects of 5-HT2A agonists (Moreno et al. 2011). The interaction between 5-HT2A receptors and mGlu2/3 receptors appears to be bidirectional. mGlu2 receptors are downregulated in 5-HT2A knockout mice (Gonzalez-Maeso et al. 2008) due to epigenetic changes in the mGlu2 promotor (Kurita et al. 2013). Benneyworth et al. (Benneyworth et al. 2008) reported that chronic treatment of mice with R-(–)-DOB decreases the behavioral response to a mGlu2/3 receptor agonist. Furthermore, although administration of either DOI or LY379268 induces ERK1/2 phosphorylation in frontal cortex, they are inactive when administered in combination, indicating they produce reciprocal inhibition (Molinaro et al. 2009).
The mechanism for the crosstalk between mGlu2 and 5-HT2A receptors remains controversial. Gonzalez-Maseo et al. proposed that mGlu2 and 5-HT2A receptors can form a heteromeric complex that mediates the crosstalk between these receptors (Gonzalez-Maeso et al. 2008; Moreno et al. 2012). These heteromers would theoretically allow 5-HT2A receptors to couple to Gαi signaling, a putative key step in the intracellular response to hallucinogens (Gonzalez-Maeso et al. 2007). Indeed, it was subsequently demonstrated that disruption of 5-HT2A-mGlu2 heterocomplex formation can abolish the HTR (Moreno et al. 2012). Alternatively, the crosstalk between mGlu2 and 5-HT2A may be purely functional, occurring at the circuit level (Delille et al. 2012; Delille et al. 2013). Many effects of 5-HT2A activation, including the HTR, are thought to be mediated by increases in cortical Glu transmission (Egashira et al. 2011; Gorzalka et al. 2005; Pei et al. 2004; Scruggs et al. 2000; Zhang and Marek 2008). Because mGlu2 receptors function primarily as presynaptic autoreceptors (Schoepp 2001), they could potentially suppress the ability of 5-HT2A receptors to enhance glutamatergic transmission.
The goal of the present investigation was to further characterize the interactions between mGlu2 and 5-HT2A receptors. Most studies of mGlu2–5-HT2A interactions have used 5-HT2 agonists such as DOI and DOB, which activate 5-HT2A receptors and 5-HT2C receptors. To minimize the potentially confounding effects of 5-HT2C activation, we used 2-([2(4-cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol (25CN-NBOH), a 5-HT2A agonist that is at least 23-fold selective versus 5-HT2C (Buchborn et al. 2018; Hansen et al. 2014; Jensen et al. 2017). Fantegrossi and colleagues have confirmed that 25CN-NBOH induces the HTR in mice by activating 5-HT2A receptors (Fantegrossi et al. 2015). As noted above, Benneyworth et al. (2008) reported that chronic treatment of mice with a 5-HT2A agonist reduces the behavioral response to a mGlu2/3 receptor agonist. Because the interaction between mGlu2 and 5-HT2A receptors is bidirectional (Gonzalez-Maeso et al. 2008), it is possible that chronic treatment with a mGlu2/3 agonist would alter the behavioral response to a 5-HT2A agonist. Indeed, it was previously shown in mice that chronic administration of the mGlu2/3 antagonist LY341495 produces 5-HT2A downregulation (Moreno et al. 2013). The current study examined whether chronic treatment with the mGlu2/3 agonist LY379268 alters the ability of 25CN-NBOH to induce the HTR. It is known that chronic administration of a mGlu2/3 agonist desensitizes the ability of the receptor to inhibit cAMP formation (Iacovelli et al. 2009) and abolishes the ability of a mGlu2/3 agonist to attenuate hyperactivity induced by the NMDA receptor (NMDAR) antagonist phencyclidine (PCP) (Galici et al. 2005). LY379268 can attenuate PCP-induced hyperactivity (Cartmell et al. 1999) and we also examined whether chronic treatment with LY379268 altered its ability to reduce the response to PCP.
MATERIALS AND METHODS
Animals and housing
Male C57BL/6J mice (6–8 weeks old) were obtained from Jackson Labs (Bar Harbor, ME, USA). The mice were housed on a reversed light-dark cycle (lights on at 1900 h, off at 0700 h,) in an AALAC-approved vivarium at the University of California San Diego. Mice were housed up to four per cage in a climate-controlled room and with food and water provided ad libitum except during behavioral testing. Testing was performed between 1000 h and 1800 h (during the dark phase of the light-dark cycle). This study was conducted in accordance with National Institutes Health (NIH) guidelines and was approved by the University of California San Diego Institutional Animal Care and Use Committee.
Drugs
2-([2-(4-Cyano-2,5-dimethoxyphenyl)ethylamino]methyl)phenol hydrochloride (25CN-NBOH; Tocris Bioscience, Minneapolis, MN, USA); LY379268 (donated by the NIMH Chemical Synthesis and Drug Supply Program, RTI International, Research Triangle Park, NC, USA); 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxamide dihydrochloride (SB242084; Cayman Chemical, Ann Arbor, MI, USA); (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100907; donated by Hoechst Marion Roussel Inc., Kansas City, MO, USA); and phencyclidine hydrochloride (PCP; Sigma-Aldrich, St. Louis, MO, USA). All drugs were dissolved in water containing 1% Tween 80 and administered SC, except for PCP, which was dissolved in saline and administered IP. Drugs were injected at a volume of 5 mL/kg. At the concentrations used in these experiments, aqueous solutions of LY379268 are relatively acidic (pH = ~3). Stress can alter the HTR induced by hallucinogens (Yamada et al. 1995); hence, any discomfort caused by injection of LY379268 could potentially influence the behavioral response produced by 25CN-NBOH. Pilot experiments, however, confirmed that the response to LY379268 and 25CN-NBOH is not affected by the pH of the pretreatment injection.
Head Twitch Response Testing
The head twitch response (HTR) was assessed using a head-mounted magnet and a magnetometer detection coil. A neodymium magnet was attached to the skull as described previously (Halberstadt and Geyer 2013). Mice were allowed to recover for at least one week prior to behavioral testing. HTR experiments were conducted in a well-lit room, and the mice were allowed to habituate to the room for at least 1 h prior to testing. The HTR was assessed in a 12-cm-diameter glass cylinder surrounded by a magnetometer coil. Head movements were recorded and analyzed for HTR as described previously (Halberstadt and Geyer 2013; 2014; Klein et al. 2018; Nichols et al. 2015). Briefly, coil voltage was low-pass filtered (2 KHz), amplified, and digitized (20 kHz sampling rate) using a Powerlab/8SP with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO, USA). The data were filtered off-line (40–200 Hz band-pass), and HTRs were identified by their waveform characteristics (sinusoidal wavelets with at least two bipolar peaks, peak spectral power between 40–120 Hz, amplitude exceeding the background noise level, and duration < 0.15 s, with stable coil voltage during the period immediately before and after each response).
Behavioral Pattern Monitor
The mouse Behavioral Pattern Monitor (mouse BPM) was used to record exploratory and investigatory behavior (Halberstadt et al. 2013; Halberstadt et al. 2009). Each mouse BPM chamber (San Diego Instruments, San Diego, CA) is a transparent Plexiglas box with an opaque 30 × 60 cm floor, enclosed in a ventilated isolation box. The position of the mouse in x,y coordinates is recorded by a grid of 12 × 24 infrared photobeams located 1 cm above the floor. A second row of 16 photobeams (parallel to the long axis of the chamber, located 2.5 cm above the floor) is used to detect rearing behavior. Holepoking behavior is detected by 11, 1.4-cm holes that are situated in the walls (3 holes in each long wall, 2 holes in each short wall) and the floor (3 holes); each hole is equipped with an infrared photobeam. The status of each photobeam is sampled every 55 ms, and data recorded for off-line analysis. The measures assessed were distance traveled (a measure of locomotor activity), total rearings and total holepokes (measures of investigatory behavior).
Experimental Design
Testing occurred immediately after treatment with 25CN-NBOH and with PCP. For Experiment 1, mice (30 total, n=5/group) were treated with vehicle or 25CN-NBOH (0.1, 0.3, 1, 3, or 10 mg/kg) and HTR assessed for 20 min. For Experiment 2, mice (31 total, n=5–6/group) were pretreated with vehicle, M100907 (0.1 mg/kg) or SB242084 (0.1, 0.3, or 1 mg/kg) 20 min prior to treatment with 1 mg/kg 25CN-NBOH, and then HTR was assessed for 20 min. An additional group of mice received two injections of vehicle at the same time points and served as a shared control group for the response to 25CN-NBOH. For Experiment 3, mice (24 total, n=6/group) were pretreated with vehicle or LY379268 (0.1, 1, or 10 mg/kg) 20 min prior to treatment with 1 mg/kg 25CN-NBOH, and then HTR was assessed for 20 min. For Experiment 4, two groups of mice (n=30/group) were treated once daily with vehicle or LY379268 (10 mg/kg) for 21 days. The mice were challenged with 25CN-NBOH (1 mg/kg) 48 h after the final LY379268 treatment and HTR monitored for 20 min. The following day (72 h after the final LY379268 treatment), the mice were pretreated with vehicle or LY379268 (3 mg/kg), treated 20 min later with vehicle or PCP (5 mg/kg), and their behavior assessed in the BPM for 60 min (n=7–8/group).
Data analysis
Data are presented as group means ± SEM. Behavioral data were analyzed using 1-, 2-, or 3-way analyses of variance (ANOVAs) with pretreatment and treatment as between-subject variables and time as a within-subject variable. Specific post hoc comparisons were made between groups using Tukey’s studentized range method. Significance was demonstrated by surpassing an α level of 0.05.
Median effective doses (ED50 values) and confidence intervals for HTR dose-response experiments were calculated by nonlinear regression (Prism 7.00, GraphPad Software, San Diego, CA, USA). A Gaussian distribution (Christopoulos et al. 2001) was used to fit biphasic HTR dose-response data:
In these equations, E is the drug effect, Baseline is the response after vehicle treatment, Range is the distance from Baseline to the top of the curve, [A] is the dose of the drug, and midA is the logarithm of the dose corresponding to the top of the curve.
RESULTS
Experiment 1. Dose response of 25CN-NBOH
25CN-NBOH produced a dose-dependent increase in HTR (Drug effect: F(5,24) = 10.83; p<0.0001), confirming previous reports (Fantegrossi et al. 2015; Halberstadt et al. 2016). As shown in Figure 1, there was a significant increase in HTR counts after administration of 1, 3, and 10 mg/kg 25CN-NBOH (p<0.01, Tukey’s test). The ED50 of 25CN-NBOH is 0.51 (95% CI = 0.28–0.93) mg/kg. The ED50 is equivalent to 1.45 (0.80–2.65) µmol/kg. Based on the dose-response of 25CN-NBOH, 1 mg/kg was used for the subsequent experiments.
Fig. 1.
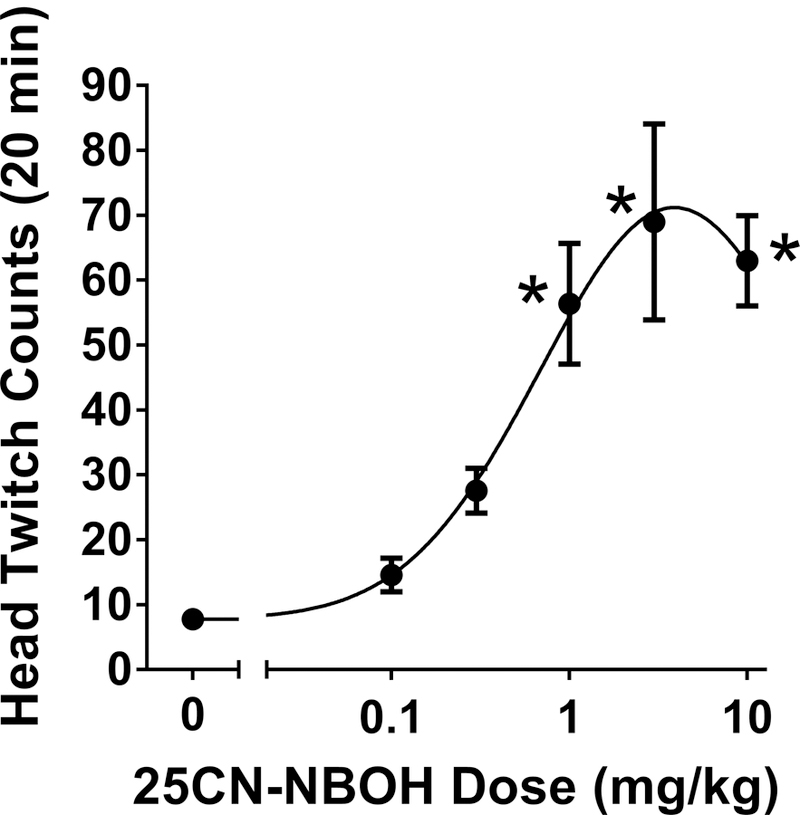
Effect of 25CN-NBOH on the head twitch response. Data are presented as group means±SEM. *p<0.05 significantly different from vehicle control group.
Experiment 2. Role of 5-HT2A and 5-HT2C receptors in 25CN-NBOH-induced HTR
The effect of pretreatment with the selective 5-HT2A antagonist M100907 and the selective 5-HT2C antagonist SB24084 were analyzed using separate 1-way ANOVAs, with the vehicle and 25CN-NBOH groups shared across the two analyses. For M100907, post-hoc tests were performed based on a significant ANOVA (F(2,13)=117.54, p<0.0001). As shown in Figure 2, M100907 completely blocked the HTR induced by 25CN-NBOH (p<0.01, Tukey’s test). Consistent with Experiment 1, 25CN-NBOH significantly increased HTR. For SB242084, a significant ANOVA (F(4,21)=12.24, p<0.001) was followed by post-hoc tests. SB242084 failed to attenuate the HTR when administered at doses ranging from 0.1 mg/kg to 1 mg/kg.
Fig. 2.
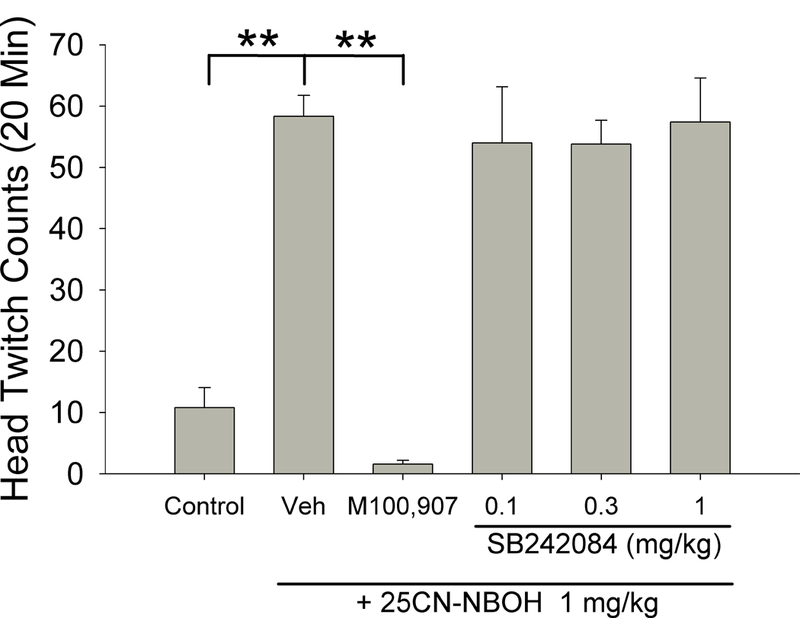
Effect of M100,907 and SB242084 on 25CN-NBOH-induced head twitch response. Data as presented as group means±SEM. **p<0.01, significantly different from 25CN-NBOH alone.
Experiment 3. LY379268 attenuates the HTR induced by 25CN-NBOH
LY379268 attenuated the HTR induced by 25CN-NBOH (Figure 3; F(3,20) = 5.00, p<0.01). Pretreatment with 10 mg/kg LY379268 (10 mg/kg; 2 mg/ml concentration injected at 5 ml/kg) significantly reduced the HTR induced by 1 mg/kg 25CN-NBOH by 52.5% (vehicle = 60.3±2.7 counts; 10 mg/kg LY379268 = 28.7±6.4; p<0.05, Tukey’s test).
Fig. 3.
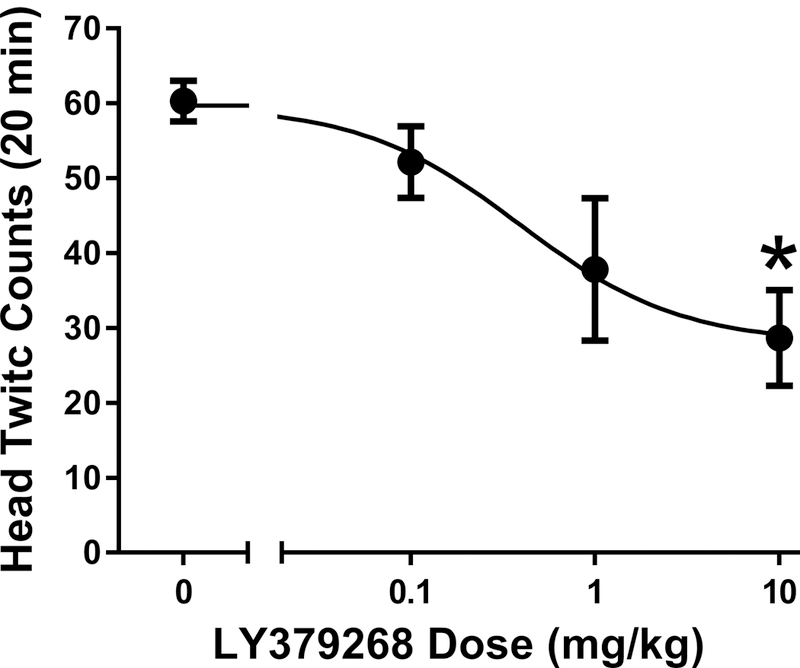
Effect of LY379268 on 25CN-NBOH-induced head twitch response (A,B). Data are presented as group means±SEM. *p<0.05 significantly different from vehicle.
Experiment 4. Effect of chronic treatment of LY379268 on 25CN-NBOH-induced HTR
Chronic treatment with LY379268 attenuated the HTR response to 25CN-NBOH (Figure 4; chronic treatment × acute treatment interaction; (F(1,54) = 30.91, p<0.001). There were also main effects of acute 25CN-NBOH (F(1,54) = 773.92, p<0.001) and chronic LY379268 (F(1,54)=16.63, p<0.001). Although 25CN-NBOH significantly increased HTR in both vehicle- and LY379268-treated mice, the number of counts was significantly greater in mice chronically treated with vehicle compared with chronic LY379368 (p<0.01, Tukey’s test).
Fig. 4.
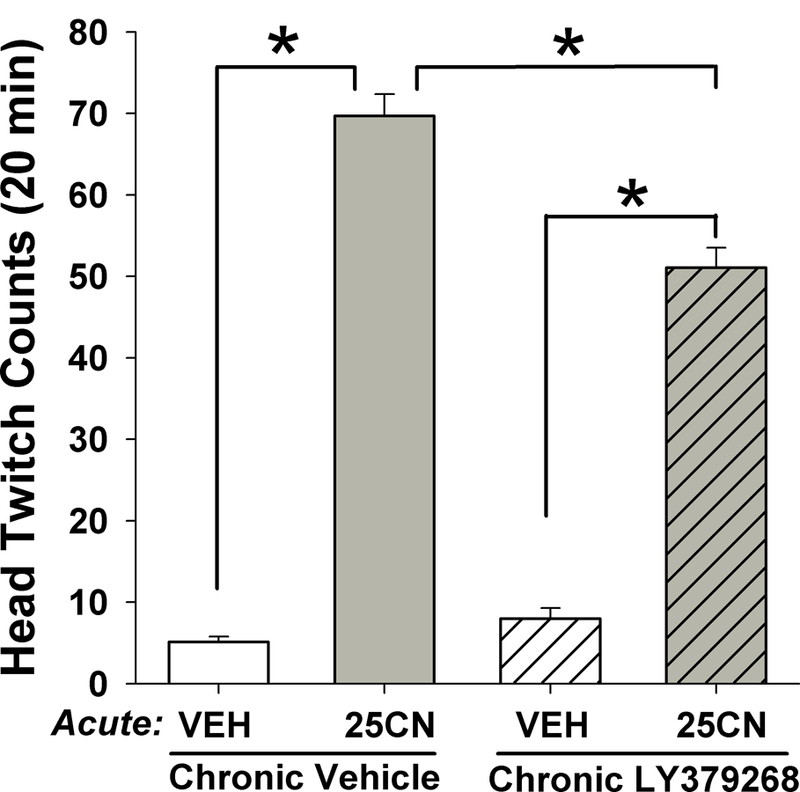
Effect of chronic LY379268 treatment on 25CN-NBOH-induced head twitch response. HTR response 48 hours after last chronic injection LY379268. Data presented as group means±SEM. *p<0.01, significantly different from comparison group.
Experiment 5. Chronic LY379268 alters the ability of acute LY379268 to block PCP-induced hyperlocomotion
This experiment tested the action of acute LY379268 on PCP-induced hyperlocomotion in mice treated chronically with either vehicle or LY379268 (Figure 5). The initial 4-way ANOVA showed that PCP significantly increased locomotor activity in both chronic groups (F(1,52) = 40.23, p<0.0001) and that LY379268 significantly attenuated PCP-induced locomotor activity as evidenced by an acute LY379268 by PCP interaction (F(1,52) = 7.98, p<0.01). There was no main effect observed of chronic LY379268, indicating no overall effects of chronic treatment on locomotor activity. Although there were no interactions with chronic treatment, the ability of acute LY379268 to reverse the effect of PCP appeared to be attenuated in the chronic LY379268 group. Thus, follow-up 3-way ANOVAs were performed on each chronic treatment group separately. In the chronic vehicle group, acute LY379268 significantly blocked the locomotor-stimulating effects of PCP (acute LY379268 vs PCP interaction: F(1,26)=4.78, p<0.05) during the last 40 min of the 1-h BPM test session. In the chronic LY379268 group, there was only a trend for the overall interaction between acute LY379268 and PCP (F(1,26)=3.23, p=0.084) with LY379268 blocking the locomotor-stimulating effects of PCP only during the last 20 min, suggesting the ability of acute LY379268 to reverse PCP effects was reduced. Table 1 summarizes the distance traveled, rearing and holepoke data from Exp. 5. PCP significantly reduced the number of rearings, whereas holepokes were unaffected. Acute pretreatment with LY379268 did not alter the effect of PCP on rearing behavior.
Fig. 5.
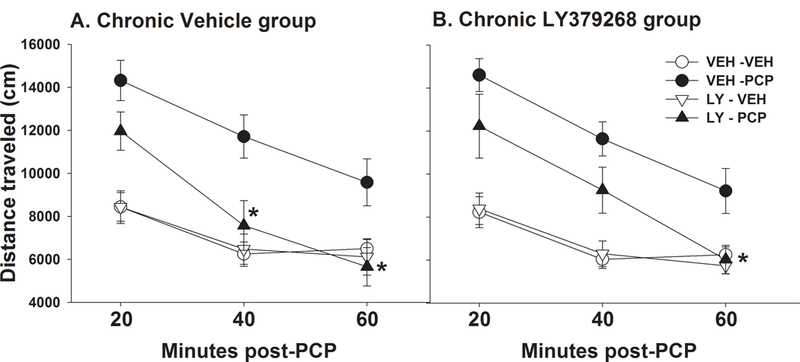
LY379268 reversal of PCP-induced hyperlocomotion in mice treated chronically with vehicle (A) or LY379268 (B). (A) Locomotor activity (distance traveled) in mice after chronic treatment with saline. (B) Locomotor activity (distance traveled) in mice after chronic treatment with LY379268. Data are presented as group means±SEM for 20-min time blocks. *p<0.05, significantly different from VEH – PCP group.
Table 1.
Effect of chronic LY379268 (10 mg/kg) on the interaction between LY379268 (3 mg/kg) and PCP (5 mg/kg) in the Behavioral Pattern Monitor.
| Chronic Treatment | Acute Treatment | Distance traveled (60 min) | Rearings (60 min) | Pokes (60 min) |
|---|---|---|---|---|
| Vehicle | VEH - VEH | 21172.1 ± 1551.7 | 294.1 ± 26.2 | 258.8 ± 19.4 |
| VEH - PCP | 35595.1 ± 2678.0** | 121.7 ± 24.0** | 210.1 ± 13.2 | |
| LY - VEH | 21006.1 ± 2195.4 | 278.9 ± 29.2 | 194.0 ± 25.1 | |
| LY - PCP | 25181.1 ± 2763.3# | 50.3 ± 23.3** | 141.5 ± 23.8** | |
| LY379268 | VEH - VEH | 20497.4 ± 1436.7 | 405.1 ± 14.1 | 245.3 ± 23.6 |
| VEH - PCP | 35445.1 ± 2344.0** | 177.9 ± 27.7** | 228.0 ± 23.9 | |
| LY - VEH | 20402.9 ± 1557.8 | 286.1 ± 29.2* | 199.9 ± 25.7 | |
| LY - PCP | 27495.1 ± 3070.0 | 138.7 ± 30.2** | 173.6 ± 6.0 | |
Data are shown as group means
SEM.
p<0.05 compared to VEH –PCP.
p<0.05
p<0.01 compared to VEH-VEH.
DISCUSSION
There is increasing evidence that 5-HT2A receptors functionally interact with mGlu2 receptors. In the present investigation, we examined whether chronic treatment with an mGlu2/3 agonist would attenuate the acute effects of a selective 5-HT2A agonist. Using an automated HTR detection system (Halberstadt and Geyer 2013), these studies demonstrated that the selective 5-HT2A agonist 25CN-NBOH increased HTR through activation of 5-HT2A receptors. Studies also demonstrated that acute administration of an mGlu2/3 agonist (LY379268) blocked HTR induced by selective activation of the 5-HT2A receptor (via 25CN-NBOH). Likewise, chronic treatment with an mGlu2/3 agonist attenuated the HTR to 25CN-NBOH. While acute LY379268 attenuated PCP-induced hyperlocomotion, these effects were somewhat blunted in mice treated chronically with LY379268, indicating a likely alteration in mGlu2/3 receptors with chronic agonist administration.
As reported in several studies, hallucinogen effects are primarily mediated by the 5-HT2A receptor in mice (Halberstadt 2015). The current study corroborates previous findings by showing that the new selective 5-HT2A agonist 25CN-NBOH increases head twitch response in mice using an automated detection system. The median effective dose of 25CN-NBOH in the present experiment (ED50 = 0.51 mg/kg) was higher than the value from our previous experiment (ED50 = 0.36 mg/kg; Halberstadt et al. 2016). Dose-response relationships for hallucinogen-induced head twitches often show biphasic inverted-U-shaped curves (Fantegrossi et al. 2010); these complex dose-effect curves can complicate analyses performed using sigmoidal distributions, as was the case in our previous report. Although the response to 25CN-NBOH did not show a clear descending arm, use of a Gaussian distribution provided a more accurate estimation of the top of the dose-response curve. Hence, compared to the earlier potency estimate, the value reported herein is likely more accurate, demonstrating the importance of fitting biphasic HTR dose-response data using a Gaussian distribution.
The dose-response curves for 25CN-NBOH generated using the automated HTR system were stable over time and across different animal cohorts. The present studies confirmed that 25CN-NBOH induces the HTR in mice and showed that 25CN-NBOH is slightly more potent than the phenethylamine hallucinogens 2C-I (ED50 = 2.42 µmol/kg) and 25I-NBMD (ED50 = 2.36 umol/kg; Halberstadt and Geyer 2014), and 2C-B (ED50=2.43 µmol/kg; Halberstadt et al., submitted). Although Fantegrossi and coworkers reported that the HTR induced by 25CN-NBOH peaks at 1 mg/kg (Fantegrossi et al. 2015), the HTR peaked at 3 mg/kg in both of our experiments. Additionally, we observed a more robust response in terms of the maximum number of HTR counts induced by the drug. These differences may be a consequence of the fact that (Fantegrossi et al. 2015) used a mouse strain (NIH Swiss) known to display relatively modest levels of HTR, whereas the present investigation was conducted with a strain (C57BL/6) that exhibits a large HTR (Canal and Morgan 2012). We were also able to confirm that the selective 5-HT2C antagonist SB-242,084 did not attenuate the 25CN-NBOH-induced HTR. By contrast, the selective 5-HT2A antagonist M100907 completely abolished the HTR induced by 25CN-NBOH. These results support the contention that the HTR is mediated by 5-HT2A and not by 5-HT2C receptors. The current studies also showed that the mGlu2/3 agonist LY379268 attenuated HTR induced by 25CN-NBOH. These findings support previous reports showing that the mGlu2/3 agonist LY379268 blocked DOI-induced HTR (Benneyworth et al. 2007; Klodzinska et al. 2002; Wieronska et al. 2013). Thus, our findings corroborate previous studies showing the ability of an mGlu2/3 agonist to attenuate hallucinogen-induced HTR and extend these findings to a moderately selective 5-HT2A agonist, 25CN-NBOH. These data support the hypothesis that mGlu2/3 receptors modulate 5-HT2A receptor-regulated behavior (Moreno et al. 2011).
We hypothesized that chronic treatment with a mGlu2/3 agonist would attenuate the behavioral response to a 5-HT2A agonist. Indeed, chronic LY379268 diminished 25CN-NBOH-induced HTR 48 hours after 21 days of repeated administration. Although blood or brain levels of LY379268 were not obtained, studies in gerbils have revealed that ~90% of the LY379268 present in the brain after administration of 10 mg/kg IP is cleared within 8 h (Bond et al. 2000), and thus the present effects probably cannot be explained by prolonged occupation of mGlu2/3 receptors by LY379268. We cannot definitively rule out the possibility that low levels of LY379268 remained in the brains of mice. There are several possible explanations that could account for the reduced response to hallucinogens after chronic treatment with a mGlu2/3 agonist, including: (1) downregulation or desensitization of mGlu2 receptors, (2) downregulation or desensitization of 5-HT2A receptors, or (3) uncoupling of 5-HT2A-mGlu2/3 heterodimers. Chronic treatment with receptor agonists often results in receptor downregulation; hence, the results may be explained by a downregulation or desensitization of mGlu2/3 receptors; however, the current study did not assess mGlu2/3 receptor levels or function.
One potential explanation for the attenuated response to 25CN-NBOH is that chronic treatment with LY379268 desensitizes 5-HT2A receptors. Potential mechanisms for 5-HT2A desensitization after LY379268 treatment include elevated 5-HT release or cross-talk between mGlu2 and 5-HT2A receptors, either through heteromeric complexes or indirectly via functional interactions. It is well known that compounds that increase 5-HT levels, including SSRIs, can induce 5-HT2A desensitization (Sanders-Bush et al. 1989). Acute administration of LY379268 to freely-moving rats has been shown to increase extracellular 5-HT levels in medial prefrontal cortex; injection of 10 mg/kg LY379268 increased the concentration of 5-HT by 79% and the effect persisted for several hours (Cartmell et al. 2001). It is not known, however, whether LY379268 would continue to increase 5-HT levels after repeated administration, or if a similar effect occurs in mice. Nevertheless, 5-HT-induced desensitization of 5-HT2A probably did not play a role in our findings because it was previously shown in mice that chronic administration of the mGlu2/3 antagonist LY341495 produces 5-HT2A downregulation (Moreno et al. 2013). It is unlikely that mGlu2/3 agonists and antagonists would have similar effects on 5-HT release and receptor downregulation.
There is evidence that chronic mGlu2/3 activation can result in behavioral tolerance to the acute effects of a mGlu2/3 agonist. In order to determine whether the behavioral response to a mGlu2/3 agonist is altered by chronic treatment with LY379268, we also assessed whether chronic treatment with this substance alters its ability to reduce the locomotor-stimulating effects of PCP. Previously, it was reported that LY379268 can decrease NMDAR antagonist-induced locomotor activity acutely in doses of 3 and 10 mg/kg (Benneyworth et al. 2007; Chartoff et al. 2005; Galici et al. 2005). We were able to corroborate these findings and show that acute LY379268 (3 mg/kg) blocked PCP-induced locomotor activity in the chronic vehicle group (Fig 5A) using a different experimental design in which mice were not habituated to the locomotor chambers prior to drug administration and thus allowing for the ability to detect decreases in locomotor activity in the LY379268 alone group. In the chronic LY379268 group, acute LY379268 also attenuated PCP-induced locomotor activity but to a lesser extent than the in chronic vehicle-treated mice. These data indicate that there may have been some degree of downregulation of mGlu2/3 receptors; however, this experiment did not definitively answer whether mGlu2/3 receptors are subject to downregulation or desensitization upon chronic administration of an mGlu2/3 agonist. Previous studies have indicated that chronic treatment with LY379268 (3 mg/kg) for 7 days decreased the ability of LY379268 to attenuate PCP- and amphetamine-induced hyperactivity was absent, indicating tolerance to mGlu2/3 receptor activation had developed (Galici et al. 2005). In the current study, LY379268 was still effective at reducing PCP-induced locomotor activity in mice administered LY379268 for 21 days; however, this effect was diminished compared to vehicle-treated mice. In contrast to the current study, Galici et al. tested whether LY379268 was able to block amphetamine- and PCP-induced hyperlocomotor activity 24 hours after a 7-day treatment period with LY379268 (Galici et al. 2005). The ability of LY379268 to decrease amphetamine- and PCP-induced hyperlocomotion was lost in animals receiving it chronically. Future studies should assess the chronic effects of mGlu2 positive allosteric modulators since these compounds may prove to be more effective therapeutics.
There is also the possibility that a downregulation or desensitization of the mGlu2/3 receptor after chronic exposure would possibly disrupt 5-HT2A–mGlu2/3 heteromeric complex functionality or other mechanisms of cross-talk between 5-HT2A and mGlu2/3 receptors. In a complementary study (Benneyworth et al. 2008), mice treated with the 5-HT2A/2C agonist, R-(−)-2,5-dimethoxy-4-bromoamphetamine (DOB) for 14 days exhibited an impaired behavioral response to LY379268. Our data shows that the opposite is also true: chronic treatment with LY379268 significantly reduced the behavioral response to the 5-HT2A agonist 25CN-NBOH, which strongly indicates that the functional interaction between mGlu2/3 and 5-HT2A receptors is bidirectional. Nevertheless, additional studies are required to determine the specific mechanism for this interaction. Further support for a functional interaction comes from chronic studies with the mGlu2/3 antagonist LY341495. Mice administered LY341495 for 21 days were less sensitive to the effects of LSD on HTR behavior and expression of immediate early genes such as c-fos and egr-2. These behavioral differences were accompanied by a reduction of 5-HT2A receptor expression in the somatosensory cortex, an effect absent in mGlu2 KO mice (Moreno et al. 2013). Thus, there is increasing evidence that the chronic administration of either mGlu2/3 ligands or 5-HT2A agonists can disrupt the functional interaction between these two receptor systems.
Acute administration of LY379268 attenuated the HTR induced by 25CN-NBOH but did not completely eliminate the response.Although some studies have observed almost complete blockade of the response to DOI by LY379268 (Benvenga et al. 2018; Gonzalez-Maeso et al. 2008), LY379268 produced ~50–60% attenuation of the HTR in most previous reports (Benneyworth et al. 2008; Gewirtz and Marek 2000; Klodzinska et al. 2002; Wieronska et al. 2013). Hence, the level of blockade observed with LY379268 in Fig. 3 is consistent with the effect of the drug in previous studies.
In conclusion, this study shows mGlu2/3 receptors play an important role in the regulation of hallucinogen-induced behavioral responses. Specifically, chronic treatment with LY379268 significantly decreased the HTR induced by 25CN-NBOH, providing additional evidence for a functional interaction between 5-HT2A and mGlu2/3 receptors. Whether these effects are mediated by a direct heteromeric complex between the receptors, or alternatively reflect downstream functional interactions, remains to be determined.
Acknowledgments
We would like to thank Landon Klein and Mahalah Buell for technical assistance. LY379268 was generously supplied by the NIMH Chemical Synthesis and Drug Supply Program (CSDSP).
Funding Source: These studies were supported by R01 DA002925, R01 DA041336, K01 MH100644, and Veteran’s Affairs VISN 22 MIRECC.
Footnotes
Disclosure/Conflict of interest
Mark Geyer holds an equity interest in San Diego Instruments.
References
- Aghajanian GK, Marek GJ (1999) Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res 825: 161–71 [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Smith RL, Sanders-Bush E (2008) Chronic phenethylamine hallucinogen treatment alters behavioral sensitivity to a metabotropic glutamate 2/3 receptor agonist. Neuropsychopharmacology 33: 2206–16 [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72: 477–84 [DOI] [PubMed] [Google Scholar]
- Benvenga MJ, Chaney SF, Baez M, Britton TC, Hornback WJ, Monn JA, Marek GJ (2018) Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice. Front Pharmacol 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Jones NM, Hicks CA, Whiffin GM, Ward MA, O’Neill MF, Kingston AE, Monn JA, Ornstein PL, Schoepp DD, Lodge D, O’Neill MJ (2000) Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: investigations into possible mechanism of action in vivo. J Pharmacol Exp Ther 294: 800–9 [PubMed] [Google Scholar]
- Buchborn T, Lyons T, Knopfel T (2018) Tolerance and Tachyphylaxis to Head Twitches Induced by the 5-HT2A Agonist 25CN-NBOH in Mice. Front Pharmacol 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 232: 275–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291: 161–70 [PubMed] [Google Scholar]
- Cartmell J, Perry KW, Salhoff CR, Monn JA, Schoepp DD (2001) Acute increases in monoamine release in the rat prefrontal cortex by the mGlu2/3 agonist LY379268 are similar in profile to risperidone, not locally mediated, and can be elicited in the presence of uptake blockade. Neuropharmacology 40: 847–55 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Heusner CL, Palmiter RD (2005) Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology 30: 1324–33 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Grant MK, Ayoubzadeh N, Kim ON, Sauerberg P, Jeppesen L, El-Fakahany EE (2001) Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J Pharmacol Exp Ther 298: 1260–8 [PubMed] [Google Scholar]
- Conn PJ, Jones CK (2009) Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology 34: 248–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M (2012) Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 62: 2184–91 [DOI] [PubMed] [Google Scholar]
- Delille HK, Mezler M, Marek GJ (2013) The two faces of the pharmacological interaction of mGlu2 and 5-HT(2)A - relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology 70: 296–305 [DOI] [PubMed] [Google Scholar]
- Downing AM, Kinon BJ, Millen BA, Zhang L, Liu L, Morozova MA, Brenner R, Rayle TJ, Nisenbaum L, Zhao F, Gomez JC (2014) A Double-Blind, Placebo-Controlled Comparator Study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry 14: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M (2011) Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol Biochem Behav 99: 52–8 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL (2015) Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT(2)A receptors, in mice. Psychopharmacology (Berl) 232: 1039–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335: 728–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD Jr., Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147: 1011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ (2005) A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther 315: 1181–7 [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23: 569–76 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52 [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Sun JC (2005) Functional role of the endocannabinoid system and AMPA/kainate receptors in 5-HT2A receptor-mediated wet dog shakes. Eur J Pharmacol 516: 28–33 [DOI] [PubMed] [Google Scholar]
- Halberstadt AL (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 277: 99–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227: 727–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77: 200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Powell SB, Geyer MA (2013) Role of the 5-HT(2)A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology 70: 218–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Sindhunata IS, Scheffers K, Flynn AD, Sharp RF, Geyer MA, Young JW (2016) Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology 107: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB (2009) 5-HT(2A) and 5-HT(2C) Receptors Exert Opposing Effects on Locomotor Activity in Mice. Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Brauner-Osborne H, Kristensen JL (2014) Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci 5: 243–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Molinaro G, Battaglia G, Motolese M, Di Menna L, Alfiero M, Blahos J, Matrisciano F, Corsi M, Corti C, Bruno V, De Blasi A, Nicoletti F (2009) Regulation of group II metabotropic glutamate receptors by G protein-coupled receptor kinases: mGlu2 receptors are resistant to homologous desensitization. Mol Pharmacol 75: 991–1003 [DOI] [PubMed] [Google Scholar]
- Jensen AA, McCorvy JD, Leth-Petersen S, Bundgaard C, Liebscher G, Kenakin TP, Brauner-Osborne H, Kehler J, Kristensen JL (2017) Detailed Characterization of the In Vitro Pharmacological and Pharmacokinetic Properties of N-(2-Hydroxybenzyl)-2,5-Dimethoxy-4-Cyanophenylethylamine (25CN-NBOH), a Highly Selective and Brain-Penetrant 5-HT2A Receptor Agonist. J Pharmacol Exp Ther 361: 441–453 [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol 31: 349–55 [DOI] [PubMed] [Google Scholar]
- Klein LM, Cozzi NV, Daley PF, Brandt SD, Halberstadt AL (2018) Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology [DOI] [PMC free article] [PubMed]
- Klodzinska A, Bijak M, Tokarski K, Pilc A (2002) Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav 73: 327–32 [DOI] [PubMed] [Google Scholar]
- Kurita M, Moreno JL, Holloway T, Kozlenkov A, Mocci G, Garcia-Bea A, Hanks JB, Neve R, Nestler EJ, Russo SJ, Gonzalez-Maeso J (2013) Repressive epigenetic changes at the mGlu2 promoter in frontal cortex of 5-HT2A knockout mice. Mol Pharmacol 83: 1166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Jamba M, Patrick C 3rd, Padmanabhan S, Brennan MD(2012) Targeted pharmacogenetic analysis of antipsychotic response in the CATIE study. Pharmacogenomics 13: 1227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117: 697–706 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD (2006) 5-Hydroxytryptamine2A (5-HT2A) receptor regulation in rat prefrontal cortex: interaction of a phenethylamine hallucinogen and the metabotropic glutamate2/3 receptor agonist LY354740. Neurosci Lett 403: 256–60 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000) Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292: 76–87 [PubMed] [Google Scholar]
- Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281: 1349–52 [DOI] [PubMed] [Google Scholar]
- Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G (2009) Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol Pharmacol 76: 379–87 [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493: 76–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Rayannavar V, Sealfon SC, Gonzalez-Maeso J (2013) Chronic treatment with LY341495 decreases 5-HT(2A) receptor binding and hallucinogenic effects of LSD in mice. Neurosci Lett 536: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, Lopez-Gimenez JF, Meana JJ, Benson DL, Gonzalez-Maeso J (2012) Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem 287: 44301–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE (2004) Hallucinogens. Pharmacol Ther 101: 131–81 [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (2015) N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem Neurosci [DOI] [PMC free article] [PubMed]
- Nisenbaum LK, Downing AM, Zhao F, Millen BA, Munsie L, Kinon BJ, Adams DH, Gomez JC, Penny MA (2016) Serotonin 2A Receptor SNP rs7330461 Association with Treatment Response to Pomaglumetad Methionil in Patients with Schizophrenia. J Pers Med 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13: 1102–7 [DOI] [PubMed] [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T (2004) Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology 46: 331–9 [DOI] [PubMed] [Google Scholar]
- Riga MS, Soria G, Tudela R, Artigas F, Celada P (2014) The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: reversal by antipsychotic drugs. Int J Neuropsychopharmacol 17: 1269–82 [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Breeding M, Knoth K, Tsutsumi M (1989) Sertraline-induced desensitization of the serotonin 5HT-2 receptor transmembrane signaling system. Psychopharmacology (Berl) 99: 64–9 [DOI] [PubMed] [Google Scholar]
- Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20 [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY (2000) DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci 20: 8846–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieronska JM, Slawinska A, Stachowicz K, Lason-Tyburkiewicz M, Gruca P, Papp M, Pilc A (2013) The reversal of cognitive, but not negative or positive symptoms of schizophrenia, by the mGlu(2)/(3) receptor agonist, LY379268, is 5-HT(1)A dependent. Behav Brain Res 256: 298–304 [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA (2004) Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology (Berl) 172: 233–40 [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M (2012) Pre-treatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology (Berl) 219: 387–400 [DOI] [PubMed] [Google Scholar]
- Yamada S, Watanabe A, Nankai M, Toru M (1995) Acute immobilization stress reduces (+/−)DOI-induced 5-HT2A receptor-mediated head shakes in rats. Psychopharmacology (Berl) 119: 9–14 [DOI] [PubMed] [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK (2003) Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28: 45–52 [DOI] [PubMed] [Google Scholar]
- Zhang C, Marek GJ (2008) AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry 32: 62–71 [DOI] [PubMed] [Google Scholar]


