Abstract
Heat shock protein 105 (HSP105) is overexpressed in many cancers, including colorectal cancer (CRC) and esophageal cancer (EC). We carried out a phase I clinical trial of HLA‐A24‐ and HLA‐A2‐restricted HSP105 peptide vaccines in patients with CRC or EC. In this additional study of the trial, we examined the immunological efficacy of the novel vaccine. Thirty patients with advanced CRC or EC underwent HSP105 peptide vaccination. Immunological responses were evaluated by ex vivo and in vitro γ‐interferon enzyme‐linked immunospot assays and their correlation with patients’ prognosis was analyzed. The HSP105 peptide vaccines induced peptide‐specific CTLs in 15 of 30 patients. Among HLA‐A24 patients (n = 15), 7 showed induction of CTLs only ex vivo, whereas among HLA‐A2 patients (n = 15), 4 showed the induction ex vivo and 6 in vitro. Heat shock protein 105‐specific CTL induction correlated with suppression of cancer progression and was revealed as a potential predictive biomarker for progression‐free survival (P = .008; hazard ratio = 3.03; 95% confidence interval, 1.34‐6.85) and overall survival (P = .025; hazard ratio = 2.72; 95% confidence interval, 1.13‐6.52). Production of cytokines by HSP105 peptide‐specific CTLs was observed at the injection sites (skin) and tumor tissues, suggesting that HSP105‐specific CTLs not only accumulated at vaccination sites but also infiltrated tumors. Furthermore, we established 2 HSP105 peptide‐specific CTL clones, which showed HSP105‐specific cytokine secretion and cytotoxicity. Our results suggest that the HSP105 peptide vaccine could induce immunological effects in cancer patients and improve their prognosis.
Keywords: cancer vaccine, cytokine, cytotoxic T lymphocyte, heat shock protein 105, human leukocyte antigen
Abbreviations
- APC
allophycocyanin
- CI
confidence interval
- CRC
colorectal cancer
- ELISPOT
enzyme‐linked immunospot
- E/T
effector/target
- HLA
human leukocyte antigen
- HR
hazard ratio
- HSP
heat shock protein
- IFNγ
γ‐interferon
- IL
interleukin
- OS
overall survival
- PD‐1
programmed cell death‐1
- PD‐L1
PD‐1 ligand‐1
- PFS
progression‐free survival
- PS
performance status
- SD
stable disease
- TAA
tumor‐associated antigen
- TCR
T‐cell receptor
- TNF
tumor necrosis factor
- TIL
tumor‐infiltrating lymphocyte
1. INTRODUCTION
Colorectal cancer is the third most common cancer type and the second leading cause of cancer‐related deaths.1 Although CRC prognosis has improved with the progress of surgery, chemotherapy, and molecular targeted drugs, it still remains poor.2 Immune checkpoint inhibitors have shown effectiveness against microsatellite instability‐high colon cancer; however, their activity is limited to this subset of cancer patients.3, 4 Esophageal cancer is the seventh most common cancer type and the sixth leading cause of cancer‐related deaths;5 however, unlike CRC, EC is sensitive to few antineoplastic agents. It is expected that for both EC and CRC, prognosis could be improved with the development of immunotherapeutic approaches.
Stress‐response HSP105 has been identified as a biomarker of colon cancer.6 Our previous results indicate that HSP105 is specifically overexpressed in various human cancers such as CRC (54/64, 84.4%), pancreatic cancer (17/17, 100%), EC (12/14, 85.7%), pharyngeal cancer (8/8, 100%), breast cancer (13/15, 86.7%), and melanoma (53/67, 79.1%), but not in normal tissues except for the testes.7, 8 Heat shock protein 105 was shown to inhibit cancer cell apoptosis9 and animal studies on the HSP105 DNA vaccine and HSP105‐pulsed dendritic cells revealed that HSP105 could be a novel promising TAA in cancer immunotherapy.10, 11
For HSP105 immunotargeting, it is necessary to identify HLA epitopes. We focused on the HLA‐A24‐encoding gene, for which 60% of Japanese (95% of whom have HLA‐A*24:02) and 20% of Caucasians are positive,12, 13 and on HLA‐A2, the most common HLA class I allele in the Caucasian population also found in 40% of Japanese.12, 14 As a result, we identified HLA‐A24‐ and HLA‐A2‐restricted HSP105 peptide epitopes: A24‐1 (NYGIYKQDL) and A24‐7 (EYVYEFRDKL) and A2‐7 (RLMNDMTAV) and A2‐12 (KLMSSNSTDL) and revealed that HSP105‐specific CTLs reactive against at least 1 of the 4 peptides were present in 67% (14/21) of CRC patients.15
Based on these data, we undertook a phase I clinical trial of the HSP105‐derived peptide vaccine for 30 patients with advanced CRC and EC positive for 1 or more of HLA‐A*24:02, 02:01, 02:06, or 02:07 alleles (manuscript in prep.). Within this trial, we undertook an additional investigation to clarify the immunological effectiveness of HSP105 peptide vaccines.
Here, we report the results of this immunological study, which indicate that HSP105 peptide‐based vaccines caused accumulation of HSP105‐specific CTLs in peripheral blood, injection sites, and tumor tissues, suggesting that CTL induction could be a prognostic biomarker in vaccinated patients with advanced CRC and EC.
2. MATERIALS AND METHODS
2.1. Study design
This research was undertaken as an additional study of a phase I clinical trial of HSP105‐derived peptide vaccine. Fifteen patients carrying the HLA‐A24 gene received HLA‐A24‐restricted HSP105‐derived peptides (A24‐1 and A24‐7) and 15 carrying the HLA‐A2 gene received HLA‐A2‐restricted HSP105‐derived peptides (A2‐7 and A2‐12) (Table S1). Peptide vaccines were given with an adjuvant (Montanide ISA‐51 VG) by intradermal injection (3 mg per time) in the axilla every 7 days for a maximum of 1 year; the treatment was discontinued if disease progression or deterioration of the patient's general condition was observed. All patients gave written informed consent; they were coded and fully anonymized. The study was approved by the Ethics Committee of the National Cancer Center and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Evaluation of clinical response
Tumor size was assessed by computed tomography or magnetic resonance imaging before vaccination and every 4 weeks after the first vaccination. Tumor responses were evaluated according to the RECIST guideline (version 1.1).16
2.3. Peripheral blood collection
Peripheral blood samples (30 mL) were obtained from CRC and EC patients before and every 2 weeks after the first vaccination. The PBMCs were isolated by density gradient centrifugation using Ficoll‐Paque (Pharmacia), frozen at −80°C, and stored in liquid nitrogen until use.
2.4. Cell lines
Human colon cancer cell line SW620 (high HSP105 expression, HLA‐A*02:01/A*24:02) and human liver cancer cell line HepG2 (low HSP105 expression, HLA‐A*02:01/A*24:02) were used as target cells. Transporter associated with antigen processing‐deficient human lymphoblastoid T2 (HLA‐A*02:01) and T2A24 (HLA‐A*24:02) cells pulsed with appropriate HSP105 peptides or HIV peptides at room temperature for 1 h were also used as CTL targets. All cell lines were preserved in our laboratory; they were cultured in RPMI‐1640 or DMEM (Sigma) supplemented with 10% heat‐inactivated FBS (Gibco) and 1% penicillin and streptomycin (Gibco) at 37°C in a humidified atmosphere containing 5% CO2.
2.5. Ex vivo IFN‐γ ELISPOT assay
The BD ELISPOT kit (BD Biosciences) was used according to the manufacturer's instructions. Noncultured PBMCs (5 × 105 per well) were incubated with peptide antigens (10 μg/mL) for 20 hours at 37°C and 5% CO2. The following HSP105 antigens were used: HLA‐A2‐restricted A2‐7 (RLMNDMTAV) or A2‐12 (KLMSSNSTDL) for HLA‐A2‐positive PBMCs and HLA‐A24‐restricted A24‐1 (NYGIYKQDL) or A24‐7 (EYVYEFRDKL) for HLA‐A24‐positive PBMCs. The PBMCs incubated with HLA‐A2‐restricted HIV19‐27 (TLNAWVKVV) or HLA‐A*24:02‐restricted HIV583‐591 (RYLKDQQLL) peptides (ProImmune) were used as negative control. The number of CTL spots was calculated automatically by the Eliphoto system (Minerva Tech). To eliminate nonspecific immune responses unrelated to HSP105 peptides, the number of spots produced by HSP105‐specific CTLs was determined by subtracting the number of spots generated against HLA‐matched HIV peptides from that against HSP105 peptides; if HIV peptides produced more than 100 spots and the difference between HSP105 and HIV peptides was less than 30 spots, the results were excluded. All analyses were carried out in duplicate.
2.6. Induction of HSP105 peptide‐specific CTLs from PBMCs and in vitro IFN‐γ ELISPOT assay
The PBMCs (2 × 106 per well) were cultured with HSP105 A2‐7, A2‐12, A24‐1, or A24‐7 (10 μg/mL) in RPMI‐1640 supplemented with 10% FBS, recombinant human IL‐2 (50 IU/mL; Novartis Pharma), and IL‐15 (10 ng/mL; PeproTech) for 14 days. CD8+ T cells were isolated using human CD8 microbeads (Miltenyi Biotec) and analyzed with the BD ELISPOT kit; T2 and T2A24 cells pulsed with the corresponding HSP105 peptides and HLA‐matched HIV peptides were used as targets. The isolated CD8+ T cells were cocultured with each group of target cells at the E/T cell ratio of 0.2 for 20 hours at 37°C and 5% CO2. The number of spots was calculated automatically by the Eliphoto system, and the results showing more than 20 spots against HIV peptides and less than 10‐spot differences between HSP105 and HIV peptides were excluded. All analyses were carried out in duplicate.
2.7. Dextramer staining
The PBMCs stimulated with each peptide were stained with HLA type‐matched Dextramer‐RPE for 10 minutes at room temperature. HLA‐A*24:02 Dextramer‐RPE (A24‐1, A24‐7, and HIV583‐591) and HLA‐A*02:01 Dextramer‐RPE (A2‐7, A2‐12, and HIV19‐27) were used. After staining with FITC‐conjugated anti‐CD8 Abs (ProImmune) for 20 minutes at 4°C, PBMCs were analyzed by flow cytometry using a FACSAria cell sorter (BD Biosciences).
2.8. CD107a staining
CD8+ T cells were incubated with T2 and T2A24 cells pulsed with HSP105 peptides or HLA type‐matched HIV peptides at the E/T ratio of 2 in the presence of APC‐conjugated CD107a‐specific Abs (BD Biosciences) for 3.5 hours at 37°C and analyzed by flow cytometry.
2.9. Analysis of TILs
Harvested tissues were cut into 2‐mm square pieces and incubated in RPMI‐1640 containing GlutaMax supplement (Thermo Fisher Scientific), 10% human AB serum (Sigma), 0.01% penicillin‐streptomycin‐glutamine mixture (Thermo Fisher Scientific), 2‐mercaptoethanol (50 μmol/L; Sigma), pyruvate (1 mmol/L; Gibco), and IL‐2 (3000 IU/mL) for 14 days at 37°C and 5% CO2. Tumor‐infiltrating lymphocytes detected by the IFN‐γ ELISPOT assay were incubated with HLA‐matched HSP105 peptide‐pulsed T2 and T2A24 cells in the presence of APC‐conjugated anti‐CD107a Abs for 3.5 hours at 37°C. After staining with FITC‐conjugated anti‐CD8 Abs for 20 minutes at 4°C, TILs were analyzed by flow cytometry. Supernatants were used to measure secretion of IL‐2, IFN‐γ, and TNF‐α (Cytometric Bead Array Enhanced Sensitivity Flex Sets; BD Biosciences) and granzyme B (Cytometric Bead Array Flex Sets; BD Biosciences). The data were analyzed with the FCAP Array Software (version 3.0; BD Biosciences).
2.10. Generation of CTL clones
The PBMC‐derived CD8+ HSP105 Dextramer+ cells and tissue‐derived CD8+CD107a+ cells were sorted, seeded on 96‐well plates (1 cell per well), and stimulated with irradiated (100 Gy) allogeneic PBMCs (8 × 104 per well) used as feeder cells in AIM‐V medium (Thermo Fisher Scientific) supplemented with 10% human AB serum, IL‐2 (100 IU/mL), IL‐15 (10 ng/mL), and phytohemagglutinin‐P (1 μg/mL; Wako) for 14‐21 days.
2.11. Cytotoxicity assay
Target cells labeled with calcein‐AM (Dojindo) for 30 minutes at 37°C were cocultured with CTL clones for 4 hours at different E/T ratios. Fluorescence was measured before and after coculture using the Terascan VPC system (Minerva Tech), and specific cytotoxic activity was calculated as previously described.17
2.12. Determination of recognition efficiency
Calcein‐AM‐labeled T2 and T2A24 cells were pulsed with different peptide concentrations (10−4‐10−14 M at log increments) and incubated with CTL clones at the E/T ratio of 10. After 4 hours, CTLs were analyzed for recognition efficiency defined as the peptide concentration at which the concentration curve crosses the 50% cytotoxicity threshold.17
2.13. Evaluation of HLA‐A2 restriction
Hepatitis B virus‐integrated human hepatocellular carcinoma cell line JHH‐7 (low HSP105 expression, HLA‐A*24:02+/A*31:01+) transfected with each HLA‐A2 gene, JHH‐7/mock (HLA‐A2−), JHH‐7/A*02:01 (HLA‐A*02:01+), JHH‐7/A*02:06 (HLA‐A*02:06+), and JHH‐7/A*02:07 (HLA‐A*02:07+) was used as the target cell line. Detailed transfection methods are in accordance with previously described methods.18 The expression of HLA‐A2 in each cell line was evaluated by flow cytometry (Figure S1). These cell lines were used as target cells after pulsing with HSP105 A2‐7 peptide.
2.14. Immunohistochemistry
Immunohistochemistry staining was carried out using mAbs against HSP105 (clone EPR4576; Epitomics), HLA class I (clone EMR8‐5; Hokudo), PD‐L1 (clone SP142; SPRING), CD8 (clone 1A5; BioGenex), and CD4 (clone 4B12; Dako) according to the manufacturers’ protocols. Quantitative analysis of HSP105 expression was carried out based on the staining intensity and the results were scored as 0, 1+, 2+, and 3+. The HLA class I expression was evaluated based on the proportion of stained cells and staining intensity; however, because all examined cancer tissues had a high proportion of stained cells (67% or more), they were classified based on the intensity. Expression of PD‐L1 was scored as – (0%) or + (1% or more) according to the percentage of positively stained areas relative to the total tumor areas. CD4+ and CD8+ T cells in tumors were counted in high power fields (×400) and the average numbers were calculated.
2.15. Sequence analysis of TCR
T‐cell receptor sequences of CTL clones were identified as previously described.19 Briefly, cDNA was amplified using 24 TCR‐BV gene family‐specific forward primers and a constant region‐specific reverse primer. Thereafter, PCR products were amplified by seminested PCR for screening the BV gene family. Polymerase chain reaction products identified using specific primers were sequenced. The international ImMunoGeneTics information system (IMGT) database site (http://imgt.cines.fr) was used to identify the human TCR‐BV gene family. Analysis of the TCR repertoire of HSP105 peptide‐specific CTLs in PBMCs was undertaken by Repertoire Genesis (Osaka, Japan).
2.16. Statistical analysis
Statistical analyses were carried out using R software (The R Foundation for Statistical Computing; http://r-project.org). Categorical data were compared by the χ2 test or Fisher's exact test, and continuous data were compared by the Mann‐Whitney U test. Prognostic factors were evaluated using the log‐rank test and Cox proportional hazard models. Spearman rank correlations were used for correlation analysis. Statistical significance was defined at P < .05.
3. RESULTS
3.1. Patient characteristics
Clinicopathological characteristics of patients are presented in Table 1. Among the 30 patients, 21 were men and 17 and 13 were diagnosed with EC and CRC, respectively. The average age was 63.0 years (range, 30‐77 years), and the median follow‐up period was 5.0 months (range, 0.8‐15.3 months). Eleven patients had SD and 16 had progressive disease 1 month after the first vaccination, according to RECIST.
Table 1.
Patients’ characteristics, heat shock protein 105 (HSP105)‐specific CTL responses, and clinical outcomes
| HLA group | No. | Age, years | Sex | Tumor type | HLA‐A | PS | No. of vaccinations | Increase in HSP105‐specific CTLs (peptide type) | Maximum spot numberc | Skin reaction | Clinical responsed | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex vivo | In vitro | |||||||||||||
| IFN‐γ ELISPOT assaya | IFN‐γ ELISPOT assayb | |||||||||||||
| A24 | 1 | 66 | M | EC | 24:02 | 0 | 4 | − | − | 27 | + | PD | ||
| 2 | 71 | M | EC | 24:02 | 0 | 5 | + | (A24‐7) | − | 54 | + | PD | ||
| 4 | 66 | M | EC | 24:02 | 0 | 13 | + | (A24‐1, A24‐7) | − | 809 | + | SD | ||
| 5 | 63 | F | EC | 24:02 | 0 | 3 | − | − | 10 | + | PD | |||
| 8 | 66 | M | EC | 24:02 | 0 | 4 | − | − | 24 | + | PD | |||
| 10 | 63 | M | EC | 24:02 | 1 | 5 | − | − | 3 | + | PD | |||
| 11 | 71 | M | EC | 24:02 | 1 | 1 | − | − | 0 | − | NE | |||
| 27 | 68 | M | EC | 24:02 | 1 | 14 | + | (A24‐1) | − | 219 | + | SD | ||
| 7 | 73 | M | CRC | 24:02 | 1 | 4 | − | − | 1 | + | PD | |||
| 13 | 65 | F | CRC | 24:02 | 0 | 13 | + | (A24‐1, A24‐7) | − | 429 | + | SD | ||
| 19 | 52 | F | CRC | 24:02 | 0 | 12 | + | (A24‐1, A24‐7) | − | 458 | + | SD | ||
| 21 | 61 | M | CRC | 24:02 | 0 | 11 | + | (A24‐1) | − | 312 | + | SD | ||
| 22 | 55 | M | CRC | 24:02 | 0 | 3 | − | − | 5 | − | NE | |||
| 24 | 56 | F | CRC | 24:02 | 0 | 4 | + | (A24‐1) | − | 558 | + | PD | ||
| 26 | 71 | M | CRC | 24:02 | 0 | 3 | − | − | 15 | + | PD | |||
| A2 | 3 | 74 | M | EC | 02:01 | 0 | 10 | − | − | 12 | − | SD | ||
| 6 | 73 | M | EC | 02:06 | 0 | 3 | − | + | (A2‐7) | 23 | − | PD | ||
| 9 | 60 | F | EC | 02:01 | 0 | 5 | − | + | (A2‐7) | 246 | + | SD | ||
| 16 | 67 | M | EC | 02:06 | 1 | 9 | − | − | 13 | + | PD | |||
| 23 | 69 | M | EC | 02:06 | 1 | 5 | − | − | 5 | − | PD | |||
| 25 | 56 | F | EC | 02:01 | 0 | 3 | − | − | 18 | − | PD | |||
| 28 | 77 | M | EC | 02:06 | 1 | 4 | − | − | 12 | − | PD | |||
| 29 | 56 | M | EC | 02:06 | 1 | 8 | + | (A2‐7) | + | (A2‐7) | 157 | + | PD | |
| 30 | 76 | M | EC | 02:01 | 0 | 28 | + | (A2‐7, A2‐12) | + | (A2‐7) | 323 | + | SD | |
| 12 | 47 | M | CRC | 02:01 | 0 | 16 | − | + | (A2‐7) | 38 | − | SD | ||
| 14 | 66 | F | CRC | 02:06 | 0 | 2 | − | − | 25 | − | NE | |||
| 15 | 30 | M | CRC | 02:01 | 0 | 12 | − | + | (A2‐7) | 45 | − | SD | ||
| 17 | 42 | F | CRC | 02:01 | 0 | 13 | + | (A2‐7) | − | 297 | + | PD | ||
| 18 | 54 | F | CRC | 02:07 | 0 | 2 | − | − | 11 | − | PD | |||
| 20 | 76 | M | CRC | 02:07 | 0 | 13 | + | (A2‐7) | − | 38 | + | SD | ||
Ex vivo γ‐interferon (IFN‐γ) enzyme‐linked immunospot (ELISPOT) assay: +, spot number of HSP105‐specific CTLs increased to ≥30 after vaccination; −, spot number of HSP105‐specific CTLs increased to <30 after vaccination.
In vitro IFN‐γ ELISPOT assay: +, spot number of HSP105‐specific CTLs increased to ≥20 after vaccination. −, spot number of HSP105‐specific CTLs increased to < 20 after vaccination.
Maximum spot number of HSP105‐specific CTLs observed in ex vivo or in vitro IFN‐γ ELISPOT assays.
Clinical responses were evaluated according to the RECIST guidelines.
CRC, colorectal cancer; EC, esophageal cancer; F, female; HLA, human leukocyte antigen; M, male; NE, not evaluable; PD, progressive disease; PS, performance status; SD, stable disease.
3.2. Immunological responses
We evaluated the expression of HSP105, HLA class I, and PD‐L1 in primary tumors and tumor infiltration of CD4+ and CD8+ T cells before and after vaccination (Tables S2 and S3 and Figure S2). The expression of HSP105 and HLA class I before the first vaccination was observed in all analyzed patients (14/14, 100%) and that of PD‐L1 was detected in 6 patients, all of whom had EC. Because of objective difficulties in obtaining matching tumor samples before and after vaccination, we could compare expression changes only in 3 patients (nos. 9, 23, and 24); nevertheless, HSP105 levels declined in all of them after vaccination. Despite the low number of analyzed patients, it can be hypothesized that the vaccination led to elimination of cancer cells with strong HSP105 expression.
To determine whether HSP105 peptide vaccines induced the functional activity of CTLs, we carried out IFN‐γ ELISPOT assays in PBMCs from the vaccinated patients (Figure 1 and Table 1). The ex vivo IFN‐γ ELISPOT results indicated that, after vaccination, the frequency of HSP105 peptide‐specific CTLs in PBMCs was increased in 11 of 30 patients (37%); among them, 7 patients received the HLA‐A24 vaccine. The in vitro IFN‐γ ELISPOT results showed that HSP105 peptide‐specific CTLs were induced in 6 patients (6/30, 20%), all of whom received the HLA‐A2 vaccine.
Figure 1.
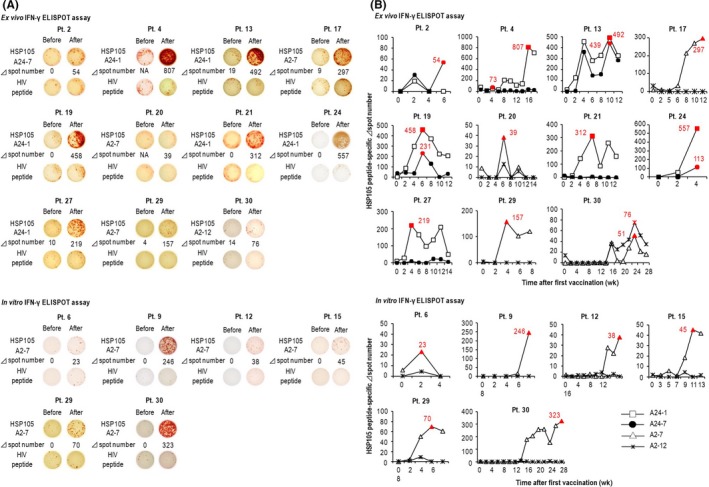
Changes in the frequencies of heat shock protein 105 (HSP105) peptide‐specific CTLs before and after vaccination. A, Representative images of samples with maximal Δ spot numbers. Patient (Pt.) numbers are given on the top. “Before” and “After” indicate samples taken before and after vaccination, respectively. B, Graphs show time‐dependent changes in Δ spot numbers of PBMCs obtained before and every 2 wk after the first vaccination. ELISPOT, enzyme‐linked immunospot; IFN‐γ, γ‐interferon
3.3. Heat shock protein 105‐specific CTLs could delay cancer progression and improve prognosis
We analyzed the correlation between HSP105‐specific CTL frequency and clinical outcomes. Mean tumor size in patients with induced HSP105‐specific CTLs (58.3 ± 33.6 mm) was smaller than that in patients without the induction (88.7 ± 47.6 mm) (P = .04; Figure 2A). The maximum increase in HSP105‐specific CTLs observed in ELISPOT assays significantly negatively correlated with the rate of tumor increase per day (r = −0.501, P < .01; Figure 2B). In patients with HSP105‐specific CTL frequencies greater than or equal to 100 (n = 10), the tumor increase per day ratio (0.32 ± 0.34) showed an especially significant decline compared to that in patients with CTL frequencies less than 100 (0.94 ± 0.74; P < .01). These data indicated that HSP105 peptide vaccines might delay cancer progression by inducing specific CTLs and that the strength of the induction was an important factor. Moreover, the CTL frequency also correlated with OS (r = .704, P < .01; Figure 2C). All patients with lower spot numbers and better OS (nos. 1, 8, 12, 15, 20, and 25) had good performance status (PS) and tended to be younger (Tables 1 and S4).
Figure 2.
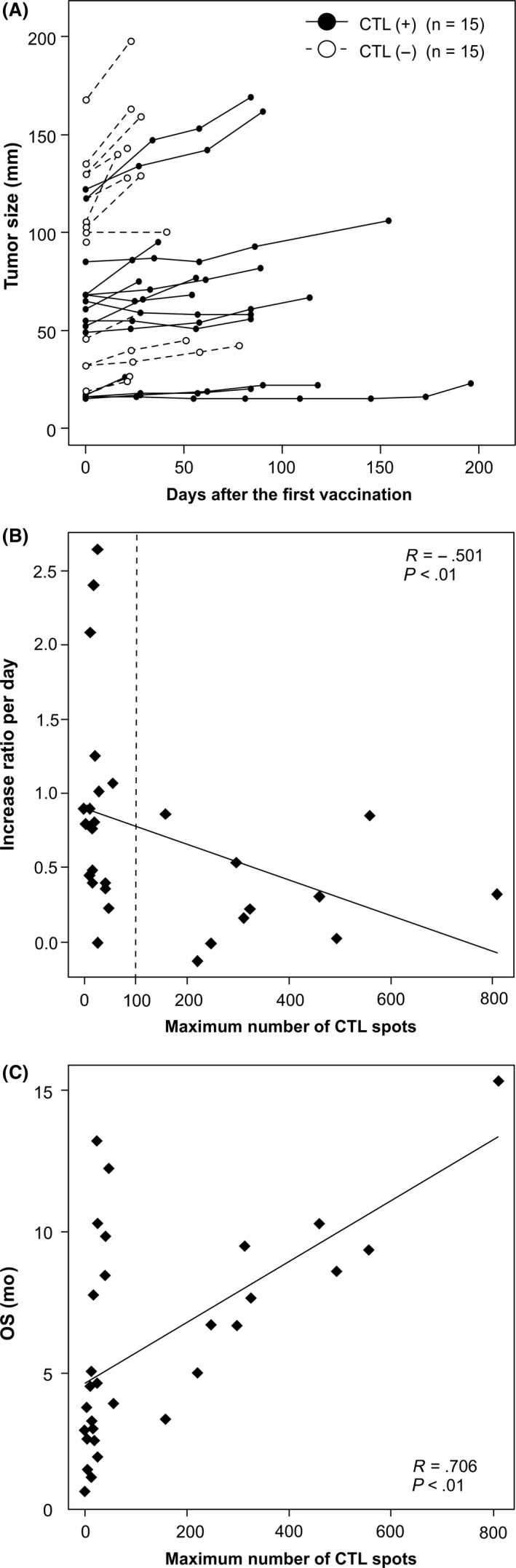
Correlation between heat shock protein 105 (HSP105)‐specific CTL frequencies and clinical outcomes. A, Spider plot of tumor size changes during HSP105 peptide vaccination. CTL(+) and CTL(−) indicate patients with and without the induction of HSP105‐specific CTLs, respectively, in ex vivo and in vitro γ‐interferon enzyme‐linked immunospot assays. B, Negative correlation observed between maximum numbers of HSP105‐specific CTL spots and the tumor increase ratio per day calculated by dividing tumor increase values by the number of observation days (r = −0.501, P < .01). C, Correlation between HSP105‐specific CTL frequency and overall survival (OS) (r = 0.706, P < .01)
We also examined prognostic factors for OS and PFS (Table 2). Univariate analysis indicated that the induction of HSP105‐specific CTLs was the only prognostic factor for PFS (–; P = .004) and PS (1; P = .004), whereas skin reaction (–; P = .021) and CTL induction (–; P = .0013) were prognostic factors for OS. Furthermore, in multivariate analysis, CTL induction was a prognostic factor for PFS (P = .008; HR = 3.03; 95% CI, 1.34‐6.85) and OS (P = .025; HR = 2.72; 95% CI, 1.13‐6.52) and PS was associated with OS (P = .010; HR = 4.39; 95% CI, 1.43‐13.50).
Table 2.
Univariate and multivariate analyses of correlations between clinical and immunological factors and overall survival (OS) and progression‐free survival (PFS) among patients who received heat shock protein 105 (HSP105) peptide vaccine
| Parameter | PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| HR | P value | HR | 95% CI | P value | HR | P value | HR | 95% CI | P value | |
| Age, years (≥65/<65) | 1.14 | .737 | 1.08 | .856 | ||||||
| Sex (male/female) | 1.89 | .143 | 1.06 | .899 | ||||||
| Tumor type (EC/CRC) | 1.54 | .261 | 1.26 | .593 | ||||||
| HLA (A24/A2) | 1.11 | .786 | 2.02 | .107 | ||||||
| Tumor size (≥50/<50) | 1.14 | .742 | 2.50 | .060 | ||||||
| Performance status (0/1) | 1.94 | .133 | 1.59 | 0.65‐3.91 | .312 | 4.84 | .004 | 4.39 | 1.43‐13.50 | .010 |
| Skin reaction (+/−) | 1.41 | .389 | 2.64 | .021 | ||||||
| Induction of HSP105‐specific CTLs in ex vivo and/or in vitro IFN‐γ ELISPOT assays (+/−) | 3.21 | .004 | 3.03 | 1.34‐6.85 | .008 | 2.97 | .013 | 2.72 | 1.13‐6.52 | .025 |
CI, confidence interval; CRC, colorectal cancer; EC, esophageal cancer; ELISPOT, enzyme‐linked immunospot; HLA, human leukocyte antigen; HR, hazard ratio; IFN‐γ, γ‐interferon.
3.4. Heat shock protein 105‐specific CTLs accumulated at vaccine inoculation sites and tumors
Next, we examined the infiltration of HSP105‐specific CTLs into vaccine inoculation sites and tumors based on cytokine production (Figure 3 and Table S5). Heat shock protein 105‐specific cytokine release after vaccination was observed in the skin (patients nos. 4, 8, 13, 17, and 23), primary tumor (patient no. 27), metastasized lymph node (patient no. 9), and lung tissue (patient no. 24), indicating that HSP105‐specific CTLs migrated not only to the injection sites but also to tumors, including metastatic lesions. In patients nos. 9, 24, and 27, the induction of HSP105‐specific CTLs was also observed in PBMCs, whereas in patients nos. 4, 13, and 17, it was detected at the injection sites and PBMCs, and in patients nos. 8 and 23 only at the injection sites.
Figure 3.
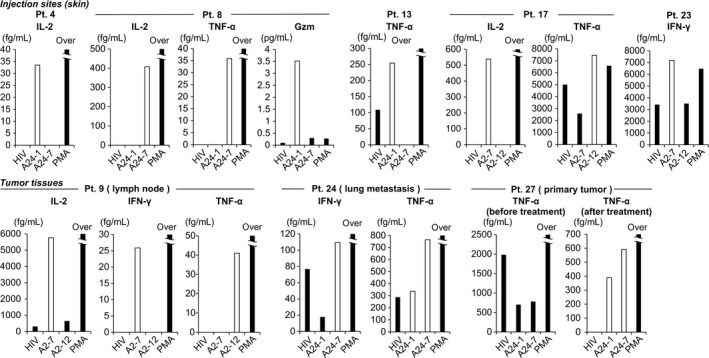
Cytokines produced by heat shock protein 105 (HSP105) peptide‐specific CTLs at vaccine injection sites and in tumor tissues. CTLs were cocultured with T2 or T2A24 cells pulsed with HIV peptide or HSP105 peptides and analyzed for cytokine secretion to supernatant; phorbol myristate acetate plus ionomycin (PMA) was added as positive control. Patient (Pt.) numbers are shown at the top. White bars indicate HSP105 peptide‐specific cytokine production. Gzm, granzyme B; IL, interleukin; TNF‐α, tumor necrosis factor‐α
3.5. Heat shock protein 105‐specific CTLs infiltrated into tumor tissue following peptide vaccination
In patient no. 9, who was diagnosed with advanced EC and had the HLA‐A*02:01 gene, the therapeutic effect was classified as SD 1 month after vaccination; nevertheless, he developed metastasis to the cervical lymph node and vaccination was terminated after the fifth injection. The patient had insignificant increase of HSP105‐specific CTLs ex vivo, but showed a remarkable induction of HSP105 A2‐7 peptide‐specific CTLs (246 of 5 × 105 PBMCs) in vitro (Figure 1). We resected the metastatic lymph node and measured cytokine release by infiltrated HSP105 peptide‐specific CTLs, which showed secretion of IL‐2, IFN‐γ, and TNF‐α (Figure 3). Furthermore, immunohistochemistry revealed increased infiltration of CD8+ lymphocytes into the lymph node after vaccination (Figure 4A and Table S3), indicating post‐vaccination changes in the tumor microenvironment.
Figure 4.
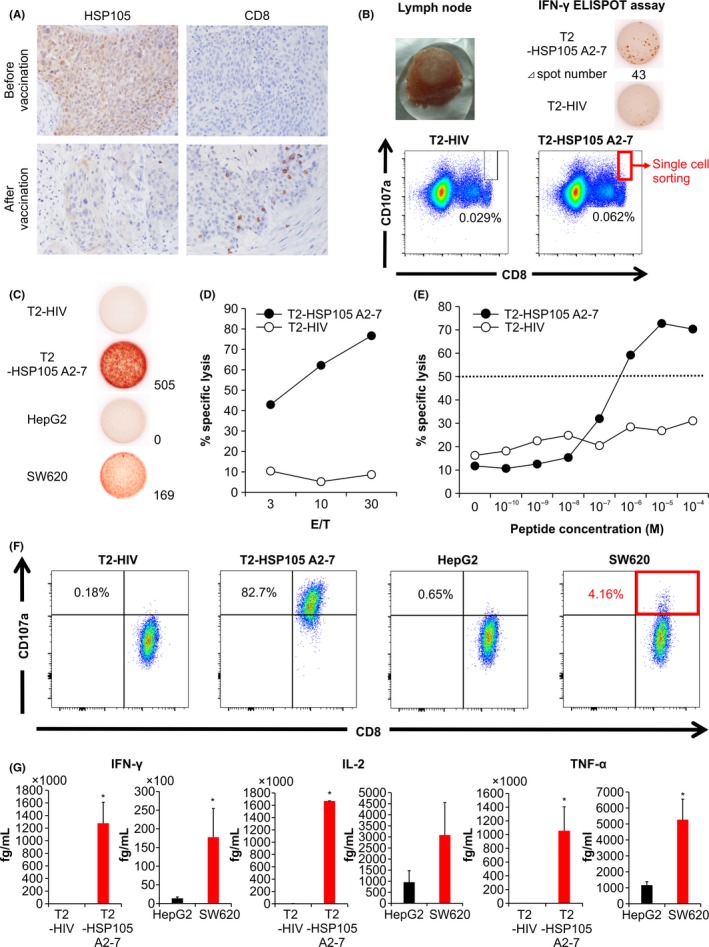
Immunological monitoring of patient no. 9 and establishment of a tissue‐derived CTL clone. A, Immunohistochemical analysis of heat shock protein 105 (HSP105) and CD8 expression before and after vaccination. Magnification, ×200. B, Cervical lymph node resected after the fifth vaccination (upper left panel) showed increased number of HSP105 A2‐7 peptide‐specific CTLs in the γ‐interferon (IFN‐γ) enzyme‐linked immunospot (ELISPOT) assay (upper right panels). FACS analysis of CD8+CD107a+ T cells showing reactivity against T2 cells pulsed with an HIV peptide (lower left panel) or the HSP105 A2‐7 peptide (lower right panel). Single CD8+CD107a+ T cells reactive against the HSP105 A2‐7 peptide‐pulsed cells were used to establish a CTL clone. C, IFN‐γ production by the CTL clone measured by the IFN‐γ ELISPOT assay. T2 cells pulsed with HSP105 A2‐7 or HIV peptides and SW620 and HepG2 cells were used as targets at the effector / target (E/T) ratio of 0.2. D, Cytotoxic activity of the CTL clone against HIV peptide‐ or HSP105 A2‐7 peptide‐pulsed T2 cells was measured at the E/T ratios of 3, 10, and 30 by the cytotoxicity assay. E, The avidity of the CTL clone was tested using T2 cells pulsed with different concentrations of HSP105 A2‐7 or HIV peptides; E/T ratio of 10. The peptide concentration at which the curve crossed the 50% cytotoxicity line (dotted) was defined as the recognition efficiency of the clone. F, Surface CD107a expression by the CTL clone was analyzed in response to the indicated target cells. G, Production of IFN‐γ, interleukin‐2 (IL‐2), and tumor necrosis factor‐α (TNF‐α) by the CTL clone (1.0 × 105 cells per well) after 24‐h of coculture with the indicated target cells (5 × 104 per well). Data represent the mean ± SD of 2 measurements. *P < .05
To assess the specificity of HSP105 recognition as an antigen, TILs from lymph node tissue were incubated with high concentration of IL‐2 and analyzed for IFN‐γ production in response to T2 cells pulsed with HSP105 A2‐7 peptide (T2‐HSP105 A2‐7). The results indicated that IFN‐γ secretion was increased, and an HSP105 A2‐7‐specific CTL clone was established from a single CD8+CD107a+ cell (Figure 4B). This CTL clone showed HSP105 peptide‐specific IFN‐γ secretion in response to T2‐HSP105 A2‐7 cells and SW620 cells (high HSP105 expression), but not in response to T2 cells pulsed with HIV19‐27 peptide (T2‐HIV) or HepG2 cells (low HSP105 expression) (Figure 4C), and also showed cytotoxicity against T2‐HSP105 A2‐7 cells (Figure 4D). The avidity of the established clone for the HSP105 A2‐7 peptide was analyzed by the ability to lyse T2‐HSP105 A2‐7 or T2‐HIV cells pulsed with decreasing concentrations of each peptide, and its recognition efficacy was determined as 10−7 M (Figure 4E). Moreover, 82.7% and 4.16% of the cloned CTLs showed CD107a expression in response to T2‐HSP105 A2‐7 and SW620 cells, respectively (Figure 4F), suggesting clone degranulation, and showed IFN‐γ, IL‐2, and TNF‐α secretion (Figure 4G). These results indicated that the established CTL clone specifically recognized the HSP105 peptide and exerted cytotoxicity against cells presenting HSP105 not only exogenously but also endogenously. Furthermore, the CTL clone produced IFN‐γ as opposed to not only JHH‐7/A*02:01 but also JHH‐7/A*02:06, showing that it could recognize not only HSP105 A2‐7 peptide‐HLA‐A*02:01 complex but also A*02:06 complex (Figure S3).
3.6. Heat shock protein 105‐specific CTL clone could recognize HSP105‐expressing cancer cells
Patient no. 21 with advanced CRC and the HLA‐A*24:02 gene received the vaccine 11 times and showed long‐term SD according to RECIST; however, the vaccination was terminated at the patient's request. In his case, we observed a remarkable increase of HSP105 A24‐1 peptide‐specific CTLs (312 of 5 × 105 PBMCs) in the ex vivo IFN‐γ ELISPOT assay (Figure 1) and established an A24‐1‐specific CTL clone from a single CD8+HSP105 A24‐1 Dextramer+ cell after the third vaccination (Figure S4A). The CTL clone, which showed 99.9% CD8/HSP105 Dextramer positivity, demonstrated cytotoxicity, cytokine secretion, and CD107a expression specifically against A24‐1‐pulsed T2A24 cells and SW620 cells; its A24‐1 recognition efficacy was 10−8 M (Figure S4B‐F).
3.7. Vaccine induced polyclonal peptide‐specific CTLs in PBMCs of vaccinated patients
We evaluated the clonality of peptide‐specific CTLs induced by vaccinations. Three other peptide‐specific CTL clones were established from the PBMCs of patient no. 30 (Figure S5A). Furthermore, we sorted peptide‐specific CTLs (CD8+CD107a+ cells) in this patient's PBMCs for TCR repertoire analysis (Figure S5B). Consequently, TCR repertoire analysis revealed that HSP105 A2‐7 peptide‐specific CTLs in this patient's PBMCs displayed numerous patterns of V‐J‐CDR3 and the frequencies of the same TCR sequence as established CTL clones were 41.5%, 8.1%, and 0.1%, respectively (Table S6), suggesting that polyclonal peptide‐specific CTLs were induced in PBMCs through vaccination.
4. DISCUSSION
Immune checkpoint inhibitors and adoptive immunotherapy with tumor‐infiltrating CTLs have shown undoubted efficacy against malignant melanoma.20, 21 Peptide vaccine therapy could prolong survival of cancer patients and prevent tumor recurrence without serious adverse effects, thus not interfering with patient's quality of life.22 However, it seems difficult to achieve an anticancer therapeutic effect using only peptide‐based vaccines. In this trial of the HSP105 peptide vaccine, we did not observe patients with complete or partial response, but there were cases with long‐term SD. Our results indicate that the HSP105 peptide vaccine induces anticancer immune reactivity and, therefore, deserves further evaluation. However, previous analyses of established CTL clones revealed their antitumor effects on HSP105+ cell lines were limited, and the clinical effect of the vaccine was inadequate, probably because these CTLs were established from the cells of patients already harboring antigen‐expressing cancer cells, suggesting the presence of an immunosuppressive mechanism in these patients or the lack of the high‐avidity T‐cell clones in these patients, based on the immunological selection for self‐antigens.
Although we observed the induction of HSP105‐specific CTLs in PBMCs in only 50% of patients (15/30), it was associated with good prognosis (higher PFS and OS); therefore, the future goal should be the increase of the CTL induction rate. The developed HSP105 peptide vaccine consists of a mixture of 2 short peptides (10 or fewer residues), and it was shown that CTLs induced by short peptide‐based vaccines do not migrate to tumors, remaining at injection sites and becoming immunologically tolerant through binding to HLA class I presented by nonprofessional APCs that do not express costimulatory molecules.23, 24 Consistent with these observations, in our study, patients nos. 8 and 23 had HSP105‐specific CTLs at the injection sites but not among PBMCs. To overcome this problem, vaccination with longer peptides that could induce more effective immune responses not only through their uptake by APCs and presentation on HLA class ΙΙ but also by cross‐presentation on HLA class Ι might be useful.24, 25 Furthermore, we targeted only one TAA, HSP105, whereas clinical trials of peptide‐based vaccines targeting several TAAs reported increased survival.26, 27, 28 In the present trial, HSP105 expression in tumors decreased after vaccination, suggesting that tumor cells with high HSP105 levels were removed, although those with low or no HSP105 expression could remain. In a clinical trial of a GPC3‐targeting peptide vaccine as adjuvant therapy for hepatocellular carcinoma, post‐vaccination recurring tumors showed low GPC3 expression,29 indicating that tumor cells lacking the targeted TAA could remain and regrow after vaccination. Although HSP105 is a very specific tumor antigen, cancer cells are highly heterogeneous and combination therapy with vaccines targeting multiple TAAs might be more effective.
It should also be considered that tumor cells reduce their antigen presentation to escape from immunological surveillance.30 Thus, HLA class I expression is decreased or absent in 16%‐50% of cancers.31 In this study, we established HSP105‐specific CTL clones that not only exerted cytotoxicity against HSP105 peptide‐pulsed cells in a concentration‐dependent manner but also recognized HSP105‐expressing cells, thus showing HSP105‐related antitumor activity. However, cytotoxicity might not be enough to achieve clinical effectiveness because of insufficient antigen presentation by cancer cells. Although we detected HSP105‐specific CTLs not only at the injection sites but also in 2 metastatic tumors and 1 primary tumor, indicating that intradermal peptide vaccination promoted tumor infiltration by CTLs, it is not sufficient to achieve the desired antitumor effects. To overcome this issue, we are developing an intratumoral injection method for peptide vaccine delivery, which could provide stronger antitumor activity than conventional skin injection and cause systemic immune responses, inhibiting the growth of metastasized tumors.32, 33 It was shown that the combination of intratumoral peptide vaccination with an Ab blocking PD‐1 highly expressed on the surface of peptide‐specific CTLs augmented anticancer effects.34, 35, 36 It is possible that vaccination efficacy can be enhanced both in primary tumors and distant metastatic sites by loading the peptide directly into HLA class I of tumor cells by intratumoral injection and using anti‐PD‐1 Abs, which we expect to test in a future trial.
It is thought difficult to obtain antigen‐specific CTL clones, especially from PBMCs of patients with cancer (except melanoma).37 Here, we established two HSP105‐specific CTL clones from PBMCs and resected tumors using HSP105‐Dextramer and CD107a assays. CD107a is a CTL degranulation marker used to isolate CTLs with high tumor antigen specificity from PBMCs after vaccination.37, 38, 39 Although our CTL clones showed similar avidity (10−7 and 10−8 M, respectively), the clone obtained using the CD107a assay tended to have higher peptide‐specific CD107a expression and cytokine secretion, suggesting that this assay could be more effective for isolation of functional CTLs. However, both clones showed insufficient cytotoxicity against cancer cells, and we are currently establishing CTL clones with higher avidity.
Furthermore, administration of CTLs with engineered TCRs targeting cancer antigens has reported significant long‐term clinical responses40, 41, 42, 43, 44; however, it requires isolation of patient‐derived high‐avidity T‐cell clones specific for a target antigen and analysis of their TCR genes, which is rarely achieved.45 In the present study, we successfully established 2 highly functional CTL clones corresponding to different HLA types and analyzed their TCRαβ genes (data not shown), suggesting that anticancer therapy using genetically engineered CTLs has a potential in patients with HSP105‐positive tumors of different HLA types.
In conclusion, we show that the HSP105 peptide vaccine could induce functional tumor‐reactive CTLs with high HSP105‐peptide recognition efficiency. Although our study had a limitation as the sample size was small, to the best of our knowledge, this is the first report regarding HSP105 as a target for cancer immunotherapy. In the future, we plan to introduce more advanced HSP105‐targeting treatment strategies, such as T‐cell therapy using engineered TCR genes from HSP105‐specific CTL clones and intratumoral injection together with immune checkpoint inhibitors.
CONFLICT OF INTEREST
The corresponding author, T. Nakatsura is the inventor of the peptide vaccine patents. T. Nakatsura and T. Yoshikawa are supported by fundamental research funding from Takeda Pharmaceutical Co., Ltd. The other authors report no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported in part by the National Cancer Center Research and Development Fund (25‐7 and 28‐A‐8), Japan Agency for Medical Research and Development (AMED, grant no. JP 16ck0106109 h0003), and by Health and Labor Science Research Grants for Research for Promotion of Cancer Control Programs from the Ministry of Health, Labor, and Welfare, Japan. The authors thank Manami Shimomura and Yukiko Kozaki for technical assistance.
Shimizu Y, Yoshikawa T, Kojima T, et al. Heat shock protein 105 peptide vaccine could induce antitumor immune reactions in a phase I clinical trial. Cancer Sci. 2019;110:3049‐3060. 10.1111/cas.14165
Shimizu and Yoshikawa contributed equally to this work.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490‐1502. [DOI] [PubMed] [Google Scholar]
- 3. Le DT, Uram JN, Wang H, et al. PD‐1 Blockade in Tumors with Mismatch‐Repair Deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐386. [DOI] [PubMed] [Google Scholar]
- 6. Nakatsura T, Senju S, Yamada K, et al. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Comm. 2001;281:936‐944. [DOI] [PubMed] [Google Scholar]
- 7. Kai M, Nakatsura T, Egami H, et al. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep. 2003;10:1777‐1782. [PubMed] [Google Scholar]
- 8. Muchemwa FC, Nakatsura T, Fukushima S, et al. Differential Expression of Heat Shock Protein 105 in melanoma and melanocytic naevi. Melanoma Res. 2008;18:166‐171. [DOI] [PubMed] [Google Scholar]
- 9. Hosaka S, Nakatsura T, Tsukamoto H, et al. Synthetic small interfering RNA targeting heat shock protein 105 induces apoptosis of various cancer cells both in vitro and in vivo. Cancer Sci. 2006;97:623‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyazaki M, Nakatsura T, Yokomine K, et al. DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4 T cells and CD8 T cells. Cancer Sci. 2005;96:695‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokomine K, Nakatsura T, Minohara M, et al. Immunization with heat shock protein 105‐pulsed dendritic cells leads to tumor rejection in mice. Biochem Biophys Res Comm. 2006;343:269‐278. [DOI] [PubMed] [Google Scholar]
- 12. Date Y, Kimura A, Kato H, et al. DNA typing of the HLA‐A gene: population study and identification of four new alleles in Japanese. Tissue Antigens. 1996;47:93‐101. [DOI] [PubMed] [Google Scholar]
- 13. Ohmori M, Yasunaga S, Maehara Y, et al. DNA typing of HLA class I (HLA‐A) and class II genes (HLA‐DR, ‐DQ and ‐DP) in Japanese patients with gastric cancer. Tissue Antigens. 1997;50:277‐282. [DOI] [PubMed] [Google Scholar]
- 14. Browning M, Krausa P. Genetic diversity of HLA‐A2: evolutionary and functional significance. Immunol Today. 1996;17:165‐170. [DOI] [PubMed] [Google Scholar]
- 15. Sawada Y, Komori H, Tsunoda Y, et al. Identification of HLA‐A2 or HLA‐A24‐restricted CTL epitopes for potential HSP105‐targeted immunotherapy in colorectal cancer. Oncol Rep. 2014;31:1051‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 17. Yoshikawa T, Nakatsugawa M, Suzuki S, et al. HLA‐A2‐restricted glypican‐3 peptide‐specific CTL clones induced by peptide vaccine show high avidity and antigen‐specific killing activity against tumor cells. Cancer Sci. 2011;102:918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tada Y, Yoshikawa T, Shimomura M, et al. Analysis of cytotoxic T lymphocytes from a patient with hepatocellular carcinoma who showed a clinical response to vaccination with a glypican‐3‐derived peptide. Int J Oncol. 2013;43:1019‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka‐Harada Y, Kawakami M, Oka Y, et al. Biased usage of BV gene families of T‐cell receptors of WT1 (Wilms’ tumor gene)‐specific CD8 + T cells in patients with myeloid malignancies. Cancer Sci. 2010;101:594‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N Engl J Med. 2013;369:134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gattinoni L, Powell DJ Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melero I, Gaudernack G, Gerritsen W, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509‐524. [DOI] [PubMed] [Google Scholar]
- 23. Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor‐specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351‐360. [DOI] [PubMed] [Google Scholar]
- 25. Van Poelgeest MI, Welters MJ, van Esch EM, et al. HPV16 synthetic long peptide (HPV16‐SLP) vaccination therapy of patients with advanced or recurrent HPV16‐induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254‐1261. [DOI] [PubMed] [Google Scholar]
- 27. Yoshitake Y, Fukuma D, Yuno A, et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin Cancer Res. 2015;21:312‐321. [DOI] [PubMed] [Google Scholar]
- 28. Aruga A, Takeshita N, Kotera Y, et al. Long‐term vaccination with multiple peptides derived from cancer‐testis antigens can maintain a specific t‐cell response and achieve disease stability in advanced biliary tract cancer. Clin Cancer Res. 2013;19:2224‐2231. [DOI] [PubMed] [Google Scholar]
- 29. Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3‐derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T‐cell immunotherapy revives an old story. Mol Med Today. 1999;5:178‐186. [DOI] [PubMed] [Google Scholar]
- 32. Nobuoka D, Yoshikawa T, Fujiwara T, et al. Peptide intra‐tumor injection for cancer immunotherapy: enhancement of tumor cell antigenicity is a novel and attractive strategy. Hum Vaccin Immunother. 2013;9:1234‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nobuoka D, Yoshikawa T, Takahashi M, et al. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen‐specific cancer immunotherapy. Cancer Immunol Immunother. 2013;62:639‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sawada Y, Yoshikawa T, Shimomura M, et al. Programmed death‐1 blockade enhances the antitumor effects of peptide vaccine‐induced peptide‐specific cytotoxic T lymphocytes. Int J Oncol. 2015;46:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fourcade J, Sun Z, Pagliano O, et al. PD‐1 and Tim‐3 regulate the expansion of tumor antigen‐specific CD8⁺ T cells induced by melanoma vaccines. Can Res. 2014;74:1045‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong RM, Scotland RR, Lau RL, et al. Programmed death‐1 blockade enhances expansion and functional capacity of human melanoma antigen‐specific CTLs. Int Immunol. 2007;19:1223‐1234. [DOI] [PubMed] [Google Scholar]
- 37. Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor‐cytolytic T cells. Nat Med. 2003;9:1377‐1382. [DOI] [PubMed] [Google Scholar]
- 38. Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen‐specific T lymphocytes. Science. 1996;274:94‐96. [PubMed] [Google Scholar]
- 39. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15‐22. [DOI] [PubMed] [Google Scholar]
- 40. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T‐cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morgan RA, Chinnasamy N, Abate‐Daga D, et al. Cancer regression and neurological toxicity following anti‐MAGE‐A3 TCR gene therapy. J Immunother. 2013;36:133‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY‐ESO‐1. J Clin Oncol. 2011;29:917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson LA, Heemskerk B, Powell DJ Jr, et al. Gene transfer of tumor‐reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor‐infiltrating lymphocytes. J Immunol. 2006;117:6548‐6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


