Abstract
Tryptophan metabolism is important to induce immune tolerance in tumors. To date, 3 types of tryptophan‐metabolizing enzymes have been identified: indoleamine 2,3‐dioxygenase 1 and 2 (IDO1 and IDO2) and tryptophan 2,3‐dioxygenase 2. Numerous studies have focused on IDO1 as its expression is enhanced in various cancers. Recently, IDO2 has been identified as a tryptophan‐metabolizing enzyme that is involved in several immune functions and expressed in cancers such as pancreatic cancer. However, the biological role of IDO2 in the induction of immune tolerance in tumors has not yet been reported. In the present study, we examined the effects of Ido2 depletion on tumor growth in a mouse model of Lewis lung carcinoma by using Ido2‐knockout mice. Ido2‐knockout mice had reduced tumor volumes compared to WT mice. Furthermore, Ido2 depletion altered the tumor microenvironment, such as tryptophan accumulation and kynurenine reduction, leading to enhancement of immune cell invasion. Finally, enzyme‐linked immunospot assay revealed that Ido2 depletion enhanced γ‐interferon secretion in the tumor. In conclusion, Ido2 is an important immune regulator in the tumor microenvironment. Our data indicate that IDO2 is a potential target for cancer treatment and drug development.
Keywords: IDO2, immune tolerance, interferon gamma, kynurenine, tryptophan metabolism
1. INTRODUCTION
The induction of immune tolerance is important to the prognosis of cancer. Recently, much attention has been devoted to the effects of tryptophan metabolism on immune tolerance. Tryptophan‐metabolizing enzymes expressed by tumor cells, dendritic cells, and macrophages in the tumor microenvironment are involved in the induction of immune tolerance.1, 2 Elevation in the activity of tryptophan‐metabolizing enzymes leads to reduced tryptophan levels and increased metabolites, such as kynurenine.3, 4 A decrease in tryptophan levels suppresses cell proliferation through the activation of general control over nonderepressible 2 (GCN2) kinase and the suppression of mTOR signals in CD8+ T cells.5 Kynurenine, an endogenous ligand of aromatic hydrocarbon receptor (AhR), then induces regulatory T cell (Treg) differentiation and cell death.6 The metabolites of kynurenine (eg, 3‐hydroxyanthranilic and quinolinic acid) induce T cell apoptosis.7 Several other metabolic products also suppress natural killer cell function and activate both dendritic cells and bone marrow‐derived immunosuppressive cells.8 Therefore, these tryptophan metabolites are involved in immune tolerances in tumors.
Elevated levels of tryptophan‐metabolizing enzymes have been observed in various cancers, where they are correlated with poor prognosis.9, 10, 11 Tryptophan 2,3‐dioxygenase (TDO), which is involved in the physiological metabolism of tryptophan in the liver, has been confirmed to be highly expressed in gliomas.6 Since then, inflammatory cytokines have been reported to induce the expression of indoleamine 2,3‐dioxygenase 1 (IDO1) in colon, hematological, liver, and lung cancers.12, 13, 14, 15 Reportedly, high IDO1 expression is associated with a poor prognosis. Another tryptophan‐metabolizing enzyme, IDO2, has recently been identified16 and is expressed in colon, gastric, and renal cancers as well as in pancreatic ductal adenocarcinomas.17, 18 However, the biological role of IDO2 in immune tolerance in tumors has not been reported.
The Lewis lung carcinoma (LLC) was isolated from a spontaneous epidermoid carcinoma of the lung in mouse in 1951, and is used to establish a tumor‐bearing animal model.19, 20 Because LLC cells do not express Ido1 or Ido2, this model is considered to be suitable for examining the function of endogenous Ido2 in tumor‐bearing mice. In the present study, we examined the effects of Ido2 depletion on tumor growth and immune function in a mouse model of LLC.
2. MATERIALS AND METHODS
2.1. Cell lines
A mouse LLC cell line was obtained from Riken BioResource Center (Saitama, Japan) and maintained at 37°C in 5% CO2 with DMEM (Nissui, Tokyo, Japan) supplemented with 10% FBS and penicillin/streptomycin (100 U/0.1 mg/mL) (Life Technologies, Grand Island, NY, USA).
2.2. Animals
All animals were mature male mice aged 6‐8 weeks old. Ido2‐knockout mice were from a C57BL/6N background obtained from the Knockout Mouse Project. Mice that were homozygous null (Ido2‐knockout) by targeted disruption of the Ido2 gene were selected from the offspring of heterozygous‐homozygous mating based on PCR of tail DNA for genotyping. We purchased the WT C57BL/6N mice from Charles River Laboratories (Yokohama, Japan). All mice were housed in the animal facilities of Fujita Health University Graduate School of Medicine under specific pathogen‐free conditions, maintained at 25°C in a 12/12‐hour light/dark cycle (lights on at 08:00), and with free access to food and water.
The protocol for all animal experiments was approved by the Animal Experimentation Committee of Fujita Health University Graduate School of Medicine. Procedures involving mice and their care were carried out in accordance with international guidelines, as described in Principles of Laboratory Animal Care (National Institutes of Health publication 85‐23, revised in 1985).
2.3. Animal experiments
Lewis lung carcinoma cells (1 × 105 in 200 μL serum‐free DMEM) were injected s.c. into the dorsal side of male WT or Ido2‐knockout mice aged 6‐8 weeks. Tumor volume was measured using calipers every 2 days, and volume was calculated as [(length × width × width) / 2]. Mice were killed 14 days after LLC cell injection. Serum and tumor tissues were stored at −80°C until analysis.
2.4. Measurement of tryptophan metabolites
Serum was diluted (1:1.25, v/v) and tumor tissue was weighed and homogenized (1:3, w/v) in 10% perchloric acid. After mixing, the precipitated proteins were removed by centrifugation (17 000 g for 15 minutes). Next, 50 μL of the resulting supernatant was subjected to HPLC (Shimadzu). Tryptophan and kynurenine were eluted from a reverse‐phase column by an isocratic method (TSKgel ODS‐100 mv 3 μm 4.6 mm [ID] × 15 cm [L]), using a mobile phase with 10 mmol/L sodium acetate and 3% acetonitrile (pH adjusted to 4.5 with acetic acid) at a flow rate of 0.9 mL/min. We used UV and visible spectrophotometry (SPD‐20A) to detect the tryptophan (UV wavelength, 230 nm) and kynurenine (UV wavelength, 344 nm).
2.5. RNA extraction and PCR analysis
Total RNA was extracted from LLC cells using Isogen (Nippon Gene, Tokyo, Japan). Real‐time PCR was carried out using Revertra Ace qPCR RT kits (Toyobo, Osaka, Japan) and reacted with cDNA. Using complex cDNA, we assayed Ido expression with SYBR Green (SsoAdvanced Universal SYBR Green SuperMix; Bio Rad, Hercules, CA, USA) using an ABI PRISM‐7900HT (Applied Biosystems, Foster City, CA, USA). The primer sequences for PCR were as follows: Gapdh, sense 5ʹ‐TGCACCACCAACTGCT‐3ʹ, antisense 5ʹ‐GGCATGGACTGTGGTC‐3ʹ; Ido1, sense 5ʹ‐CCCACACTGAGCACGG‐3ʹ, antisense 5ʹ‐TTGCGGGGCAGCACCT‐3ʹ; and Ido2, sense 5ʹ‐GCCCAGAGCTCCGTGC‐3ʹ, antisense 5ʹ‐TGGGAAGGCGGCATGT‐3ʹ.
2.6. Immunohistochemistry
Tissues were fixed in 10% formalin in PBS overnight and then embedded in paraffin. Sections (thickness, 4 μm) were used for both H&E and immunohistochemical staining. The primary Abs were rabbit polyclonal CD3 Ab (ab 5690; Abcam, Cambridge, UK), rabbit polyclonal CD8 Ab (ab 203035; Abcam), rabbit polyclonal Foxp3 Ab (ab 54501; Abcam), and rabbit polyclonal Ido2 Ab (ab 214214; Abcam). Histofine Simple Stain MAX PO peroxidase‐labeled anti‐rabbit IgG polyclonal Ab (Nichirei Biosciences, Tokyo, Japan) was used as the secondary Ab.
2.7. Immunofluorescent staining
The deparaffinized sections were used for immunofluorescent staining. The primary Abs were rat monoclonal F4/80 Ab (ab16911; Abcam) and rabbit polyclonal Ido2 Ab (ab 214214; Abcam). The secondary Abs, Alexa Fluor 488 goat anti‐rabbit Ab (A31628; Invitrogen, Carlsbad, CA, USA) and Alexa Fluor 568 goat anti‐rat IgG Ab (A11077; Invitrogen), were used to visualize the signal.
2.8. Enzyme‐linked immunospot assay
Single‐cell suspensions were prepared from the resected tumor tissues of Ido2‐knockout mice or WT mice using a gentleMACS Octo Dissociator with the Tumor Dissociation Kit, according to the manufacturer's instructions. Single‐cell suspensions were cultured without stimulation, and 72 hours later, the amount of γ‐interferon (IFN‐γ) in the culture supernatant was measured using an enzyme‐linked immunospot assay kit (Mabtech, Nacka Strand, Sweden).
2.9. Statistical analysis
All data are expressed as mean ± SD. Statistically significant differences between groups were determined by Student's t test or one‐way ANOVA. A P value of <.05 was considered statistically significant. In animal survival experiments, the Kaplan‐Meier method was used to generate P values and calculate the log‐rank (Mantel‐Cox). GraphPad Prism software, version 7, was used for all statistical analyses.
3. RESULTS
3.1. Depletion of Ido2 suppressed tumor growth in mice
First, we confirmed by real‐time PCR that neither Ido1 nor Ido2 was expressed in LLC cells (Figure S1). However, immunohistochemical analysis revealed that Ido2‐positive cells had infiltrated WT mice tumor tissue 14 days after LLC cells were inoculated (Figure S2). Immunofluorescence images confirmed the colocalization of F4/80 and Ido2 immunoreactivity in the tumor sites (Figure S2). The colocalization of F4/80 and Ido2 indicated that Ido2 is expressed in macrophages. In the present mouse model, we observed the role of endogenous Ido2 in immune cells but not in tumor cells, when LLC cells were s.c. inoculated into Ido2‐knockout or WT mice (Figure 1A). Of note, there was no significant difference in body weight between the groups (Figure S3), whereas tumor volume was significantly decreased in the Ido2‐knockout mice compared to the WT mice 12‐14 days after tumor inoculation (Figure 1B). Survival rate was examined in tumor‐bearing WT and Ido2‐knockout mice. The Ido2‐knockout mice showed a slight improvement in the survival rate (Figure 1C), but there was no statistically significant difference.
Figure 1.

Effect of indoleamine 2,3‐dioxygenase 2 (Ido2) depletion on tumor growth in mice. A, Protocol for preparing the s.c. tumor model. Lewis lung carcinoma (LLC) cells (1 × 105/mouse) were s.c. inoculated in the dorsal side of WT or Ido2‐knockout (KO) mice. B, After inoculation, volume of tumor were measured. Volume was calculated as (length × width × width) / 2. Data are shown as mean ± SD (n = 5‐8/group). C, After inoculation, survival rates were examined (n = 16‐17/group). *P < .05
3.2. Effect of Ido2 depletion on tryptophan metabolism in serum and tumor
Previous reports have shown that tryptophan metabolism is related to cancer progression through its control of immune cell function. In the tumor microenvironment, decreased tryptophan levels and accumulated kynurenine catabolites enhance the immune escape of tumor cells.4 Therefore, we investigated whether tryptophan metabolites were involved in a reduction of tumor volumes in the Ido2‐knockout mice. There was no significant difference in serum tryptophan concentrations between the WT and Ido2‐knockout mice in either the control or tumor group (Figure 2A). In the control group, serum kynurenine concentrations were significantly decreased in Ido2‐knockout mice compared to the WT mice; in the tumor group, kynurenine concentrations tended to decrease in Ido2‐knockout mice compared to the WT mice (Figure 2B). At the tumor site, tryptophan concentrations in Ido2‐knockout mice were significantly higher than in WT mice (Figure 2C), whereas kynurenine concentrations were significantly decreased in Ido2‐knockout mice (Figure 2D). At the tumor site of Ido1 expression, there was no difference between WT and Ido2‐knockout mice (Figure 2E). Thus, Ido2 depletion induced the accumulation of tryptophan and the decrease of kynurenine at the tumor site.
Figure 2.
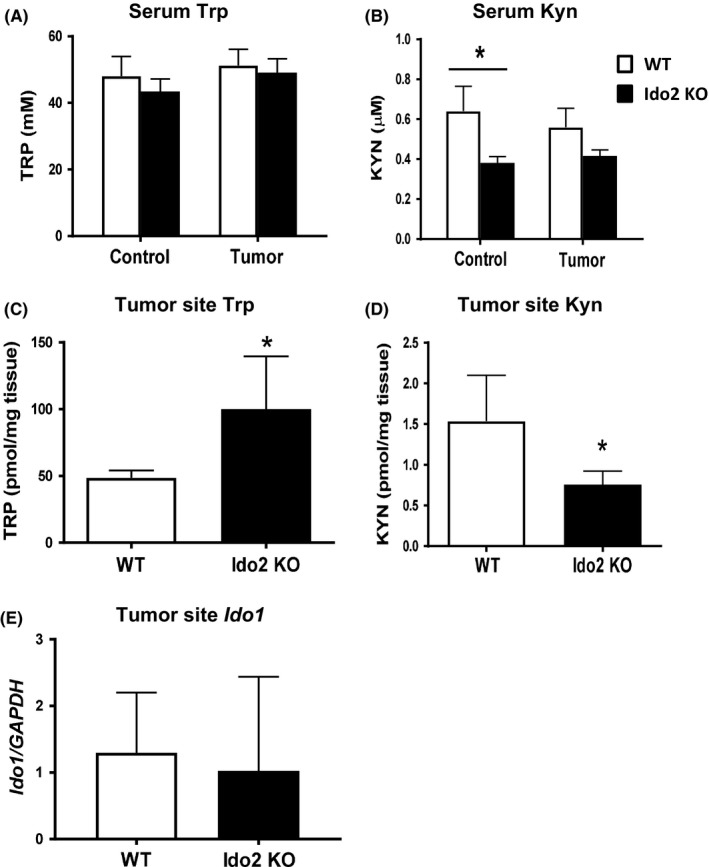
Tryptophan metabolite levels in WT and indoleamine 2,3‐dioxygenase 2‐knockout (Ido2 KO) mice after tumor inoculation. Serum and tumor‐site concentrations of tryptophan (Trp) and kynurenine (Kyn) were measured using HPLC. A, Serum Trp. B, Serum Kyn. C, Tumor‐site Trp. D, Tumor‐site Kyn. E, Tumor‐site Ido1 expression. Data are shown as mean ± SD (n = 5‐8/group). *P < .05
3.3. Depletion of Ido2 increased the number of tumor‐infiltrating immune cells in mice
The number of immune cells that infiltrate the tumor locally is important for tumor growth suppression.21 Some reports show that tryptophan metabolites affect tumor‐infiltrating immune cells.22 A decrease in tryptophan levels suppresses cell proliferation in CD8+ T cells.23 Furthermore, kynurenine induces Treg differentiation and cell death and the metabolites of kynurenine induce T cell apoptosis.6, 24 To confirm that Ido2 depletion affects tumor‐infiltrating immune cells, we analyzed the number of these immune cells by histopathological staining. Hematoxylin‐eosin staining revealed that numbers of infiltrating immune cells increased in the Ido2‐knockout mice compared to the WT mice (Figure 3A).
Figure 3.
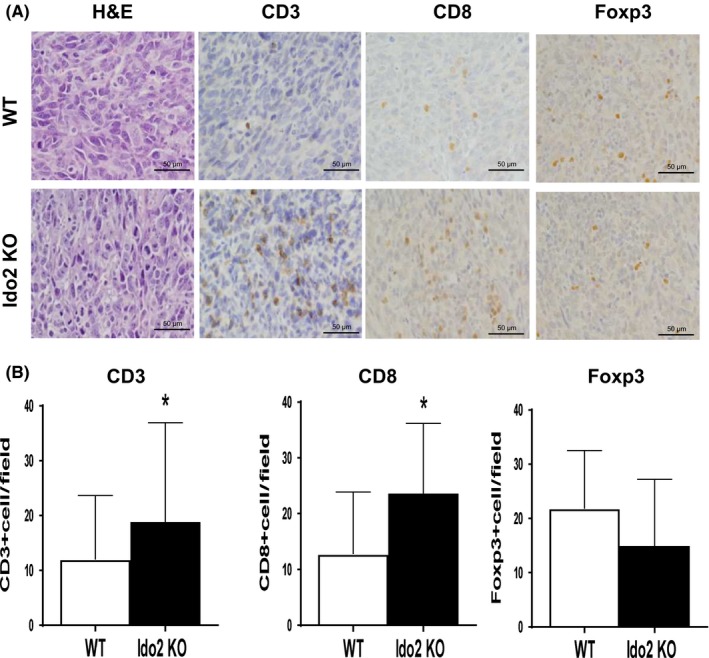
Levels of infiltrating immune cells at tumor sites in WT and indoleamine 2,3‐dioxygenase 2‐knockout (Ido2 KO) mice after tumor inoculation. Histopathological analysis was undertaken on day 14 after tumor inoculation to assess the effect of Ido2 deficiency on immune cell induction. A, Representative macroscopic H&E staining and immunostaining results for CD3, CD8, and Foxp3. Scale bar = 50 μm. B, Positive cells are quantified for the immunostaining results. Data are shown as mean ± SD (n = 5/group). *P < .05
The level of T cell infiltration into the tumor microenvironment is an important factor in tumor progression.25 We examined the number of T cells in the tumor microenvironment using CD3, which is a T cell marker. In the Ido2‐knockout mice, the number of CD3+ T cells significantly increased compared to WT mice (Figure 3A). To confirm the subtypes of T cells, we examined the expression of CD8 and Foxp3, which are the markers for effector T cells and Tregs, respectively. Compared to WT mice, the Ido2‐knockout mice showed significantly increased numbers of CD8+ cells, which have antitumor activity. In contrast, Foxp3+ Tregs, which suppress immune activity, tended to decrease (Figure 3B), but there was no statistical difference.
3.4. Effect of Ido2 depletion on IFN‐γ secretion in the tumor microenvironment
CD8+ cells, also known as CTLs, are the key immune cells for killing cancer cells through IFN‐γ secretion.26 The IFN‐γ enzyme‐linked immunospot assays can detect both CTL frequency and function by measuring IFN‐γ secretion. Therefore, IFN‐γ secretion was examined in Ido2‐knockout mice inoculated with LLC. As shown in Figure 4, IFN‐γ secretion was enhanced in the Ido2‐knockout mice compared with the WT mice. These results show that Ido2 has an immune modulatory function in tumor growth.
Figure 4.
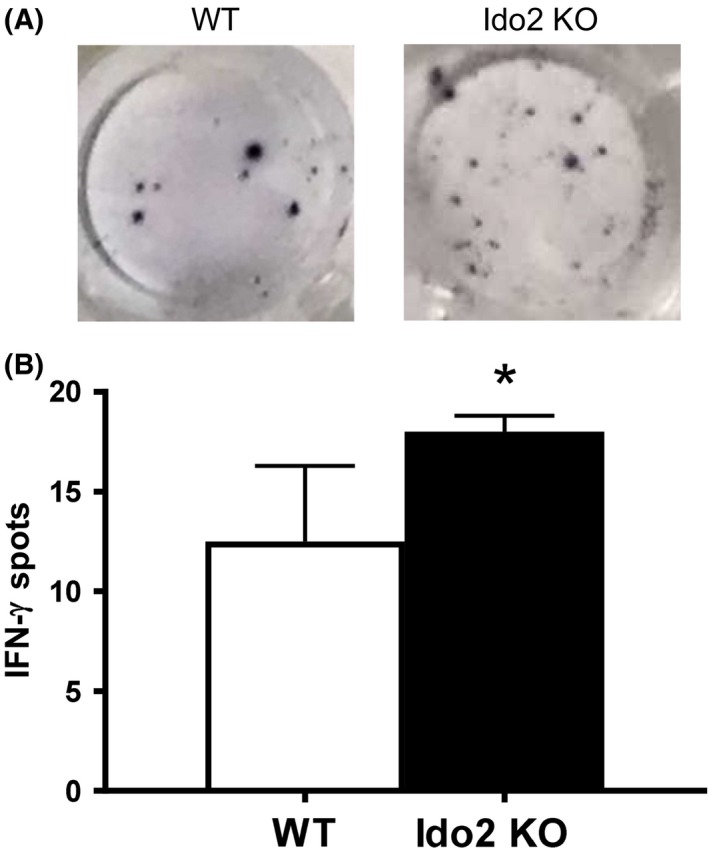
Effect of indoleamine 2,3‐dioxygenase 2 (Ido2) deletion on γ‐interferon (IFN‐γ) secretion. A, IFN‐γ production in the tumor site was examined by enzyme‐linked immunospot assay. B, Number of spots of IFN‐γ per well are quantified (n = 5/group). *P < .05. KO, knockout
4. DISCUSSION
It has been clarified that tryptophan‐metabolizing enzymes are important to induce immune tolerance, as tryptophan depletion causes T cell anergy through GCN2 activation5, 24 and mTOR inhibition.27 Further, kynurenine is an endogenous ligand of AhR, so its accumulation can be expected to induce AhR signaling, which, in turn, promotes naïve CD4+ T cell differentiation into Tregs.6, 24 Therefore, the change in metabolites due to the inhibition of the tryptophan metabolism enzyme leads to activation of CD8+ effector T cell responses and suppression of Tregs.4
Previous reports have indicated that Ido1‐knockout mice have reduced tumor growth in an LLC model.28 Furthermore, the Ido1 inhibitor, 1 methyl tryptophan (1‐MT), inhibits tumor growth in vivo.29 In addition, the treatment with a Tdo2 inhibitor improves dendritic cell function and T cell response significantly, leading to decreased tumor metastasis in mice.30
In the present study, we showed that Ido2 deletion enhanced CD8+ cell invasion in the tumor microenvironment, and inhibited tumor growth in an LLC mouse model. This resulted from increases in tryptophan and decreases in kynurenine levels in the tumor microenvironment, which confirmed the results of previous reports,28, 29, 30 although the enzyme activity of Ido2 is much lower than that of Ido1. From these results the change in tryptophan metabolism caused by not only Ido1 and Tdo2, but also Ido2, was important in the induction of immune tolerance in tumors.
Interestingly, recently Nevler et al31 have shown that Ido2‐knockout mice are less likely to have pancreatic ductal adenocarcinoma. Furthermore, in patients, the biallelic occurrence of either of the 2 IDO2‐inactivating SNPs is also significantly associated with markedly improved disease‐free survival in response to adjuvant radiotherapy.
Furthermore, we showed that Ido2 depletion enhanced IFN‐γ secretion in the tumor microenvironment, consistent with previous results of IFN‐γ32: (i) IFN‐γ plays a key immunomodulatory role in cancer immunosurveillance;32 (ii) IFN‐γ is produced mainly by CD8+ cells for tumor control;33 and (iii) IFN‐γ shows anticancer activity by attenuating cancer cell growth,32 whereas IFN‐γ‐deficient mice spontaneously develop lung epithelial malignancies and lymphoma.34 Therefore, exogenous IFN‐γ is used to treat patients with ovarian cancer, adult T‐cell leukemia, and malignant melanoma.35
Various inhibitors that target tryptophan‐metabolizing enzymes have been developed based on the evidence that upregulation of enzymes correlates with poor prognosis and poor quality of life in various cancers.36 Epacadostat is an oral IDO1‐specific competitive inhibitor that has recently been developed and that can activate T cells and natural killer cells while suppressing Treg production.37 However, no antitumor effects have been observed with its single use in clinical trials, resulting in reductions and discontinuations of clinical trials for IDO1 inhibitors. However, considering the favorable results in mouse models and usefulness in some tumors, combining an IDO1 inhibitor and an immunity checkpoint inhibitor might be effective, depending on the carcinoma and the patient immunity. Further studies investigating the usefulness of IDO1 inhibitors are awaited. Recently, compounds that simultaneously inhibit IDO1, TDO2, and IDO2 have been identified, and these could prove to be beneficial.38
Our data showed that Ido2 depletion in immune cells effectively reduces cancer burden, indicating that Ido2 plays an important role in tumor progression. Thus, IDO2 could be an attractive target for cancer treatment and drug development. In the future, individualized therapy might be possible based on the tryptophan‐metabolizing enzyme that is expressed in cancer cells and on differences in the responsiveness of the immune system.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
The work of the authors is partly supported by the Japan Society for the Promotion of Science (KAKENHI Grant Numbers 15H03086 [KS], 17H04252 [TN], and 17H07222 [YY]) and by the Private University Research Branding Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Yamasuge W, Yamamoto Y, Fujigaki H, et al. Indoleamine 2,3‐dioxygenase 2 depletion suppresses tumor growth in a mouse model of Lewis lung carcinoma. Cancer Sci. 2019;110:3061–3067. 10.1111/cas.14179
REFERENCES
- 1. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762‐774. [DOI] [PubMed] [Google Scholar]
- 2. Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242‐248. [DOI] [PubMed] [Google Scholar]
- 3. Munn DH, Mellor AL. Indoleamine 2,3‐dioxygenase and tumor‐induced tolerance. Journal of Clinical Investigation. 2007;117:1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munn DH, Sharma MD, Baban B et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3‐dioxygenase. Immunity. 2005;22:633‐642. [DOI] [PubMed] [Google Scholar]
- 6. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour‐promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197‐203. [DOI] [PubMed] [Google Scholar]
- 7. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 8. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan‐derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3‐dioxygenase. J Exp Med. 2002;196(4):459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fangxuan L, Rupeng Z, Shixia L, Juntian L. IDO1: an important immunotherapy target in cancer treatment. Int Immunopharmacol. 2017;47:70‐77. [DOI] [PubMed] [Google Scholar]
- 10. Zhai L, Spranger S, Binder DC, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427‐5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li S, Han X, Lyu N, et al. Mechanism and prognostic value of indoleamine 2,3‐dioxygenase 1 expressed in hepatocellular carcinoma. Cancer Sci. 2018;109(12):3726‐3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshikawa T, Hara T, Tsurumi H, et al. Serum concentration of L‐kynurenine predicts the clinical outcome of patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Eur J Haematol. 2010;84(4):304‐309. [DOI] [PubMed] [Google Scholar]
- 13. Taguchi A, Niwa M, Hoshi M, et al. Indoleamine 2,3‐dioxygenase 1 is upregulated in activated microglia in mice cerebellum during acute viral encephalitis. Neurosci Lett. 2014;564:120‐125. [DOI] [PubMed] [Google Scholar]
- 14. Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitra D, Horick NK, Brackett DG, et al. High IDO1 expression is associated with poor outcome in patients with anal cancer treated with definitive chemoradiotherapy. Oncologist. 2019;24(6):e275‐e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ball HJ, Yuasa HJ, Austin CJD, Weiser S, Hunt NH. Indoleamine 2,3‐dioxygenase‐2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467‐471. [DOI] [PubMed] [Google Scholar]
- 17. Löb S, Königsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: Levo‐ but not dextro‐1‐methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58(1):153‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208(5):781‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugiura K, Stock CC. Studies in a tumor spectrum III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Can Res. 1955;15:38‐51. [PubMed] [Google Scholar]
- 20. O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell. 1994;79(2):315‐328. [DOI] [PubMed] [Google Scholar]
- 21. Frey AB, Monu N. Signaling defects in anti‐tumor T cells. Immunol Rev. 2008;222:192‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3‐dioxygenase. Immunol Lett. 2007;111:69‐75. [DOI] [PubMed] [Google Scholar]
- 23. Liu Z, Dai H, Wan N, et al. Suppression of memory CD8 T cell generation and function by tryptophan catabolism. Journal of Immunology. 2007;178(7):4260‐4266. [DOI] [PubMed] [Google Scholar]
- 24. Grohmann U, Puccetti P. The coevolution of IDO1 and AhR in the emergence of regulatory T‐Cells in mammals. Front Immunol. 2015;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trend Cancer. 2017;3:797‐808. [DOI] [PubMed] [Google Scholar]
- 26. Farhood B, Najafi M, Mortezaee K. CD8 + cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509‐8521. [DOI] [PubMed] [Google Scholar]
- 27. Metz R, Rust S, DuHadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D‐1‐methyl‐tryptophan. OncoImmunology. 2012;1(9):1460‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schafer CC, Wang Y, Hough KP, et al. Indoleamine 2,3‐dioxygenase regulates anti‐tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget. 2016;7(46):75407‐75424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu KT, Liu YH, Liu HL, Chong IW, Yen MC, Kuo PL. Neutrophils are essential in short hairpin RNA of indoleamine 2,3‐ dioxygenase mediated‐antitumor efficiency. Molecular Therapy ‐ Nucleic Acids. 2016;5(12):e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu YL, Hung JY, Chiang SY, et al. Lung cancer‐derived galectin‐1 contributes to cancer associated fibroblast‐mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7(19):27584‐27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nevler A, Muller AJ, Sutanto‐Ward E, et al. Host IDO2 gene status influences tumor progression and radiotherapy response in KRAS‐driven sporadic pancreatic cancers. Clin Cancer Res. 2019;25(2):724‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin CF, Lin CM, Lee KY, et al. Escape from IFN‐γ‐dependent immunosurveillance in tumorigenesis. J Biomed Sci. 2017;24(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoenborn JR, Wilson CB. Regulation of Interferon‐γ during innate and adaptive immune responses. Adv Immunol. 2007;41‐101. [DOI] [PubMed] [Google Scholar]
- 34. Shankaran V, et al. IFNγ, and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107‐1111. [PubMed] [Google Scholar]
- 35. Miller CHT, Maher SG, Young HA. Clinical use of interferon‐γ. Ann N Y Acad Sci. 2009;1182:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurz K, Schroecksnadel S, Weiss G, Fuchs D. Association between increased tryptophan degradation and depression in cancer patients. Curr Opin Clin Nutr Metab Care. 2011;14:49‐56. [DOI] [PubMed] [Google Scholar]
- 37. Jochems C, Fantini M, Fernando RI, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen‐specific T cells. Oncotarget. 2016;7(25):37762‐37772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winters M, DuHadaway JB, Pham KN, et al. Diaryl hydroxylamines as pan or dual inhibitors of indoleamine 2,3‐dioxygenase‐1, indoleamine 2,3‐dioxygenase‐2 and tryptophan dioxygenase. Eur J Med Chem. 2019;162:455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


