Abstract
Delphinidin, one of the main anthocyanidins, has potent anti‐cancer properties. In this study, we investigated the effect of delphinidin on 1‐methyl‐1‐nitrosourea (MNU)‐induced breast carcinogenesis on rats and the mechanism of delphinidin via negative regulation of the HOTAIR/microRNA‐34a axis. We found administration of delphinidin could effectively suppress MNU‐induced mammal breast carcinogenesis. Delphinidin downregulated the level of HOTAIR and upregulated miR‐34a in breast carcinogenesis. Western blot analysis confirmed that delphinidin treatment can significantly decrease the expression of β‐catenin, glycogen synthase kinase‐3β (Gsk3β), c‐Myc, cyclin‐D1, and matrix metalloproteinase‐7(MMP‐7) expression in breast cancer cells, and inhibition of miR‐34a significantly reduced the effect of delphinidin on c‐Myc, cyclin‐D1, and MMP‐7. HOTAIR overexpression also blocked the effect of delphinidin on miR‐34a and the Wnt/β‐catenin signaling pathway in MDA‐MB‐231 cells. RNA immunoprecipitation (RIP) assay and chromatin immunoprecipitation (ChIP) assay results showed that delphinidin upregulated miR‐34a by inhibiting HOTAIR, coupled with enhancement of the zeste homolog 2 (EZH2) and histone H3 Lys27 trimethylation (H3K27me3). This study indicated that delphinidin may potentially suppress breast carcinogenesis and exert its anti‐cancer effect through the HOTAIR/miR‐34a axis. These findings provided new evidence for the use of delphinidin in preventing breast carcinogenesis.
Keywords: breast carcinogenesis, delphinidin, HOTAIR, microRNA‐34a, Wnt/β‐catenin signaling pathway
Abbreviations
- Gsk‐3β
glycogen synthase kinase‐3β
- HOTAIR
HOX transcript antisense RNA
- MMP‐7
matrix metalloproteinase‐7
- MNU
1‐methyl‐1‐nitrosourea
1. INTRODUCTION
Breast cancer is the most common cancer and the leading cause of cancer deaths in women globally.1 Surgery, hormone therapy, radiation therapy, and chemotherapy are conventional treatments used in breast cancer treatment.2 However, side effects, such as multidrug resistance, increase the challenge of breast cancer treatment. Many researchers are attempting to find alternative treatments for breast cancer. Clinical research concluded that the co‐administration of phytochemicals could be a promising approach to reduce the side effects and complications of chemotherapy and radio therapy and improve immunologic function in patients with breast cancer.3 Considerable attention has been focused on dietary approaches that may be beneficial for the prevention and treatment of breast cancer.
Many natural products derived from dietary sources benefit breast cancer prevention and treatment.4 Phytotherapeutic products, such as Echinacea, Salvia, Allium sativum, and green tea, have been used as anti‐cancer agents, based on their rich content of phytochemicals.5 Anthocyanin, a kind of natural polyphenolic, is abundant in many fruits and vegetables including berries, red grapes, and red cabbages.6 Anthocyanins have antioxidant, anti‐aging, anti‐inflammatory, and anti‐cancer functions.7 Researchers have determined that anthocyanins can induce tumor cell differentiation, decrease tumor malignancy, and block carcinogenesis.8, 9 Delphinidin is one of the main anthocyanidins and has potent anti‐cancer properties.10, 11, 12 In our previous research, we found that delphinidin suppressed carcinogenic transformation in breast MCF10A cells induced by carcinogens. We also found that delphinidin downregulated HOX transcript antisense RNA (HOTAIR) expression in xenografted tumors of athymic mice and breast cancer cells.13
HOTAIR is a 2.2‐kb long noncoding RNA transcribed from the HOXC gene cluster on chromosome 12q13.13. High expression of HOTAIR has been implicated in various types of malignancies, and it plays a critical role in most biologic processes of cancer.14 Patients who have breast cancer and high HOTAIR expression have poor prognosis and survival, and HOTAIR has been regarded as an oncogene and a potential new target in cancer therapy.15 miR‐34a, as a tumor suppressor, has been shown to play an important role in carcinogenesis and has low expression in breast cancer tissues and cell lines.16 Researchers have found that HOTAIR knockdown in gastric cancer cells could increase miR‐34a levels and inhibit drug resistance.17 Studies have shown that HOTAIR physically interacts with the miR‐34a promoter to silence miR‐34a, indicating that HOTAIR can function as competing endogenous RNA for miR‐34a.18, 19
Our previous study showed that HOTAIR causes significant upregulation during cellular breast carcinogenesis and was significantly decreased by delphinidin treatment. Only a few studies have investigated the mechanism by which delphinidin inhibits breast carcinogenesis through HOTAIR. In the current study, we evaluated the effects of delphinidin on breast carcinogenesis in rats and further investigated the anti‐cancer mechanism of delphinidin through regulating HOTAIR/miR‐34a axis.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Delphinidin (98%) was purchased from Sigma‐Aldrich Co., LLC, and delphinidin (25%) was purchased from DaXingAnLing Lingonberry Boreal Biotech Co., Ltd. 1‐methyl‐1‐nitrosourea (MNU) was obtained from Sigma‐Aldrich Co., LLC. DMEM/F12 medium and fetal bovine serum (FBS) were purchased from HyClone; a cell counting kit‐8 (CCK‐8), dimethyl sulfoxide (DMSO), phosphate‐buffered saline (PBS), and other chemicals were purchased from Sigma‐Aldrich. Sprague‐Dawley rats were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. The EZ‐ChIP Chromatin Immunoprecipitation Kit, the Magna RIP™ RNA‐Binding Protein Immunoprecipitation Kit, anti‐human argonaute 2 (Ago2) antibodies, IgG, antibody EZH2, and trimethylating histone H3 lysine 27 (H3K27me3) were purchased from Millipore. Other antibodies were obtained from Cell Signaling Technology Inc. and Sigma‐Aldrich.
2.2. Experimental model of carcinogenesis in rats
Female Sprague‐Dawley rats (age, 42‐48 d; weight, 145‐165 g) were bred to an AIN‐93G diet and maintained in accordance with our institutional guidelines for the use of laboratory animals (SYXK [chuan] 2015‐196). After 3 d, the rats were divided into three groups: normal, control, and delphinidin administration groups. At 1 wk later, the rats in the control and delphinidin administration groups were given a single intraperitoneal injection of MNU (50 mg/kg) dissolved in physiologic saline containing 0.05% acetic acid prepared within 30 min before use. The rats in the normal group were injected with an equal volume of physiologic saline. In our pretest, we found 100 mg/kg/d delphinidin had no side effects on rats. During the experiment, the rats in the delphinidin administration group were fed 100 mg/kg/d delphinidin. The rats in the normal and control groups were orally fed normal saline alone. The rats were weighed twice per week and monitored once per day. The following parameters were calculated for each group: tumor incidence, the percentage of rats bearing at least one palpable malignant mammary tumor; tumor latency, the average time of tumor appearance; and tumor multiplicity, the total number of malignant mammary tumors.
2.3. Hematoxylin–eosin staining and immunohistochemistry
Samples were embedded in paraffin and cut into 4‐mm‐thick sections. After they were dehydrated with gradient ethanol, the sections were stained with hematoxylin‐eosin and assessed using an optical microscope. For immunohistochemistry experiments, tissue sections were treated with 3% hydrogen peroxide to block endogenous peroxidase activity, blocked with 20% goat serum, and incubated with antibody at 4°C overnight. Negative controls were performed by omitting the primary antibody. After application of a biotinylated secondary antibody, the signal was developed with a modified avidin‐biotin complex immunoperoxidase staining procedure. Positive cells were quantified per high‐power field, and 10 fields were averaged for each case. Three sections were analyzed for each sample.
2.4. Cells and culture
Breast cancer cells MDA‐MB‐231, MCF‐7, and MDA‐MB‐453 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. MDA‐MB‐231 and MCF‐7 cells were grown in DMEM/F12 medium. MDA‐MB‐453 cells were grown in RPMI 1640 medium, supplemented with 10% FBS. All the cultures were maintained in a humidified atmosphere of 5% CO2/95% air at 37°C. In our previous research, we found that 40 μmol/L delphinidin significantly reduced cells viability in breast cancer cells.13 So we used 40 μmol/L delphinidin for the breast cancer cell lines in this study.
2.5. Cell viability assay
Cell viability was assessed with a cell counting kit‐8 (CCK‐8) assay according to the protocol. Cells were planted in 96‐well plates at a density of 1 × 105/mL and then treated with delphinidin for 48 h. Then 10% CCK‐8 solution was added to the wells and incubated for 2 h at 37°C. Optical density (OD) was measured at 450 nm. The assay was repeated three times.
2.6. Plasmids, oligonucleotides, and transfections
To express HOTAIR ectopically, PCR fragments of HOTAIR were cloned into pcDNA3.1(+) (Invitrogen, Beijing, China) using the following primers: HOTAIR, sense, 5′‐CATGGATCCACATTCTGCCCTGATTTCCGGAACC‐3′; reverse, 5′‐ACTCTCGAGCCACCAC‐ACACACACAACCTACAC‐3′. To inhibit miR‐34a expression, anti‐miR‐34a oligonucleotides (5′‐ACAACCAGCTAAGACACTGCC‐3′) (Exiqon) were used. The cells were transfected with Lipofectamine 2000 in Opti‐Mem according to the manufacturer's protocol. The medium was replaced 8 h later, and cells were collected for subsequent experiments 48 h after transfection.
2.7. Cell migration and invasion assay
The migration and invasion abilities of breast cancer cells were observed with wound healing and transwell assays. Scratch/wound healing assay was used to determine cell migration. Cells were seeded onto 6‐cm dishes and grown to form a monolayer. After washing with PBS, the cell monolayer was scraped with a sterile cell scraper to create a cell‐free zone to produce wounded cultures. The distance traveled by the cells was measured between the two boundaries of the cellular area at 0 and 48 h. All experiments were performed in triplicate.
A cell invasion assay kit (Chemicon) was used to examine the capability for cell invasion: the extracellular matrix layer was rehydrated for 1 to 2 h at room temperature, and 300 μL cell suspension (1.0 × 106 cells/mL) was added to each insert. After incubation for 24 h, non‐invading cells were removed with a cotton‐tipped swab. The cells were stained and counted by photographing the membrane through the microscope.
2.8. Western blot analysis
Cells were lysed with a buffer containing 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate supplemented with protease and phosphatase inhibitors. Equal amounts of cellular proteins were resolved by electrophoresis in 10% or 12% sodium dodecyl sulfate‐polyacrylamide gels and transferred to a nylon filter for western immunoblotting with specific antibodies. The antigen‐antibody complexes on the filters were detected by chemiluminescence.
2.9. qRT‐PCR analysis of HOTAIR
Total RNA was extracted with the TRIzol reagent, and reverse transcription was performed with oligo(dT) 20 as the primer and M‐MLV reverse transcriptase (Promega) at 42°C for 30 min. HOTAIR levels were quantified using a Light Cycler 480 Probes Master kit (Roche) according to the manufacturer's protocol, with the following specific HOTAIR primers (forward, 5′‐ACGGAACCCATGGACTCATA‐3′, reverse, 5′‐TTGGGGAAGCATTTTCTGAC‐3′). All samples were read in triplicate, and values were normalized to β‐actin (forward, 5′‐TGACAGGATGCAGAAGGAGA‐3′; reverse, 5′‐TAGAGCCACCAATCCACACA‐3′). The relative expression level of each miRNA was calculated with the comparative CT method.
2.10. qRT‐PCR analysis for miR‐34a
Total RNA was extracted. The miRNA First‐Strand cDNA Synthesis Kit and the miRNA Real‐Time PCR Assay Kit (Aidlab) were used to quantify the miRNA transcripts. U6 small nucleolar RNA was used as the reference. The primers for miR‐34a were: stem‐loop RT primer, 5′‐GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACAAC‐3′; forward, 5′‐CGGTATCATTTGGCAGTGTCT‐3′; and reverse, 5′‐GTGCAGGGTCCGAGGT‐3′. Each reaction sample had been run in triplicate. The relative expression level of miRNA was calculated with the comparative CT method.
2.11. ChIP‐qPCR analysis
Chromatin immunoprecipitation (ChIP) was performed according to the instructions given in the EZ‐ChIP Chromatin Immunoprecipitation Kit (Millipore). Briefly, after it was sonicated into 200‐ to 500‐bp fragments, cross‐linked chromatin DNA was captured with primary antibody EZH2 or H3K27me3. IgG was used as the negative control. Precipitated DNA fragments were quantified by qPCR. The result was calculated in the form of percentage input. miR‐34a primers20 were used in the PCR assay: forward, 5′‐GGGCTACGAGGGACACCCGA‐3′; reverse, 5′‐CTCAGCCCGCAGGATAGC G‐3′.
2.12. RNA immunoprecipitation assay
An RNA immunoprecipitation (RIP) assay was performed according to the manufacturer's protocol. Cells were collected and lysed with RIP lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). MDA‐MB‐231 cells and MDA‐MB‐231 cells transfected with pcDNA‐HOTAIR (TCHOTAIR) were lysed with RNA lysis buffer containing protease inhibitor and RNase inhibitor. Lysis solutions were incubated with the RIP buffer containing magnetic beads coated with anti‐human argonaute 2 (Ago2) antibodies. IgG was used as a negative control (input group). After incubation with gentle rotation for 2 h at 4°C and removal of the unbound material by washing, co‐precipitated RNA was isolated. Levels of HOTAIR and miR‐34a were presented as fold enrichment in Ago2 relative to IgG immunoprecipitates.
2.13. Statistical analysis
Results are presented as mean ± SD from at least three independent experiments. The tumor incidence of rats in different groups was compared using the χ2 test. Other data were analyzed by one‐way analysis of variance followed by Tukey's test for multiple comparisons. Significance was set at P < .05. All statistical analysis was conducted using IBM SPSS Statistics 21.0. GraphPad Prism software was used for data presentation.
3. RESULTS
3.1. Chemopreventive effects of delphinidin against breast carcinogenesis in rats
We used the model of MNU‐induced breast carcinogenesis in female Sprague‐Dawley rats to evaluate the chemopreventive effect of delphinidin in vivo. As shown in Table 1, at the end of the study, the cancer incidence in the delphinidin administration group was 43.7% lower than that in the control group (P < .05), indicating that oral administration of delphinidin can effectively suppress MNU‐induced mammal breast carcinogenesis. The rats in the delphinidin administration group grew at the same rate as the control rats. No evidence of adverse effects was observed with delphinidin administration.
Table 1.
Effect of delphinidin on tumorigenesis endpoints and body weight
| Group | Numbers of rats | Numbers of rats with tumor | Incidence (%) | Multiplicity (average number/rat) | Latency (d) | Final body weight (g) |
|---|---|---|---|---|---|---|
| Normal | 9 | 0 | 0 | ‐ | ‐ | 301.66 ± 15.97 |
| Control | 16 | 12 | 75 | 2.42 ± 1.61 | 84.66 ± 22.47 | 284.33 ± 21.14 |
| Dp | 16 | 5 | 31.3* | 1.66 ± 0.57 | 90.85 ± 7.15 | 280.75 ± 11.47 |
Values are means ± SD. The tumor incidence in delphinidin (100 mg/kg/d) group was significantly lower than that in control group.
*P < .05 compared with control group.
The histopathologic sections of breast tissue and tumors showed evidence of carcinogenesis (Figure 1A). All mammary tumors were histologically confirmed and classified as mammary cancers.
Figure 1.
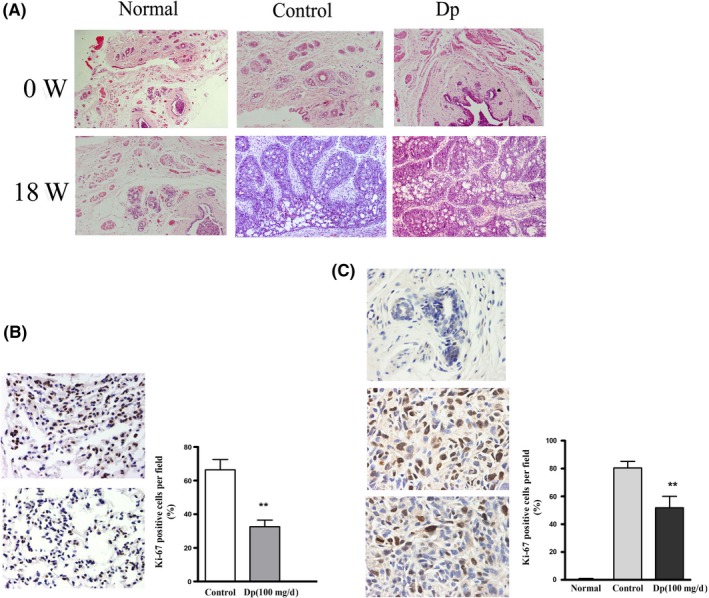
Delphinidin downregulates 1‐methyl‐1‐nitrosourea (MNU)‐induced mammary cancers proliferation in breast carcinogenesis. A, Histopathological sections of breast tissue (0 wk) and tumors (18 wk) in MNU‐treated rats stained with hematoxylin and eosin. Magnification, ×100. B, Immunohistochemical analysis of Ki‐67 expression in pulmonary metastatic tumor tissues of different groups. C, Immunohistochemical analysis of Ki‐67 expression in breast cancer tissues or normal mammary gland tissues of different groups. Positive cells were counted in three high‐powered fields per tumor and represent three tumors per experimental group. Magnification, ×400. The data are presented as means ± SD (n = 9). **P < .01 compared with control group
3.2. Delphinidin downregulates the proliferation of MNU‐induced mammary cancer in breast carcinogenesis
To study the effect of delphinidin on proliferation of MNU‐induced mammary cancer and pulmonary metastatic tumor in rats, positive expression rates of Ki‐67 in breast cancer tissue and pulmonary metastatic tumor are shown in Figure 1. As shown in Figure 1B, the positive expression rates of Ki‐67 in pulmonary metastatic tumor tissue in the delphinidin administration group were significantly decreased compared with that of the control group. In MNU‐induced mammary cancer tissue, the positive expression rates of Ki‐67 in the control group were significantly greater than in the delphinidin administration group. Ki‐67 expression in mammary gland tissues was not detected in normal group (Figure 1C). The results indicated that delphinidin administration significantly suppressed breast tumor cell and pulmonary metastatic tumor cell proliferation in MNU‐treated rats.
3.3. Delphinidin regulates the level of HOTAIR in breast carcinogenesis and modulates breast cancer cell proliferation and migration via negative regulation of HOTAIR
The mRNA level of HOTAIR was examined in breast and cancer tissues in the normal, control, and delphinidin administration groups. As shown in Figure 2A, cancer tissues in the delphinidin administration group exhibited lower HOTAIR expression compared with the control group. The results showed that oral administration of delphinidin can decreased the level of HOTAIR in cancer tissue.
Figure 2.
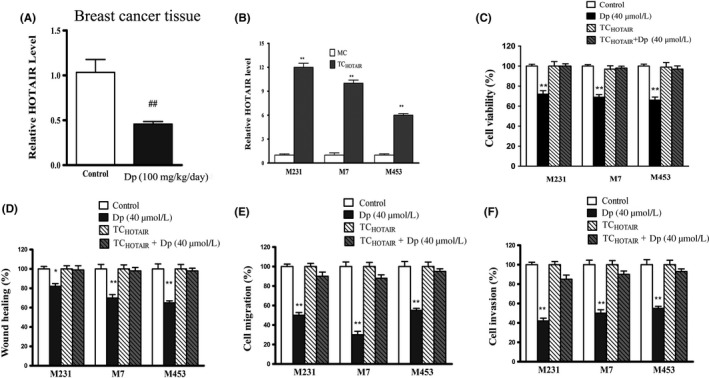
Delphinidin regulates the level of HOX transcript antisense RNA (HOTAIR) in breast tissues of different groups. A, The levels of HOTAIR in breast cancer tissues were detected by qRT‐PCR; ## P < .01 compared with the control group. B, The levels of HOTAIR in breast cancer cells MDA‐MB‐231(M231), MCF‐7 (M7), MDA‐MB‐453(M453) transfected with blank plasmids (mock control, MC) or transfected with pcDNA‐HOTAIR(TCHOTAIR) were detected by qRT‐PCR. **P < .01 compared with MC. C, Cell viability was determined by CCK‐8 assay in breast cancer cells and TCHOTAIR with or without delphinidin (40 μmol/L) treatment. D, Wound healing assay in breast cancer cells and TCHOTAIR with or without delphinidin treatment. E, Transwell invasion assay in breast cancer cells and TCHOTAIR with or without delphinidin treatment. F, Transwell migration assay in breast cancer cells and TCHOTAIR with or without delphinidin treatment. The data are presented as the means ± SD (n = 3). *P < .05 and **P < .01 compared with Control
In previous research, we found that delphinidin treatment effectively decreased the expression of HOTAIR in breast cancer cells MDA‐MB‐231, MCF‐7, and MDA‐MB‐453.13 In this study, we blocked delphinidin‐induced inhibition of HOTAIR by transfecting cells with pcDNA‐HOTAIR(TCHOTAIR). As shown in Figure 2B, qRT‐PCR analysis results showed that introduction of the pcDNA‐HOTAIR plasmids led to substantial production of HOTAIR in breast cancer cells MDA‐MB‐231, MCF‐7, and MDA‐MB‐453. Results of the cell proliferation assay showed that delphinidin decreased proliferation of breast cancer cells and HOTAIR overexpression abrogated the effect of delphinidin on cell proliferation (Figure 2C).The wound healing assay also showed that delphinidin decreased proliferation and mobility to heal the wound of breast cancer cells effectively, which suppressed by HOTAIR overexpression (Figure 2D). As shown in Figure 2E,F, transwell invasion and transwell migration assay results showed that delphinidin decreased the invasion and migration of breast cancer cells, but HOTAIR overexpression abrogated the effect of delphinidin.
3.4. Delphinidin upregulates miR‐34a by inhibiting HOTAIR
Because miR‐34a is a major component of HOTAIR‐miRNA‐cancer cross‐talk,21 we investigated whether delphinidin inhibited breast cancer through HOTAIR/miR‐34a axis. We detected the level of miR‐34a in breast and cancer tissues and found that miR‐34a expression was decreased in breast and tumor tissues in MNU‐treated rats, and delphinidin upregulated miR‐34a in breast and tumor tissues (Figure 3A). The qRT‐PCR data also showed that delphinidin treatment effectively upregulated the expression of miR‐34a in breast cancer cells MDA‐MB‐231, MCF‐7, and MDA‐MB‐453.HOTAIR overexpression blocked the effect of delphinidin on miR‐34a in MDA‐MB‐231 breast cancer cells (Figure 3B).
Figure 3.
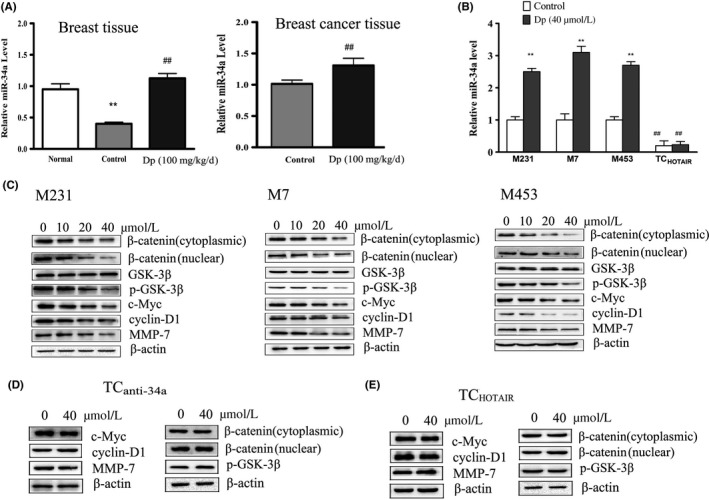
Delphinidin upregulates miR‐34a through the β‐catenin signaling pathway in breast and cancer tissues and breast cancer cells. A, The levels of miR‐34a in breast and breast cancer tissues were detected by qRT‐PCR. **P < .01 compared with normal group; ## P < .01 compared with control group. B, Effects of delphinidin (40 μmol/L) on the level of miR‐34a in MDA‐MB‐231(M231), MCF‐7 (M7), MDA‐MB‐453(M453) and MDA‐MB‐231 cells transfected with pcDNA‐HOTAIR (TCHOTAIR) were detected by qRT‐PCR; The data are presented as means ± SD (n = 3). *P < .05 and **P < .01 compared with breast cancer cells. C, Effects of delphinidin (40 μmol/L) treatment for 24 h on the level of β‐catenin, p‐GSK‐3β, c‐Myc, cyclin‐D1, and MMP‐7 in MDA‐MB‐231, MCF‐7, and MDA‐MB‐453 were detected by western blot. D,E, Effects of delphinidin (40 μmol/L) treatment for 24 h on the levels of c‐Myc, cyclin‐D1, MMP‐7, β‐catenin, and p‐GSK‐3β in MDA‐MB‐231 cells transfected with anti‐miR‐34a oligonucleotides (TC anti‐34a) and TCHOTAIR cells
Because miR‐34a inactivated the Wnt/β‐catenin signaling pathway in breast cancer cells, we conducted western blot analysis to detect β‐catenin, glycogen synthase kinase‐3β (Gsk3β), c‐Myc, cyclin‐D1, and MMP‐7 expression. As shown in Figure 3C, delphinidin treatment decreased the expression of β‐catenin, p‐GSK‐3β, c‐Myc, cyclin‐D1, and MMP‐7 in breast cancer cells significantly.
With a locked nucleic acid oligonucleotide complementary to the miR‐34a sequence, we blocked miR‐34a function in MDA‐MB‐231 cells (TCanti‐34a). The results showed that inhibition of miR‐34a significantly reduced the effect of delphinidin on β‐catenin, p‐GSK‐3β, c‐Myc, cyclin‐D1, and MMP‐7. HOTAIR overexpression also blocked the effect of delphinidin on β‐catenin, p‐GSK‐3β, c‐Myc, cyclin‐D1, and MMP‐7 in MDA‐MB‐231 cells (Figure 3D,E).
Taken together, these results indicated that delphinidin suppresses the Wnt/β‐catenin signaling pathway partially by modulating miR‐34a and HOTAIR.
3.5. Delphinidin regulates expression of miR‐34a through HOTAIR
In this study, RIP assay was performed in MDA‐MB‐231 cells and MDA‐MB‐231 cells transfected with pcDNA‐HOTAIR (TCHOTAIR) with antibodies against Ago2. The results showed that delphinidin treatment decreased HOTAIR and miR‐34a in an Ago2 pellet (Figure 4A,B). EZH2 and HOTAIR underlies the silencing of miR‐34a through induction of heterochromatin formation,22 and miR‐34a could be repressed through H3K27me3 on the miR‐34a promoter.20 ChIP assay was conducted to measure the enrichment of EZH2 and H3K27me3 in breast cancer cells. We found that delphinidin treatment decreased the occupancy of EZH2 and H3K27me3 at the miR‐34a promoter in MDA‐MB‐231 cells and that HOTAIR overexpression could inhibit the effect of delphinidin (Figure 4C,D). Taken together, these findings indicated that delphinidin can increase miR‐34a by inhibiting HOTAIR coupled with EZH2 and H3K27me3.
Figure 4.

Delphinidin regulates the expressions of miR‐34a through HOX transcript antisense RNA (HOTAIR) (A) and (B) RNA immunoprecipitation (RIP) assay was performed to determine the effect of delphinidin (40 μmol/L) on HOTAIR and miR‐34a in MDA‐MB‐231 cells and TCHOTAIR. RNA levels in immunoprecipitates were determined by qRT‐PCR. IgG was used as a negative control. C,D, Chromatin immunoprecipitation (ChIP) analysis in MDA‐MB‐231 cells and MDA‐MB‐231 cells transfected with pcDNA‐HOTAIR (TCHOTAIR) on the miR‐34a promoter regions using anti‐H3K27me3 and EZH2 antibodies. The bar graphs of the TCHOTAIR stand for MDA‐MB‐231 cells transfected with pcDNA‐HOTAIR. Enrichment was determined relative to the input controls. The data are presented as means ± SD (n = 3). *P < .05 and **P < .01 compared with the control
4. DISCUSSION
This study adds to the evidence that delphinidin has cancer prevention and anti‐cancer activity. Delphinidin exerts its anti‐cancer effect through inducing apoptosis and anti‐angiogenesis, and delphinidin inhibits the migration, invasion, and epithelial‐to‐mesenchymal transition of breast cancer cells.23, 24, 25, 26 This study was designed to investigate the anti‐cancer effects of delphinidin on MNU‐induced breast carcinogenesis in rats and breast cancer cells. Our study showed that dietary delphinidin effectively suppresses breast carcinogenesis in vivo and inhibits the proliferation and migration of breast cancer cells. These results indicated that delphinidin is an effective chemopreventive agent against breast cancer and provided useful insight into the role of delphinidin in breast cancer prevention.
HOTAIR, regarded as an oncogene, plays a critical role in most biologic processes of cancer and is a potential new target in cancer therapy.27 High expression of HOTAIR was associated with advanced stage of disease, lymphatic node metastasis, and poor overall survival.28 We found that, in a previous study, delphinidin treatment effectively decreased the expression of HOTAIR in breast cancer cells.13 In this study, we investigated the role of HOTAIR in breast carcinogenesis. These results showed that HOTAIR was overexpressed in breast cancer tissues, and oral administration of delphinidin in rats could decrease the level of HOTAIR in cancer tissues. We also found that delphinidin modulated proliferation and migration of breast cancer cells via negative regulation of HOTAIR. Based on these data, we concluded that delphinidin decreased the level of HOTAIR in breast carcinogenesis and that delphinidin may exert its anti‐cancer effect by inhibiting HOTAIR.
miR‐34a, regarded as a tumor suppressor, is decreased in breast cancer cell lines and tissues.29 In the current study, we detected that the level of miR‐34a was decreased in breast and tumor tissues in MNU‐treated rats, and that delphinidin treatment effectively upregulated the level of miR‐34a in tumor tissues. miR‐34a has been reported to inhibit the proliferation, invasion, and migration of breast cancer through decreased activity of the Wnt/β‐catenin signaling pathway.30 In this study, we found that delphinidin treatment effectively inhibited the expression of β‐catenin, p‐GSK‐3β, c‐Myc, cyclin‐D1, and MMP‐7 in breast cancer cells. Overexpression of HOTAIR could block the effect of delphinidin on miR‐34a in breast cancer cells.
Studies have shown that EZH2 and H3K27me3 play a major role in silencing miR‐34a and that HOTAIR directly binds to the miR‐34a promoter and plays a critical role in EZH2‐mediated repression of miR‐34a. Using RIP and ChIP assays, we found that delphinidin treatment decreased the binding of miR‐34a and HOTAIR, delphinidin also decreased the occupancy of EZH2 and H3K27me3 at the miR‐34a promoter in MDA‐MB‐231 cells. These findings indicated that delphinidin could increase miR‐34a by inhibiting HOTAIR and EZH2 occupancy at the miR‐34a promoter.
In conclusion, this study indicated that delphinidin may potentially suppress breast carcinogenesis. The results also suggested that delphinidin exerts its anti‐cancer effect through the HOTAIR/miR‐34a axis. These findings provided new evidence for the use of delphinidin in preventing breast carcinogenesis. Further studies could be conducted in the effect of delphinidin on human breast cancer prevention and the level of HOTAIR in the primary tumor of breast cancer patients.
DISCLOSURE
The authors declare no conflict of interest for this article.
ACKNOWLEDGMENTS
The study was financially supported by a research grant from the National Natural Science Foundation of China (81402675). It was also supported by the program for innovative research teams in Chengdu Medical College, Scientific Research and Foundation of the Sichuan Health and Family Planning Commission (17PJ438), Scientific Research and Foundation of the Sichuan Education Department (17ZA0114).
Han B, Peng X, Cheng D, et al. Delphinidin suppresses breast carcinogenesis through the HOTAIR/microRNA‐34a axis. Cancer Sci. 2019;110:3089–3097. 10.1111/cas.14133
Han and Peng as co‐first authors contributed equally to this study.
REFERENCES
- 1. Abood RA. Breast cancer in basra oncology center: a clinico‐ epidemiological analysis. Asian Pac J Cancer Prev. 2018;19:2943‐2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moo TA, Sanford R, Dang C, et al. Overview of breast cancer therapy. PET Clin. 2018;13:339‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Israel BB, Tilghman SL, Parker‐Lemieux K, et al. Phytochemicals: current strategies for treating breast cancer. Oncol Lett. 2018;15:7471‐7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Li S, Meng X, et al. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shareef M, Ashraf MA, Sarfraz M. Natural cures for breast cancer treatment. Saudi Pharm J. 2016;24:233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Różańska D, Regulska‐Ilow B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv Clin Exp Med. 2018;27:135‐142. [DOI] [PubMed] [Google Scholar]
- 7. Lin BW, Gong CC, Song HF, et al. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 2017;174:1226‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimes KL, Stuart CM, McCarthy JJ, et al. Enhancing the cancer cell growth inhibitory effects of table grape anthocyanins. J Food Sci. 2018;83:2369‐2374. [DOI] [PubMed] [Google Scholar]
- 9. Zhou L, Wang H, Yi J, et al. Anti‐tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: in vitro and in vivo study. Biomed Pharmacother. 2018;104:520‐529. [DOI] [PubMed] [Google Scholar]
- 10. Rahman N, Jeon M, Kim YS. Delphinidin, a major anthocyanin, inhibits 3T3‐L1 pre‐adipocyte differentiation through activation of Wnt/β‐catenin signaling. BioFactors. 2016;42:49‐59. [DOI] [PubMed] [Google Scholar]
- 11. Lee W, Yun JM. Suppression of β‐catenin signaling pathway in human prostate cancer PC3 cells by delphinidin. J Cancer Prev. 2016;21(2):110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Zhu Y, Zhang W, et al. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER‐2 positive breast cancer cells. BMC Cancer. 2018;18:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X, Luo E, Liu X, et al. Delphinidin‐3‐glucoside suppresses breast carcinogenesis by inactivating the Akt/HOTAIR signaling pathway. BMC Cancer. 2016;16:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balas MM, Johnson AM. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018;3:108‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang Q, Hann SS. HOTAIR: an oncogenic long non‐coding RNA in human cancer. Cell Physiol Biochem. 2018;47:893‐913. [DOI] [PubMed] [Google Scholar]
- 16. Li ZH, Weng X, Xiong QY, et al. miR‐34a expression in human breast cancer is associated with drug resistance. Oncotarget. 2017;8:106270‐106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng C, Qin Y, Zhi Q, et al. Knockdown of long non‐coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β‐catenin signaling pathways by up‐regulating miR‐34a. Int J Biol Macromol. 2018;107:2620‐2629. [DOI] [PubMed] [Google Scholar]
- 18. Yu Y, Zhang X, Li Z, et al. LncRNA HOTAIR suppresses TNF‐α induced apoptosis of nucleus pulposus cells by regulating miR‐34a/Bcl‐2 axis. Biomed Pharmacother. 2018;107:729‐737. [DOI] [PubMed] [Google Scholar]
- 19. Pawłowska E, Szczepanska J, Blasiak J. The long noncoding RNA HOTAIR in breast cancer: does autophagy play a role? Int J Mol Sci. 2017;18:2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng Y, Huang W, Meng W, et al. Heat shock improves Sca‐1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR‐34a/HSP70 pathway. Stem Cells. 2014;32:462‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao L, Wang X, Guo S, et al. LncRNA HOTAIR functions as a competing endogenous RNA to upregulate SIRT1 by sponging miR‐34a in diabetic cardiomyopathy. J Cell Physiol. 2019;234(4):4944‐4958. [DOI] [PubMed] [Google Scholar]
- 22. Li CH, Xiao Z, Tong JH, et al. EZH2 coupled with HOTAIR to silence microRNA‐34a by the induction of heterochromatin formation in human pancreatic ductal adenocarcinoma. Int J Cancer. 2017;140:120‐129. [DOI] [PubMed] [Google Scholar]
- 23. Kang HM, Park BS, Kang HK, et al. Delphinidin induces apoptosis and inhibits epithelial‐to‐mesenchymal transition via the ERK/p38 MAPK‐signaling pathway in human osteosarcoma cell lines. Environ Toxicol. 2018;33:640‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee DY, Park YJ, Hwang SC, et al. Cytotoxic effects of delphinidin in human osteosarcoma cells. Acta Orthop Traumatol Turc. 2018;52:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim WC, Kim H, Kim YJ, et al. Delphinidin inhibits BDNF‐induced migration and invasion in SKOV3 ovarian cancer cells. Bioorg Med Chem Lett. 2017;27:5337‐5343. [DOI] [PubMed] [Google Scholar]
- 26. Kim MH, Jeong YJ, Cho HJ, et al. Delphinidin inhibits angiogenesis through the suppression of HIF‐1α and VEGF expression in A549 lung cancer cells. Oncol Rep. 2017;37:777‐784. [DOI] [PubMed] [Google Scholar]
- 27. Quan J, Pan X, Zhao L, et al. LncRNA as a diagnostic and prognostic biomarker in bladder cancer: a systematic review and meta‐analysis. Onco Targets Ther. 2018;11:6415‐6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weng SL, Wu WJ, Hsiao YH, et al. Significant association of long non‐coding RNAs HOTAIR genetic polymorphisms with cancerrecurrence and patient survival in patients with uterine cervical cancer. Int J Med Sci. 2018;15:1312‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imani S, Zhang X, Hosseinifard H, et al. The diagnostic role of microRNA‐34a in breast cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:23177‐23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun N, Zhang G, Liu Y. Long non‐coding RNA XIST sponges miR‐34a to promotes colon cancer progression via Wnt/β‐catenin signaling pathway. Gene. 2018;665:141‐148. [DOI] [PubMed] [Google Scholar]


