Abstract
Skeletal muscle volume is associated with prognosis of cancer patients. Maintenance of skeletal muscle is an essential concern in cancer treatment. In nutritional intervention, it is important to focus on differences in metabolism between tumor and skeletal muscle. We examined the influence of oral intake of glucose (0%, 10%, 50%) and 2% medium‐chain fatty acid (lauric acid, LAA, C12:0) on tumor growth and skeletal muscle atrophy in mouse peritoneal metastasis models using CT26 mouse colon cancer cells and HT29 human colon cancer cells. After 2 weeks of experimental breeding, skeletal muscle and tumor were removed and analyzed. Glucose intake contributed to prevention of skeletal muscle atrophy in a sugar concentration‐dependent way and also promoted tumor growth. LAA ingestion elevated the level of skeletal muscle protein and suppressed tumor growth by inducing tumor‐selective oxidative stress production. When a combination of glucose and LAA was ingested, skeletal muscle mass increased and tumor growth was suppressed. Our results confirmed that although glucose is an important nutrient for the prevention of skeletal muscle atrophy, it may also foster tumor growth. However, the ingestion of LAA inhibited tumor growth, and its combination with glucose promoted skeletal muscle integrity and function, without stimulating tumor growth. These findings suggest novel strategies for the prevention of skeletal muscle atrophy.
Keywords: cachexia, glucose, medium‐chain fatty acid, sarcopenia, Warburg effect
Abbreviations
- LAA
lauric acid
- MCFA
medium‐chain fatty acid
- MYL1
myosin light chain 1
- QFM
quadriceps femoris muscle
- QOL
quality of life
1. INTRODUCTION
Cancer cachexia develops in 40% of all‐stage cancer patients,1 while in the elderly and in patients with advanced cancers it is even more frequent.2 Cancer cachexia causes a decline in QOL and impairment of physical function.3 Possible determinants include cancer‐related inflammatory cytokines,4, 5 increased oxidative stress,6 increased catabolism,7 and anorexia.8, 9
As defined in the cachexia consensus conference in 2008, “cachexia, is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass”.10 Thus, the skeletal muscle atrophy accompanying cancer cachexia is considered a major pathogenic factor.11, 12 Importantly, a strong positive correlation has been reported between skeletal muscle mass and survival rate in patients with cancer.13, 14 It was reported that survival is remarkably maintained when skeletal muscle mass was increased using an activin IIB receptor inhibitor in a mouse cachexia model.14 Despite no significant difference in tumor weight between the two groups, the survival rate was 80% in the mice in the skeletal muscle increase group whereas all the mice in the cachexia group become moribund.
Impaired tolerability to cancer treatments, such as chemotherapy, has been identified as one of the possible causes of decreased survival in sarcopenic patients.9 Thus, therapeutic suppression of skeletal muscle atrophy during cancer cachexia is considered vitally important.5, 9 Moreover, Bye et al15 reported the effects of skeletal muscle mass on performance and quality of life in cancer patients. It has also been reported that skeletal muscle mass correlates with high physical function and retention of QOL; maintenance of skeletal muscle by nutritional intervention and resistance training has also been reported to improve the survival prognosis and QOL of cancer patients.16, 17
In the treatment of cachexia, measures for the management of reduced food intake and for tackling metabolic changes as a result of inflammation are considered necessary.9 In recent years, nutritional intervention has been proposed as a treatment for cancer patients with cachexia and malnutrition.18, 19, 20 However, some nutrients may promote tumors, such as linoleic acid21 and carbohydrates;22, 23 therefore, the impact of nutritional intervention on both muscles and tumors should be carefully considered. However, few reports have addressed this problem in cancer patients.
In the present study, we focused on the distinct metabolic requirements of tumor and skeletal muscle cells and evaluated the impact of two types of nutrients, a sugar and a lipid, on skeletal muscle atrophy. Sugars are generally metabolized by glycolysis, whereas MCFA are promptly incorporated into mitochondria, stimulating oxidative phosphorylation. Carbohydrates have been reported to promote cell proliferation in both tumor and skeletal muscle cells in vitro.24, 25 In contrast, MCFA were shown to inhibit the proliferation of tumor cells,26, 27 while there is no evidence of any MFCA‐related suppressive effect on skeletal muscle.
Several open questions remain on the mechanism of cancer cachexia, and clinical trials still lag behind.28 Therefore, animal models are an indispensable tool in research on cachexia.28, 29 Syngeneic rodent models are thought to elicit reasonable immune and metabolic responses and are useful to study cachexia.28 Herein, a syngeneic mouse peritoneal dissemination model strongly inducing cachexia was used to examine the effects of carbohydrates and MCFA on tumor growth and skeletal muscle atrophy within the same individuals.
2. MATERIALS AND METHODS
2.1. Cell culture
CT26 mouse colon cancer cell line was a kind gift from Professor I.J. Fidler (MD Anderson Cancer Center, Houston, TX, USA).27, 30 Human colon cancer cell line HT29 was purchased from Dainihon Pharmacy Co.31, 32 The cells were cultured in DMEM (Wako Pure Chemical Industries, Ltd) supplemented with 10% FBS (Sigma‐Aldrich Chemical Co.).
2.2. Animals
Five‐week‐old male BALB/c mice were purchased from SLC Japan. The animals were maintained in a pathogen‐free animal facility under a 12/12 hours light/dark cycle in a temperature (22°C)‐ and humidity‐controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval nos. 11812, 11857, 11916, 12043 and 12262). Animals were acclimated to their housing for 7 days before the start of the experiment.
For the subcutaneous tumor model, CT26 cancer cells (1 × 107 in 0.2 mL per mouse) were inoculated into the mouse scapular tissue. For the peritoneal dissemination tumor model, CT26 cancer cells (1 × 107 in 0.2 mL per mouse) were inoculated into the mouse peritoneal cavity.
To measure tumor weight, mice were killed and the subcutaneous tumors were excised, whereas the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, grossly removing non‐tumoral tissues.
For preparation of skeletal muscles, the QFM was cut at the muscle end on the upper edge of the patella, peeled off from the femur, and separated at the muscle origin on the frontal surface of the anterior lower iliac spine. The excised QFM was weighed immediately, avoiding drying. After measurement, QFM was stored frozen at −80°C.
2.3. Diet and drink
Glucose solution (50% glucose for injection, Otsuka Pharmaceutical Co. Ltd) was used directly or diluted to 10% with distilled water for drinking.
CE‐2 diet (containing 5% crude fat, mainly derived from soybean oil; CLEA Japan, Inc.) was used as control diet.
Lauric acid diet was prepared by mixing 0%, 2%, or 5% (w/w) LAA (12‐carbon saturated MCFA; Tokyo Chemical Industry Co., Ltd) with control diet (CE‐2). Dietary nutrients of LAA diets are indicated in Table 1. Intakes of food, LAA, and calories per mouse were calculated based on the total daily intake of three mice in each group.
Table 1.
Dietary nutrients in lauric acid (LAA) diets
| Diet | |||
|---|---|---|---|
| Control | 2% LAA | 5% LAA | |
| LAA (%) | 0 | 2 | 5 |
| Moisture (%) | 8.83 | 8.6534 | 8.3885 |
| Crude protein (%) | 25.13 | 24.6274 | 23.8735 |
| Crude fat (%) | 4.92 | 4.8216 | 4.674 |
| Crude fiber (%) | 4.42 | 4.3316 | 4.199 |
| Crude ash (%) | 6.86 | 6.7228 | 6.517 |
| NFE (%) | 49.84 | 48.8432 | 47.348 |
| Energy (kcal) | 344.2 | 355.316 | 371.99 |
NFE, nitrogen‐free extract.
2.4. Protein extraction
The muscle mass stored at −80°C was crushed with a hammer to remove tendons and fascia. Only the muscle tissue was washed with cold PBS and pelleted with a sonicator (QSONICA; WakenBtech Co. Ltd). Whole‐cell lysates were prepared as previously described using 0.1% SDS‐added RIPA‐buffer (Thermo Fisher Scientific).33 Protein assay was carried out using a Protein Assay Rapid Kit (Wako Pure Chemical Corporation).
2.5. Enzyme‐linked immunosorbent assay
An ELISA kit was used to measure the concentration of myosin light chain 1/3 isoform, myosin light chain 1 (MYL1) (CSB‐EL015305MO; Cusabio Biotech Co., Ltd), according to the manufacturer's instructions.
2.6. Statistical analysis
Statistical significance was calculated using two‐tailed Fisher's exact test, χ2 test, unpaired Student's t test with Bonferroni correction, and ANOVA using InStat software (version 3.0; GraphPad Software, Inc.). Data are expressed as mean ± SD of three independent experiments. P values <.05 (two‐sided) were considered indicative of statistically significant differences.
3. RESULTS
3.1. Effect of glucose on tumor and skeletal muscle
First, we examined the effect of glucose intake on tumor growth and skeletal muscle atrophy in the syngeneic mouse subcutaneous tumor model (Figure 1). Glucose was given by free drinking as shown in Figure 1A. Glucose intake (Figure 1B) and mean body weight (Figure 1C) were not different between groups. Blood sugar levels were higher in tumor‐bearing mice in each glucose concentration group (Figure 1D).
Figure 1.
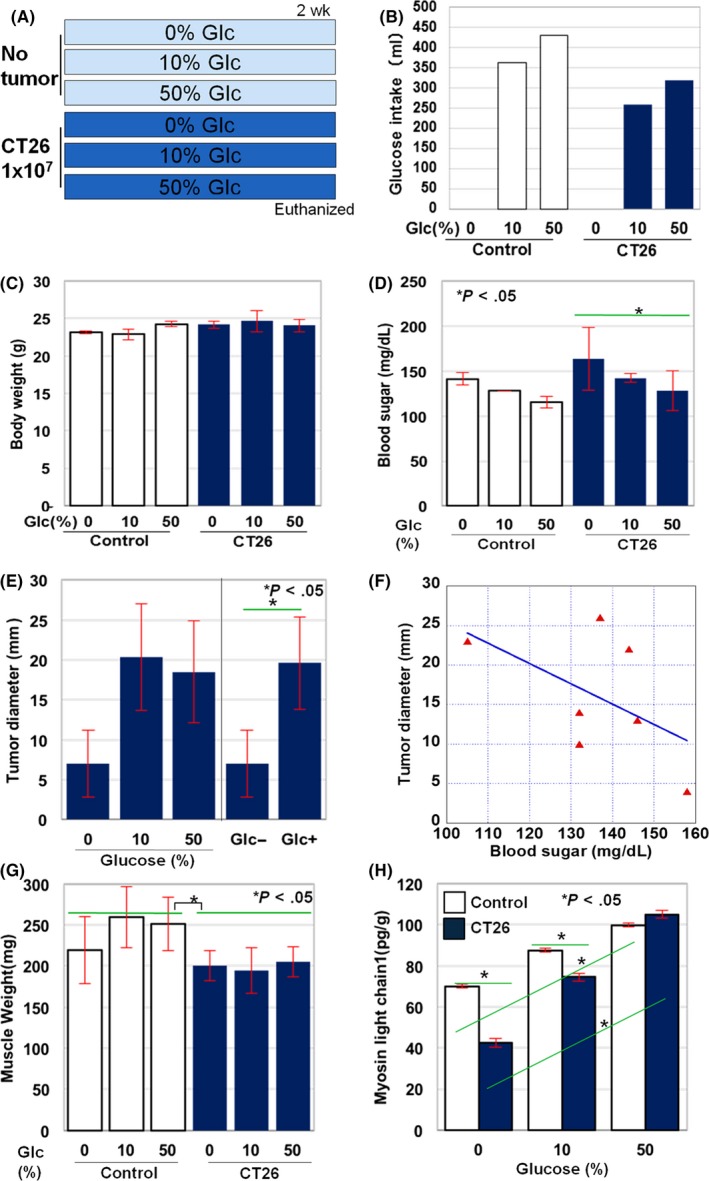
Effect of glucose on tumor and skeletal muscle in mice. A, Effect of giving glucose on tumor growth and skeletal muscle atrophy was examined after ad libitum access to glucose water (0%, 10% and 50%) in BALB/c mice with or without subcutaneous tumors deriving from CT26 mouse colon cancer cells. Each group consisted of three mice. B, Total glucose intake in each group. C, Body weight; D, blood sugar; E, tumor diameter; and G, weight of quadriceps femoris muscle (QFM, two muscles per mouse) in each group at death. F, Correlation between blood sugar level and tumor diameter. The correlation was evaluated by Spearman regression test (R = −.5345, P < .05). H, Content of 1% SDS‐soluble myosin light chain in QFM tissues was examined by ELISA. Error bar, standard error. Statistical significance was calculated by Student's t test
Tumor size increased by approximately threefold compared to control mice in either group of 50% and 10% glucose drinking. In addition, a significant difference was observed between the control group and the glucose‐drinking groups (Figure 1E). Moreover, in tumor‐bearing mice, a significant negative correlation was observed between the blood glucose level and tumor diameter, which suggests that tumor growth may result in a decrease in blood sugar (Figure 1F).
Next, the effect of glucose intake on skeletal muscle was examined. As shown in Figure 1G, the weight of the QFM was significantly lower in tumor‐bearing (both normal diet and glucose loaded) than in non‐tumor‐bearing mice. Notably, glucose loading tended to increase muscle weight in non‐cancer‐bearing, but not in cancer‐bearing mice. In order to examine the functional maturity of skeletal muscle, the concentration of SDS‐soluble MYL1 was measured in the QFM (Figure 1H). Glucose was found to increase the MYL1 level in both non‐tumor and tumor‐bearing groups.
3.2. Effect of LAA intake on skeletal muscle in non‐tumor‐bearing mice
We examined the effects of LAA on the skeletal muscle of BALB/c mice (Table 2). No significant changes in body and QFM weight were observed after giving 2% LAA.
Table 2.
Effect of lauric acid (LAA) intake on skeletal muscle of BALB/c mice
| Groupa | Food intakeb (g/d per mouse) | LAA intakeb (g/d per mouse) | Body weight (g) | Muscle weight (g) |
|---|---|---|---|---|
| Control | 7 | 0 | 26.5 ± 1.5 | 0.2 ± 0.01 |
| LAA 2% | 6.8 | 0.09 | 27 ± 1.8 | 0.22 ± 0.01* |
| LAA 5% | 4.9 | 0.18 | 22 ± 1.6 | 0.18 ± 0.01 |
aEach group contained three mice. bThe value per mouse was calculated from the intake in one cage (three mice). *P < .05, calculated by ANOVA.
3.3. Mouse peritoneal dissemination model for cancer cachexia
As shown in Figure 2A, CT26 cells were inoculated i.p. into syngeneic BALB/c mice to induce peritoneal dissemination. As a result, after an average of 14 days, the mice became moribund and were killed. Macroscopically, tumor‐bearing mice showed deterioration of coat and reduced level of exercise. Overall body weight was significantly decreased in tumor‐bearing mice in comparison with that in non‐tumor‐bearing mice (Figure 2C). Loss of adipose tissue (Figure 2D) and ascites retention was more pronounced in tumor‐bearing mice (Figure 2E). In non‐tumor‐bearing mice, ascites was not measurable. Moreover, the decrease in both skeletal muscle weight and mature (SDS‐soluble) myosin was found in tumor‐bearing in comparison with non‐tumor‐bearing mice (Figure 2G,H). Based on these findings, the CT26 colon cancer peritoneal dissemination mouse model proved to be a suitable system to explore cancer‐related sarcopenia and cachexia.
Figure 2.
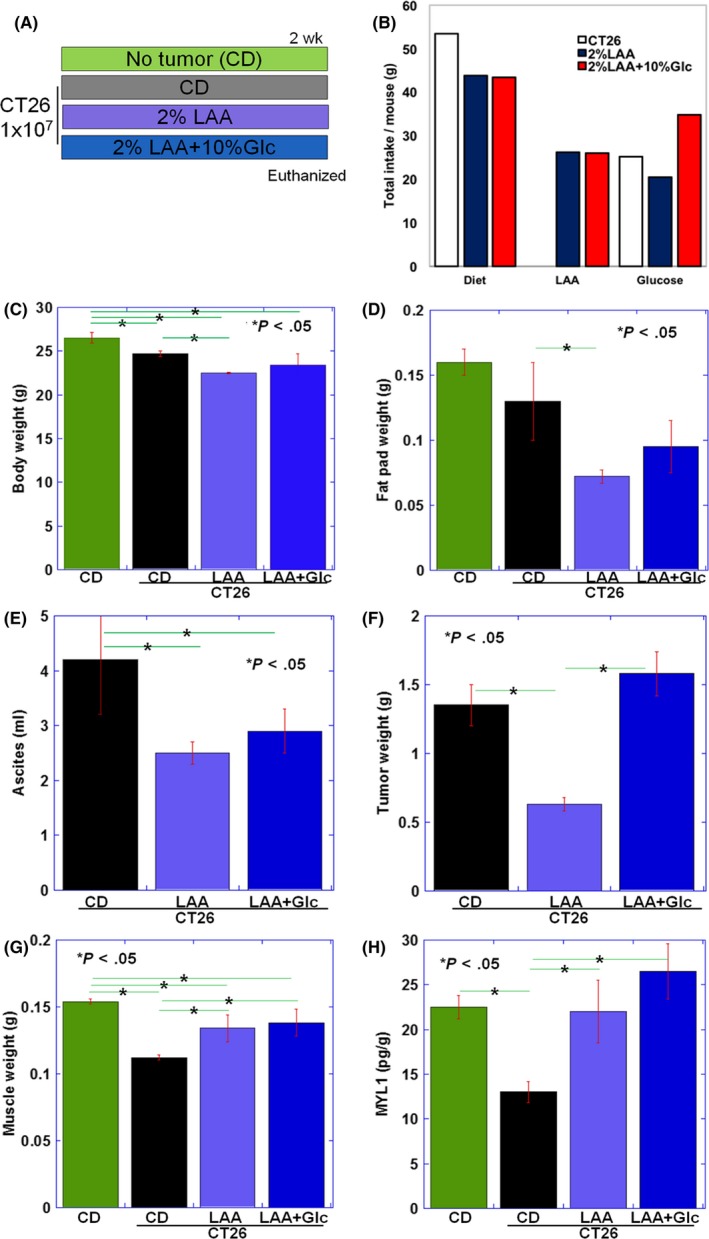
Effect of lauric acid (LAA) or LAA and glucose on tumor and skeletal muscle in CT26 tumor‐bearing BALB/c mice. A, Effect of LAA or LAA + glucose administration on tumor growth and skeletal muscle atrophy was examined after ad libitum access to LAA diet (2% LAA w/w in CE‐2 control diet [CD]), with or without 10% glucose (Glc), in BALB/c mice with peritoneal tumors deriving from CT26 mouse colon cancer cells. Each group consisted of three mice. B, Total dietary intake, LAA, and glucose in each group. C, Body weight; D, fat pad weight; E, ascites; F, tumor weight; and G, weight of quadriceps femoris muscle (QFM, two muscles per mouse) in each group at death. H, Content of 1% SDS‐soluble myosin light chain 1 (MYL1) in QFM tissues was examined by ELISA. Error bar, standard error. Statistical significance was calculated by Student's t test with Bonferroni correction
3.4. Effects of giving LAA or LAA plus glucose on tumor and skeletal muscle in CT26 tumor‐bearing mice
Next, the effects of giving dietary 2% LAA or 2% LAA + 10% glucose on tumor and skeletal muscle were examined in the CT26 mouse colon cancer cachexia model (Figure 2A,B). In the LAA group, body weight including ascites significantly decreased compared to the control diet (CD) group (Figure 2C). However, body weight excluding ascites did not differ among CD, LAA and LAA + glucose groups. Notably, the weight of adipose tissue decreased in the LAA group in comparison with the CD group (Figure 2D).
In the LAA group, ascitic fluid volume and tumor weight were significantly lower than in the CD group. In the LAA + glucose group, ascites was decreased in comparison with the CD group; however, tumor weight was not significantly different from the CD group (Figure 2E,F). Body weight excluding ascites and skeletal muscle weight were higher in the LAA and the LAA + glucose groups compared to the CD group. There was no difference between LAA and the LAA + glucose groups (Figure 2G). Mature myosin (SDS‐soluble MYL1) was significantly increased in the LAA and the LAA + glucose groups compared to the CD group (Figure 2H) and its level was higher in the LAA + glucose group than in the LAA group.
3.5. Effects of giving LAA or LAA plus glucose on tumor and skeletal muscle in HT29 tumor‐bearing mice
Finally, the effects of giving dietary 2% LAA or 2% LAA + 10% glucose on tumor and skeletal muscle were examined in the HT29 human colon cancer cachexia model (Figure 3A,B). In the LAA group, body weight including ascites significantly decreased compared to the control diet (CD) group (Figure 3C). However, body weight excluding ascites did not differ among CD, LAA and LAA + glucose groups. The weight of adipose tissue decreased in the LAA group in comparison with the CD groups (Figure 3D).
Figure 3.
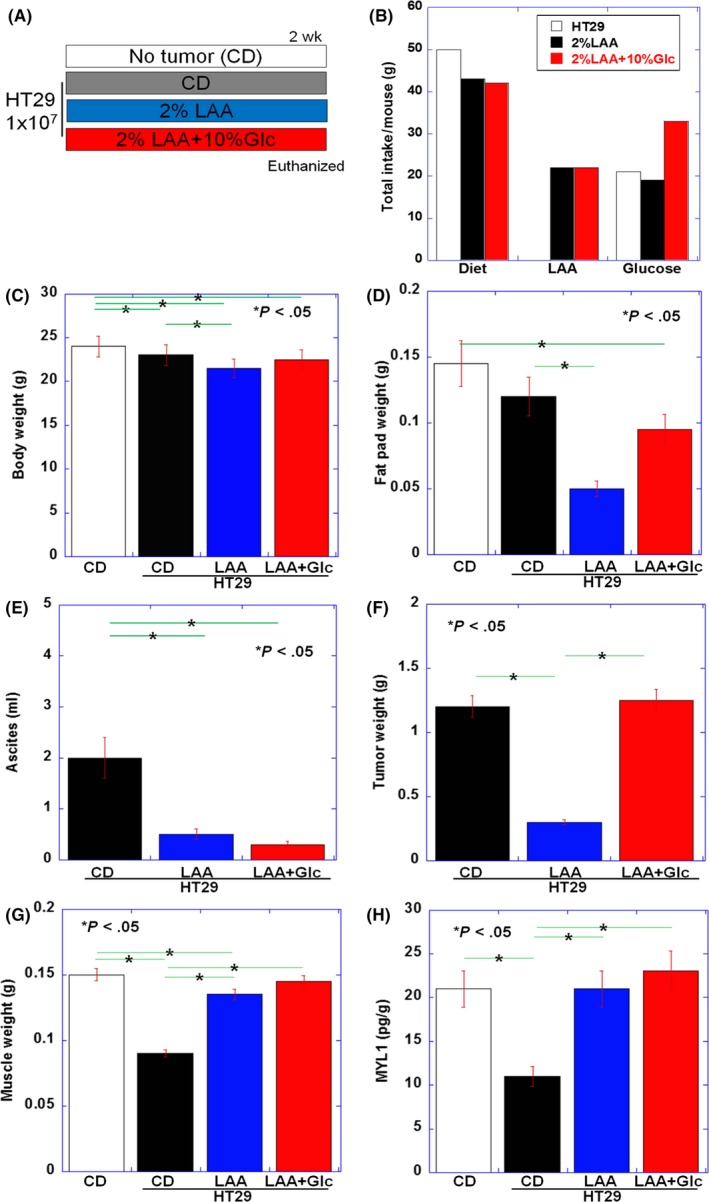
Effect of lauric acid (LAA) or LAA and glucose on tumor and skeletal muscle in HT29 tumor‐bearing nude mice. A, Effect of LAA or LAA + glucose administration on tumor growth and skeletal muscle atrophy was examined after ad libitum access to LAA diet (2% LAA w/w in CE‐2 control diet [CD]), with or without 10% glucose (Glc), in nude mice with peritoneal tumors deriving from HT29 human colon cancer cells. Each group consisted of three mice. B, Total dietary intake, LAA, and glucose in each group. C, Body weight; D, fat pad weight; E, ascites; F, tumor weight; and G, weight of quadriceps femoris muscle (QFM, two muscles per mouse) in each group at death. H, Content of 1% SDS‐soluble myosin light chain 1 (MYL1) in QFM tissues was examined by ELISA. Error bar, standard error. Statistical significance was calculated by Student's t test with Bonferroni correction
In the LAA group, ascitic fluid volume and tumor weight were significantly lower than in the CD group (Figure 3E,F). Body weight excluding ascites and skeletal muscle weight were higher in the LAA and the LAA + glucose groups compared to the CD group. There was no difference between LAA and the LAA + glucose groups (Figure 3G). SDS‐soluble MYL1 was significantly increased in the LAA and the LAA + glucose groups compared to the CD group (Figure 3H).
4. DISCUSSION
In the present study, we used a mouse cachexia model to examine the ability of glucose and medium‐chain fatty acids to suppress skeletal muscle atrophy. Although glucose had a strong muscle‐protective action, it also promoted tumor growth, whereas medium‐chain fatty acids inhibited muscle atrophy and exerted an antitumor effect.
Glucose loading caused an increase in skeletal muscle weight and SDS‐soluble MYL1, which was indicative of skeletal muscle maturation. In mouse myoblasts and myotubes, glucose was reported to promote skeletal muscle growth by enhancing myoblast proliferation and differentiation in a concentration‐dependent way.24, 34 Conversely, glucose restriction suppresses skeletal muscle differentiation and results in muscle atrophy.35 Moreover, insulin is important for myocyte proliferation and differentiation along with glucose intake.24 In this study, as the mice showed normal blood glucose levels, it was thought that glucose was taken into skeletal muscle and metabolized for muscle remodeling. These findings also suggested that glucose was important for the suppression of skeletal muscle atrophy in tumor‐bearing mice.
However, increased tumor growth was observed upon glucose loading. According to the Warburg effect, tumors are characterized by a shift in energy production towards glycolysis and lactate fermentation.25, 36, 37 Therefore, glucose is used for the growth of cancer cells.38 Interestingly, our data showed a negative correlation between tumor size and blood glucose levels. Decreased blood glucose in tumor‐bearing mice is thought to be as a result of dysregulation of sugar utilization, which is excessive sugar intake by tumors. However, glucose utilization in skeletal muscle is also considered to increase in correlation with glucose load, and is considered to have resulted in an increase in MYL1 levels. Thus, glucose suppresses muscle atrophy, but it may promote tumor growth. This makes it hard to define the appropriate glucose dosage in cancer patients. This study did not examine glucose uptake into tumor and skeletal muscle and requires further exploration.
K‐ras is mutated in CT26 mouse colon cancer cells.30 K‐ras mutant tumors are characterized by enhanced Warburg effect: increased glucose uptake, enhanced glycolysis and glutaminolysis.39 Then the question arises whether the antitumoral effect of LAA can be seen in tumors with K‐ras‐specific or other driver genes. We then examined HT29 human colon cancer cells, which have a V600E B‐raf mutation.31 Our data showed that HT29 tumors were reduced by LAA but enhanced by glucose as shown in CT26 tumors. V600E B‐raf mutation also shifts energy metabolism to aerobic glycolysis from oxidative phosphorylation in melanomas.40 In spite of the differences in driver genes affecting energy metabolism, Warburg‐deviated cancers might be impaired by LAA. As cancers generally show the Warburg effect,36, 37 antitumoral effect of LAA is expected to be effective for cancers other than colorectal cancer.
Lauric acid resulted in an increase in skeletal muscle weight, as well as in SDS‐soluble MYL1, albeit these effects were not as pronounced as with glucose. Medium‐chain fatty acids are taken into mitochondria without carnitine shuttle, undergo β‐oxidation, and are used for oxidative phosphorylation in the tricarboxylic acid (TCA) cycle.41 Therefore, in skeletal muscle cells, medium‐chain fatty acids lead to efficient ATP production by oxidative phosphorylation without increasing oxidative stress or inhibiting glycolytic metabolism.42, 43 Thus, medium‐chain fatty acids are considered an effective energy source for skeletal muscle.
Notably, LAA suppressed tumor growth. We have previously reported that LAA induces a marked increase in oxidative stress in CT26 cells in vitro, resulting in cell death.27 CT26 cells were found to have an imbalance in the expression of mitochondrial genes of the electron transport system,27 and this is thought to be the reason for LAA‐induced oxidative stress through the promotion of oxidative phosphorylation. As imbalanced expressions in mitochondrial genes are frequently found in cancer cells,44, 45 the antitumor effect of LAA is considered to be a general phenomenon. In skeletal muscle cells and other non‐cancer cells, there are no mutations in mitochondrial genes and hence no imbalanced expression; thus LAA is unlikely to increase oxidative stress, and cytotoxicity is considered a tumor‐specific event. However, we have reported that when a high concentration of 5% or 10% LAA diet, but not 2% LAA diet, is given to a normal myocardium, oxidative stress and myocardial atrophy occurs.46 Overload of long‐chain fatty acids provided dysregulation of mitochondrial oxidative phosphorylation in skeletal muscle.47 In the present study, 2% LAA increased skeletal muscle weight, whereas 5% LAA did not. Thus, an appropriate dosage should be established before applying LAA‐supplemented diets to humans.
We further tested a combination of LAA and glucose loading. LAA/glucose combination resulted in a significant increase in both skeletal muscle weight and SDS‐MYL1 level as compared to LAA alone. Tumor growth was increased by glucose loading alone but was suppressed by the LAA/glucose combination. Thus, a single combined administration of LAA and glucose resulted in skeletal muscle preservation without fostering tumor growth. Interestingly, one study reported that LAA promotes glycolytic myofiber formation by Toll‐like receptor‐4 signaling,48 and suggested that the combined use of LAA and carbohydrates may be beneficial for such a process.
The present study suggested that the combination of glucose loading and medium‐chain fatty acids may alleviate cancerous muscle atrophy without promoting tumor growth. These findings are expected to result in future clinical application.
DISCLOSURE
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported by MEXT KAKENHI grant numbers 16H05164 (HK), 17K15648 (RF‐T), 17K19923 (HK), 18K16671 (SK), 18K17726 (IK), 18K10788 (KF). The authors thank Ms Tomomi Masutani for expert assistance with the preparation of this manuscript.
Mori T, Ohmori H, Luo Y, et al. Giving combined medium‐chain fatty acids and glucose protects against cancer‐associated skeletal muscle atrophy. Cancer Sci. 2019;110:3391–3399. 10.1111/cas.14170
REFERENCES
- 1. Bozzetti F, SCRINIO Working Group . Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. 2009;17:279‐284. [DOI] [PubMed] [Google Scholar]
- 2. Paillaud E, Caillet P, Campillo B, Bories PN. Increased risk of alteration of nutritional status in hospitalized elderly patients with advanced cancer. J Nutr Health Aging. 2006;10:91‐95. [PubMed] [Google Scholar]
- 3. Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo Y, Yoneda J, Ohmori H, et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014;74:330‐340. [DOI] [PubMed] [Google Scholar]
- 5. Lucia S, Esposito M, Rossi Fanelli F, Muscaritoli M. Cancer cachexia: from molecular mechanisms to patient's care. Crit Rev Oncog. 2012;17:315‐321. [DOI] [PubMed] [Google Scholar]
- 6. Bjorklund G, Dadar M, Aaseth J, Chirumbolo S, Pen JJ. Cancer‐associated cachexia, reactive oxygen species, and nutrition therapy. Curr Med Chem. 2018, in press, [E-pub Ahead of Print]. 10.2174/0929867325666180629123817 [DOI] [PubMed] [Google Scholar]
- 7. Shyh‐Chang N. Metabolic changes during cancer cachexia pathogenesis. Adv Exp Med Biol. 2017;1026:233‐249. [DOI] [PubMed] [Google Scholar]
- 8. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle. 2015;6:287‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90‐99. [DOI] [PubMed] [Google Scholar]
- 10. Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793‐799. [DOI] [PubMed] [Google Scholar]
- 11. Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124‐1132. [DOI] [PubMed] [Google Scholar]
- 12. Acharyya S, Butchbach ME, Sahenk Z, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421‐432. [DOI] [PubMed] [Google Scholar]
- 13. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer. 2016;57:58‐67. [DOI] [PubMed] [Google Scholar]
- 14. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531‐543. [DOI] [PubMed] [Google Scholar]
- 15. Bye A, Sjoblom B, Wentzel‐Larsen T, et al. Muscle mass and association to quality of life in non‐small cell lung cancer patients. J Cachexia Sarcopenia Muscle. 2017;8:759‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanchez‐Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non‐small‐cell lung cancer: a prospective study. Nutr Cancer. 2012;64:526‐534. [DOI] [PubMed] [Google Scholar]
- 17. Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2016;25:784‐794. [DOI] [PubMed] [Google Scholar]
- 18. Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J. 2017;474:2663‐2678. [DOI] [PubMed] [Google Scholar]
- 19. Laviano A, Di Lazzaro Giraldi G, Koverech A. Does nutrition support have a role in managing cancer cachexia? Curr Opin Support Palliat Care. 2016;10:288‐292. [DOI] [PubMed] [Google Scholar]
- 20. Mantovani G, Madeddu C. Cancer cachexia: medical management. Support Care Cancer. 2010;18:1‐9. [DOI] [PubMed] [Google Scholar]
- 21. Ohmori H, Luo Y, Fujii K, et al. Dietary linoleic acid and glucose enhances azoxymethane‐induced colon cancer and metastases via the expression of high‐mobility group box 1. Pathobiology. 2010;77:210‐217. [DOI] [PubMed] [Google Scholar]
- 22. Shimomoto T, Luo Y, Ohmori H, et al. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane‐injected Fischer 344 rats fed with a high‐linoleic acid and high‐glucose diet. J Gastroenterol. 2012;47:1073‐1083. [DOI] [PubMed] [Google Scholar]
- 23. Shimomoto T, Ohmori H, Luo Y, et al. Diabetes‐associated angiotensin activation enhances liver metastasis of colon cancer. Clin Exp Metastasis. 2012;29:915‐925. [DOI] [PubMed] [Google Scholar]
- 24. Grabiec K, Gajewska M, Milewska M, Blaszczyk M, Grzelkowska‐Kowalczyk K. The influence of high glucose and high insulin on mechanisms controlling cell cycle progression and arrest in mouse C2C12 myoblasts: the comparison with IGF‐I effect. J Endocrinol Invest. 2014;37:233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891‐899. [DOI] [PubMed] [Google Scholar]
- 26. Hao GW, Chen YS, He DM, Wang HY, Wu GH, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega‐3 fatty acids and medium‐chain triglycerides. Asian Pac J Cancer Prev. 2015;16:2061‐2068. [DOI] [PubMed] [Google Scholar]
- 27. Kadochi Y, Mori S, Fujiwara‐Tani R, et al. Remodeling of energy metabolism by a ketone body and medium‐chain fatty acid suppressed the proliferation of CT26 mouse colon cancer cells. Oncol Lett. 2017;14:673‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ballaro R, Costelli P, Penna F. Animal models for cancer cachexia. Curr Opin Support Palliat Care. 2016;10:281‐287. [DOI] [PubMed] [Google Scholar]
- 29. Penna F, Busquets S, Argiles JM. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin Cell Dev Biol. 2016;54:20‐27. [DOI] [PubMed] [Google Scholar]
- 30. Castle JC, Loewer M, Boegel S, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genom. 2014;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calleros L, Sanchez‐Hernandez I, Baquero P, Toro MJ, Chiloeches A. Oncogenic Ras, but not (V600E)B‐RAF, protects from cholesterol depletion‐induced apoptosis through the PI3K/AKT pathway in colorectal cancer cells. Carcinogenesis. 2009;30:1670‐1677. [DOI] [PubMed] [Google Scholar]
- 32. Fujii K, Luo Y, Fujiwara‐Tani R, et al. Pro‐metastatic intracellular signaling of the elaidic trans fatty acid. Int J Oncol. 2017;50:85‐92. [DOI] [PubMed] [Google Scholar]
- 33. Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end‐products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163‐170. [DOI] [PubMed] [Google Scholar]
- 34. Elkalaf M, Andel M, Trnka J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS One. 2013;8:e70772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dugdale HF, Hughes DC, Allan R, et al. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol Cell Biochem. 2018;444:109‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirayama A, Kami K, Sugimoto M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time‐of‐flight mass spectrometry. Cancer Res. 2009;69:4918‐4925. [DOI] [PubMed] [Google Scholar]
- 39. Kawada K, Toda K, Sakai Y. Targeting metabolic reprogramming in KRAS‐driven cancers. Int J Oncol. 2017;22:651‐659. [DOI] [PubMed] [Google Scholar]
- 40. Haq R, Fisher DE, Widlund HR. Molecular pathways: BRAF induces bioenergetic adaptation by attenuating oxidative phosphorylation. Clin Cancer Res. 2014;20:2257‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Metges CC, Wolfram G. Medium‐ and long‐chain triglycerides labeled with 13C: a comparison of oxidation after oral or parenteral administration in humans. J Nutr. 1991;121:31‐36. [DOI] [PubMed] [Google Scholar]
- 42. Montgomery MK, Osborne B, Brown SH, et al. Contrasting metabolic effects of medium‐ versus long‐chain fatty acids in skeletal muscle. J Lipid Res. 2013;54:3322‐3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abe T, Hirasaka K, Kohno S, et al. Capric acid up‐regulates UCP3 expression without PDK4 induction in mouse C2C12 myotubes. J Nutr Sci Vitaminol (Tokyo). 2016;62:32‐39. [DOI] [PubMed] [Google Scholar]
- 44. Caudron‐Herger M, Diederichs S. Mitochondrial mutations in human cancer: curation of translation. RNA Biol. 2018;15:62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YK, Jee BA, Kwon SM, et al. Identification of a mitochondrial defect gene signature reveals NUPR1 as a key regulator of liver cancer progression. Hepatology. 2015;62:1174‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyagawa Y, Mori T, Goto K, et al. Intake of medium‐chain fatty acids induces myocardial oxidative stress and atrophy. Lipids Health Dis. 2018;17:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen PB, Yang JS, Park Y. Adaptations of skeletal muscle mitochondria to obesity, exercise, and polyunsaturated fatty acids. Lipids. 2018;53:271‐278. [DOI] [PubMed] [Google Scholar]
- 48. Wang L, Luo L, Zhao W, et al. Lauric acid accelerates glycolytic muscle fiber formation through TLR4 signaling. J Agric Food Chem. 2018;66:6308‐6316. [DOI] [PubMed] [Google Scholar]


