Abstract
The programmed cell death 1/programmed cell death 1 ligand 1 pathway was successfully targeted in cancer immunotherapy. Elevated interleukin‐17 (IL‐17), which is known in autoimmune diseases, has recently been recognized in cancer patients. We investigated the role of IL‐17 in the regulation of expression of programmed cell death 1 ligand 1 in ovarian cancer by evaluating changes in the number of IL‐17‐producing cluster of differentiation 4 helper T cells (Th17) and γδT cells (γδT17) in PBMC of 52 gynecological cancer patients (including 30 ovarian cancer patients) and 18 healthy controls. The occupancy ratio of Th17 and γδT17 was higher in ovarian cancer and endometrial cancer patients than in controls, determined by multi‐color flow cytometry (Th17: P < 0.0001 and P = 0.0002, respectively; γδT17: P = 0.0020 and P = 0.0084, respectively). IL‐17 mRNA level was elevated in PBMC of ovarian cancer patients (P = 0.0029), as measured by RT‐PCR. The neutrophil‐to‐lymphocyte ratio, which is a prognostic biomarker of ovarian cancer, correlated with Th17 occupancy ratio in patients (P = 0.0068). We found that programmed cell death 1 ligand 1 expression and its associated factors (IL‐6 and phospho‐signal transducer and activator of transcription 3) were induced by IL‐17 in an ovarian cancer cell line. These results suggest that increased Th17 counts and IL‐17 level, which correlated with high neutrophil‐to‐lymphocyte ratio and programmed cell death 1 ligand 1 expression, are potential biomarkers for poor prognosis in ovarian cancer and likely indications for application of programmed cell death 1 ligand 1 pathway inhibitors.
Keywords: IL‐17, neutrophil‐to‐lymphocyte ratio, ovarian cancer, PD‐L1, Th17
1. INTRODUCTION
Ovarian cancer (OC) is a common gynecological disease. In Japan, the number of patients affected with ovarian cancer exceeded 10 000 in 2014 and the cumulative mortality risk of OC was 1 in 198 cases.1 Detecting OC in the early stages is difficult due to lack of obvious symptoms. Therefore, postoperative chemotherapy is required in many cases, but outcomes are not always favorable. A global surveillance of cancer survival conducted between 2010 and 2014 concluded that the 5‐year survival rate was <50% in diagnosed cases of OC in most countries.2
Interleukin‐17 (IL‐17) is a pro–inflammatory cytokine produced by the cluster of differentiation 4+ (CD4+) helper T cells (Th17), which differ from the Th1 cells that produce interferon‐γ (IFN‐γ) or Th2 cells that produce IL‐4.3 Stimulation by IL‐6 and transforming growth factor β (TGF‐β) induces Th17 differentiation of the naïve T cells.4 IL‐17 signal transduction activates and promotes migration of neutrophils, macrophages and monocytes, which play important roles in host defense against bacterial infection.5, 6 Th17 cells induce inflammation and are implicated in autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and asthma,7 while also contributing to host defense against pathogens. IL‐6, IL‐23, IL‐27, retinoic acid receptor‐related orphan receptor‐γt (ROR‐γt) and IFN‐γ are important for inducing and maintaining Th17 populations. IL‐6 and TGF‐β activate signal transducer and activator of transcription 3 (STAT3); this upregulates ROR‐γt, a transcription factor that induces differentiation of the naïve T cells into Th17 cells. ROR‐γt also activates the IL‐23 receptor on Th17 cells. These differentiation processes are inhibited by IFN‐γ and IL‐27.7, 8, 9 Tumor formation was reportedly enhanced in an IL‐17‐dependent manner in mice inoculated with Bacteroides fragilis, a human‐resident bacterium.10
γδT cells are another type of T cell that produce IL‐17 (γδT17). These cells were first reported in pulmonary infection by Mycobacterium tuberculosis 11 and are now known to play important roles in host defense against infection. However, γδT17 differ from Th17 cells in that they recognize non‐peptide antigens independent of human leukocyte antigen molecules and antigen‐presenting cells.12 Although IL‐17/Th17 expression has been confirmed in various human tumors including OC,13, 14, 15, 16 the significance of this observation is still debated.17
The capacity for immunosuppression is altered in cancer patients. Normally, the T cell response is initiated by recognition by the T cell receptor of an antigen expressed on a major histocompatibility complex. The action of T cells is positively or negatively regulated by co‐stimulatory molecules such as CD28, which causes T cell activation, and cytotoxic T lymphocyte‐associated antigen 4, which suppresses T cell function by binding to the same ligands (ie, CD80 or CD86).18
The programmed cell death 1 (PD‐1)/programmed cell death 1 ligand 1 (PD‐L1) pathway inhibits T cell activation against tumors; blockade of PD‐1/PD‐L1 signaling leads to antitumor efficacy in melanoma, non‐small‐cell lung cancer and renal‐cell carcinoma.19 However, identifying patients who may benefit from such inhibitory drugs remains challenging due to lack of appropriate biomarkers. PD‐L1 is known to be highly expressed under inflammatory conditions, such as in the presence of IFN‐γ. PD‐L1 is also upregulated through STAT3 signaling initiated by IL‐6, a major pro–inflammatory cytokine.20, 21
We speculated that factors related to IL‐17/Th17 and PD‐L1 may be involved in the immune response caused by inflammation. In fact, IL‐17 has reportedly been positively associated with PD‐L1 upregulation in colon and breast cancers,22 but IL‐17–PD‐L1 correlation in OC has not been studied. We aimed to investigate the changes in IL‐17 expression and Th17 numbers in gynecological cancer, especially in OC, and to clarify their roles in regulating PD‐L1 expression.
2. MATERIALS AND METHODS
2.1. Patients and tissue samples
Fifty‐two patients with gynecological cancer were recruited from the Tokyo University Hospital, Tokyo with the approval of the institutional research ethics committee. Subjects included OC (n = 30), endometrial cancer (n = 12) and cervical cancer (n = 10) patients. The histopathological classifications of the OC patients were serous (n = 17), mucinous (n = 3), endometrioid (n = 6) and clear‐cell (n = 4) carcinoma. Of the 30 OC patients, 27 (70%) had not yet received chemotherapy, and none of the patients had received radiotherapy. According to the International Federation of Gynecology and Obstetrics classification, patients were grouped as stage I (n = 6), stage II (n = 2), stage III (n = 5) and stage IV (n = 17). Peripheral blood and tumor samples were obtained from all gynecological cancer patients. Women in the healthy control (HC) group, who ranged in age from 24 to 79 years (mean age = 50 years, n = 18), voluntarily provided peripheral blood samples. All participants provided informed consent. Patients’ and tumor characteristics are listed in Table 1.
Table 1.
Number of each category of gynecological cancer patients (upper table) and characteristics of ovarian cancer (OC) patients, including cancer stage, histopathological type and adherence to chemotherapy (lower table)
| Classification | (n) |
|---|---|
| Patient characteristics | |
| Healthy control people (HC) | 18 |
| Ovarian cancer patients | 30 |
| Endometrial cancer patients | 12 |
| Cervical cancer patients | 10 |
| Characteristics of ovarian cancer patient samples (n = 30) | |
| Age | (Median/range) |
| 50/24‐79 | |
| Histopathology | (n) |
| Serous | 17 |
| Mucinous | 3 |
| Endometrioid | 6 |
| Clear cell | 4 |
| Prior chemotherapy | (n) |
| One regimen | 9 |
| No chemotherapy | 21 |
| Stage | (n) |
| I | 6 |
| II | 2 |
| III | 5 |
| IV | 17 |
2.2. Preparation of plasma and PBMC
Peripheral blood (20 mL) was collected from patients or HC and divided into 2 parts. Using the first part, plasma was separated and stored at −80°C immediately after centrifugation to determine cytokine levels by ELISA. PBMC were isolated from the other half of each blood sample using Ficoll Hypaque (Sigma‐Aldrich)23 and stored at −80°C for RNA extraction or stimulation.
2.3. Cell culture and stimulation
The OVSAHO human serous carcinoma cell line and the MCAS mucinous carcinoma cell line were purchased from the Japanese Collection of Research Bioresources. OVSAHO cells were cultured in RPMI‐1640 (Thermo Fisher Scientific) with 10% (v/v) FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin. MCAS cells were grown in DMEM (Thermo Fisher Scientific) containing 10% FBS and 1% penicillin/streptomycin. Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C; the cells were seeded in 6‐well plates (2 × 105 cells/well) or in 10‐cm plates (2.0 × 106 cells/well) and incubated with/without IL‐17A (20 ng/mL; BioLegend), anti–IL‐6 mAb (mAbIL‐6; 20 ng/mL; Abcam; ab9324), the nuclear factor‐κB (NF‐κB) inhibitor Bay11‐7082 (15 μmol/L; Santa Cruz Biotechnology), and/or the STAT3 inhibitor S31‐201 (50, 100 and 200 ng/mL; Santa Cruz Biotechnology) for 4,16 or 24 hours at 37°C and 5% CO2 for ELISA, quantitative RT‐PCR (qRT‐PCR), or western blotting. BAY11‐7082 was dissolved in DMSO (Sigma‐Aldrich) as a 10 mmol/L stock solution.
2.4. Flow cytometry
PBMC suspended in 2 mL of RPMI‐1640 were incubated for 4 hours at 37°C with PMA (25 ng/mL; Sigma‐Aldrich) and ionomycin (1 μg/mL; Sigma‐Aldrich) in the presence of 2 μL Golgi Stop (BD Biosciences). Cells were washed and incubated in fixation and permeabilization buffers (Thermo Fisher Scientific)8 and then labeled with the following Ab: fluorescein isothiocyanate‐conjugated anti–γδT (isotype mIgG1; test IO; IM1571U), phycoerythrin‐conjugated anti–CD3 (isotype mIgG1; Beckman Coulter; IM1282) and anti–CD4 (isotype mIGg1κ, PerCP5.5, BD Biosciences; 560650) were used for cell‐surface labeling; and anti–IL‐17 conjugated with Alexa Fluor 647 (isotype mIgG1κ; BD Biosciences; 560490) was used for intracellular labeling. At least 30 000 events were recorded using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using Kaluza software (Beckman Coulter).
2.5. RNA isolation and quantitative RT‐PCR
PBMC, OVSAHO and MCAS cells were lysed in 350 μL/well of FARB buffer (Favorgen) containing 3.5 μL/well of 98% 2‐mercaptoethanol (Sigma‐Aldrich), and the Blood/Cultured Cell Total RNA Mini Kit (Favorgen) was used to extract total RNA from the cells. cDNA was synthesized using 5 × RT Master Mix (Toyobo) according to the manufacturer's instructions, and qRT‐PCR was performed in duplicate using SYBR Green I Master Mix and a LightCycler 480 (Roche) according to the manufacturer's protocols. Forward and reverse primer sequences were: PD‐L1, 5′‐GGCATTTGCTGAACGCAT‐3′ and 5′‐CAATTAGTGCAGCCAGGT‐3′; IL‐6, 5′‐ACAAGCCAGAGCTGTGCAGATG‐3′ and 5′‐GTGCCCATGCTACATTTGCCGA‐3′; TGF‐β, 5′‐GCTGCCTGTGTGACTTTGG‐3′ and 5′‐TCCTGGATTCTAGCACTTCTGG‐3′; ROR‐γt, 5′‐GTGGGGACAAGTCGTCTGG‐3′ and 5′‐AGTGCTGGCATCGGTTTCG‐3′; IFN‐γ, 5′‐CGAGGGTTGAATGAGAGCTT‐3′ and 5′‐CAGACGGCTGCCTTTATAGC‐3′; GAPDH, 5′‐GAAAGGTGAAGGTCGGAGTC‐3′ and 5′‐GAAGATGGTGATGGGATTTC‐3′; IL‐17, 5′‐AGAGATATCCCTCTGTGATC‐3′ and 5′‐CACCCCAAAATTGTCTCAGG‐3′; IL‐27, 5′‐GAGCAGCTCCCTGATGTTTC‐3′ and 5′‐AGCTGCATCCTCTCCATGTT‐3′; tumor necrosis factor α (TNF‐α), 5′‐GGCGTGGAGCTGAGAGATAAC‐3′ and 5′‐GGTGTGGGTGAGGAGCACAT‐3′; and IL‐23A, 5′‐CAGTTCTGCTTGCAAAGGAT‐3′ and 5′‐ATCTGCTGAGTCTCCCAGTG‐3′. All the Ab were from Sigma‐Aldrich. The ratio of each gene product to GAPDH was used to determine mRNA expression according to the cycle‐threshold method.
2.6. Plasma cytokine measurement
Interleukin‐17, IL‐23 and IL‐6 concentrations in plasma samples from study subjects or cell‐culture supernates were measured by ELISA (Bio‐Techne for IL‐17; BioLegend and Bio‐Techne for IL‐23; and Thermo Fisher Scientific for IL‐6) according to the manufacturers’ instructions.
2.7. Western blotting
Cultured cells were lysed in lysis buffer (Cell Signaling Technology; #9803) containing the protease inhibitor cocktail for general use (Nakalai Tesque) and PhosSTOP (Roche). Equal amounts of protein were separated by electrophoresis on an Any kD Mini‐PROTEAN TGX Precast Gel (Bio‐Rad), which was probed, after blotting, with Abs against the following proteins: α‐tubulin (clone TU‐02, mouse monoclonal IgM) and STAT1 (clone E‐23, rabbit polyclonal IgG (both from Santa Cruz Biotechnology); pSTAT1 (Tyr701, rabbit polyclonal IgG), STAT3 (clone 124H6, mouse monoclonal IgM), and pSTAT3 (Tyr705, rabbit polyclonal IgG) (all from Cell Signaling Technology); and PD‐L1 (rabbit monoclonal IgM; Abcam; ab205921). Protein bands were detected using the ECL Western Blotting Detection Reagent (GE Healthcare Life Sciences).
2.8. Immunohistochemistry
For immunohistochemistry, we used an anti–PD‐L1 IgG (Abcam, clone 28‐8; dilution, 1:250 v/v, ab205921) and a polyclonal anti–IL‐17 IgG (Bioss Antibodies; 1:200 v/v, bs‐2140R). Slides bearing sections of the OC samples were heated at 121°C for 10 minutes in a diluted universal HIER antigen‐retrieval reagent (Abcam; pH 6.0, 1:10 v/v, ab208572) before deparaffinization in xylene and rehydration in graded ethanol. After complete rehydration, endogenous peroxidase activity was blocked by incubation in 0.3% H2O2 in methanol for 20 minutes at room temperature. Slides were then incubated with primary antibodies at room temperature overnight. After being washed using TBS, slides were incubated for 60 minutes at room temperature with a rabbit‐specific IHC polymer detection kit HRP/DAB (Abcam, ab209101) for detecting PD‐L1, and the Dako EnVision+ System, HRP‐Labelled Polymer, Anti‐Rabbit (Dako, K4003), for detecting IL‐17, as the respective secondary antibodies. Specific antigen–antibody reactions were visualized after using the ImmPACT DAB (Vector Laboratories, SK‐4105) and counterstaining with Mayer's hematoxylin solution.
2.9. Neutrophil‐to‐lymphocyte ratio
Preoperative leukocyte counts were documented by reviewing clinical charts and were used to calculate neutrophil‐to‐lymphocyte ratio (NLR) by dividing neutrophil count (%) by lymphocyte count (%).
2.10. Statistical analyses
Differences between groups were evaluated using the non‐parametric Mann‐Whitney U test. Correlations were determined using Spearman's correlation analysis. Statistical analyses were performed using the JMP v.13.2 software (SAS Institute). P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Th17 and γδT17 cell counts were elevated in ovarian cancer and endometrial cancer patients
We collected blood samples from HC, OC, endometrial cancer and cervical cancer patients and isolated PBMC, which were then stimulated with PMA and ionomycin before adding Golgi Stop and incubating at 37°C for 4 hours. CD3+ cells were identified among lymphocytes; of these, CD4+ and IL‐17+ cells were identified as Th17 (Figure 1A). Among γδT+ cells, those that were IL‐17+ were identified as γδT17 cells. Th17 and γδT17 counts in PBMC from each subject were used to calculate the occupancy ratios of Th17 to CD4+ cells and of γδT17 to γδT+ cells, respectively. Both ratios were significantly higher in OC and endometrial cancer patients than in HC (Figure 1B); however, no differences were observed between cervical cancer patients and HC. The Th17 and γδT17 occupancy were positively correlated in OC patients (Figure 1C).
Figure 1.
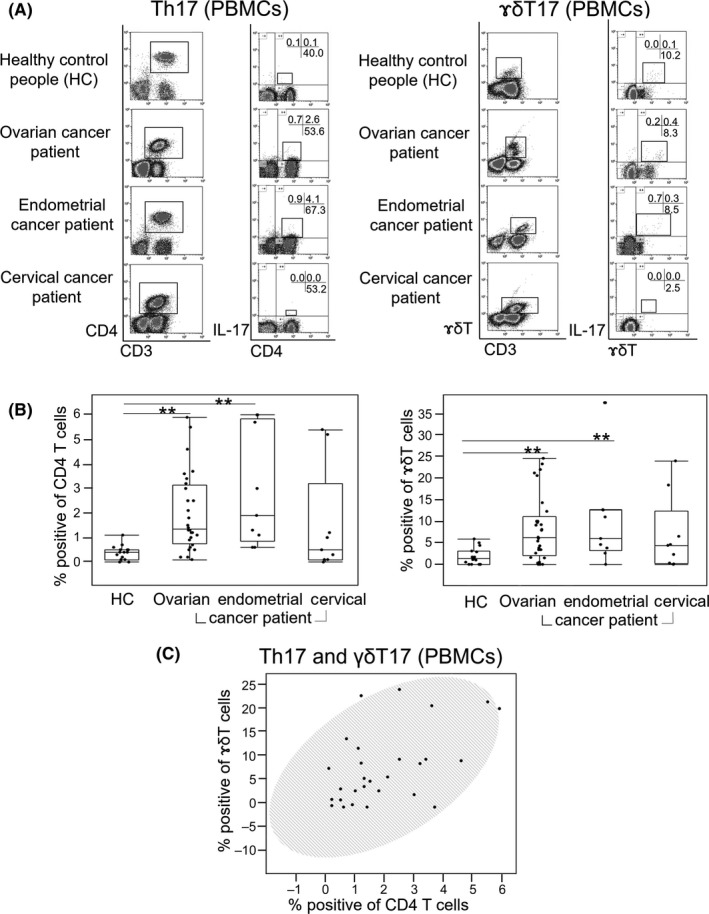
Ratio of Th17 and γδT17 cells in gynecological cancer patients. A, Squares in the left column indicate CD3+CD4+ and CD3+γδT+ cells; those in the right column indicate CD4+IL‐17+ and γδT+IL‐17+ cells. The proportion of CD3+CD4+IL‐17+ cells in CD3+CD4+ cells or the proportion of CD3+γδT+IL‐17+ cells in CD3+γδT+ cells was defined as the occupancy ratio of Th17 and γδT17, respectively. Numbers on cross bars in each column represent the percentage of the Th17 or γδT17 cells in CD3+ cell population. B, Occupancy ratio of Th17 or γδT17 for each carcinoma type (ovarian cancer [OC], n = 30; endometrial cancer, n = 12; and cervical cancer, n = 10) and 18 healthy control (HC) subjects. Lines in the middle of each box represent the median value and the lower and upper boundaries show the 5th and 95th percentiles, respectively. Results were analyzed using the Mann‐Whitney U test. *P < 0.05, **P < 0.01. C, Correlation between Th17 and γδT17 in PBMC of OC patients. Data were analyzed using the Spearman's test, and shaded ellipses represent 95% confidence level (r = 0.50, P = 0.0052)
3.2. Th17‐associated cytokines were elevated in PBMC but not in plasma of ovarian cancer patients
The PBMC mRNA levels of IL‐23, ROR‐γt, TNF‐α, IFN‐γ, IL‐6 and IL‐17 (factors associated with differentiation of the Th17 cells from the naïve T cells) were analyzed by qRT‐PCR. We also examined the PD‐L1 transcript expression to determine the relevance of these factors. IL‐17 and IL‐23 were upregulated in OC patients as compared to HC, with a positive correlation observed between their expression levels (Figure 2A,B). Levels of PD‐L1, IL‐6, IL‐27, TNF‐α, ROR‐γt and IFN‐γ mRNA did not differ between OC patients and HC. However, IL‐17 mRNA expression correlated positively with that of the upstream factor ROR‐γt, whereas IL‐23 transcript level correlated positively with that of TNF‐α produced by Th17 cells (Figure 2C). Plasma concentrations of IL‐17, IL‐23 and IL‐6 were similar between OC patients and HC, as determined by ELISA (Figure 2D).
Figure 2.
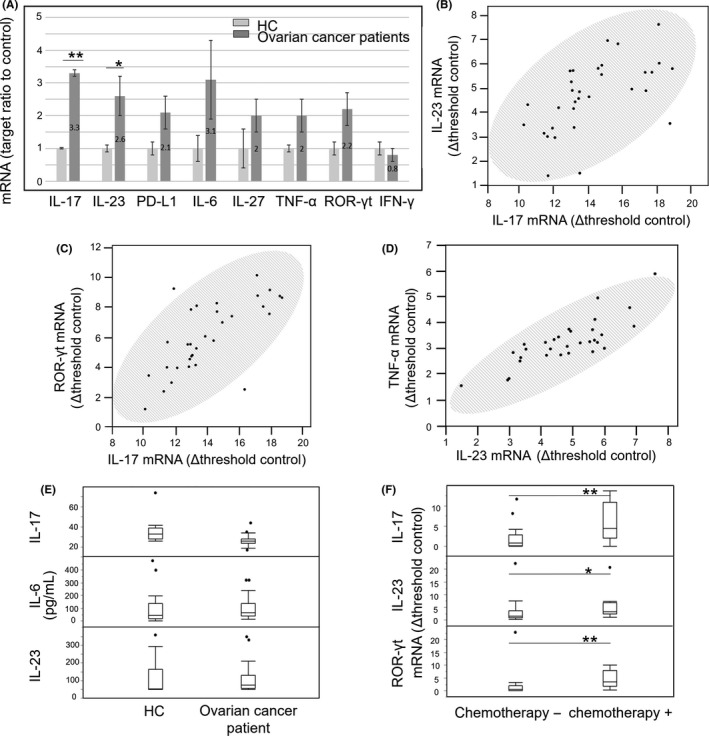
mRNA and proteins levels of interleukin‐17 (IL‐17)‐related factors. A, Relative mRNA expression of IL‐17‐related genes in PBMC of ovarian cancer (OC) patients, as determined by RT‐PCR. Right and left bars represent OC patients and healthy control (HC) respectively. B‐D, Correlation between IL‐17 and IL‐23 mRNA levels in PBMC of OC patients. (C) and (D) show the correlation between IL‐17 and ROR‐γt mRNA and between IL‐23 and TNF‐α mRNA levels, respectively, in PBMC of OC patients (Spearman's test, b: r = 0.64, P < 0.0001; c: r = 0.69, P < .0001; and d: r = 0.84, P < .0001). Shaded ellipses represent 95% confidence level. E, Plasma IL‐17, IL‐23 and IL‐6 protein levels in HC and OC patients, as determined by ELISA. F, Comparative analysis of IL‐17, IL‐23 and ROR‐γt mRNA levels in PBMC of OC patients with or without chemotherapy. *P < 0.05, **P < 0.01 (Mann‐Whitney test). Black circles in (E) and (F) indicate outliers
3.3. Th17‐associated cytokines were elevated in patients who received chemotherapy
To assess the effect of chemotherapy, IL‐17, IL‐23 and ROR‐γt mRNA levels were compared in PBMC of patients who underwent surgery without chemotherapy and those who underwent interval debulking surgery (IDS) after chemotherapy. All of these factors were upregulated in patients who received chemotherapy as compared to those who did not (Figure 2E). The chemotherapy consisted of a combination of paclitaxel and carboplatin for all IDS cases.
3.4. Th17 and γδT17 cell counts were elevated in mucinous carcinoma of ovarian cancer patients
We examined whether the histopathological types of OC affect the occupancy ratio of the Th17 and γδT17 cells in PBMC and found that all histopathological types of OC showed increased Th17 occupancy ratios relative to HC, whereas for γδT17, the serous and mucinous carcinoma subtypes differed from HC. In addition, the occupancy ratio of γδT17 cells in PBMC of mucinous carcinoma patients was higher than that of serous carcinoma patients (Figure 3A).
Figure 3.
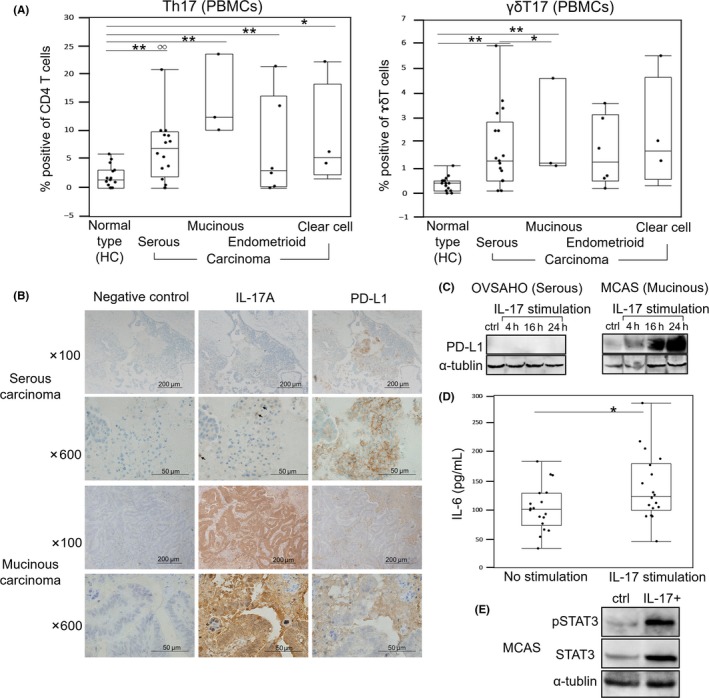
Analysis of factors related to interleukin‐17 (IL‐17) according to ovarian cancer (OC) histopathological type. A, Occupancy ratio of Th17 and γδT17 cells in PBMC of OC patients or healthy controls (HC). B, Immunodetection of IL‐17A and programmed cell death 1 ligand 1 (PD‐L1) in surgical tumor specimens from OC patients. C, Effect of IL‐17 on PD‐L1 expression, as detected by western blotting in OVSAHO and MCAS cell lines. D, IL‐6 expression following stimulation with IL‐17A (right), as determined by ELISA; untreated cells served as control (left). In A‐D, lines in the middle of each box indicate the median value, and the lower and upper boundaries represent the 5th and 95th percentiles, respectively. E, STAT3 phosphorylation in MCAS cells evaluated 24 h after IL‐17A stimulation by ELISA. *P < .05, **P < .01 (Mann‐Whitney test). White circles in panel indicate outliers
3.5. Programmed death ligand 1, interleukin‐6 and phospho‐signal transducer and activator of transcription 3 were induced by interleukin‐17A in mucinous carcinoma cells
Surgical specimens from OC patients were analyzed for IL‐17A and PD‐L1 expression by immunohistochemistry. Both serous and mucinous carcinoma specimens were immunopositive for IL‐17A and PD‐L1, with the former highly expressed in T lymphocyte (Figure 3B). Based on these observations, we investigated the relationship between IL‐17A and PD‐L1 expression using OVSAHO serous carcinoma and MCAS mucinous carcinoma cells stimulated with recombinant IL‐17A. PD‐L1 expression was examined after 4, 16 and 24 hours. In OVSAHO cells, IL‐17A did not induce PD‐L1 expression, but in MCAS cells, high levels of PD‐L1 were detected at 16 and 24 hours (Figure 3C). As mentioned above, IL‐17 expression requires IL‐6 activation and STAT3 phosphorylation and activation, which are also associated with PD‐L1 expression. To investigate the interaction between IL‐17, IL‐6, STAT3 and PD‐L1, MCAS cells were stimulated with IL‐17A and the concentration of each factor in the supernates was measured by ELISA. IL‐6 expression (Figure 3D) and STAT3 phosphorylation (Figure 3E) were markedly altered 24 hours after IL‐17A treatment.
3.6. Interleukin‐6, phospho‐signal transducer and activator of transcription 3 and nuclear factor κB inhibition suppressed programmed death 1 ligand 1 expression induced by interleukin‐17A
To investigate the relationship between PD‐L1 expression and IL‐17A‐induced IL‐6 and pSTAT3 upregulation, we evaluated PD‐L1 levels in IL‐17A‐stimulated cells treated with inhibitors of IL‐6 and pSTAT3. Application of a blocking mAb against IL‐6 (mAbIL‐6) abolished PD‐L1 expression even in the presence of IL‐17A (Figure 4A), although the Ab had no effect in unstimulated cells. BAY11‐7082 does not directly inhibit IL‐6 but abolishes NF‐κB binding to DNA and thereby blocks IL‐6 expression. MCAS cells stimulated with IL‐17A and treated with BAY11‐7082 showed decreased expression of PD‐L1 24 hours later (Figure 4B), while DMSO did not reduce PD‐L1 expression. Similarly, application of STAT3 inhibitor (S31‐201) reduced STAT3 phosphorylation in IL‐17A‐stimulated cells in a concentration‐dependent manner (Figure 4C) and reduced the PD‐L1 protein level. qRT‐PCR analysis of IL‐6 and PD‐L1 mRNA expression in PBMC of OC patients showed that IL‐6 and PD‐L1 were positively correlated (Figure 4D). Furthermore, plasma IL‐6 concentration in OC patients correlated with the Th17 occupancy ratio in PBMC (Figure 4E).
Figure 4.
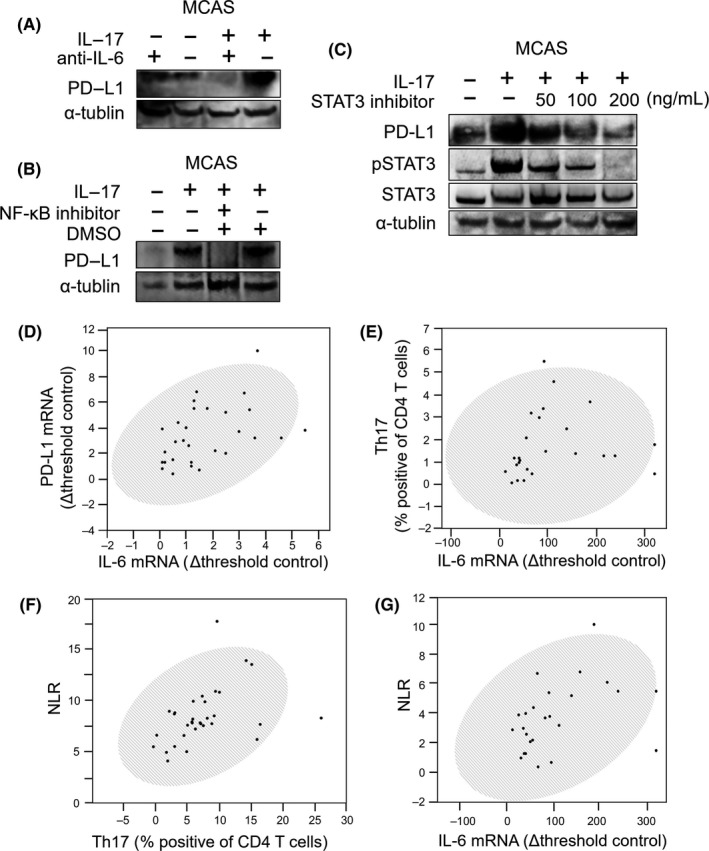
Programmed cell death 1 ligand 1 (PD‐L1) expression induced by interleukin‐17A (IL‐17A) and its inhibition by IL‐6, phospho‐signal transducer and activator of transcription (pSTAT3) and nuclear factor‐κB (NF‐κB) inhibitors. A, Expression of IL‐17‐related genes in MCAS cells following IL‐17 stimulation in the presence of anti–IL‐6 Ab (mAbIL‐6), as determined by western blotting. B, Change in PD‐L1 expression upon IL‐17 stimulation in the presence of NF‐κB inhibitor (BAY11‐7082) and 0.1% DMSO, as determined by western blotting. C, Western blotting of changes in PD‐L1 and pSTAT3 expression following IL‐17 stimulation in the presence of STAT3 inhibitor (S31‐201: 0, 50, 10 and 200 μg/mL). Investigation of the relationship between PD‐L1, IL‐6, Th17 and neutrophil‐to‐lymphocyte ratio (NLR). D, Correlation between IL‐6 and PD‐L1 mRNA levels in PBMC of ovarian cancer (OC) patients. E, Relationship between IL‐6 mRNA and Th17 occupancy ratio in PBMC of OC patients. F,G, Relationship between NLR in preoperative blood collected from OC patients and Th17 occupancy ratio in PBMC (F) or plasma IL‐6 protein level (G). Results in (D) to (G) were analyzed using Spearman's test and shaded ellipses represent 95% confidence level ((D) ρ = 0.52, P = 0.0023; (D) ρ = 0.51, P = 0.0098; (F) ρ = 0.49, P = 0.0068; (G) ρ = 0.48, P = 0.0184)
3.7. Neutrophil‐to‐lymphocyte ratio is positively correlated with Th17 counts in PBMC and with plasma interleukin‐6 of ovarian cancer patients
We determined the NLR of OC patients immediately before surgery and found that the occupancy ratio of Th17 to CD4+ cells in PBMC (Figure 4F) and plasma IL‐6 concentration (Figure 4G) positively and significantly correlated with NLR in OC patients.
4. DISCUSSION
Previous studies have reported elevated Th17 counts in various cancers, including acute myeloid leukemia,24 hepatocellular carcinoma,25 breast cancer15 and gallbladder cancer,23 with increased numbers of Th17 and γδT17, which both promote IL‐17 expression, thought to be associated with decreased survival. Thus, IL‐17‐producing cells likely play important roles in the pathogenic immunity changes of cancer. In this study, we demonstrated that the occupancy ratios of Th17 and γδT17 were increased in PBMC of OC patients, especially in those with the mucinous subtype. Moreover, NLR positively correlated with the Th17 occupancy ratio. This is significant because in a meta‐analysis of 4910 OC patients, elevated NLR was associated with shorter overall survival (OS) and worse progression‐free survival (PFS).26 Another meta‐analysis including 12 studies of 1630 OC patients revealed that PD‐L1 protein expression was not associated with OS or PFS,27 and Mesnage et al28 observed that upregulation of PD‐L1 expression after chemotherapy in OC patients was not associated with patients’ OS. In contrast, the meta‐analysis study concluded that PD‐L1 mRNA expression was significantly associated with worse PFS in 1228 patients with OC (P < 0.001).27 This discrepancy may not be reconciled presently because further confirmatory studies are needed. In our study, the short observation period precluded investigation of the relationship between high Th17 occupancy and poor OS or PFS in OC patients, but a correlation between these parameters is possible as in other cancers such as hepatocellular and gallbladder carcinomas.23, 25
The IL‐17 mRNA level was upregulated in OC patients compared to HC. In addition, the mRNA expression of IL‐23, which is required for Th17 maintenance and proliferation, was increased and positively correlated with that of IL‐17. In contrast, plasma IL‐17 concentration was unaltered. This is presumably because protein production is mainly controlled at the translation stage and is not dictated by the amount of transcript.29 However, immunohistochemistry of tumor specimens clearly showed increased expression of IL‐17 in OC. Our objective in these experiments was to examine how PD‐L1 expression is altered by IL‐17‐induced inflammation, because PD‐L1 expression is known to be enhanced by inflammatory cytokines such as IFN‐γ30 and IL‐6.31 PD‐L1 mRNA level did not correlate with that of IL‐17 or the Th17 occupancy ratio in PBMC of OC patients, although tumor specimens were positive for both IL‐17 and PD‐L1. However, a relationship possibly exists between IL‐17 and PD‐L1 given that PD‐L1 expression was induced in vitro by IL‐17 stimulation, albeit only in the mucinous ovarian carcinoma cell line. Interestingly, although IL‐17 production was initially induced by IL‐6 and TGF‐β, IL‐17 itself increased the levels of IL‐6 mRNA and protein.
Recent studies have described a positive feedback loop involving IL‐17 and IL‐6 that induces STAT3 phosphorylation.32, 33, 34 IL‐6 and STAT3 promote production of IL‐17, which, in turn, activates IL‐6 and STAT3. As stated above, PD‐L1 is produced through IL‐6 activation and STAT3 phosphorylation. Our results suggest that IL‐6 and STAT3 activation in tumor cells by IL‐17 stimulation induces PD‐L1 expression. This was supported by the observation that treatment with IL‐6 and STAT3 inhibitors abolished the IL‐17‐induced expression of PD‐L1 (Figure 4A,C).
One of the functions of IL‐17 is NF‐κB activation. STAT3‐binding and NF‐κB‐binding sites are found in the promoters of thousands of genes, and the 2 proteins may control overlapping genes during tumorigenesis (specifically, those encoding chemokines) to regulate immune responses within the tumor microenvironment. It also coordinates various cytokines involved in the immune response, but, interestingly, some of these are produced not only by hematopoietic cells but also by tumor cells. STAT3 and NF‐κB in tumor cells activate IL‐6, which, in turn, activates STAT3, forming a positive feedback loop.33, 35 TNF‐α is another cytokine controlled by NF‐κB22 in tumor cells; TNF‐α also activates NF‐κB through a positive feedback loop.35 We found that treatment with NF‐κB inhibitor abolished IL‐17‐induced PD‐L1 expression. Given that IL‐17 activates NF‐κB, then IL‐17 causes PD‐L1 expression by activating NF‐κB, which produces IL‐6 and phosphorylates STAT3 in tumor cells.
Notably, mRNA levels of IL‐17 and associated factors were upregulated in the plasma of OC patients after chemotherapy. Paclitaxel has been shown to induce PD‐L1 overexpression via NF‐κB activation in OC;28, 36 our results showed that IL‐17 caused an effect similar to paclitaxel; that is, it induced PD‐L1 expression via NF‐κB activation. Upregulation of IL‐17 and associated factors in PBMC of OC patients who received chemotherapy including paclitaxel does not contradict these findings because these pathways could further stimulate IL‐17 production through a feedback mechanism (Figure 5).
Figure 5.
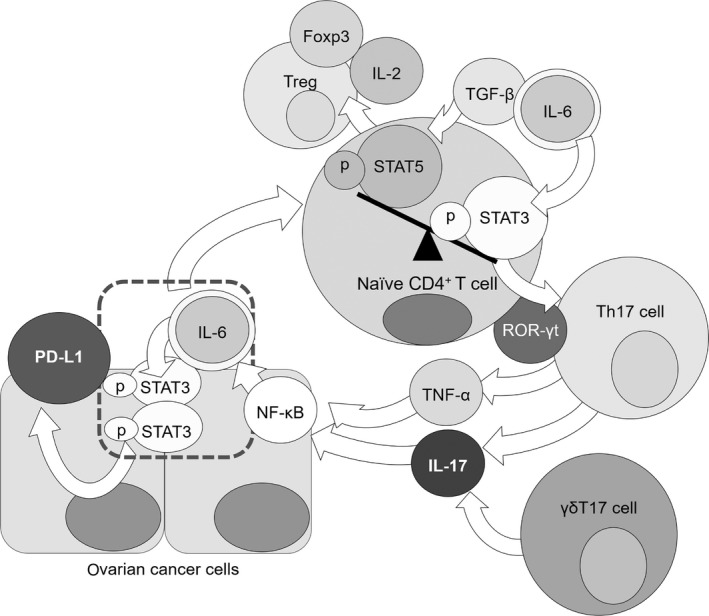
Interleukin‐6 (IL‐6) and transforming growth factor beta (TGF‐β) phosphorylate STAT3 in naïve T cells. Naïve T cells are induced to differentiate into regulatory T cells by TGF‐β in the absence of IL‐6. ROR‐γt is activated to induce differentiation of the naïve T cells into Th17 cells, which produce IL‐17 and TNF‐α. Nuclear factor‐κB (NF‐κB) activated by IL‐17 or TNF‐α stimulation induces expression of IL‐6, which phosphorylates STAT3 in cancer cells. These steps result in programmed cell death 1 ligand 1 (PD‐L1) expression in ovarian cancer (OC) cells and reactivation of IL‐6 and STAT3 through a positive feedback loop
Next, we will discuss the potential functions of STAT3 in T cell differentiation. Regulatory T cells (Treg) also differentiate from naïve T cells and maintain peripheral tolerance. Foxp3, which is essential for development and maintenance of Treg in the immune periphery, is induced also by TGF‐β and IL‐2 through STAT5‐dependent and STAT3‐dependent mechanisms.37, 38, 39 The Foxp3 promoter contains STAT3‐binding domains through which IL‐6 can attenuate Foxp3 expression under inflammatory conditions.37 Differentiation of Th17 and Treg are reportedly modified by the balance between active STAT3 and STAT5.40, 41, 42 That is, naïve T cells are likely to differentiate into Treg when STAT5 expression is increased, while suppressing STAT3 expression. Importantly, IL‐6 expression alters the balance between active STAT3 and STAT5, suppresses differentiation of the naïve T cells into Treg, and promotes naïve T cell differentiation into Th17. Treg numbers have been reported to be significantly higher in peripheral circulation of OC patients than that in healthy individuals.43 High Th17 occupancy ratio in PBMC of OC patients suggests that high STAT3 levels are not always observed in peripheral blood of OC patients. In contrast, pSTAT3 has been reported to be expressed in OC tissues more strongly than ovarian samples from healthy individuals; pSTAT3 expression level was found by immunohistochemistry to be increased in advanced compared to early cancers.44, 45, 46 In addition, pSTAT3 expression level was found to be significantly higher in chemo‐resistant OC cells in vivo.47 This suggests that high numbers of Th17 cells do not always correlate with high STAT3 levels in peripheral blood; however, the tumorigenic STAT3 activity may contribute to high numbers of Th17 cells in OC patients (Figure 5).
Based on our results, we propose that the following changes occur in OC patients. IL‐6 and TGF‐β phosphorylate STAT3 in the naïve T cells, while ROR‐γt is activated to induce differentiation of the naïve T cells into Th17 cells, which then produce IL‐17 and TNF‐α, thereby eliciting an inflammatory response in patients. NF‐κB activation by IL‐17 or TNF‐α stimulation promotes IL‐6, which phosphorylates STAT3; these changes induce PD‐L1 expression in OC cells while reactivating IL‐6 and STAT3 by feedback (Figure 5).
In conclusion, elevation of Th17/IL‐17, which is correlated with NLR or stimulates PD‐L1, is a potential biomarker for predicting prognosis and PD‐L1 overexpression in OC patients.
DISCLOSURE
The authors have no conflict of interest or financial ties to disclose.
Aotsuka A, Matsumoto Y, Arimoto T, et al. Interleukin‐17 is associated with expression of programmed cell death 1 ligand 1 in ovarian carcinoma. Cancer Sci. 2019;110:3068–3078. 10.1111/cas.14174
REFERENCES
- 1. Cancer Registry and Statistics . Cancer Information Service, National Cancer Center, Japan. 2015.
- 2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391:1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong C. IL‐23/IL‐17 biology and therapeutic considerations. J Immunotoxicol. 2008;5:43‐46. [DOI] [PubMed] [Google Scholar]
- 4. Kimura A, Kishimoto T. IL‐6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830‐1835. [DOI] [PubMed] [Google Scholar]
- 5. Ma J, Wang J, Wan J, et al. Morphine disrupts interleukin‐23 (IL‐23)/IL‐17‐mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun. 2010;78:830‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker KH, Beury DW, Ostrand‐Rosenberg S. Myeloid‐derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin‐17 family members. Immunity. 2011;34:149‐162. [DOI] [PubMed] [Google Scholar]
- 8. Duan M, Ning Z, Fu Z, et al. Decreased IL‐27 negatively correlated with Th17 cells in non‐small‐cell lung cancer patients. Mediators Inflamm. 2015;2015:802939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang SC, Fan XH, Pan QM, Sun QS, Liu Y. Decreased expression of IL‐27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. J Neurol Sci. 2015;348:174‐180. [DOI] [PubMed] [Google Scholar]
- 10. Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lockhart E, Green AM, Flynn JL. IL‐17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662‐4669. [DOI] [PubMed] [Google Scholar]
- 12. Korn T, Petermann F. Development and function of interleukin 17‐producing γδ T cells. Ann N Y Acad Sci. 2012;1247:34‐45. [DOI] [PubMed] [Google Scholar]
- 13. Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL‐17‐producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105(40):15505‐15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang B, Rong G, Wei H, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533‐537. [DOI] [PubMed] [Google Scholar]
- 15. Yang L, Qi Y, Hu J, Tang L, Zhao S, Shan B. Expression of Th17 cells in breast cancer tissue and its association with clinical parameters. Cell Biochem Biophys. 2012;62:153‐159. [DOI] [PubMed] [Google Scholar]
- 16. He S, Fei M, Wu Y, et al. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424‐7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilke CM, Kryczek I, Wei S, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD‐1/PD‐L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu L, Chen X, Shen M, et al. Inhibition of IL‐6‐JAK/Stat3 signaling in castration‐resistant prostate cancer cells enhances the NK cell‐mediated cytotoxicity via alteration of PD‐L1/NKG2D ligand levels. Mol Oncol. 2018;12:269‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD‐L1 expression via the IL‐6/JAK/STAT3 signaling pathway in EGFR‐mutated non‐small cell lung cancer. Int J Oncol. 2016;49:1360‐1368. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Yang L, Huang F, et al. Inflammatory cytokines IL‐17 and TNF‐α up‐regulate PD‐L1 expression in human prostate and colon cancer cells. Immunol Lett. 2017;184:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869‐881. [DOI] [PubMed] [Google Scholar]
- 24. Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin‐17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci. 2014;105:933‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL‐17‐producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980‐989. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Q, Hong L, Zuo MZ, He Z. Prognostic significance of neutrophil to lymphocyte ratio in ovarian cancer: evidence from 4,910 patients. Oncotarget. 2017;8:68938‐68949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin W. Prognostic effect of programmed death ligand 1 (PD‐L1) in ovarian cancer: a systematic review, meta‐analysis and bioinformatics study. J Ovarian Res. 2019;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mesnage SJL, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death‐ligand 1 (PD‐L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28:651‐657. [DOI] [PubMed] [Google Scholar]
- 29. Schwanhäusser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337‐342. [DOI] [PubMed] [Google Scholar]
- 30. Abiko K, Matsumura N, Hamanishi J, et al. IFN‐γ from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD‐L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43:1893‐1906. [DOI] [PubMed] [Google Scholar]
- 32. Ogura H, Murakami M, Okuyama Y, et al. Interleukin‐17 promotes autoimmunity by triggering a positive‐feedback loop via interleukin‐6 induction. Immunity. 2008;29:628‐636. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL‐17 can promote tumor growth through an IL‐6‐Stat3 signaling pathway. J Exp Med. 2009;206:1457‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camporeale A, Poli V. IL‐6, IL‐17 and STAT3: a holy trinity in auto‐immunity? Front Biosci (Landmark Ed). 2012;17:2306‐2326. [DOI] [PubMed] [Google Scholar]
- 35. Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF‐κ collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death‐ligand 1 overexpression via the nuclear factor‐κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034‐5045. [DOI] [PubMed] [Google Scholar]
- 37. Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. 2009;47:149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41‐51. [DOI] [PubMed] [Google Scholar]
- 39. Zorn E, Nelson EA, Mohseni M, et al. IL‐2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT‐dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pallandre JR, Brillard E, Créhange G, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft‐versus‐host disease and antitumor immunity. J Immunol. 2007;179:7593‐7604. [DOI] [PubMed] [Google Scholar]
- 41. Zheng Y, Wang Z, Deng L, et al. Modulation of STAT3 and STAT5 activity rectifies the imbalance of Th17 and Treg cells in patients with acute coronary syndrome. Clin Immunol. 2015;157:65‐77. [DOI] [PubMed] [Google Scholar]
- 42. Laurence A, Tato CM, Davidson TS, et al. Interleukin‐2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371‐381. [DOI] [PubMed] [Google Scholar]
- 43. Cannioto RA, Sucheston‐Campbell LE, Hampras S, et al. The association of peripheral blood regulatory T‐cell concentrations with epithelial ovarian cancer: a brief report. Int J Gynecol Cancer. 2017;27:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004;94:630‐635. [DOI] [PubMed] [Google Scholar]
- 45. Min H, Wei‐hong Z. Constitutive activation of signal transducer and activator of transcription 3 in epithelial ovarian carcinoma. J Obstet Gynaecol Res. 2009;35:918‐925. [DOI] [PubMed] [Google Scholar]
- 46. McCann GA, Naidu S, Rath KS, et al. Targeting constitutively‐activated STAT3 in hypoxic ovarian cancer, using a novel STAT3 inhibitor. Oncoscience. 2014;1:216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duan Z, Foster R, Bell DA, et al. Signal transducers and activators of transcription 3 pathway activation in drug‐resistant ovarian cancer. Clin Cancer Res. 2006;12:5055‐5063. [DOI] [PubMed] [Google Scholar]


