Abstract
Chemoprevention began to be considered as a potential strategy for lowering the incidence of cancer and cancer‐related deaths in the 1970s. For clinical chemoprevention trials against cancer, including colorectal cancer (CRC), well‐established biomarkers are necessary for use as reliable endpoints. Difficulty in establishing validated biomarkers has delayed the start of CRC chemoprevention development. Chemoprevention trials for CRC have only recently been initiated thanks to the identification of reliable biomarkers, such as colorectal adenomas and aberrant crypt foci. Some promising agents have been developed for the prevention of CRC. The chemopreventive effect of selective cyclooxygenase 2 inhibitors has been shown, although these inhibitors are associated with cardiovascular toxicity as a crucial adverse effect. Aspirin, which is a unique agent among non‐steroidal anti‐inflammatory drugs (NSAIDs) showing minimal gastrointestinal toxicity and no cardiovascular risk, has prevented adenoma recurrence in some randomized controlled trials. More recently, metformin, which is a first‐line oral medicine for type 2 diabetes, has been shown to be safe and to prevent adenoma recurrence. A recommendation of the United States Preventive Services Task Force published in 2016 provides a Grade B recommendation for the use of aspirin for chronic prophylaxis against diseases, including CRC, in certain select populations. However, the roles of other agents have yet to be determined, and investigations to identify novel “post‐aspirin” agents are also needed. The combined use of multiple drugs, such as aspirin and metformin, is another option that may lead not only to stronger CRC prevention, but also to improvement of other obesity‐related diseases.
Keywords: aspirin, calcium, chemoprevention, colorectal cancer, metformin
Abbreviations
- ACF
aberrant crypt foci
- AMPK
AMP‐activating protein kinase
- CRC
colorectal cancer
- PUFA
polyunsaturated fatty acids
- RCT
randomized controlled trial
- T2DM
type 2 diabetes mellitus
1. INTRODUCTION
Cancer is a major health problem and a leading cause of death. In 2012, an estimated 14.1 million new cancer cases and 8.2 million cancer deaths were reported worldwide.1 Over the last two decades, although there have been great advances in cancer treatment, including the development of more effective drugs with better safety and more precise molecular targeting, adverse effects remain a major problem. Newer cancer treatments are also extremely expensive. Thus, as a cheaper and more effective strategy towards the goal of decreasing cancer mortality, chemoprevention is drawing more attention.
Cancer chemoprevention has been defined as a pharmacological intervention to arrest or reverse the process of carcinogenesis. As a chemopreventive agent is intended to be taken for long periods of time, it needs to meet certain requirements (Table 1).
Table 1.
Requirements of chemopreventive agents
| 1. Low toxicity |
| 2. Few or no side‐effects |
| 3. Easy to take |
| 4. Easy to administer |
| 5. Cost‐effective |
Regarding CRC, the removal of colorectal polyps has been shown to reduce the risk of future development of advanced colorectal adenomas and colorectal cancers, thereby reducing colorectal cancer death.2 Although the resection of polyps is an effective tool for reducing CRC, patients with colorectal polyps also constitute a high‐risk group for the development of CRC.3 Therefore, newer strategies for prevention are needed to lower the burden of this disease.
Herein, recent progress in the chemoprevention of sporadic CRC will be discussed and the potential for chemoprevention and future opportunities will be highlighted.
2. PROGRESS OF CRC CHEMOPREVENTION
Both genetic and environmental factors, such as smoking and high meat consumption, are known to contribute to the risk of developing CRC.4 Thus, primary prevention strategies focusing on modifying these lifestyle factors have already been tested. Chemoprevention has been a subject of intensive research for the last 4 decades. For a better understanding of progress in CRC chemoprevention, a comparison with the chemoprevention of cardiovascular events is helpful (Figure 1). A successful method to measure blood pressure, which is a useful biomarker for the risk of cardiovascular events, was first established in the 1900s; since then, it has taken more than 80 years to implement chemoprevention for ischemic cardiovascular disease. After a delay of 8 decades, the development of CRC chemoprevention has also now begun with the demonstration of the adenoma‐carcinoma sequence in colorectal carcinogenesis.5
Figure 1.
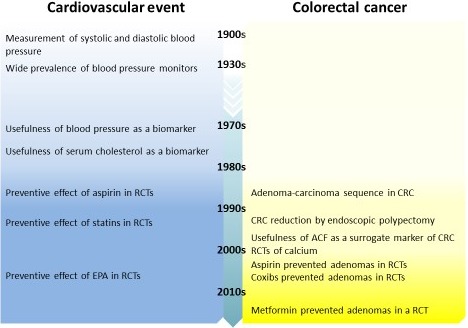
Timeline of progress in chemoprevention. ACF, aberrant crypt foci; CRC, colorectal carcinoma; EPA, eicosapentaenoic acid; RCT, randomized controlled trial
One of the important tasks in the development of cancer chemoprevention is the establishment of a valid surrogate endpoint. Although the incidence of cancer itself is the most reliable endpoint in clinical trials, setting it as the endpoint is unsuitable because of the relatively low occurrence rate of cancer in the general population and the need for a prolonged observation period. The surrogate endpoint should be an intermediate event along the carcinogenesis pathway, and the modulation of this event should be reflected in modifications to the ultimate outcome, which is the incidence of cancer. Difficulty in establishing a valid surrogate endpoint has delayed the start of progress in CRC chemoprevention. For cardiovascular events, reliable endpoints, including blood pressure and serum cholesterol, were established much earlier and permitted the accelerated development of chemoprevention.6, 7 Moreover, setting the cardiovascular event incidence itself as the endpoint may not be unreasonable because of the higher incidence of cardiovascular events in the general population, compared with that of cancer. In colorectal carcinogenesis, the development of an adenoma is considered to be a reliable surrogate endpoint. Another available surrogate endpoint is the development of ACF. Takayama et al8 reported the existence of a relationship between number of ACF, presence of dysplastic foci, size of the foci, and number of adenomas. In fact, the usefulness of ACF as an endpoint in chemoprevention trials has already been reported.9
3. CANDIDATE AGENTS FOR CRC CHEMOPREVENTION
3.1. Non‐steroidal anti‐inflammatory drugs and aspirin
Non‐steroidal anti‐inflammatory drugs, including aspirin, are the best‐investigated class of drugs as chemopreventive agents against CRC. The first description of inverse association between aspirin use and the risk of CRC was published in 1988.10 Although the underlying mechanism has yet to be defined, NSAIDs are believed to prevent CRC mainly by inhibiting COX‐2.11 Although COX‐2 is undetectable in the normal gastrointestinal epithelium, it is detectable in 40% of colorectal adenomas and in more than 80% of CRC.12 In clinical studies, the non‐selective COX inhibitor sulindac reduced the number and size of colorectal adenomas.13
Thus, newly developed NSAIDs that selectively inhibit COX‐2 were expected to be useful as chemopreventive agents and have been used in some RCT.14 In the Adenomatous Polyp Prevention on Vioxx (APPROVe) trial, rofecoxib significantly reduced the risk of the development of colorectal adenomas by 24%.15 In another trial (Adenoma Prevention with Celecoxib [APC] study), celecoxib reduced the number of adenomas in a dose‐dependent method.16 Although these results provide clear evidence of the beneficial effect of coxibs on the risk of recurrent colorectal adenomas, coxibs are unsuitable for use as chemopreventive agents because they also cause significant cardiovascular toxicity. Based on observations of an increased risk of cardiovascular events, the data and safety monitoring board recommended the early termination of both trials.17, 18
Among the NSAIDs, aspirin is the only agent with a rather low gastrointestinal risk and no cardiovascular risk. Although remarkably consistent epidemiological data have shown that aspirin intake is associated with a decreased risk of either colorectal adenomas or cancer,19 RCT for aspirin in which the cancer incidence was set as the endpoint yielded discouraging results (Tables 2, 3).20, 21 However, a follow‐up study of four trials of aspirin versus a control for the prevention of vascular events showed that aspirin taken for several years reduced the long‐term (median follow‐up duration, 18.3 years) incidence and mortality arising from CRC.34 In high‐risk patients, Ishikawa et al22 carried out an RCT and reported that aspirin decreased the incidence of adenomas by 40%. Several other RCT using the incidence of colorectal adenomas as the study endpoint have also supported the ability of aspirin to reduce the recurrence of adenomas.23, 24, 25, 26
Table 2.
Representative RCT for chemoprevention of CRC
| First author | Agent | Length (years) | Endpoint | Subjects | ||
|---|---|---|---|---|---|---|
| N (M:F) | Age, y (mean) | |||||
| Sturmer21 | Aspirin, 325 mg | 12 | CRC incidence | Healthy male physicians | 22 071 (22 071:0) | NA |
| Cook20 | Aspirin, 100 mg | 10 | CRC incidence | Healthy women | 39 876 (0:39 876) | 54.6 |
| aBenamouzig24 | Aspirin, 160/300 mg | 4 | Adenoma recurrence | Patients with a history of adenoma | 272 (191:81) | 58 |
| aLogan25 | Aspirin, 300 mg | 3 | Adenoma recurrence | Patients with a history of adenoma resection within 6 months | 853 (534:319) | 57.8 |
| aIshikawa22 | Aspirin, 100 mg | 2 | Colorectal tumor recurrence | Patients with endoscopic resection of adenoma or CRC | 311 (246:65) | 60.3 |
| aSandler26 | Aspirin, 325 mg | 3 | Adenoma incidence | Patients with a previous history of CRC | 635 (332:303) | NA |
| aBaron23 | Aspirin, 81/325 mg | 3 | Adenoma recurrence | Patients with a recent history of adenoma resection | 372 (235:137) | 57.7 |
| aArber14 | Celecoxib, 400 mg | 3 | Adenoma recurrence | Patients with a history of adenoma removal | 1561 (1033:528) | NA |
| aBaron,15 Bresalier17 | Rofecoxib, 25 mg | 3 | Adenoma recurrence | Patients with a recent history of adenoma diagnosis | 2587 (1604:983) | 59.4 |
| aBertagnolli,16 Solomon18 | Celecoxib, 400/800 mg | 3 | Adenoma recurrence | Patients with a history of adenoma removal | 2035 (1387:648) | 59 |
| aHigurashi27 | Metformin, 250 mg | 1 | Adenoma recurrence | Patients with a history of adenoma removal | 133 (103:30) | 63.8 |
| aTokudome28 | Advised to increase intake of PUFA | 2 | Adenoma recurrence | Polypectomized patients | 205 (151:54) | 58.9 |
| aBonithon‐Kopp29 | Calcium carbonate, 2000 mg | 3 | Adenoma recurrence | Patients with a history of adenoma | 354 (223:131) | 59.1 |
| aBaron30 | Calcium carbonate, 1200 mg | 4 | Adenoma recurrence | Patients with a recent history of adenoma | 930 (672:258) | 61.0 |
| Wactawski‐Wende31 | Calcium carbonate, 1000 mg and vitamin D3, 400 IU | 7 | Colorectal cancer | Postmenopausal women | 36 282 (0:36 282) | NA |
| aBaron32 | Calcium carbonate, 1200 mg Vitamin D3, 1000 IU | 3‐5 | Adenoma recurrence | Patients with a history of adenoma | 1675 (1423:252) | 58.4 |
| Pommergaard33 | Aspirin, 75 mg; Calcitriol, 0.5 μg; and calcium carbonate, 1250 mg | 3 | Adenoma recurrence | Patients with a history of adenoma removal | 427 (247:180) | 59.5 |
Abbreviations: 95% CI, 95% confidence interval; CRC, colorectal cancer; RCT, randomized controlled trial; RR, relative risk.
Study included in a meta‐analysis.
Table 3.
Results of representative RCT for chemoprevention of CRC
| Study | Events/evaluated | Results | Adverse events | |
|---|---|---|---|---|
| Treatment | Control | |||
| Sturmer21 | NA | NA | Negative (RR, 1.07; 95% CI, 0.75‐1.53) | Various gastrointestinal symptoms and diseases |
| Cook20 | 133/19 934 | 136/19 942 | Negative (RR, 0.97; 95% CI, 0.77‐1.24) | Not reported |
| aBenamouzig24 | 42/102 | 33/83 | Negative (RR, 0.96; 95% CI, 0.75‐1.22) | Insignificant difference |
| aLogan25 | 99/434 | 121/419 | Positive (RR, 0.79; 95% CI, 0.63‐0.99) | Insignificant difference |
| aIshikawa22 | 56/152 | 73/159 | Positive (RR, 0.60; 95% CI, 0.36‐0.98) | No serious adverse effects |
| aSandler26 | 43/259 | 70/258 | Positive (RR, 0.65; 95% CI, 0.46‐0.91) | Insignificant difference |
| aBaron23 | 300/721 | 171/363 | Positive with aspirin, 81 mg (RR, 0.81; 95% CI, 0.69‐0.96) | Insignificant difference |
| aArber14 | 95/589 | 83/334 | Positive (RR, 0.64; 95% CI, 0.56‐0.75) | Insignificant increase in cardiovascular events (RR, 1.30; 95% CI, 0.65‐2.62) |
| aBaron,15 Bresalier17 | 460/1158 | 646/1218 | Positive (RR, 0.76; 95% CI, 0.57‐0.83) | Increased cardiovascular events (HR, 1.92; 95% CI, 1.19‐3.11) |
| aBertagnolli,16 Solomon18 | 548/1356 | 421/679 | Positive for both doses (RR, 0.67 and 0.55; 95% CI, 0.59‐0.77 and 0.48‐0.64) | Increased death from cardiovascular causes (HR, 2.3 and 3.4; 95% CI, 0.9‐5.5 and 1.4‐7.8) |
| aHigurashi27 | 22/71 | 32/62 | Positive (RR, 0.60; 95% CI, 0.39‐0.92) | No serious adverse events |
| aTokudome28 | 56/96 | 54/85 | Negative (RR, 0.81; 95% CI 0.54‐1.21) | No adverse events |
| aBonithon‐Kopp29 | 28/176 | 36/178 | Negative (RR, 0.66; 95% CI, 0.38‐1.17) | More frequent side‐effects (26/176 vs 12/178; P = .043) |
| aBaron30 | 33/459 | 24/454 | Positive (RR, 0.85; 95% CI, 0.74‐0.98) | Insignificant difference |
| Wactawski‐Wende31 | 168/18 716 | 154/18 106 | Negative (RR, 1.08; 95% CI, 0.86‐1.34) | Insignificant difference |
| aBaron32 | 345/762 | 362/761 | Negative (RR, 0.91; 95% CI, 0.75‐1.12) | Insignificant difference |
| 438/1024 | 442/1035 | Negative (RR, 1.00; 95% CI, 0.84‐1.19) | Insignificant difference | |
| Pommergaard33 | 52/209 | 58/218 | Negative (RR, 0.95; 95% CI, 0.61‐1.48) | Insignificant difference |
Abbreviations: 95% CI, 95% confidence interval; CRC, colorectal cancer; RCT, randomized controlled trial; RR, relative risk.
Study included in a meta‐analysis.
3.2. Metformin
Metformin, a member of the biguanide family, is an insulin‐sensitizing drug and is currently a first‐line oral drug for the control of hyperglycemia in patients with T2DM, according to both national and international guidelines.35
In addition to the therapeutic effect of metformin on T2DM, there has been increasing evidence supporting its anticancer potential. The antitumor effects of metformin are mainly twofold: one is an indirect effect resulting from systemic metabolic changes, including decreases in plasma glucose and insulin levels. Insulin decreases the expression of insulin‐like growth factor binding protein (IGFBP) and induces the expression of insulin‐like growth factor‐1 (IGF1). Both insulin and IGF‐1 are proliferating factors that promote cell proliferation and suppress apoptosis. The other is a direct effect on tumor cells, which can mainly be explained by activation of AMPK. This latter mechanism involves the AMPK/mTOR signaling pathway, which inhibits protein synthesis and gluconeogenesis in tumor cells. A number of epidemiological findings suggest that exposure to metformin may lead to a reduction of cancer incidence.36 Recently, meta‐analysis data have shown that the use of metformin in patients with T2DM is associated with a significantly lower risk of colon neoplasia.37 Interestingly, in a clinical trial of non‐diabetic patients, giving short‐term metformin at a small dose suppressed ACF formation in the rectum and colonic epithelial proliferation.38 More recently, Higurashi et al carried out a double‐blind RCT to investigate the efficacy of metformin. They found that giving low‐dose metformin for 1 year to non‐diabetic patients after a polypectomy for a clean colon reduced the incidence of adenomas by 40% and the total number of colon polyps by 33% without causing any serious adverse events.27 This result suggests that the antitumor efficacy of metformin is not merely due to improved T2DM control.
3.3. Omega‐3 polyunsaturated fatty acids
Based on epidemiological evidence suggesting that populations with a high intake of fish have a low rate of colorectal cancer mortality, some studies have evaluated the influence of PUFA on colorectal carcinogenesis.39 Although some observational studies suggested an inverse relationship between higher fish consumption and colorectal cancer,40 others found no such consistent association.41 An observational study suggested that the intake of marine n‐3 polyunsaturated fatty acids may be inversely related to the risk of colon cancer, particularly at proximal sites of the large bowel.42 A meta‐analysis showed that fish consumption decreased the risk of colorectal cancer by 12%.43
The mechanisms underlying the antitumor activity of omega‐3 PUFA remain unclear. Recently, some likely mechanisms have been proposed, including (i) the inhibition of cyclooxygenase activity, (ii) the production of novel anti‐inflammatory lipid mediators, (iii) direct fatty acid signaling through G protein‐coupled receptors, (iv) alteration of membrane dynamics and cell surface receptor function, and (v) increased cellular oxidative stress.44
However, interventional trials providing encouraging evidence have been sparse. An RCT carried out for polypectomized patients found a tendency towards a reduction in colorectal tumor incidence after 24 months of an advice‐based intervention to increase the intake of omega‐3 PUFA.28
3.4. Calcium and vitamin D
There are several observational studies suggesting that calcium may have a chemopreventive effect against colon adenoma and CRC.45, 46 One possible mechanism underlying the protective effect of dietary calcium on colorectal carcinogenesis is its ability to bind to toxic secondary bile acids and ionized fatty acids to form insoluble soaps in the lumen of the colon.47 An alternative explanation is that calcium decreases the cell proliferative activity, stimulates cell differentiation, and induces apoptosis. Modulation of calcium‐sensing receptors and the subsequent activation and its direct action as an activator/cofactor for activation of protein kinase C are essential roles of calcium in cell growth and proliferation. Some basic and clinical studies have shown that calcium has direct antiproliferative, differentiation‐stimulating and apoptosis‐inducing effects on normal and transformed colonic cells.48, 49
An analysis of two large prospective cohort studies that examined the association between calcium intake and the risk of colon cancer showed an inverse association between a higher total calcium intake (>1250 mg/day vs ≤500 mg/day) and the incidence of distal colon cancer.50 Despite these findings of observational studies, the results of randomized trials are somewhat controversial.29, 30 An RCT found no significant effect of calcium treatment; the adjusted odds ratio for recurrent adenoma was 0.66. However, a meta‐analysis of three randomized trials concluded that the risk of recurrence of colorectal adenoma was significantly lower for patients randomized to the calcium supplementation arm of the study.51
Recent case‐controlled studies have shown an inverse association between the serum levels of vitamin D and the incidence of colon polyps.52 Despite these observational findings suggesting a preventive effect of vitamin D against CRC, the targets and molecular basis for the antitumor activity of vitamin D remain poorly understood. One possible mechanism is that vitamin D and vitamin D receptors activated by vitamin D repress β‐catenin signaling, which is universally activated very early in colon cancer.53 Regarding interventional trials, however, the largest RCT of postmenopausal women failed to show any marked effect of vitamin D on the incidence of CRC.31 Furthermore, RCT giving calcium and vitamin D exerted no effect on the rate of invasive CRC.31, 32 More recently, an RCT showed no significant efficacy of a combination pill containing aspirin, calcitriol (hormonally active vitamin D metabolite) and calcium, perhaps because of the effects of smoking or the low doses of the tested agents.33
3.5. Summary of RCT
In Tables 2, 3 and Figure 2, we have summarized the results of a meta‐analysis of placebo‐controlled RCT including colorectal adenoma occurrence as an endpoint. Metformin, selective COX‐2 inhibitors, and aspirin reduced the risk of colorectal adenoma, whereas calcium and vitamin D did not. Among the agents with a preventive effect, the efficacy of aspirin was rather mild. Although metformin was comparable in efficacy to the selective COX‐2 inhibitors, additional research is needed, as the sample size was relatively small compared with those in the trials for aspirin and NSAIDs.
Figure 2.
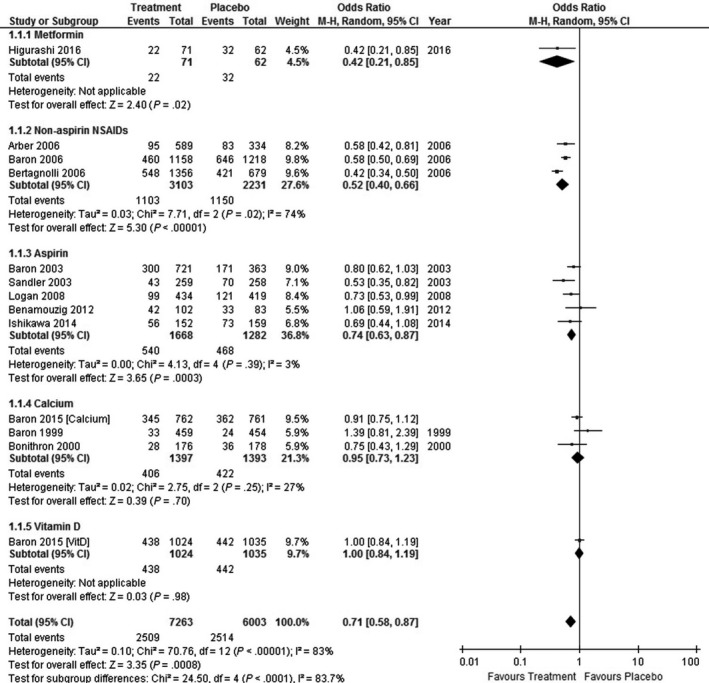
Forest plot of placebo‐controlled randomized trials examining chemoprevention for colorectal adenoma. We found 12 reports of placebo‐controlled trials for the chemoprevention of colorectal adenoma. One of them used a two‐arm study design. Thus, we ultimately included 13 comparisons. A random‐model meta‐analysis that collectively evaluated all the treatment regimens suggested that the incidence of adenoma was marginally decreased with an OR of 0.71 (95% CI, 0.58‐0.87; I2 = 83%). According to the subgroup analyses, metformin (OR, 0.42; 95% CI, 0.21‐0.85), non‐aspirin NSAIDs (OR, 0.52; 95% CI, 0.40‐0.66; I2 = 74%), and aspirin (OR, 0.74; 95% CI, 0.63‐0.87; I2 = 3%) decreased the incidence of adenoma. However, calcium and vitamin D did not decrease the risk of adenoma
4. CONCLUSION AND FUTURE PERSPECTIVES
Chemoprevention of CRC has developed rapidly in the last 4 decades, and promising agents have been identified. Among these, aspirin appears to be the closest to actual clinical application. However, many problems remain to be resolved before aspirin can be used as a chemopreventive agent against CRC in clinical practice. First, we need to define an appropriate target population that is most likely to benefit from chemoprevention. The United States Preventive Services Task Force (USPSTF) has published a recommendation regarding the use of aspirin for the prevention of CRC in its updated draft guidelines published in 2016.54 The USPSTF provides a Grade B recommendation (“high or moderate certainty that the net benefit is moderate to substantial”) for the use of low‐dose aspirin for chronic disease prophylaxis, including CRC prevention, in adult residents of the US between the ages of 50 and 59 years with a >10% 10‐year risk of cardiovascular events. Of note, the updated USPSTF recommendation is mainly based on the benefits of aspirin use, including the prevention of myocardial infarction and ischemic stroke, and a more conservative view of a possible reduction in the incidence of CRC. Thus, in countries where cardiovascular events are not as severe a problem as they are in the USA, the usefulness of aspirin might be more limited. In regard to detection of appropriate target, use of genomic information can be helpful. For example, gene polymorphism with a high sensitivity to aspirin is known, for example, SNP of COX‐1, A842G, and C50T.55 Second, studies examining combinations of chemoprevention and screening are necessary, as aspirin use is not a substitute for screening.56 Particularly in countries where CRC screening is already highly recommended and widely prevalent, the question of how chemoprevention should work in the context of screening should be resolved.
Most of all, showing the preventive effect of aspirin in RCT that include the incidence of CRC itself as an endpoint is an urgent challenge. A clinical foundation for the chemoprevention of CRC itself will only be realized once agents with suppressive effects on the incidence of adenoma are approved.
Furthermore, exploration of novel “post‐aspirin” agents is also needed. Low‐dose aspirin intake reduced the risk of recurrent adenoma by only 20% even in a high‐risk population,22, 23, 24, 25, 26 which is a rather modest effect compared with the preventive effect of aspirin against cardiovascular events. Further research is therefore warranted to identify more effective and safer agents to enable CRC chemoprevention to become clinically applicable. Metformin, which has been shown to reduce adenoma recurrence after 1 year of treatment in a recent RCT,27 could be a candidate agent. If a large‐scale RCT of metformin yielded positive results, the use of metformin to prevent not only other cancers, but also other obesity‐associated outcomes could be examined in large RCT.57
The combined use of multiple agents is also an option, as the concomitant use of more than one preventive drug (eg, antihypertensive drugs, statins, fibrates, and antiplatelet drugs) has a synergistic effect on cardiovascular event prevention. For example, the combined use of aspirin and metformin, which have different preventive mechanisms, may work synergistically. Thus, this should be assessed in an RCT for CRC prevention. Moreover, the combined use of these agents may lead not only to greater CRC prevention, but also to improvement in other obesity‐related diseases. Similar to the case of aspirin, the efficacy of metformin treatment should be evaluated from the viewpoint of the total risk reduction for these diseases, which would reflect the true impact on a broader range of targets.
DISCLOSURE
Authors declare no conflicts of interest for this article.
Umezawa S, Higurashi T, Komiya Y, et al. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci. 2019;110:3018‐3026. 10.1111/cas.14149
Funding information
This work was performed at Yokohama City University School of Medicine, Department of Gastroenterology and Hepatology (3‐9 Fukuura, Kanazawa‐ku, Yokohama 236‐0004, Japan).
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N Engl J Med. 2012;366(8):687‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maisonneuve P, Botteri E, Lowenfels AB. Five‐year risk of colorectal neoplasia after negative colonoscopy. N Engl J Med. 2008;359(24):2611‐2612; author reply 2. [PubMed] [Google Scholar]
- 4. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029‐2043.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal‐tumor development. N Engl J Med. 1988;319(9):525‐532. [DOI] [PubMed] [Google Scholar]
- 6. Stamler J, Rhomberg P, Schoenberger JA, et al. Multivariate analysis of the relationship of seven variables to blood pressure: findings of the Chicago Heart Association Detection Project in Industry, 1967‐1972. J Chronic Dis. 1975;28(10):527‐548. [DOI] [PubMed] [Google Scholar]
- 7. Reid DD, Hamilton PJ, McCartney P, Rose G, Jarrett RJ, Keen H. Smoking and other risk factors for coronary heart‐disease in British civil servants. Lancet. 1976;2(7993):979‐984. [DOI] [PubMed] [Google Scholar]
- 8. Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339(18):1277‐1284. [DOI] [PubMed] [Google Scholar]
- 9. Sakai E, Takahashi H, Kato S, et al. Investigation of the prevalence and number of aberrant crypt foci associated with human colorectal neoplasm. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1918‐1924. [DOI] [PubMed] [Google Scholar]
- 10. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Can Res. 1988;48(15):4399‐4404. [PubMed] [Google Scholar]
- 11. Grossman HB. Selective COX‐2 inhibitors as chemopreventive and therapeutic agents. Drugs Today (Barc). 2003;39(3):203‐212. [DOI] [PubMed] [Google Scholar]
- 12. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up‐regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183‐1188. [DOI] [PubMed] [Google Scholar]
- 13. Matsuhashi N, Nakajima A, Fukushima Y, Yazaki Y, Oka T. Effects of sulindac on sporadic colorectal adenomatous polyps. Gut. 1997;40(3):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885‐895. [DOI] [PubMed] [Google Scholar]
- 15. Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674‐1682. [DOI] [PubMed] [Google Scholar]
- 16. Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873‐884. [DOI] [PubMed] [Google Scholar]
- 17. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092‐1102. [DOI] [PubMed] [Google Scholar]
- 18. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071‐1080. [DOI] [PubMed] [Google Scholar]
- 19. Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila). 2012;5(2):164‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cook NR, Lee IM, Gaziano JM, et al. Low‐dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47‐55. [DOI] [PubMed] [Google Scholar]
- 21. Sturmer T, Glynn RJ, Lee IM, Manson JE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post‐trial follow‐up data from the Physicians’ Health Study. Ann Intern Med. 1998;128(9):713‐720. [DOI] [PubMed] [Google Scholar]
- 22. Rothwell PM, Wilson M, Elwin CE, et al. Long‐term effect of aspirin on colorectal cancer incidence and mortality: 20‐year follow‐up of five randomised trials. Lancet. 2010;376(9754):1741‐1750. [DOI] [PubMed] [Google Scholar]
- 23. Ishikawa H, Mutoh M, Suzuki S, et al. The preventive effects of low‐dose enteric‐coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63(11):1755‐1759. [DOI] [PubMed] [Google Scholar]
- 24. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891‐899. [DOI] [PubMed] [Google Scholar]
- 25. Benamouzig R, Uzzan B, Deyra J, et al. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4‐year results of the APACC randomised trial. Gut. 2012;61(2):255‐261. [DOI] [PubMed] [Google Scholar]
- 26. Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29‐38. [DOI] [PubMed] [Google Scholar]
- 27. Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883‐890. [DOI] [PubMed] [Google Scholar]
- 28. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540‐559. [DOI] [PubMed] [Google Scholar]
- 29. Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta‐analysis. Cancer Prev Res (Phila). 2010;3(11):1451‐1461. [DOI] [PubMed] [Google Scholar]
- 30. Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: a meta‐analysis. Eur J Intern Med. 2016;33:60‐66. [DOI] [PubMed] [Google Scholar]
- 31. Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short‐term clinical trial. Cancer Prev Res (Phila). 2010;3(9):1077‐1083. [DOI] [PubMed] [Google Scholar]
- 32. Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post‐polypectomy patients without diabetes: a multicentre double‐blind, placebo‐controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475‐483. [DOI] [PubMed] [Google Scholar]
- 33. Caygill CP, Hill MJ. Fish, n‐3 fatty acids and human colorectal and breast cancer mortality. Eur J Cancer Prev. 1995;4(4):329‐332. [DOI] [PubMed] [Google Scholar]
- 34. Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97(12):906‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kampman E, Verhoeven D, Sloots L, van‘t Veer P. Vegetable and animal products as determinants of colon cancer risk in Dutch men and women. Cancer Causes Control. 1995;6(3):225‐234. [DOI] [PubMed] [Google Scholar]
- 36. Sasazuki S, Inoue M, Iwasaki M, et al. Intake of n‐3 and n‐6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center‐based prospective study. Int J Cancer. 2011;129(7):1718‐1729. [DOI] [PubMed] [Google Scholar]
- 37. Wu S, Feng B, Li K, et al. Fish consumption and colorectal cancer risk in humans: a systematic review and meta‐analysis. Am J Med. 2012;125(6):551‐559.e5. [DOI] [PubMed] [Google Scholar]
- 38. Hull MA. Omega‐3 polyunsaturated fatty acids. Best Pract Res Clin Gastroenterol. 2011;25(4–5):547‐554. [DOI] [PubMed] [Google Scholar]
- 39. Tokudome S, Kuriki K, Yokoyama Y, et al. Dietary n‐3/long‐chain n‐3 polyunsaturated fatty acids for prevention of sporadic colorectal tumors: a randomized controlled trial in polypectomized participants. Prostaglandins Leukot Essent Fatty Acids. 2015;94:1‐11. [DOI] [PubMed] [Google Scholar]
- 40. Cho E, Smith‐Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96(13):1015‐1022. [DOI] [PubMed] [Google Scholar]
- 41. Ishihara J, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr. 2008;88(6):1576‐1583. [DOI] [PubMed] [Google Scholar]
- 42. Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72(6):1323‐1325. [PubMed] [Google Scholar]
- 43. Kallay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium‐sensing receptor. Cancer Detect Prev. 2000;24(2):127‐136. [PubMed] [Google Scholar]
- 44. Lipkin M. Preclinical and early human studies of calcium and colon cancer prevention. Ann N Y Acad Sci. 1999;889:120‐127. [DOI] [PubMed] [Google Scholar]
- 45. Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94(6):437‐446. [DOI] [PubMed] [Google Scholar]
- 46. Bonithon‐Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356(9238):1300‐1306. [DOI] [PubMed] [Google Scholar]
- 47. Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 48. Shaukat A, Scouras N, Schunemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol. 2005;100(2):390‐394. [DOI] [PubMed] [Google Scholar]
- 49. Gandini S, Boniol M, Haukka J, et al. Meta‐analysis of observational studies of serum 25‐hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414‐1424. [DOI] [PubMed] [Google Scholar]
- 50. Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev Endocr Metab Disord. 2012;13(1):31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wactawski‐Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684‐696. [DOI] [PubMed] [Google Scholar]
- 52. Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373(16):1519‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150(1):114‐122.e4. [DOI] [PubMed] [Google Scholar]
- 54. USPSTF . Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Preventive Medication 2016. [Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed April 27, 2016.
- 55. van Oijen MG, Laheij RJ, Koetsier M, et al. Effect of a specific cyclooxygenase‐gene polymorphism (A‐842G/C50T) on the occurrence of peptic ulcer hemorrhage. Dig Dis Sci. 2006;51(12):2348‐2352. [DOI] [PubMed] [Google Scholar]
- 56. Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, Fendrick AM. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer. A cost‐effectiveness analysis. Ann Intern Med. 2001;135(9):769‐781. [DOI] [PubMed] [Google Scholar]
- 57. Chan AT. Metformin for cancer prevention: a reason for optimism. Lancet Oncol. 2016;17(4):407‐409. [DOI] [PubMed] [Google Scholar]


