Abstract
Anaplastic lymphoma kinase (ALK) fusions have been recognized as a therapeutic target in non‐small cell lung cancer (NSCLC). However, molecular signatures and clinical characteristics of the Chinese population with ALK‐rearranged NSCLC are not well elucidated. In the present study, we carried out targeted next‐generation sequencing on tissue and plasma ctDNA samples in 1688 patients with NSCLC. Overall, ALK fusions were detected in 70 patients (4.1%), and the frequencies of ALK fusions detected in tissue and plasma samples were 5.1% and 3.3%, respectively. Additionally, the prevalence of breakpoint locations for EML4‐ALK fusions in ctDNA was significantly correlated with that in tumor tissues (R 2 = .91, P = .045). According to age, the incidence rates of ALK fusions among young (age <45 years), middle‐aged (between 45 and 70 years) and elderly (>70 years) patients were significantly different (P < .001). In 70 ALK‐rearranged cases, coexistence of epidermal growth factor receptor (EGFR) alterations and ALK fusions was detected in 12 cases (17.1%) and EGFR mutations tended to coexist with non‐EML4‐ALK rearrangements. Notably, novel ALK fusion partners, including TRIM66,SWAP70,WNK3,ERC1,TCF12 and FBN1 were identified in the present study. Among EML4‐ALK fusion variants, patients with variant V1 were younger than patients with variant V3 (P = .023), and TP53 mutations were more frequently concurrent with variant V3 compared with variant V1 (P = .009). In conclusion, these findings provide new insights into the molecular‐clinical profiles of patients with ALK‐rearranged NSCLC that may improve the treatment strategy of this population.
Keywords: ALK, circulating tumor DNA, next‐generation sequencing, non‐small cell lung cancer, tissue
1. INTRODUCTION
Non‐small cell lung cancer (NSCLC) is the most common type of cancer and a leading cause of cancer‐associated mortality worldwide.1 Detection of driver genes in patients with NSCLC has become standard for clinical decision‐making. Rearrangement of the anaplastic lymphoma kinase (ALK) gene has been identified as a specific molecular subtype in NSCLC and patients with this rearrangement respond to ALK tyrosine kinase inhibitors (TKI).2, 3, 4 Although ALK‐TKI can dramatically improve the clinical efficacy, responses have been shown to widely vary between patients.5 Therefore, it is critical to determine the potential mechanisms associated with the heterogeneous outcomes.
In addition to ALK, several other oncogenic driver genes, including epidermal growth factor receptor ( EGFR), ROS1, BRAF, MET, RET and ERBB2, have been defined as important targets in NSCLC.6 Traditional detection methods of ALK rearrangements, including FISH and immunohistochemistry (IHC), are limited by certain technical limitations, including signal instability and scoring difficulties.7, 8 Additionally, these detection methods are unable to interrogate different driver gene mutations at the same time. Next‐generation sequencing (NGS) has become an alternative method for determining ALK fusions in NSCLC with high sensitivity and specificity,9 which can also simultaneously detect multiple gene alterations and complete a comprehensive analysis of genomic alterations, including single nucleotide variations, insertions, deletions, copy number variations and chromosomal rearrangements. At present, detection of genomic alterations predominantly relies on the analysis of cancer tissues obtained by surgical excision or biopsy. However, tumor tissues are not always available from all patients and a tissue biopsy of a single site may not fully reflect the tumor genomic landscape due to tumor genetic heterogeneity, which limits the utility of tissue‐based sequencing.10, 11 Circulating tumor DNA (ctDNA), released into the plasma from apoptotic and necrotic tumor cells originating from primary tumors and metastatic lesions, comprises tumor‐specific sequence alterations and may be an alternative to tissue sample biopsies.12, 13 A number of studies have indicated that a ctDNA assay could be used for patients without acquirable tissue samples to screen for genetic variations and thus guide treatment decisions for patients with NSCLC.14, 15, 16 Plasma ctDNA is not suitable for the detection of ALK rearrangements by IHC or FISH methods as it is highly fragmented DNA of approximately 150 bp and cannot provide protein information.17, 18 In contrast, ctDNA‐based NGS assays serve a crucial role in simultaneously identifying ALK arrangements and other driver gene alterations in patients with NSCLC whose tumor samples are insufficient or unobtainable.
Fusion partners of the ALK gene are variable, including EML4, KIF5B, HIP1, TFG and more.19, 20, 21 EML4 is the most common partner in ALK translocation‐positive patients with NSCLC, but breakpoints within the EML4 gene often occur at different sites. Among the EML4‐ALK variants identified in previous studies, variant V1 [exon 13 of EML4 fuses to exon 20 of ALK (E13;A20)] and variant V3 [exon 6 of EML4 fuses to exon 20 of ALK (E6;A20)] have been shown to be the most predominant.22, 23, 24 Variant V3 has recently been identified as a high‐risk factor in ALK translocation‐positive lung adenocarcinoma, which is correlated with metastasis and short overall survival (OS).25, 26, 27
Although a few studies have evaluated the frequency of ALK rearrangements in Chinese patients with NSCLC,28, 29 the genetic profile of ALK fusions has not been particularly well studied. Using NGS‐based tissue and ctDNA assays with a large NSCLC cohort, the current study investigated the genomic landscape and characterized the heterogeneity of ALK fusions. In addition, the clinical and molecular characteristics of ALK‐positive patients were further analyzed, which may provide comprehensive knowledge of the complexity of this population.
2. MATERIALS AND METHODS
2.1. Sample collection
The present study enrolled 1688 patients with NSCLC who were treated at multiple hospitals across China, including Affiliated Sir Run Run Shaw Hospital of Zhejiang University (Hangzhou), Zhejiang Cancer Hospital (Hangzhou), Quzhou People's Hospital (Quzhou), Lishui Municipal Central Hospital (Lishui) and First Affiliated Hospital of Wenzhou Medical University (Wenzhou) between September 2017 and February 2019. The specimens obtained included 885 plasma and 803 tissue samples. Inclusion criteria for the patients were as follows: (i) patients were diagnosed with NSCLC; and (ii) at least 150 ng and 15 ng DNA from each tissue and ctDNA sample, respectively, were successfully extracted. Patients who had other malignant tumors and serious mental illness were excluded. All experiments were carried out in accordance with the relevant guidelines and regulations of the ethical committees of the participating hospitals. Written informed consent was obtained from all participants.
2.2. DNA extraction and library preparation
ctDNA was isolated from at least 2 mL plasma with a QIAamp Circulating Nucleic Acid kit (Qiagen GmbH), according to the manufacturer's protocol. Tissue DNA was extracted using the QIAamp Genomic DNA kit (Qiagen GmbH). Quality and quantification of the DNA were assessed using the Agilent 2100 BioAnalyzer (Agilent Technologies, Inc.) and Qubit ds DNA HS assay kit (Thermo Fisher Scientific, Inc.). Sequencing libraries were constructed according to the Illumina standard library construction instructions (Illumina, Inc.).
2.3. Next‐generation sequencing
The various libraries were hybridized with a nine‐gene panel, which was enriched for the coding regions and selected introns of genes with known relevance to NSCLC. The target‐enriched libraries were pooled and sequenced on an Illumina HiSeq2500 NGS platform (Illumina, Inc.). Sequencing depth was >10 000×. Genomic data were then processed by a relevant bioinformatics platform. Multiple types of genomic alterations, including single‐nucleotide variants, small insertions or deletions, copy number variants and rearrangements of ALK, RET and ROS1 were identified.
2.4. Statistical analysis
SPSS 21.0 statistical software (IBM Corp.) was used to analyze the data. Associations between ALK rearrangements and clinical characteristics were analyzed using Fisher's exact test or chi‐squared test. Differences in continuous variables were assessed with Student's t‐test. A two‐sided P < .05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Identification and incidence rate of ALK rearrangements in NSCLC
In the present study, a total of 1688 patients diagnosed with NSCLC were enrolled. NGS was carried out for 885 plasma ctDNA and 803 tissue samples. In total, 70 cases with ALK rearrangements were identified, with an overall incidence rate of 4.1%. According to the sample category, the frequencies of ALK fusions detected in tissue and plasma samples were 5.1% and 3.3%, respectively. Although the frequency of ALK fusions detected by ctDNA profiling was lower compared with that detected by tissue sequencing, no statistically significant differences were observed (P = .067) (Table 1).
Table 1.
Frequencies of ALK rearrangements among 1688 patients with NSCLC
| Histological type | Total | ALK rearrangements status | |||
|---|---|---|---|---|---|
| Positive | Negative | Freq (%) | P value | ||
| Tissue | 803 | 41 | 762 | 5.1 | .067 |
| ctDNA | 885 | 29 | 856 | 3.3 | |
ALK, anaplastic lymphoma kinase; ctDNA, circulating tumor DNA; Freq, frequency of ALK rearrangements; NSCLC, non‐small cell lung cancer.
3.2. Molecular and clinical characteristics of patients with ALK rearrangements
Among all patients, median age at diagnosis was 62 years (range: 24‐89 years) and 57.0% of the patients were male. Patients with ALK fusions were significantly younger than those without ALK fusions (median age: 53 vs 62 years, P < .001). Previous studies have reported age cutoff points of 45 and 70 years to determine a patient as young or elderly, respectively.30, 31 In the present study, the frequencies of ALK fusions between young (<45 years), middle‐aged (between 45 and 70 years) and elderly (>70 years) patients were compared. As presented in Figure 1, the prevalence of ALK fusions in young patients was significantly higher compared with the other two groups (P < .001). Additionally, middle‐aged patients showed higher frequencies of ALK fusions compared with elderly patients (3.8% vs 2.2%), whereas no statistically significant difference was observed (P = .160). Further analysis showed that ALK rearrangements were significantly associated with females (P = .019). Patients without EGFR or KRAS mutations more frequently presented with ALK rearrangements compared with patients with EGFR or KRAS mutations (7.0% vs 1.5%, P < .001; 4.8% vs 0.4%, P = .001, respectively). Furthermore, a potentially statistical difference was identified between the frequencies of ALK rearrangements in patients with and without ERBB2 mutations (1.3% vs 4.4%, respectively, P = .058) (Table 2).
Figure 1.
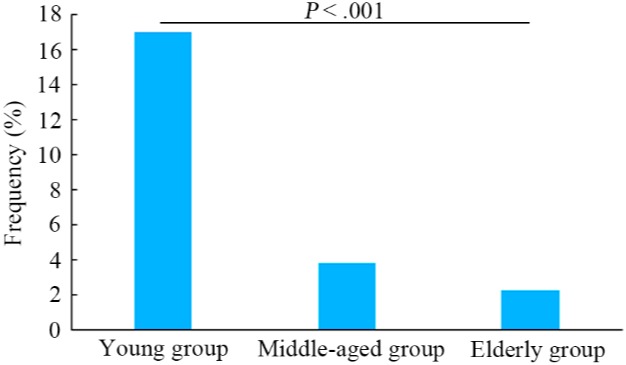
Prevalence of anaplastic lymphoma kinase (ALK) rearrangements in young (<45 y), middle‐aged (between 45 and 70 y) and elderly patients (>70 y) with non‐small cell lung cancer. Statistical significance was defined as P < .05
Table 2.
Association analysis between ALK rearrangements and clinical or molecular characteristics
| Characteristic | Total | ALK rearrangements status | Negative | Freq (%) | P value |
|---|---|---|---|---|---|
| Positive | |||||
| Age, y, median (range) | 62 (24‐89) | 53 (32‐83) | 62 (24‐89) | <.001 | |
| Gender | |||||
| Male | 963 | 31 | 932 | 3.2 | .039 |
| Female | 703 | 39 | 664 | 5.5 | |
| Unknown | 22 | 0 | 22 | 0 | |
| EGFR | |||||
| Wild type | 813 | 58 | 755 | 7.1 | <.001 |
| Mutations | 875 | 12 | 863 | 1.4 | |
| KRAS | |||||
| Wild type | 1429 | 69 | 1360 | 4.8 | <.001 |
| Mutations | 259 | 1 | 258 | 0.4 | |
| ERBB2 | |||||
| Wild type | 1531 | 68 | 1463 | 4.4 | .058 |
| Mutations | 157 | 2 | 155 | 1.3 | |
ALK, anaplastic lymphoma kinase; Freq, frequency of ALK rearrangements.
3.3. Genomic heterogeneity of ALK rearrangements
For ALK translocation‐positive patients, the frequency of the EML4 gene as the predominant partner of ALK was 84.3% (Figure 2A). Previously reported partners of ALK identified in the present study included KIF5B, HIP1 and TFG, and novel ALK fusion partners included TRIM66, SWAP70, WNK3, ERC1, TCF12 and FBN1 (Table 3). Among the EML4‐ALK fusion variants, frequencies of variant V1, variant V2 and variant V3 were 38.9%, 15.3% and 30.5%, respectively (Figure 2B). An increasing number of studies have reported that patients with different EML4‐ALK variants show heterogeneous clinical outcomes to ALK‐TKI for NSCLC.25, 26, 27 Therefore, we hypothesized that molecular or clinical characteristics associated with different variants might show some difference. In the present study, the age of patients with variant V1 was significantly younger compared with that of patients with variant V3. Additionally, the frequency of TP53 concurrent with variant V3 was markedly higher compared with that of TP53 concurrent with variant V1 (Figure 2C,D). Further analysis indicated that prevalence of breakpoint locations for EML4‐ALK fusions found in ctDNA was strongly correlated with that found in tumor tissues (R 2 = .91, P = .045) (Figure 3).
Figure 2.
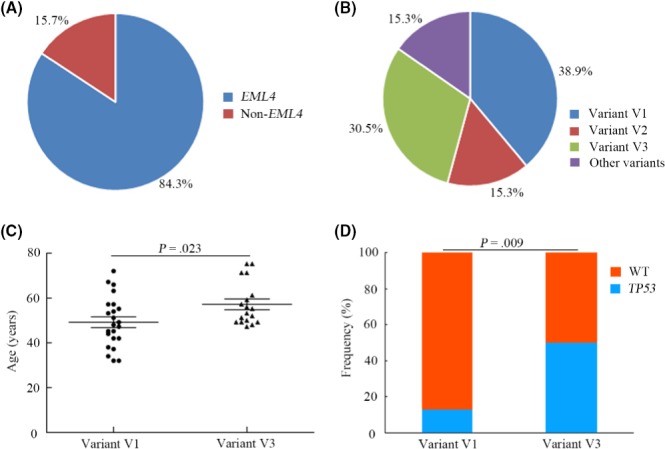
Genomic heterogeneity of anaplastic lymphoma kinase (ALK) rearrangements and characteristics of patients with different EML4‐ALK variants. A, Frequency of EML4 and non‐EML4 partners of ALK fusions. B, Frequency of different EML4‐ALK fusion variants. C, Age difference between EML4‐ALK fusion variant V1 and variant V3 patients. D, Distribution of TP53 mutations between EML4‐ALK fusion variant V1 and variant V3 patients. WT, wild type
Table 3.
Distribution of non‐EML4 partners of ALK fusions in the present study
| Non‐EML4 fusion partners | No. of patients |
|---|---|
| KIF5B | 2 |
| HIP1 | 2 |
| TFG | 1 |
| TRIM66 | 1 |
| SWAP70 | 1 |
| WNK3 | 1 |
| ERC1 | 1 |
| TCF12 | 1 |
| FBN1 | 1 |
ALK, anaplastic lymphoma kinase.
Figure 3.
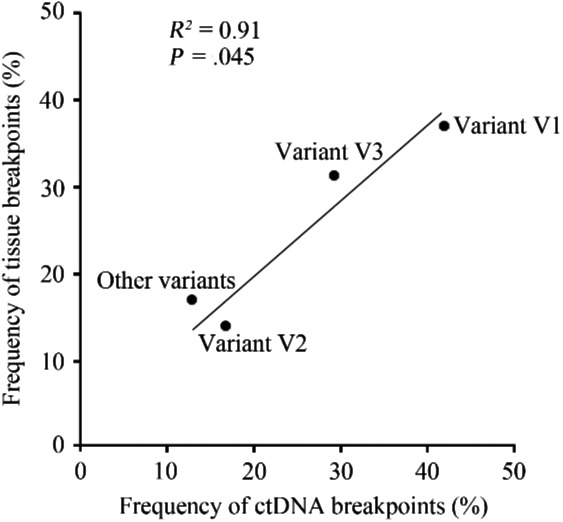
Correlation analysis between frequencies of different variants of EML4‐ALK fusions in ctDNA and tissue specimens. Variant V1, exon 13 of EML4 fuses to exon 20 of ALK; variant V2, exon 20 of EML4 fuses to exon 20 of ALK; variant V3, exon 6 of EML4 fuses to exon 20 of ALK. ALK, anaplastic lymphoma kinase; ctDNA, circulating tumor DNA
3.4. Case with longitudinal ctDNA ALK analysis
During the present study, a 52‐year‐old male patient was diagnosed with brain metastases of lung adenocarcinoma in June 2016 and was treated with six cycles of chemotherapy including cisplatin and gemcitabine. After 8 months, the patient presented with progressive disease (PD) and analysis of the tissue specimen by Sanger sequencing and FISH showed that the patient carried EML4‐ALK fusion but no EGFR mutation; therefore, the patient received crizotinib treatment. However, disease progression was observed after 7 months and the patient showed a poor performance status. The patient's plasma ctDNA sample was then evaluated by NGS to investigate the potential resistance mechanisms and guide the subsequent therapy. EML4‐ALK fusion, ALK G1202R, and TP53 A74 fs mutations were identified, and the mutant allele frequencies of these were 2.68%, 0.85%, and 3.01%, respectively. Based on the current National Comprehensive Cancer Network (NCCN) Guidelines for NSCLC (version 8.2017), the patient was given brigatinib after crizotinib treatment. However, the patient experienced rapid disease progression within 3 months, at which time the plasma ctDNA sample was reassessed. ALK rearrangement, ALK G1202R, and TP53 A74 fs mutations were identified again; however, no novel mutations were detected. These data showed that the abundance of EML‐ALK fusions and TP53 A74 fs mutations decreased dramatically (to 0.81% and 1.06%, respectively), whereas the abundance of ALK G1202R mutations increased and reached a relatively high level (to 1.94%) (Figure 4). Based on the NGS result, further analysis showed that the variant type of EML4‐ALK fusion in this case was variant V3, which was consistent with the finding that variant V3 of EML4‐ALK fusion tended to coexist with TP53 mutation.
Figure 4.
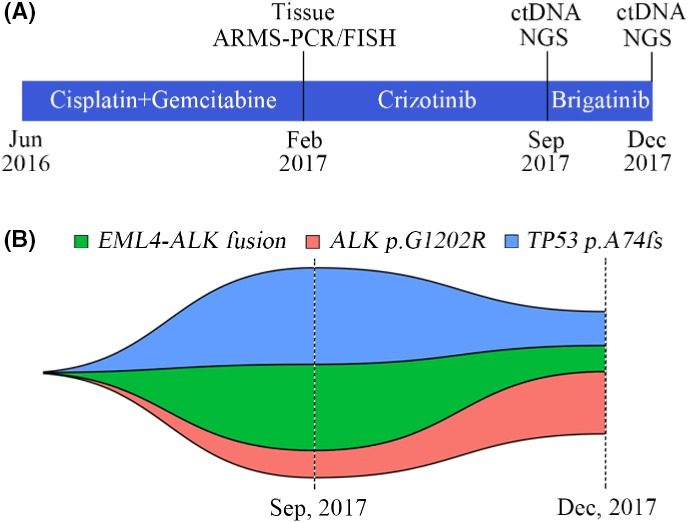
Longitudinal analysis of plasma ctDNA ALK alterations from a patient. A, Timeline indicates different treatments. B, Map of clonal evolution. Two plasma ctDNA samples analyzed by next‐generation sequencing (NGS) were collected during different treatments. Different colors represent different gene mutations. ALK, anaplastic lymphoma kinase; ARMS, amplification refractory mutation system; ctDNA, circulating tumor DNA; fs, frameshift mutation
3.5. Analysis of concurrent ALK rearrangements and other driver gene mutations
Although previous studies have reported that ALK rearrangements are mutually exclusive with other driver gene mutations in NSCLC, a number of studies have identified that ALK fusions coexist with other genomic alterations, and such co‐alterations may affect the therapeutic outcome of patients.32, 33, 34 As NGS can simultaneously detect multiple gene alterations, the concurrent pattern of ALK fusions with other driver gene alterations, including EGFR, ROS1, BRAF, MET, ERBB2, RET and KRAS, were analyzed in the present study. Among 70 ALK‐positive cases, the coexistence of ALK fusions and EGFR mutations was detected in 12 cases (17.1%) (Figure 5). According to EGFR mutant categories, ALK fusions primarily coexisted with EGFR general mutant types, including EGFR exon 19 deletion and EGFR exon 21 L858R mutation (Figure S1). The frequency of co‐alteration between ALK rearrangements and EGFR exon 19 deletion tended to be higher compared with that between ALK rearrangements and EGFR exon 21 L858R mutation, but no significant statistical difference was identified (1.46% vs 0.67%, P = .341). According to the types of ALK fusion partner, non‐EML4‐ALK fusions tended to coexist with EGFR alterations (P = .065) (Figure S2). In addition, concurrent MET amplification, and ERBB2 and KRAS mutations were also observed in ALK rearrangement‐positive patients (Figure 5). No BRAF, ROS1 and RET‐associated variations concurrently presented with ALK fusions.
Figure 5.
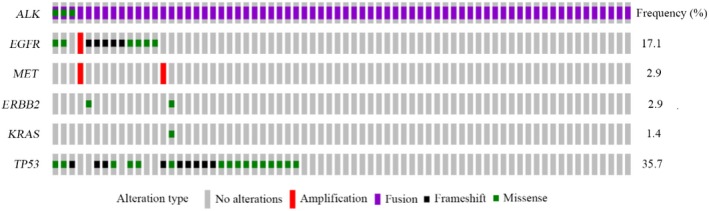
Genetic profiles in patients with ALK‐rearranged non‐small cell lung cancer. Samples of 70 patients harboring ALK fusions were analyzed by targeted next‐generation sequencing assays. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor
4. DISCUSSION
The present study provided an overview of the prevalence and features of genomic heterogeneity of ALK fusions in a large cohort of Chinese patients with NSCLC. Previous studies have reported that the frequency of ALK fusion is ~2%‐7% in NSCLC.35, 36, 37 The incidence rate of ALK rearrangements in the current study was 4.1%, which is comparable with the previously published data. Frequencies of ALK fusions detected in tissue and plasma samples were 5.1% and 3.3%, respectively. To the best of our knowledge, the present study is the first to simultaneously evaluate the prevalence of ALK rearrangements in a large number of tissue and plasma ctDNA samples, and comprehensively analyze the concurrent status of ALK rearrangements and other driver gene mutations in NSCLC. Although the frequency of ALK rearrangement in ctDNA was lower compared with that in tissue samples, the present data indicated that detection of ALK translocations by ctDNA‐based NGS was required to guide treatment decisions in patients with NSCLC with inadequate or unacquirable tissue samples. Moreover, breakpoint patterns of EML4‐ALK fusions between ctDNA and tissue samples were also compared, and frequencies of different EML4‐ALK variants in ctDNA samples showed a strong similarity with those observed in tumor tissues, further supporting the feasibility and use of plasma ctDNA for detection of ALK fusions among patients with NSCLC. Incidence rates of ALK fusions among young, middle‐aged, and elderly patients showed significant differences, which indicates that in NSCLC the genomic landscape among different age categories is diverse. According to previous studies, ALK fusions either do not differ among different genders or generally occur more frequently in females,38 whereas Kang et al39 reported that ALK‐positive patients were more commonly male. The present results also showed a markedly higher prevalence of ALK fusions in female patients.
All samples were profiled by targeted sequencing with a panel of nine of the most common driver genes in NSCLC, including EGFR, ALK, ROS1, BRAF, MET, RET, ERBB2, KRAS and TP53. Among the ALK‐positive cases, 17.1% of patients were identified to show concurrent EGFR mutations and ALK fusions. Although a number of studies have reported that ALK rearrangements are not frequently concomitant with EGFR mutations,40, 41 the current results showed that such co‐alterations accounted for a relatively high proportion of ALK rearrangement‐positive patients with NSCLC. EGFR‐TKI and ALK‐TKI have been approved for EGFR mutation‐positive and ALK translocation‐positive advanced NSCLC, respectively.42 However, the clinical activity of EGFR‐TKI and ALK‐TKI among patients with such co‐alterations remains largely unknown and how to use these TKI remains controversial. Limited studies found that the response rate to both TKI in this population appears to be slightly lower compared with that in EGFR mutation‐positive or ALK rearrangement‐positive patients.43 Additionally, several studies have reported that different orders of treatment lines of EGFR‐TKI and ALK‐TKI affect the therapeutic outcomes of this subgroup of patients.32, 33, 34 Moreover, numerous studies reported that EGFR mutation and ALK rearrangements may coexist in the same tumor cells44 or may occur in different lesions of the tumor,45 which may have an effect on the treatment strategies.46 As ctDNA was released from tumor lesions of the whole body, the result of ALK/EGFR co‐alteration from ctDNA should be further verified by tissue samples from different tumor foci in patients with multifocal NSCLC if possible, in order to clarify the intratumoral or intertumoral coexistence of such co‐alteration and guide the subsequent therapy. Therefore, the treatment strategies for patients with NSCLC harboring such co‐alterations deserve more attention. NGS can simultaneously detect multiple genomic alterations with tissue or plasma ctDNA samples; therefore, the present study comprehensively and simultaneously assessed EGFR mutations and ALK fusions, with the aim of establishing optimal treatment decisions for EGFR and ALK mutation‐positive patients with NSCLC. In addition to EGFR mutations, this study also showed that ALK rearrangements were concurrent with KRAS, ERBB2, and MET mutations, which may have important consequences regarding treatment. Therefore, the selection of optimal targeted therapy is crucial for this population.
FISH is approved as the gold standard for detecting ALK fusions and IHC is an effective routine examination approach; however, neither of these methods can distinguish between different ALK translocation variants. NGS has increasingly been applied for the analysis of gene fusions in clinical cancer samples, particularly for identifying novel partner genes of ALK fusions. Although numerous studies have reported that the ALK gene fuses with a number of genes in NSCLC,19, 20, 21 new fusion partner genes are increasingly being discovered. In the present study, novel ALK fusion partner genes, including TRIM66, SWAP70, WNK3, ERC1, TCF12 and FBN1 were identified, which encompass specific domains such as coiled‐coil, basic helix‐loop‐helix (bHLH) and proline/glycine‐rich regions mediating the dimerization of these genes.47, 48, 49, 50, 51, 52 This dimerization might result in activation of the tyrosine kinase function of ALK and thereby confers marked tumorigenic activity. Additionally, the result also indicated that a technical limitation of FISH and IHC may be the identification of novel ALK fusion partners, which affects the personal therapy of patients. It is well known that EML4 is the most frequent and studied ALK fusion partner gene. Among the EML4‐ALK fusion variants evaluated in the current study, variant V1 (37.2%) and variant V3 (32.2%) were the most prevalent, which was similar to previous reports.24 An increasing number of studies have indicated that different EML4‐ALK variants have an impact on the prognosis of EML4‐ALK‐positive NSCLC patients. According to these publications, variant V3 tends to be a higher risk feature associated with poor prognosis compared with variant V1.25, 26, 27 The present study identified that the age of patients with variant V3 was significantly older compared with that of patients with variant V1. According to previous studies, young patients with lung cancer show improved survival.53, 54 The present data provide a novel insight into the differences in prognosis between EML4‐ALK variant V1 and variant V3 patients. A number of studies have demonstrated that TP53 mutations predict a poor outcome following systemic therapy for ALK fusion NSCLC, and co‐alterations between EML4‐ALK variant V3 and TP53 mutations define a patient subset with worse prognosis.55, 56, 57 The present study demonstrated that the frequency of concurrent TP53 mutations and EML4‐ALK variant V3 was markedly higher compared with that of concurrent TP53 mutations and EML4‐ALK variant V1, which indicates that TP53 mutation may be one of the critical reasons for the differences in prognosis between patients with EML4‐ALK variant V1 and variant V3 NSCLC.
Among the ALK translocation‐positive patients, there was a case with longitudinal plasma ctDNA samples. An EML4‐ALK fusion was identified at the time of initial diagnosis by FISH with a tissue specimen and the patient was then given crizotinib. However, disease progression was observed after 7 months with crizotinib treatment. Further molecular characterization with a plasma ctDNA sample showed EML4‐ALK fusion, and ALK G1202R and TP53 A74 fs mutations. According to the current guidelines for NSCLC, the patient was subsequently given brigatinib; however, the patient experienced rapid disease progression within 3 months. Further investigation using ctDNA samples based on NGS assays showed that the abundance of EML‐ALK fusions and TP53 A74 fs mutations decreased markedly, whereas the abundance of ALK G1202R mutations increased to a relatively high level, which indicates that the ALK G1202R mutation may be associated with brigatinib resistance. Although limited studies have previously reported that ctDNA could be used for ALK fusion detection,58, 59 the present results further demonstrated the clinical utility of ctDNA for the detection of ALK fusions and the investigation of molecular mechanisms of ALK inhibitor resistance in NSCLC. According to the current guidelines for NSCLC, second‐generation ALK‐TKI, including brigatinib and ceritinib, are recommended for ALK‐positive patients following failure of crizotinib therapy. However, the patient in the present study experienced rapid progression following brigatinib treatment. At the time of identification of ALK G1202R, several studies reported that the ALK G1202R mutation is associated with first‐generation and second‐generation ALK‐TKI resistance, and third‐generation ALK‐TKI, including lorlatinib, potentially overcome this resistance.60, 61 The unsatisfactory clinical response of brigatinib treatment in the current case also suggests that there should be more focus on individualized medicine in cancer treatment, rather than a total dependence on relevant guidelines for the guidance of treatment decisions.
In conclusion, the present study evaluated a large prospective cohort to investigate the prevalence of ALK fusions in Chinese patients with NSCLC using targeted sequencing technology with tissue and plasma samples. The results showed that plasma ctDNA samples can serve crucial roles in detecting ALK fusions or resistant mutations among patients with inadequate or unacquirable tissue samples for molecular testing, which greatly contributes to the guidance of treatment decisions for these patients. Identification of novel ALK fusion partners, coexistence between ALK fusions and variants of other oncogenic drivers, and the characterization of EML4‐ALK‐postive patients support the critical use of molecular profiling by NGS in NSCLC.
DISCLOSURE
Huanqing Cheng, Huina Wang, Feng Lou and Shanbo Cao are employees of AcornMed Biotechnology. The other authors are not employees of AcornMed Biotechnology, but they cooperate with AcornMed Biotechnology in certain fields. This work was completed by the cooperation of AcornMed Biotechnology and the other authors.
Supporting information
ACKNOWLEDGMENTS
This study was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LQ16H160003, No. LY17H160029, and No. LQ17H160011) and Medical Scientific Research Foundation of Zhejiang Province of China (No. 2016ZDB007, No. 2017ZD021, and No. 2019RC027).
Zhou X, Shou J, Sheng J, et al. Molecular and clinical analysis of Chinese patients with anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer. Cancer Sci. 2019;110:3382–3390. 10.1111/cas.14177
Xiaoyun Zhou and Jiawei Shou contributed equally to this study.
Contributor Information
Shanbo Cao, Email: shanbocao@acornmed.com.
Hongming Pan, Email: panhongming@zju.edu.cn.
Yong Fang, Email: fangyong@zju.edu.cn.
REFERENCES
- 1. Torr LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med. 2010;363:1693‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of Crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368:2385‐2394. [DOI] [PubMed] [Google Scholar]
- 5. Solomon BJ, Mok T, Kim DW, et al. First‐line Crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371:2167‐2177. [DOI] [PubMed] [Google Scholar]
- 6. Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11:613‐638. [DOI] [PubMed] [Google Scholar]
- 7. Dacic S, Villaruz LC, Abberbock S, Mahaffey A, Incharoen P, Nikiforova MN. ALK FISH patterns and the detection of ALK fusions by next generation sequencing in lung adenocarcinoma. Oncotarget. 2016;7:82943‐82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu CW, Wang WX, Chen YP, et al. Simultaneous VENTANA IHC and RT‐PCR testing of ALK status in Chinese non‐small cell lung cancer patients and response to Crizotinib. J Transl Med. 2018;16:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Letovanec I, Finn S, Zygoura P, et al. Evaluation of NGS and RT‐PCR methods for ALK rearrangement in European NSCLC Patients: results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2018;13:413‐425. [DOI] [PubMed] [Google Scholar]
- 10. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early‐ and late‐stage human malignancies. Sci Transl Med. 2014;6:224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks JK, Saller J, Wang E, Boyle T, Gray JE. Cell‐free circulating tumor DNA supplementing tissue biopsies for identification of targetable mutations: Implications for precision medicine and considerations for reconciling results. Lung Cancer. 2017;111:135‐138. [DOI] [PubMed] [Google Scholar]
- 14. Xu S, Lou F, Wu Y, et al. Circulating tumor DNA identified by targeted sequencing in advanced‐stage non‐small cell lung cancer patients. Cancer Lett. 2016;370:324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pisapia P, Pepe F, Smeraglio R, et al. Cell free DNA analysis by SiRe® next generation sequencing panel in non small cell lung cancer patients: focus on basal setting. J Thorac Dis. 2017;9:S1383‐S1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mayo‐de‐Las‐Casas C, Jordana‐Ariza N, Garzón‐Ibañez M, et al. Large scale, prospective screening of EGFR mutations in the blood of advanced NSCLC patients to guide treatment decisions. Ann Oncol. 2017;9:2248‐2255. [DOI] [PubMed] [Google Scholar]
- 17. Mouliere F, Robert B, Arnau Peyrotte E, et al. High fragmentation characterizes tumor‐derived circulating DNA. PLoS ONE. 2011;6:e23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer‐genetics in the blood. Nat Rev Clin Oncol. 2013;10:472‐484. [DOI] [PubMed] [Google Scholar]
- 19. Takeuchi K, Choi YL, Togashi Y, et al. KIF5B‐ALK, a novel fusion oncokinase identified by an immunohistochemistry‐based diagnostic system for ALK‐positive lung cancer. Clin Cancer Res. 2015;15:3143‐3149. [DOI] [PubMed] [Google Scholar]
- 20. Fang DD, Zhang B, Gu Q, et al. HIP1‐ALK, a novel ALK fusion variant that responds to Crizotinib. J Thorac Oncol. 2014;9:285‐294. [DOI] [PubMed] [Google Scholar]
- 21. Evangelista AF, Zanon MF, Carloni AC, et al. Detection of ALK fusion transcripts in FFPE lung cancer samples by NanoString technology. BMC Pulm Med. 2017;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription‐PCR screening for EML4‐ALK fusion transcripts. Clin Cancer Res. 2008;14:6618‐6624. [DOI] [PubMed] [Google Scholar]
- 23. Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4‐ALK transforming gene in non‐small cell lung cancer. Cancer Res. 2008;68:4971‐4976. [DOI] [PubMed] [Google Scholar]
- 24. Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4‐ALK non‐small cell lung cancer. Eur J Cancer. 2010;46:1773‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4‐ALK fusion variants to various ALK inhibitors in advanced ALK‐rearranged non‐small cell lung cancer. Ann Oncol. 2017;28:791‐797. [DOI] [PubMed] [Google Scholar]
- 26. Christopoulos P, Endris V, Bozorgmehr F, et al. EML4‐ALK fusion variant V3 is a high‐risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non‐small cell lung cancer. Int J Cancer. 2018;142:2589‐2598. [DOI] [PubMed] [Google Scholar]
- 27. Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4‐ALK variant on resistance mechanisms and clinical outcomes in ALK‐positive lung cancer. J Clin Oncol. 2018;36:1199‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu Y, Ding Z, Zhu L, Teng H, Lu S. Frequencies of ALK rearrangements in lung adenocarcinoma subtypes: a study of 2299 Chinese cases. SpringerPlus. 2016;5:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao R, Zhang J, Han Y, et al. Clinicopathological features of ALK expression in 9889 cases of non‐small‐cell lung cancer and genomic rearrangements identified by capture‐based next‐generation sequencing: a Chinese retrospective analysis. Mol Diagn Ther. 2019;23(3):395‐405. [DOI] [PubMed] [Google Scholar]
- 30. Aridgides PD, Janik A, Bogart JA, Duffy S, Rosenbaum P, Gajra A. Radiotherapy for stage III non‐small‐cell lung carcinoma in the elderly (age ≥ 70 years). Clin Lung Cancer. 2013;14:674‐679. [DOI] [PubMed] [Google Scholar]
- 31. Hsu CH, Tseng CH, Chiang CJ, et al. Characteristics of young lung cancer: analysis of Taiwan's nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status. Oncotarget. 2016;7:46628‐46635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77:460‐463. [DOI] [PubMed] [Google Scholar]
- 33. Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26:348‐354. [DOI] [PubMed] [Google Scholar]
- 34. Sahnane N, Frattini M, Bernasconi B, et al. EGFR and KRAS mutations in ALK‐positive lung adenocarcinomas: biological and clinical effect. Clin Lung Cancer. 2016;17:56‐61. [DOI] [PubMed] [Google Scholar]
- 35. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448:561‐566. [DOI] [PubMed] [Google Scholar]
- 36. Solomon B, Varella‐Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non‐small cell lung cancer. J Thorac Oncol. 2009;4:1450‐1454. [DOI] [PubMed] [Google Scholar]
- 37. Yokoyama A, Tamura A, Miyakawa K, et al. Pulmonary adenocarcinoma, harboring both an EGFR mutation and ALK rearrangement, presenting a stable disease to erlotinib and a partial response to alectinib. Intern Med. 2018;57:2377‐2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Li Y, Yang T, et al. Clinical significance of EML4‐ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non‐small cell lung cancer. PLoS ONE. 2013;8:e52093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang HJ, Lim HJ, Park JS, et al. Comparison of clinical characteristics between patients with ALK‐positive and EGFR‐positive lung adenocarcinoma. Respir Med. 2014;108:388‐394. [DOI] [PubMed] [Google Scholar]
- 40. Shaw AT, Yeap BY, Mino‐Kenudson M, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27:4247‐4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR‐TKI and Crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20:1383‐1392. [DOI] [PubMed] [Google Scholar]
- 42. Clark JW, Longo DL. Recent progress in systemic treatment for lung cancer. Curr Opin Pulm Med. 2018;24:355‐366. [DOI] [PubMed] [Google Scholar]
- 43. Zhao N, Zheng SY, Yang JJ, et al. Lung adenocarcinoma harboring concomitant EGFR mutation and EML4‐ALK fusion that benefits from three kinds of tyrosine kinase inhibitors: a case report and literature review. Clin Lung Cancer. 2015;16:e5‐e9. [DOI] [PubMed] [Google Scholar]
- 44. Baldi L, Mengoli MC, Bisagni A, Banzi MC, Boni C, Rossi G. Concomitant EGFR mutation and ALK rearrangement in lung adenocarcinoma is more frequent than expected: report of a case and review of the literature with demonstration of genes alteration into the same tumor cells. Lung Cancer. 2014;86:291‐295. [DOI] [PubMed] [Google Scholar]
- 45. Cai W, Lin D, Wu C, et al. Intratumoral heterogeneity of ALK‐rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol. 2015;33:3701‐3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhuang X, Zhao C, Li J, et al. Clinical features and therapeutic options in non‐small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019;8:2858‐2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esposito D, Koliopoulos MG, Rittinger K. Structural determinants of TRIM protein function. Biochem Soc Trans. 2017;45:183‐191. [DOI] [PubMed] [Google Scholar]
- 48. Chacón‐Martínez CA, Kiessling N, Winterhoff M, Faix J, Müller-Reichert T, Jessberger R. The switch‐associated protein 70 (SWAP‐70) bundles actin filaments and contributes to the regulation of F‐actin dynamics. J Biol Chem. 2013;288:28687‐28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moniz S, Jordan P. Emerging roles for WNK kinases in cancer. Cell Mol Life Sci. 2010;67:1265‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Munro S. The golgin coiled‐coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol. 2011;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sjögren H, Wedell B, Meis‐Kindblom JM, Kindblom LG, Stenman G. Fusion of the NH2‐terminal domain of the basic helix‐loop‐helix protein TCF12 to TEC in extraskeletal myxoid chondrosarcoma with translocation t(9;15)(q22;q21). Cancer Res. 2000;60:6832‐6835. [PubMed] [Google Scholar]
- 52. Ashworth JL, Kelly V, Wilson R, Shuttleworth CA, Kielty CM. Fibrillin assembly: dimer formation mediated by amino‐terminal sequences. J Cell Sci. 1999;112:3549‐3558. [DOI] [PubMed] [Google Scholar]
- 53. Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non‐small cell lung cancer (NSCLC): a California Cancer Registry analysis. Lung Cancer. 2014;85:264‐269. [DOI] [PubMed] [Google Scholar]
- 54. Arnold BN, Thomas DC, Rosen JE, et al. Lung cancer in the very young: treatment and survival in the national cancer data base. J Thorac Oncol. 2016;11:1121‐1131. [DOI] [PubMed] [Google Scholar]
- 55. Wang WX, Xu CW, Chen YP, et al. TP53 mutations predict for poor survival in ALK rearrangement lung adenocarcinoma patients treated with Crizotinib. J Thorac Dis. 2018;10:2991‐2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kron A, Alidousty C, Scheffler M, et al. Impact of TP53 mutation status on systemic treatment outcome in ALK‐rearranged non‐small‐cell lung cancer. Ann Oncol. 2018;29:2068‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Christopoulos P, Kirchner M, Bozorgmehr F, et al. Identification of a highly lethal V3+ TP53+ subset in ALK+ lung adenocarcinoma. Int J Cancer. 2019;144:190‐199. [DOI] [PubMed] [Google Scholar]
- 58. Yang Y, Qin SK, Zhu J, et al. A rare STRN‐ALK fusion in lung adenocarcinoma identified using next‐generation sequencing‐based circulating tumor DNA profiling exhibits excellent response to Crizotinib. Mayo Clin Proc Innov Qual Outcomes. 2017;1:111‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCoach CE, Blakely CM, Banks KC, et al. Clinical utility of cell‐free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non‐small cell lung cancer. Clin Cancer Res. 2018;24:2758‐2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qiao H, Lovly CM. Cracking the code of resistance across multiple lines of ALK inhibitor therapy in lung cancer. Cancer Discov. 2016;6:1084‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6:1118‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


