Abstract
The Centers for Disease Control and Prevention recommend annual influenza vaccination of persons ≥ 6 months old. However, in 2016–17, only 43.3% of U.S. adults reported receiving an influenza vaccination. Limited awareness about the cost-effectiveness (CE) or the economic value of influenza vaccination may contribute to low vaccination coverage. In 2017, we conducted a literature review to survey estimates of the CE of influenza vaccination of adults compared to no vaccination. We also summarized CE estimates of other common preventive interventions that are recommended for adults by the U.S. Preventive Services Task Force. Results are presented as costs in US$2015 per quality-adjusted life-year (QALY) saved. Among adults aged 18–64, the CE of influenza vaccination ranged from $8000 to $39,000 per QALY. Assessments for adults aged ≥ 65 yielded lower CE ratios, ranging from being cost-saving to $15,300 per QALY. Influenza vaccination was cost-saving to $85,000 per QALY for pregnant women in moderate or severe influenza seasons and $260,000 per QALY in low-incidence seasons. For other preventive interventions, CE estimates ranged from cost-saving to $170,000 per QALY saved for breast cancer screening among women aged 50–74, from cost-saving to $16,000 per QALY for colorectal cancer screening, and from $27,000 to $600,000 per QALY for hypertension screening and treatment. Influenza vaccination in adults appears to have a similar CE profile as other commonly utilized preventive services for adults. Efforts to improve adult vaccination should be considered by adult-patient providers, healthcare systems and payers given the health and economic benefits of influenza vaccination.
Keywords: Adult, Influenza vaccines, Preventive medicine, Literature review
1. Introduction
Millions of influenza-related illnesses, lost work days and outpatient medical visits - including an estimated 140,000 to 710,000 influenza-related hospitalizations and 12,000 to 56,000 deaths - occur in the United States (U.S.) during influenza seasons (Centers for Disease Control and Prevention, 2018). The Centers for Disease Control and Prevention recommend that all people aged ≥ 6 months receive the influenza vaccination each year (Grohskopf et al., 2017). However, only 59.0% of children 6 months to 17 years old and 43.3% of adults ≥18 years old were vaccinated in the U.S. in the 2016–17 influenza season (Centers for Disease Control and Prevention, 2017). Other health prevention services for adults appear to have higher levels of utilization, with 71.5% of women aged 50–74 undergoing mammography (Centers for Disease Control and Prevention/National Center for Health Statistics, 2016), 62.4% of adults aged ≥50 undergoing colorectal cancer screening (White et al., 2017), and about 82.8% of adults aged ≥ 18 screened for high blood pressure (Mozaffarian et al., 2015). The relatively lower utilization of influenza vaccination may in part be due to limited awareness of the cost-effectiveness (CE) profile of adult influenza vaccination and the health burden that can be avoided by increasing the influenza vaccine uptake, as providers tend to have better knowledge of recommendations for higher risk groups (Centers for Disease Control and Prevention, 2017; Nowak et al., 2018).
CE analysis is one way to estimate and quantify the economic value of different health services and public health interventions. CE studies provide information that can be used by healthcare providers, healthcare systems, payers, and policy makers who are making decisions regarding which services to prioritize and how to allocate additional health-related investments. This study collects and summarizes results of research on the CE of adult influenza vaccinations as well as other preventive services relevant to adults - specifically breast cancer screening, colorectal cancer screening, and hypertension screening and treatment. The results from this study provide support for the ongoing use and promotion of influenza vaccinations as well as additional context for future CE analyses of influenza vaccination.
2. Methods
In 2017, we conducted a literature review identifying and synthesizing CE analyses that evaluated the use of influenza vaccination in the U.S. adult population. To summarize selected CE findings for other common preventive services for U.S. adults, we reviewed the economic literature related to colorectal cancer for persons ≥ 50 years old, blood pressure screening for adults of all ages, and breast cancer screening for women aged 50–74. The selection of these three other preventive services was informed by recommendations made by the U.S. Preventive Services Task Force (USPSTF), which indicates the quality of a recommendation using a scale of letter grades, with A and B grades signaling a “high certainty that the benefit is moderate to substantial”(Drummond et al., 2007).
We searched all major medical and public health research literature databases - PubMed, ProQuest, EBSCO, Thomson Reuters, ProQuest Science & Technology, ScienceDirect, Web of Science, EBSCO, Thomson Reuters, Applied Science & Technology Abstracts, Business Source Complete, and MEDLINE - for publications dated from 1996 to 2016 using a combination of terms that were designed to identify economic evaluations and CE analyses of influenza vaccinations, colorectal cancer screening, mammography, and hypertension screening and treatment (see Appendix A). Note that the USPSTF only refers to “hypertension screening,” while our searches identified studies that assessed the CE of screening in combination with treatment for hypertension. A professional librarian conducted these searches in January 2017. To capture additional studies that may not have been identified with our database searches, we added studies to our final list by reviewing the reference and citation lists in each of the articles identified by the database searches. The original searches and subsequent additions yielded 1089 peer-reviewed articles.
These 1089 articles were then subjected to an initial review of the titles and abstracts, during which we excluded 978 articles that did not appear to be relevant to our study objectives. In particular, we excluded studies with titles and abstracts that did not contain any relevant terminology, such as quality-adjusted life-year (QALY), CE ratio (CER), life-years saved (LYS), cost, or benefit. Following the initial title and abstract review, there were 70 research articles related to influenza vaccination in the United States and 41 articles related to the other preventive services that we considered for the comparator group (see Fig. 1).
Fig. 1.
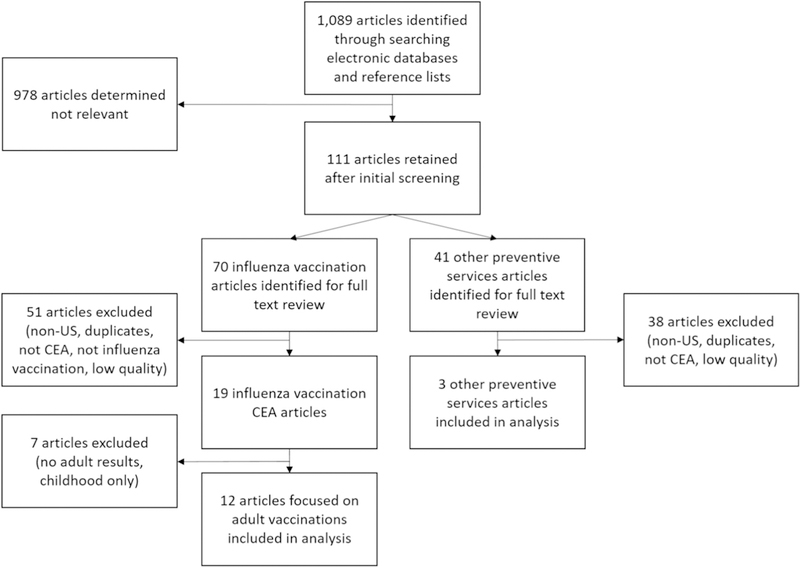
Diagram of search, identification, screening, and eligibility of included studies.
Of the 70 influenza vaccination articles, 51 were excluded because they either focused exclusively on non-U.S. populations, were duplicates, compared two distinct types of influenza vaccines but did not have a “no vaccination” comparison, were not CE analyses, were not from the societal perspective, or did not meet our criteria for quality. The evaluation of quality for the remaining CE studies was based on a checklist developed by Drummond and colleagues (see Appendix B). In the assessment of quality, one point was given for each of the ten questions in the Drummond check list that was effectively answered by the study being assessed (see Appendix C). Questions consisting of multiple parts were assessed as a one, receiving one point if both parts were answered or zero if any part of the was unanswered. Studies that received six or more points, out of a total of ten, were included in the final analysis. These quality scores were determined by two independent reviewers, with any discrepancies in the two reviews resolved by discussion. Another 7 articles were then excluded because they focused on childhood vaccinations, leaving a total of 12 studies that assessed adult influenza vaccination CE for inclusion in the final analysis. Of the 41 other preventive services studies that remained following the title and abstract review, three were retained based on their relevance and quality scores.
From the 12 influenza vaccination CE articles and the three studies of CE of other preventive services that were included in our final analysis, we extracted and quantified relevant study characteristics. These characteristics included the specific types of interventions being assessed, the age ranges of the study population, the study perspective, the types of outcomes assessed, and the estimated cost per outcome (or the CE ratios). All monetary values were adjusted to US$2015 using the U.S. consumer price index (The World Bank, 2018). CE ratios from the societal perspective were utilized and the units of outcome focused on in this review included final outcome measures such as persons vaccinated, LYS, and QALYs saved, to maintain comparability across studies.
3. Results
The type of outcomes used in the CE ratios varied across the 12 studies on influenza CE that were included in the final dataset. While cost per QALY saved was the most common CE ratio measure used, several studies reported costs per outcome using other health outcomes, such as LYS (Drummond et al., 2007; Patel and Davis, 2006), illnesses averted (Luce et al., 2001), and costs per person vaccinated (White et al., 1999; Nichol, 2001; Jordan et al., 2006; Gibson et al., 2016; Bridges et al., 2000).
The influenza vaccination studies looking at adult populations reported CE ratios that would likely be considered CE by traditional standards (Grosse, 2008). Among the studies that estimated CE ratios in terms of cost per QALY saved, CE estimates for adult influenza vaccination ranged from being cost-saving (Prosser et al., 2008) to $39,000 Maciosek et al., 2006 per QALY saved adults, excluding pregnant women. Among pregnant women, the CE ranged from being cost-saving (Xu et al., 2016; Beigi et al., 2009) during a moderate influenza season to $260,000 per QALY saved (Xu et al., 2016) during an influenza season with little influenza activity (Fig. 2, details available in Appendices).
Fig. 2.

Summary of cost-effectiveness estimates of influenza vaccinations and other preventive services among U.S. adults.
QALY = quality-adjusted life-year. Cost-effectiveness ratios that were cost-saving are represented as a point on the x-axis. The “X” indicates a value that was truncated to simplify presentation, where the upper range of this value was $600,000/QALY saved.
The three studies of other preventive services yielded a range of preventive service interventions, age groups, and outcome measures. The CE ranged from being cost-saving to $170,000 per QALY saved for breast cancer screening interventions (Stout et al., 2014), from cost-saving to $16,000 per QALY saved for colorectal cancer screening interventions (Kingsley et al., 2016) and from $27,000 to $600,000 per QALY saved for hypertension screening and treatment (Dehmer et al., 2017). Among adult populations, the CE estimates for influenza vaccination were comparable with the estimates for these other preventive services considered (Fig. 2).
4. Discussion
We found that influenza vaccination compared favorably with other commonly accepted and well-implemented preventive services for adults in terms of CE. This comparability is noteworthy given the differences observed in implementation coverage and implementation rates between other preventive services and influenza vaccinations among adult populations. While at least one other recent study has summarized the cost-effectiveness of many types of adult vaccinations (Leidner et al., 2018), our study goes further by focusing on the cost-effectiveness of influenza vaccinations with comparisons to other preventive health interventions in adults. In particular, this study highlights the difference in utilization rates between influenza vaccination, which suggests that additional utilization of influenza vaccinations could benefit adult patients.
A recent Cochrane review found influenza vaccination of healthy adults protects against experiencing a case of laboratory-confirmed influenza and influenza-like-illness (Demicheli et al., 2018a). This large systematic review of randomized-control and quasi-randomized studies also concluded that the influenza vaccine has a minimal or modest effect in reducing hospitalizations (risk-ratio = 0.96 with 95% confidence interval from 0.85 to 1.08) and days off work (−0.04 days with 95% confidence interval from - 0.14 days to 0.06 days). Both of these findings were characterized as having low-certainty of evidence. Recent observational studies that were not included in the Cochrane review found evidence of vaccine effectiveness against hospitalizations that ranged 45% and 57% (Havers et al., 2016; Ferdinands et al., 2018). The majority of CEA studies summarized in our review assumed a moderate level of vaccine effectiveness against all influenza-related disease outcomes. Base case assumptions of vaccine effectiveness ranged from 13% to 75% with differences related to outcomes of interest (Maciosek et al., 2006), patient type (Xu et al., 2016; Beigi et al., 2009), and vaccine match with the circulating types of influenza (Nichol, 2001).
The cost-effectiveness ratios that were estimated by the studies summarized in this review looked at the differences in costs and health outcomes under scenarios with and without influenza vaccination. We focused on results that utilized QALYs saved by vaccination as the primary health outcome of interest. Prevented influenza-related deaths and disease resulted in QALYs saved, where the types of disease included in the models varied across studies. Looking across all studies, disease states included influenza cases that were not medically attended, outpatient cases, hospitalizations or inpatient cases, deaths, preterm births, as well as adverse events from vaccinations.
Across the studies we reviewed, a number of estimates of cost-effectiveness for influenza vaccination identified cost-saving results. Under a variety of modeling assumptions and across several target populations, estimates of influenza vaccination cost-effectiveness varied from being cost-saving to $260,000 per QALY saved. The majority of estimates costing less than equal to $85,000 per QALY saved. In addition, influenza vaccination appears favorable in terms of CE relative to other commonly recommended preventive services given A or B grades by the USPSTF (U.S. Preventive Services Task Force, 2018): screening for colon cancer for adults 50–74 years, breast cancer for women aged 50–74 years, and hypertension for adults ≥18 years (U.S. Preventive Services Task Force, 2018). Relative economic favorability may depend on a number of factors, including emerging data and scientific consensus on the effectiveness of clinical interventions such as influenza vaccination (Demicheli et al., 2018b) and mammography (Gotzsche and Nielsen, 2013). Among influenza vaccination CEA studies reviewed here, the greatest variability in influenza vaccination economic outcomes is associated with pregnant women, with less variation observed among the other, strictly age-based population groups among studies included in our analyses. Variation among cost-effectiveness results could be due to multiple factors that vary across population groups and across influenza seasons, including influenza antigenic match between vaccine strains and circulating influenza viruses, and influenza illness rates and severity of influenza illnesses in a given season and age groups most impacted.
USPSTF formulates recommendations based on evidence of effectiveness, but their recommendations do not explicitly include economic CE U.S. Preventive Services Task Force, 2018. The recommended use of vaccines in the United States are not evaluated by the USPSTF, but rather by the Advisory Committee on Immunization Practices (ACIP). The ACIP, in contrast to the USPSTF, considers cost-effectiveness analyses in their deliberations of vaccine recommendations (Smith, 2010; Pike et al., 2019), which may explain, in part, the larger number of CE studies found on influenza vaccination relative to other preventive services. The effectiveness of the influenza vaccine varies across age groups, influenza seasons, and measurable disease outcomes which also impacts that range of CE estimates (Demicheli et al., 2018b; Cowling et al., 2016; Ohmit et al., 2013; Kostova et al., 2013); however, the relatively favorable CE results associated with this vaccine are likely related to the low cost of influenza vaccines - with a cost between $11 and $22 per dose (Centers for Disease Control and Prevention, 2019) -as well as the substantial disease burden associated with influenza (Reed et al., 2015).
Based on a survey conducted in 2016, approximately 53% of influenza vaccinations among adults appear to occur at health-care locations, such as a physician’s office, hospital, or public health clinic (Centers for Disease Control and Prevention, 2016). This finding is consistent with an earlier study that used data from the Behavioral Risk Factor Surveillance System that found 70% of influenza vaccinations among adults aged 18 years and older were administered in physician offices and other traditional settings (Singleton et al., 2005). This is also consistent with a more recent study that investigated the factors associated with receiving influenza vaccinations in non-traditional settings, in which 60% of individuals in this study that received an influenza vaccination did so in a traditional setting (Kim and Mountain, 2017). The challenge to allocate time during routine patient visits to implement all vaccine and USPSTF A and B recommended preventive services (Hurley et al., 2016) may force healthcare providers to prioritize specific preventive services at each visit. Concerns about payment adequacy and patient insurance coverage and patient out-of-pocket costs may also influence preventive services decisions (Hurley et al., 2016). Utilization of preventive visits for adults, such as Medicare annual wellness visits, may help providers expand the implementation of preventive services by patients, including influenza vaccination when visits are timed to coincide with influenza vaccination timing.
Influenza vaccination has been demonstrated to offer multiple benefits to patients of all ages, including reduced risk of influenza-related hospitalization in both children age 6 months to 17 years and adults aged ≥ 18 (Havers et al., 2016), reduced risk of major acute cardiovascular events among persons with existing cardiovascular disease (Udell et al., 2013; Barnes et al., 2015), reduced risk of influenza in pregnant women and infants born to vaccinated pregnant women (Madhi et al., 2014), and finally, reduced risk of deaths in children (Flannery et al., 2017) and adults (The World Bank, 2018; Shay et al., 2017; Grohskopf, 2016). A 2018 Cochrane review found influenza vaccination of healthy adults protects against experiencing a case of laboratory-confirmed influenza - with a risk ratio of 0.41 (confidence interval (CI): 0.36 to 0.47) - and protects against influenza-like-illness - with a risk ratio of 0.84 (CI: 0.75 to 0.95) (Demicheli et al., 2018a). Similar results were also found among the elderly, where influenza vaccinations protect against laboratory-confirmed influenza illness - with a risk ratio of 0.42 (CI: 0.27 to 0.66) - and against influenza-like-illness - with a risk ratio of 0.59 (CI: 0.47 to 0.73) (Demicheli et al., 2018b). While these results confirm the vaccine confers substantial benefits in terms of protection against illness, the review did not identify significant protection from certain specific outcomes, such as hospitalization or work loss (Demicheli et al., 2018b). These outcomes were also associated with substantial uncertainty around effectiveness estimates. Our review of the economic literature on influenza vaccinations of adults also identifies uncertainty across studies, which depended on attributes of the population under consideration as well as the severity of the influenza season. Across the studies we reviewed, the substantial burden of influenza among persons of all ages and the benefits of vaccination suggest that providers and health systems should include influenza vaccination in routine care.
This study is subject to several limitations. The rigor of our study criteria and of our assessments of economic evaluations - in addition to the relative scarcity of intervention CE studies - yielded few eligible studies. A number of high-quality economic evaluation studies we found utilized different economic perspectives (i.e. provider), study types (i.e. cost benefit analysis), and denominator units (i.e. illness averted). We therefore could not compare these studies. The final studies we included, however, are high quality and representative of the respective preventive health interventions. The small number of CE studies underscores the need for additional economic evaluations of health prevention interventions that - among other criteria - focus on the societal perspective, utilize a consistent economic metric, and compare interventions to no intervention. We were not able to assess the potential impact of herd immunity on CE since herd immunity was not included in the majority of studies we reviewed. Omitting herd immunity impact is likely to underestimate the CE of influenza vaccination (Bauch et al., 2009). The severity of an influenza season as well as the efficacy of an influenza vaccine can vary substantially across different years. In this review, we found that many influenza vaccination studies reported only single-year data which may limit generalizability of the results (Bridges et al., 2000; Havers et al., 2016; Shay et al., 2017; Grohskopf, 2016). Multi-year data analyses would offer additional evidence useful to CE studies. We were unable to fully investigate the impact of adverse events on the different interventions; however, the incidence and cost of adverse events from the interventions were included in the CE analyses. While we consider this issue to be important, it is beyond the scope of the current review. We also limited our review to influenza vaccination and a few selected other USPSTF A and B grade prevention interventions. The relative CE of influenza vaccination when compared to other preventive services recommended by USPSTF that were not included in this study may differ from the preventive services that were included.
5. Conclusion
Our literature review found several studies that demonstrate that influenza vaccination among adult populations would likely be considered cost-effective by many standards. The CE results for influenza vaccination among adults was comparable to the CE of other USPTF grade A and B preventive health interventions targeting adults, including colorectal screening, breast cancer screening, and hypertension screening and treatment. Healthcare practitioners and healthcare systems may want to consider these findings in making decisions regarding their level of effort and investment in promoting, implementing, and incentivizing influenza vaccination programs. Adoption of the National Vaccine Advisory Committee Standards for Adult Immunization Practice (National Vaccine Advisory Committee, 2014) is recommended so that all adults may benefit from influenza and other vaccines and to reduce the health burden of vaccine preventable illnesses. Adoption of these standards, especially as they apply to influenza vaccination of adults, may require system- and policy-level changes to ensure all patients receive a strong provider recommendation for vaccination and have the opportunity to benefit from influenza vaccination.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We thank the Centers for Disease Control and Prevention’s Immunization Services Division - who contracted Battelle’s Public Health and Advanced Analytics division for this project - for their guidance and funding. This study was designed by ND, ES, HK, SG, AL, and CB. ND, ES, and HK conducted the literature search, assessed study quality, and conducted primary data abstraction. AL and IF assisted with the analysis. The manuscript was drafted and reviewed by ND, ES, HK, SG, AL, IF, DJ, and CB. We thank Dr. Joy Schwerzmann for project management assistance and Dr. Gavin Hougham for edits made in early drafts. No conflicts of interest were reported by authors of this paper.
Footnotes
Financial disclosures
There are no financial disclosures to report.
Declaration of Competing Interest
There are no conflicts of interest to report.
Appendices. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2019.05.022.
References
- Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR, 2015. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart 101 (21), 1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch CT, Anonychuk AM, Van Effelterre T, Pham BZ, Merid MF, 2009. Incorporating herd immunity effects into cohort models of vaccine cost-effectiveness. Med. Decis. Mak. 29 (5), 557–569. [DOI] [PubMed] [Google Scholar]
- Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY, 2009. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin. Infect. Dis. 49 (12), 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. , 2000. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA 284 (13), 1655–1663. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. National Early-season Flu Vaccination Coverage, United States, November 2016. 2016. December 9. [Google Scholar]
- Centers for Disease Control and Prevention, 2017. Flu vaccination coverage, United States, 2016–17 influenza season. updated September 28, 2017 Available from. https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm.
- Centers for Disease Control and Prevention, 2018. Disease Burden of Influenza. Available from: https://www.cdc.gov/flu/about/disease/burden.htm.
- Centers for Disease Control and Prevention, 2019. CDC Vaccine Price List, Adult Influenza Caccine Price List. [Google Scholar]
- Centers for Disease Control and Prevention/National Center for Health Statistics, 2016. Public-use data files and documentation. updated July 25, 2016 Available from: https://www.cdc.gov/nchs/data_access/ftp_data.htm.
- Cowling BJ, Feng S, Finelli L, Steffens A, Fowlkes A, 2016. Assessment of influenza vaccine effectiveness in a sentinel surveillance network 2010–13, United States. Vaccine 34 (1), 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmer SP, Maciosek MV, LaFrance AB, Flottemesch TJ, 2017. Health benefits and cost-effectiveness of asymptomatic screening for hypertension and high cholesterol and aspirin counseling for primary prevention. Ann. Fam. Med. 15 (1), 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C, 2018a. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2, CD001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas R, et al. , 2018b. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. (2), CD004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M, Chevat C, Lothgren M, 2007. Do we fully understand the economic value of vaccines? Vaccine 25 (32), 5945–5957. [DOI] [PubMed] [Google Scholar]
- Ferdinands J, Gaglani M, Martin E, Middleton D, Monto A, Murthy K, et al. , 2018. Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J. Infect. Dis. 10.1093/infdis/jiy723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B, Reynolds SB, Blanton L, Santibanez TA, O’Halloran A, Lu P-J, et al. , 2017. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics 139 (5), e20164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E, Begum N, Sigmundsson B, Sackeyfio A, Hackett J, Rajaram S, 2016. Economic evaluation of pediatric influenza immunization program compared with other pediatric immunization programs: a systematic review. Hum. Vaccin. Immunother. 12 (5), 1202–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzsche P, Nielsen M, 2013. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 4 (6), CD001877. [DOI] [PubMed] [Google Scholar]
- Grohskopf LA, 2016. Prevention and control of seasonal influenza with vaccines. MMWR Recomm. Rep. 65. [DOI] [PubMed] [Google Scholar]
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, et al. , 2017. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017–18 influenza season. MMWR Recomm. Rep. 66 (2), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, 2008. Assessing Cost-Effectiveness in Healthcare: History of the $50,000 per QALY Threshold. [DOI] [PubMed] [Google Scholar]
- Havers F, Sokolow L, Shay DK, Farley MM, Monroe M, Meek J, et al. , 2016. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–2011. Clin. Infect. Dis. 63 (10), 1304–1311. [DOI] [PubMed] [Google Scholar]
- Hurley LP, Bridges CB, Harpaz R, Allison MA, Leary STO, Crane LA, et al. , 2016. Physician attitudes toward adult vaccines and other preventive practices, United States, 2012. Public Health Rep. 131 (2), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R, Connock M, Albon E, Fry-Smith A, Olowokure B, Hawker J, et al. , 2006. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 24 (8), 1047–1062. [DOI] [PubMed] [Google Scholar]
- Kim N, Mountain T, 2017. Role of non-traditional locations for seasonal flu vaccination: empirical evidence and evaluation. Vaccine 35 (22), 2943–2948. [DOI] [PubMed] [Google Scholar]
- Kingsley J, Karanth S, Revere FL, Agrawal D, 2016. Cost effectiveness of screening colonoscopy depends on adequate bowel preparation rates-a modeling study. PLoS One 11 (12), e0167452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova D, Reed C, Finelli L, Cheng P, Gargiullo P, Shay DK, et al. , 2013. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PLoS One 8 (6), e66312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidner A, Murthy N, Chesson H, Biggerstaff M, Stoecker C, Harris A, et al. , 2018. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine 37 (2), 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, et al. , 2001. Cost-effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics 108 (2), E24. [DOI] [PubMed] [Google Scholar]
- Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ, 2006. Influenza vaccination: health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am. J. Prev. Med. 31 (1), 72–79. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. , 2014. Influenza vaccination of pregnant women and protection of their infants. N. Engl. J. Med. 371 (10), 918–931. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. , 2015. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131 (4), e29–e322. [DOI] [PubMed] [Google Scholar]
- National Vaccine Advisory Committee, 2014. Recommendations from the National Vaccine Advisory Committee: standards for adult immunization practice. Public Health Rep. 129 (2), 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KL, 2001. Cost-benefit analysis of a strategy to vaccinate healthy working adults against influenza. Arch. Intern. Med. 161 (5), 749–759. [DOI] [PubMed] [Google Scholar]
- Nowak G, Sheedy K, Bursey K, Smith T, Basket M, 2018. Promoting influenza vaccination: insights from a qualitative meta-analysis of 14 years of influenza-related communications research by U.S. Centers for Disease Control and Prevention (CDC). Vaccine 33 (24), 2741–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. , 2013. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 56 (10), 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Davis MM, 2006. Could a federal program to promote influenza vaccination among elders be cost-effective? Prev. Med. 42 (3), 240–246. [DOI] [PubMed] [Google Scholar]
- Pike J, Leidner A, MacNeil J, Cohn A, 2019. Review of the economic evidence presented to the United States advisory committee on immunization practices, 2012–2016 Vaccine 37 (1), 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser LA, O’Brien MA, Molinari NA, Hohman KH, Nichol KL, Messonnier ML, et al. , 2008. Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. PharmacoEconomics 26 (2), 163–178. [DOI] [PubMed] [Google Scholar]
- Reed C, Chaves SS, Kirley PD, Emerson R, NAragon D, Hancock EB, et al. , 2015. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 10 (3), e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DK, Chillarige Y, Kelman J, Forshee RA, Foppa IM, Wernecke M, et al. , 2017. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J. Infect. Dis. 215 (4), 510–517. [DOI] [PubMed] [Google Scholar]
- Singleton JA, Poel AJ, Lu PJ, Nichol KL, Iwane MK, 2005. Where adults reported receiving influenza vaccination in the United States. Am. J. Infect. Control 33 (10), 563–570. [DOI] [PubMed] [Google Scholar]
- Smith JC, 2010. The structure, role, and procedures of the US Advisory Committee on Immunization Practices (ACIP). Vaccine 28, A68–A75. [DOI] [PubMed] [Google Scholar]
- Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, et al. , 2014. Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J. Natl. Cancer Inst. 106 (6), dju092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank, 2018. Consumer Price Index: United States. Available from: https://data.worldbank.org/indicator/FP.CPI.TOTL?locations=US.
- U.S. Preventive Services Task Force, 2018. USPSTF A and B recommendations. updated April, 2018 Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/.
- Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. , 2013. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 310 (16), 1711–1720. [DOI] [PubMed] [Google Scholar]
- White T, Lavoie S, Nettleman MD, 1999. Potential cost savings attributable to influenza vaccination of school-aged children. Pediatrics 103 (6), e73. [DOI] [PubMed] [Google Scholar]
- White A, Thompson T, White MC, Sabatino S, Doria-Rose P, Geiger A, et al. , 2017. Cancer screening test use-United States, 2015. MMWR Morb. Mortal. Wkly Rep. 66 (8), 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhou F, Reed C, Chaves SS, Messonnier M, Kim IK, 2016. Cost-effectiveness of seasonal inactivated influenza vaccination among pregnant women. Vaccine 34 (27), 3149–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


