Abstract
Although the proteasome inhibitor bortezomib (BTZ) shows excellent efficacy in multiple myeloma (MM), a fraction of patients has a suboptimal or no response to this agent. In addition, BTZ‐induced peripheral neuropathy (BiPN), a frequent side‐effect of this therapy, limits its use in some patients. This study aimed to explore serum lipid biomarker candidates to predict the response to BTZ and the severity of BiPN. Fifty‐nine serum samples were collected from patients with MM prior to receiving BTZ plus low‐dose dexamethasone therapy. Serum levels of phospholipids, sphingolipids, neutral lipids, and polyunsaturated fatty acids and their oxidation products were measured by a comprehensive lipidomic study. Overall, 385 lipid metabolites were identified in patients’ sera; lower levels of several glycerophospholipids, sphingolipids, and cholesteryl esters were associated with a poor treatment response. Metabolites related to platelet‐activating factor biosynthesis and cholesterol metabolism appeared particularly relevant. Furthermore, several lysophosphatidylcholines, phosphatidylcholines, ceramides, neutral lipids, and oxidative fatty acids were significantly increased or decreased in patients with BiPN grades ranging from G0 to G3. Among these compounds, mediators reportedly inducing myelin breakdown and stimulating inflammatory responses were prominent. Although further study is necessary to validate these biomarker candidates, our results contribute to the development of predictive biomarkers for response to BTZ treatment, or ensuing severe BiPN, in patients with MM.
Keywords: bortezomib, lipidomics, multiple myeloma, peripheral neuropathy, response rate
1. INTRODUCTION
The proteasome inhibitor bortezomib (BTZ) is widely used in the treatment of multiple myeloma (MM). This agent strongly inhibits proteasome activity, which results in homeostasis disruption between protein synthesis and destruction. Bortezomib treatment often yields excellent responses, both partial response (PR) and complete response (CR), not only in newly diagnosed MM, but also in patients who have relapsed or are refractory to other treatment. However, not all patients treated with this agent experience such a favorable outcome. Suboptimal or no responses to BTZ are seen in a fraction of patients, and the efficacy of the agent is unpredictable. In addition, BTZ‐induced peripheral neuropathy (BiPN), an accompanying neuropathic pain and reflex suppression, is a frequent side effect of the therapy. Bortezomib‐induced peripheral neuropathy is thought to occur at a certain treatment threshold (within 5 cycles, but rarely beyond) in 40%‐60% of patients, of whom 15%‐40% will develop severe peripheral neuropathy (grade 2 or higher).1, 2, 3, 4, 5 As no effective prophylactic treatment is available, the onset of BiPN limits the use of BTZ in some patients. Therefore, identifying patients who have a potential drug resistance or high risk of developing BiPN is an important issue.
To date, a few potential biomarkers associated with the efficacy and toxicity of BTZ treatment have been proposed. A genome‐wide association study identified that genetic polymorphisms of PKNOX1 (transcription factor PBX/knotted 1 homeobox 1) and those in the immediate vicinity of CBS (cystathionine‐β‐synthetase) were associated with severe BTZ‐induced toxicity.6 Comparative proteomic profiling of sera from patients with refractory MM revealed 54 proteins that differentiated each analyzed experimental group, dividing them based on their response to therapy.7 Zaal et al8 aimed to identify the altered metabolic pathways that drove resistance in BTZ‐resistant MM cells. As a result, they indicated that interfering with the serine metabolism could be a novel strategy to improve BTZ therapy, and identified 3‐phosphoglycerate dehydrogenase, the rate‐limiting enzyme of serine synthesis, as a potential biomarker for BTZ resistance.
Metabolomics is a powerful approach for exploring diagnostic biomarkers and druggable targets, because endogenous metabolites are responsible for phenotypic manifestations as a final expression of genomic information. Lipidomics, a lipid‐targeting subarea of metabolomics, is an especially promising approach to biomarker discovery, because lipids are important players in a variety of complex physiological processes, executing important functions by acting as cellular membrane components, signaling mediators, energy reserve molecules, and endocrine regulators. We have already established global lipidomic analytical systems using liquid chromatography (LC)‐time‐of‐flight mass spectrometry (TOFMS), or triple quadrupole mass spectrometry (MS/MS) to measure lipid (eg, phospholipids) metabolites in biological samples.9 Applying this system to the study of disease metabolomics using clinical samples, the lipidomic signatures of clear cell renal cell carcinoma were obtained to reveal the metabolic changes in cancerous tissue and renal cell carcinoma pathophysiology.10 In this study, we undertook a lipid metabolomics analysis of pretreatment sera from patients with MM who received BTZ plus low‐dose dexamethasone (Bd) therapy, to identify predictive response markers for Bd therapy and BiPN severity.
2. MATERIALS AND METHODS
2.1. Clinical serum specimens
A total of 59 patients with MM were recruited between October 2007 and December 2014 at the Nagoya City University Hospital (Nagoya, Japan). Their venous blood was collected before Bd therapy in the morning after 14 hours fasting. Samples were centrifuged according to the manufacturer's instructions, and the serum was separated within 2 hours after blood sample collection. Patients were diagnosed according to the International Myeloma Working Group uniform response criteria11 and divided into 6 response subcategories: CR, very good partial response (VGPR), PR, minor response (MR), stable disease (SD), and progressive disease (PD). The BiPN grades (grades 0‐3) of the study subjects were diagnosed according to CTCAE version 4.0. The patient characteristics are summarized in Table 1, and their details are shown in Table S1. The Institutional Review Boards of the Nagoya City University and the National Institute of Health Sciences, Doshisha Women's College of Liberal Arts (Kyoto, Japan), approved this study. Informed consent was obtained from all patients.
Table 1.
Clinical characteristics of multiple myeloma patients treated with bortezomib and low‐dose dexamethasone
| Characteristic | No. of patients (%) |
|---|---|
| Total number of patients | 59 |
| Age (y) | |
| Median | 63 |
| Range | 36‐85 |
| Sex | |
| Male | 30 (51) |
| Female | 29 (49) |
| Bortezomib administration | |
| Intravenous injection | 26 (44) |
| Subcutaneous injection | 33 (56) |
| Best response to treatmenta | |
| CR | 1 (2) |
| VGPR | 14 (24) |
| PR | 21 (36) |
| MR | 5 (8) |
| SD | 7 12) |
| PD | 6 (10) |
| NEa | 5 (8) |
| Peripheral neuropathy | |
| Grade 0 | 34 (58) |
| Grade 1 | 11 (19) |
| Grade 2 | 9 (15) |
| Grade 3 | 5 (8) |
| Prior therapy | |
| Yes | 36 (61) |
| No | 23 (39) |
| M component | |
| IgG | 27 (46) |
| IgA | 14 (24) |
| IgD | 2 (3) |
| BJP | 16 (27) |
| ISS stage | |
| I | 11 (19) |
| II | 16 (27) |
| III | 28 (47) |
| NE | 4 (7) |
According to the International Uniform Response Criteria (Durie et al11).
Five patients not evaluable for response were excluded.
BJP, Bence Jones protein; CR, complete response; ISS, International Staging System; MR, minor response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
2.2. Metabolomic analysis of clinical serum samples
Detailed information regarding the extraction and measurement of lipid metabolites was described in our previous paper.12 Briefly, lipid metabolites were extracted from 100 μL serum using the method described by Bligh and Dyer (BD),13 with a few modifications in the presence of internal standards (IS). The lower organic layers were measured by ultraperformance liquid chromatography (UPLC)‐TOFMS (Micromass LCT Premier XE; Waters) to analyze for glycerophospholipids (GPLs), sphingolipids (SLs), and neutral lipids (NLs). To distinguish plasmalogen and alkylacyl PL species with the same exact mass, such as 34:1 ethanolamine plasmalogen and 34:2 alkylacyl phosphatidylethanolamine (PE), a small aliquot of each BD sample was hydrolyzed using 1 N HCl as previously described and analyzed by UPLC‐TOFMS. The upper aqueous layers were subjected to solid extraction to obtain polyunsaturated fatty acids (PUFAs) and their oxidative fatty acids (oxFAs), and then measured by UPLC‐MS/MS using a 5500QTRAP quadrupole‐linear ion trap hybrid mass spectrometer (AB Sciex) interfaced with an ACQUITY UPLC System (Waters). Structural analysis of PLs and SLs was carried out by LC‐Fourier Transform Mass Spectrometry (LTQ Orbitrap XL; Thermo Fisher Scientific) as previously described.12 The UPLC‐TOFMS data were processed using the 2DICAL software (Mitsui Knowledge Industry), and the extracted ion peak heights were normalized using IS. The data for PUFAs and oxFAs from the UPLC‐MS/MS were processed using MultiQuant software version 2.1 (AB Sciex), and the integrated peak area of each metabolite was normalized to IS.
2.3. Statistical and multiple classification analyses
The lipid metabolite levels were relatively quantified as the ion count ratio of each metabolite to the internal standard. Statistical analyses were carried out using the Jonckheere‐Terpstra trend test to examine ordered differences in metabolite levels for the 3 response subcategories (PD + SD, PR + MR, and CR + VGPR) or the 4 BiPN grades (G0, G1, G2, and G3). The R statistical environment (http://r-project.org/) software was used for the statistical analyses. Differences with P values <.005 were considered significant in accordance with the recent proposal on P value threshold for statistical significance by Benjamin et al.14 The multiple testing correction was not applied, as metabolite levels are not exclusive but rather related to each other, and we focused on revealing the contributions of overall metabolic changes (such as pathways or metabolite groups) to the responses to Bd therapy in the serum of MM patients. Differences in the distribution of patient background characteristics between the analytical groups were assessed by the χ2 test or Fisher's exact test and the Mann‐Whitney U test for categorical and continuous variables, respectively. Differences with P values <.005 were considered statistically significant.14
3. RESULTS
3.1. Distribution of patient background characteristics
We first evaluated whether the patients’ background characteristics were differently associated with the response to Bd therapy and BiPN severity. Concerning efficacy, there were no significant differences (P < .005) in background characteristics between responders (CR, VGPR, PR, and MR) and nonresponders (SD and PD), although both body mass index (BMI) (P = .0296) and serum albumin (P = .0095) tended to be higher in responders than in nonresponders (Table S2). For BiPN severity, the presence or absence of prior therapy showed a different distribution for patients with grade 2 or higher BiPN and patients with no or grade 1 BiPN (Table S3).
3.2. Profiling of lipid metabolites measured by UPLC‐TOFMS and UPLC‐MS/MS
In total, 385 detected lipids were classified into 4 classes (120 GPLs, 49 SLs, 177 NLs, and 39 oxFAs), which were further divided into 20 subclasses (Table S4). The relative quantification levels of the GPLs, SLs, and NLs for each patient are shown in Table S5, and those of the FAs in Table S6.
3.3. Lipid metabolites associated with response to Bd therapy
First, to identify the lipid metabolites associated with the response to Bd therapy, their serum levels were statistically compared among the 3 response groups (CR + VGPR, PR + MR, and SD + PD) (Table S7). Among the 346 lipid metabolites detected by LC‐TOFMS, levels of 2 phosphatidylcholines (PCs), 5 ether‐type PEs, 1 sphingomyelin (SM), and 2 cholesterolesters (ChEs) were increased progressively from the nonresponders (PD and SD), across the minor/partial responders (MR and PR), to the good responders (VGPR and CR) (Table 2 and Figure 1); their serum levels were 1.33 to 2.31‐fold higher in the good responders than in the nonresponders. When focusing on the fatty acid compositions of GPLs showing significantly different levels, 2 PCs and 4 ether PEs contained PUFAs such as FA(20:3), FA(20:4), and FA(20:5). Regarding the 39 oxFAs (Table S8), none of the metabolites showed significantly different levels between nonresponders, minor/partial responders, and good responders.
Table 2.
Lipid metabolites with significant P values in multiple myeloma patients classified as nonresponders, minor/partial responders, and good responders to treatment with bortezomib
| Class | Subclass | Metabolites | Fatty acid composition of GPL and SL | P value | Median fold change (MR + PR vs PD + SD) | Median fold change (VGPR + CR vs PD + SD) |
|---|---|---|---|---|---|---|
| GPL | PC | PC(38:3) + H | PC(18:0/20:3) | .00433 | 1.41 | 2.31 |
| GPL | PC | PC(38:5) + H | PC(16:0/22:5), PC(18:1/20:4) | .00482 | 1.05 | 1.33 |
| GPL | Ether PE | PE(36:4e) + H | PE(18:2e/18:2) | .00003 | 1.22 | 1.75 |
| GPL | Ether PE | PE(36:4p) + H | PE(16:0p/20:4) | .00457 | 1.68 | 2.06 |
| GPL | Ether PE | PE(38:4p) + H | PE(18:0p/20:4) | .00312 | 1.70 | 2.12 |
| GPL | Ether PE | PE(38:5p) + H | PE(18:1p/20:4) | .00176 | 1.22 | 1.79 |
| GPL | Ether PE | PE(38:7e) + H | PE(18:1p/20:5) or PE(18:2e/20:5) | .00368 | 1.19 | 2.30 |
| SL | SM | SM(39:1) + H | SM(d17:1/22:0), SM(d16:1/23:0) | .00457 | 1.21 | 1.77 |
| NL | ChE | ChE(18:3) + NH4 | — | .00037 | 1.31 | 2.06 |
| NL | ChE | ChE(20:3) + NH4 | — | .00166 | 1.40 | 1.71 |
Statistical analysis was carried out using the Jonckheere‐Terpstra trend test for the 3 response subcategories: progressive disease (PD) + stable disease (SD), minor response (MR) + partial response (PR), and very good partial response (VGPR) + complete response (CR). P values <.005 were considered statistically significant.
ChE, cholesterolester; GPL, glycerophospholipid; NL, neutral lipid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SL, sphingolipid; SM, sphingomyelin.
Figure 1.
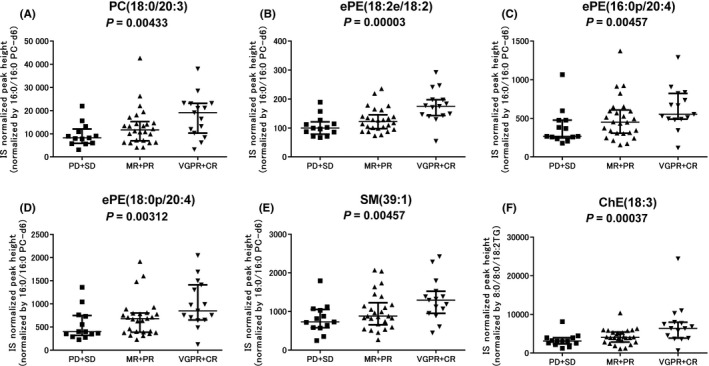
Comparison of lipid metabolite levels in multiple myeloma patients according to the response to bortezomib and low‐dose dexamethasone therapy. Six representative metabolites associated with the response to bortezomib and low‐dose dexamethasone therapy are shown. A, Phosphatidylcholine (PC)(18:0/20:3). B, Ether‐type phosphatidylethanolamine (ePE)(18:2e/18:2). C, ePE(16:0p/20:4). D, ePE(18:0p/20:4). E, Sphingomyelin (SM)(39:1). F, Cholesterol ester (ChE)(18:3). Graphs show the medians and interquartile ranges. Statistical significance was assessed using the Jonckheere‐Terpstra trend test. CR, complete response; IS, internal standard; MR, minor response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response
3.4. Lipid metabolites associated with severity of BiPN
Next, the association of lipid metabolite levels with the peripheral neuropathy grades was examined (Tables S7 and S8). When the levels of each metabolite were compared between the groups of patients with the 4 ordered BiPN grades (G0‐G3), 14 metabolites showed significantly different levels (Table 3). They comprised 1 ether‐type lysophosphatidylcholine (LPC), 1 PC, 1 ceramide, 1 diacylglycerol, 1 triacylglycerol, and 9 oxFAs. Their levels were increased progressively in patients with BiPN grades from G0 to G3, except for 8,9‐diHETrE levels, which were decreased over the BiPN grades. The median fold change for the G3 group in comparison with the G0 group ranged from 1.24 to 3.46 for the 13 significantly increased metabolites and was 0.17 for 8,9‐diHETrE. The representative metabolites with large fold changes in their levels are shown in Figure 2.
Table 3.
Lipid metabolites with significant P values between groups of multiple myeloma patients with 4 ordered grades (G0‐G3) of peripheral neuropathy due to treatment with bortezomib and low‐dose dexamethasone (BiPN)
| Class | Subclass | Metabolites | Fatty acid composition of GPL | P value | Median fold change (G1/G0) | Median fold change (G2/G0) | Median fold change (G3/G0) |
|---|---|---|---|---|---|---|---|
| GPL | Ether LPC | LPC(16:0e) + H | — | .000534 | 1.34 | 1.57 | 1.29 |
| GPL | PC | PC(36:6) + H | PC(14:0/22:6) | .003393 | 2.23 | 1.67 | 3.46 |
| SL | Cer | Cer(41:2) + H | N.D. | .003228 | 1.56 | 1.43 | 1.24 |
| NL | DG | DG(32:2) + NH4 | — | .003228 | 1.48 | 2.19 | 1.28 |
| NL | TG | TG(54:8) + NH4 | — | .002378 | 1.74 | 1.20 | 2.35 |
| oxFA | ALA | 9‐HOTrE | — | .000135 | 1.44 | 1.74 | 2.02 |
| oxFA | AA | 5‐HETE | — | .002378 | 4.45 | 3.95 | 1.41 |
| oxFA | AA | 14,15‐EpETrE | — | .001255 | 2.51 | 2.13 | 2.00 |
| oxFA | AA | 8,9‐diHETrE | — | .003069 | 0.24 | 0.75 | 0.17 |
| oxFA | AA | 5,15‐diHETE | — | .001188 | 2.21 | 2.00 | 1.44 |
| oxFA | EPA | Eicosapentaenoic acid | — | .001646 | 1.65 | 1.66 | 1.77 |
| oxFA | EPA | 5‐HEPE | — | .000045 | 2.50 | 1.92 | 2.89 |
| oxFA | EPA | 14,15‐diHETE | — | .001063 | 1.33 | 1.35 | 1.65 |
| oxFA | DHA | 4‐HDoHE | — | .001560 | 1.48 | 1.34 | 2.32 |
Statistical analysis was carried out using the Jonckheere‐Terpstra trend test for 4 grades of BiPN (G0‐G3). P values <.005 were considered statistically significant.
14,15‐diHETE, 14,15‐dihydroxyeicosatetraenoic acid; 14,15‐EpETrE, 14,15‐epoxyeicosatrienoic acid; 4‐HDoHE, 4‐hydroxyldocosahexaenoic acid; 5,15‐diHETE, 5,15‐dihydroxyeicosatetraenoic acid; 5‐HEPE, 5‐hydroxyeicosapentaenoic acid; 5‐HETE, 5‐hydroxyeicosatetraenoic acid; 8,9‐diHETrE, 8,9‐dihydroxyeicosatrienoic acid; 9‐HOTrE, 9‐hydroxyoctadecatrienoic acid; AA, arachidonic acid; ALA, alpha‐linolenic acid; Cer, ceramide; DG, diacylglycerol; DHA, docosahexsaenoic acid; EPA, eicosapentaenoic acid; GPL, glycerophospholipid; LPC, lysophosphatidylcholine; N.D., MS2 or MS3 fragment ion was not detected in the structural analysis using LTQ Orbitrap; NL, neutral lipid; oxFA, oxidative fatty acid; PC, phosphatidylcholine; SL, sphingolipid; TG, triacylglycerol.
Figure 2.
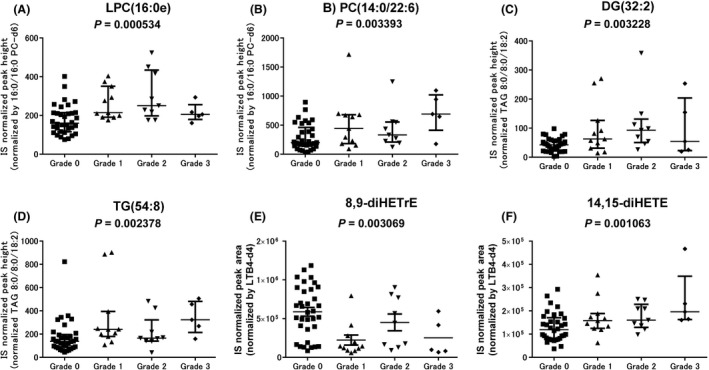
Comparison of lipid metabolite levels in multiple myeloma patients according to grades of bortezomib‐induced peripheral neuropathy BiPN during treatment with bortezomib and low‐dose dexamethasone. Six representative metabolites associated with BiPN severity are shown. A, Lysophosphatidylcholine (LPC)(16:0e). B, Phosphatidylcholine (PC)(14:0/22:6). C, Diacylglycerol (DG)(32:2). D, Triacylglycerol (TG)(54:8); E, Dihydroxyeicosatrienoic acid (8,9‐diHETrE). F, Dihydroxyeicosatetraenoic acid (14,15‐diHETE). Graphs show the medians and interquartile ranges. Statistical significance was assessed using the Jonckheere‐Terpstra trend test. IS, internal standard
4. DISCUSSION
Our study is the first comprehensive lipidomics analysis of pretreatment sera in order to explore which biomarkers could predict an optimal response to BTZ‐based chemotherapy and BiPN. The levels of 10 lipid metabolites, 2 PCs, 5 ether PEs, 1 SM, and 2 ChEs in the pretreatment sera were increased progressively from the nonresponders, across the minor/partial responders, to the good responders (P < .005). To identify potential confounding factors influencing lipid metabolite levels in the blood for our biomarker exploration study, we examined the association between patients’ backgrounds and their response to Bd therapy. There were no differences in the total cholesterol and triglyceride levels and hemoglobin concentrations between responders and nonresponders, whereas serum albumin levels showed a trend towards decrease (P = .0095, not significant) in nonresponders. Serum albumin is the carrier of fatty acids in the blood and reflects the patients’ nutritional status, suggesting that in nonresponders both the nutritional status was inferior, and fatty acids might not be supplied in sufficient amount for the biosynthesis of GPLs and NLs. Then, to assess the influence of the different nutritional status between responders and nonresponders on the lipid biomarker exploration for Bd response, we examined the association between serum albumin and the levels of 10 lipid biomarker candidates. There was no significant correlation (P > .005, Spearman rank correlation test) between the specific lipid levels and the serum albumin, with the exception of PE(38:7e) (P = .0045) and SM(39:1) (P = .0014), indicating that at least 8 biomarker candidates are associated with the Bd response and not with the patients’ nutritional status. In addition to serum albumin levels, the BMI tended to be lower in nonresponders than in responders (P = .0296). As the BMI has been extensively correlated with metabolite levels such as lipoprotein cholesterols and triacylglycerols,15 we cannot rule out the possibility that our findings were confounded by the BMI. However, the levels of these 10 lipids and BMI were not significantly correlated (P > .005, Spearman rank correlation test). Therefore, the lower levels of both albumin and BMI in nonresponders are unlikely to be a confounding factor for the decreased levels of these lipid species, although a careful validation taking into account patients’ backgrounds is needed for a few metabolites.
Several reports have indicated that MM pathophysiology is supported by a strong interaction between clonal plasma cells and the surrounding bone marrow microenvironment. Marrow fat might participate in the lipid metabolism by clearing and storing circulating triglycerides, thereby providing a localized energy reservoir for osteogenesis.16 Ether PE species appear to be potential biomarker candidates for predicting BD responsiveness, whereas ether PC species and lyso platelet‐activating factors (PAFs), including LPC(16:1e) and LPC(18:1e), are not, because they were present at similar levels between nonresponders and responders. The synthesis of ether PEs and PCs from 1‐alkyl‐2‐acylglycerol shares a common enzyme, choline/ethanolamine phosphotransferase. Ether PCs are converted into lyso PAF by phospholipase A2 cleavage of the acetyl group at their sn‐2 position, and its subsequent acetylation generates PAF. Platelet‐activating factor is an inflammatory phospholipid present in human bone marrow and reported to stimulate the Ig secretion of plasma cell lines initially derived from myeloma patients.17 Serum ether PE levels seem to be an indicator of PAF production in the clonal plasma cells and stromal cells, which might affect Bd responsiveness.
Of the 14 ChE species detected in the patient sera, low levels of ChE(18:3) and ChE(20:3), which esterifies PUFA, might be possible biomarker candidates for predicting Bd nonresponders. Hypocholesterolemia has previously been described in MM patients, likely representing an increase in low‐density lipoprotein clearance and lipid and cholesterol utilization by myeloma cells.18 Łuczak et al7 reported by comparative proteomic analysis that a higher abundance of apolipoprotein C1 in the pretreatment sera of patients with refractory/relapsed MM positively correlated with a better response to BTZ‐containing therapy. Their results are in agreement with ours, because both apolipoprotein C1 and ChEs are major constituents of the lipoprotein particles carried in the blood, including high‐density lipoprotein, low‐density lipoprotein, and very low‐density lipoprotein.
Higher levels of an ether‐type LPC (lyso PAF), LPC(16:0e), were strongly associated with a more severe BiPN grade (P = .0005). In addition, other LPCs, such as LPC(17:1) (P = .0058), LPC(18:1e) (P = .0055), and LPC(20:5) (P = .0087), showed borderline significant association. These results implicate phospholipase A2, which hydrolyzes phospholipids to produce lysophospholipids and a free fatty acid, in BiPN.19 Phospholipase A2 provides precursors for eicosanoid generation, when the cleaved fatty acid is arachidonic acid, or for PAF formation from ether‐type PC, playing key roles in the inflammatory responses. Furthermore, LPC as a bioactive lipid, has been shown to enhance intracellular Ca2+ levels in many cell types, resulting in LPC‐induced demyelination and axonal degeneration.20, 21 In addition, LPCs are known to cause neuropathic pain through the increase of neuronal nitric oxide synthase in the dorsal root ganglion.22
It was notable that differences in the levels of cytochrome P450 (P450) and/or the soluble epoxide hydrolase‐mediated metabolites (14,15‐epoxyeicosatrienoic acid [14,15‐EpETrE], 8,9‐dihydroxyeicosatrienoic acid [8,9‐diHETrE], and 14,15‐dihydroxyeicosatetraenoic acid [14,15‐diHETE]) were associated with the BiPN grade. Interestingly, levels of 14,15‐EpETrE and 14,15‐diHETE were higher, whereas 8,9‐diHETrE levels were lower in patients with severe BiPN. As endogenous signaling molecules, EpETrEs produce diverse effects in multiple biological systems including vasodilation, ion channel modulation, anti‐inflammatory effects, and angiogenesis, which are attenuated following conversion to diHETrEs by soluble epoxide hydrolase.23 8,9‐EpETrE, derived from arachidonic acid, is a natural agonist of the calcium channel transient receptor potential vanilloid‐4 (TRPV4), and 8,9‐diHETrE is a degradation product of 8,9‐EpETrE.23 Transient receptor potential vanilloid‐4 has an important role through its expression in sensory neurons for the mechanotransduction of peripheral neuropathy‐associated pain.24 Furthermore, TRPV4 inhibition prevents paclitaxel‐induced neurotoxicity in preclinical models.25 In contrast, epoxides from arachidonic acid and eicosapentaenoic acid, such as 14,15‐EpETrE and 14,15‐epoxy‐eicosatetraenoic acid (14,15‐EpETE), respectively, mediate cell signaling with anti‐inflammatory, antihypertensive, and analgesic properties.26 Although we could not detect 14,15‐EpETE and 8,9‐EpETrE in the patients’ sera, because of their instability, high and low levels of their degradation products, 14,15‐diHETE and 8,9‐diHETrE, respectively, together with 14,15‐EpETrE, could be plausible biomarkers of BiPN.
In conclusion, a comprehensive lipidomic study using pretreatment sera from MM patients undergoing Bd therapy revealed several biomarker candidates for predicting responses to BTZ and BiPN severity. The lipid metabolites that differentiated nonresponders from responders to Bd therapy were mainly involved in PAF biosynthesis and cholesterol metabolism. On the other hand, lysophosphatidylcholines and several oxFAs were examined as lipids associated with BiPN severity. Further studies are needed to validate these biomarker candidates together with their roles in MM pathophysiology and as Bd targets of action.
DISCLOSURE
MR received research funding from Celgene and declares honoraria from Janssen Pharmaceutical. SI received research funding and declares honoraria from Ono Pharmaceutical, Janssen Pharmaceutical, Celgene, Bristol‐Myers Squibb, Takeda Yakuhin, Novartis Pharma, and Daiichi Sankyo. SI also received research funding from Kyowa Hakko Kirin, Chugai Pharmaceutical, Sanofi, MSD, Gilead Sciences, and AbbVie. RU received remuneration from Terumo and research funding from Kyowa Hakko Kirin. RU is an endowed chair funded by Kyowa Hakko Kirin, Chugai Pharmaceutical, Ono Pharmaceutical, Rikaken, and Medical & Biological Laboratories. MT has accepted researchers from Daiichi Sankyo, MSD, and Ono Pharmaceutical.
Supporting information
ACKNOWLEDGMENTS
This work was partly supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (16K07179 and 16K09855), the National Cancer Center Research and Development Fund (26‐A‐4), the Accelerating Regulatory Science Initiative from the Ministry of Health, Labour and Welfare of Japan, the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (15ck0106077h0002), and the research fund from the Hoansha Foundation in Japan.
Maekawa K, Ri M, Nakajima M, et al. Serum lipidomics for exploring biomarkers of bortezomib therapy in patients with multiple myeloma. Cancer Sci. 2019;110:3267‐3274. 10.1111/cas.14178
Keiko Maekawa and Masaki Ri contributed equally to this work.
Contributor Information
Yoshiro Saito, Email: yoshiro@nihs.go.jp.
Shinsuke Iida, Email: iida@med.nagoya-cu.ac.jp.
REFERENCES
- 1. Mateos M‐V, Oriol A, Martínez‐López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934‐941. [DOI] [PubMed] [Google Scholar]
- 2. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non‐inferiority study. Lancet Oncol. 2011;12:431‐440. [DOI] [PubMed] [Google Scholar]
- 3. Ogawa Y, Suzuki K, Sakai A, et al. Phase I/II study of bortezomib‐melphalan‐prednisolone for previously untreated Japanese patients with multiple myeloma. Cancer Sci. 2013;104:912‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogawa Y, Tobinai K, Ogura M, et al. Phase I and II pharmacokinetic and pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci. 2008;99:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906‐917. [DOI] [PubMed] [Google Scholar]
- 6. Magrangeas F, Kuiper R, Avet‐Loiseau H, et al. A genome‐wide association study identifies a novel locus for bortezomib‐induced peripheral neuropathy in European patients with multiple myeloma. Clin Cancer Res. 2016;22:4350‐4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Łuczak M, Kubicki T, Rzetelska Z, et al. Comparative proteomic profiling of sera from patients with refractory multiple myeloma reveals potential biomarkers predicting response to bortezomib‐based therapy. Pol Arch Intern Med. 2017;127:392‐400. [DOI] [PubMed] [Google Scholar]
- 8. Zaal EA, Wu W, Jansen G, Zweegman S, Cloos J, Berkers CR. Bortezomib resistance in multiple myeloma is associated with increased serine synthesis. Cancer Metab. 2017;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maekawa K, Hirayama A, Iwata Y, et al. Global metabolomic analysis of heart tissue in a hamster model for dilated cardiomyopathy. J Mol Cell Cardiol. 2013;59:76‐85. [DOI] [PubMed] [Google Scholar]
- 10. Saito K, Arai E, Maekawa K, et al. Lipidomic signatures and associated transcriptomic profiles of clear cell renal cell carcinoma. Sci Rep. 2016;6:28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467‐1473. [DOI] [PubMed] [Google Scholar]
- 12. Ishikawa M, Maekawa K, Saito K, et al. Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects’ gender and age. PLoS ONE. 2014;9:e91806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911‐917. [DOI] [PubMed] [Google Scholar]
- 14. Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2:6‐10. [DOI] [PubMed] [Google Scholar]
- 15. Schutte BA, van den Akker EB, Deelen J, et al. The effect of standardized food intake on the association between BMI and 1H‐NMR metabolites. Sci Rep. 2016;6:38980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lecka‐Czernik B, Stechschulte LA. Bone and fat: a relationship of different shades. Arch Biochem Biophys. 2014;561:124‐129. [DOI] [PubMed] [Google Scholar]
- 17. Desplat V, Dulery C, Faucher JL, Praloran V, Denizot Y. Metabolism and effect of platelet‐activating factor on the growth of human myeloma cell lines. Cancer Lett. 2000;149:7‐13. [DOI] [PubMed] [Google Scholar]
- 18. Yavasoglu I, Tombuloglu M, Kadikoylu G, Donmez A, Cagirgan S, Bolaman Z. Cholesterol levels in patients with multiple myeloma. Ann Hematol. 2008;87:223‐228. [DOI] [PubMed] [Google Scholar]
- 19. Glaser KB. Regulation of phospholipase A2 enzymes: selective inhibitors and their pharmacological potential. Adv Pharmacol. 1995;12:31‐66. [DOI] [PubMed] [Google Scholar]
- 20. Li XH, Long DX, Li W, Wu YJ. Different mechanisms of lysophosphatidylcholine‐induced Ca(2+) mobilization in N2a mouse and SH‐SY5Y human neuroblastoma cells. Neurosci Lett. 2007;424:22‐26. [DOI] [PubMed] [Google Scholar]
- 21. Okajima F, Sato K, Tomura H, et al. Stimulatory and inhibitory actions of lysophosphatidylcholine, depending on its fatty acid residue, on the phospholipase C/Ca2+ system in HL‐60 leukaemia cells. Biochem J. 1998;336(Pt 2):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang HY, Tsai YJ, Chen SH, Lin CT, Lue JH. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol Biochem Behav. 2013;106:47‐56. [DOI] [PubMed] [Google Scholar]
- 23. Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996‐C1012. [DOI] [PubMed] [Google Scholar]
- 24. Nazıroğlu M, Braidy N. Thermo‐sensitive TRP channels: novel targets for treating chemotherapy‐induced peripheral pain. Front Physiol. 2017;8:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boehmerle W, Huehnchen P, Lee SLL, et al. TRPV4 inhibition prevents paclitaxel‐induced neurotoxicity in preclinical models. Exp Neurol. 2018;306:64‐75. [DOI] [PubMed] [Google Scholar]
- 26. Morisseau C, Inceoglu B, Schmelzer K, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481‐3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


