Abstract
Background
Idiopathic toe walking (ITW) is an exclusionary diagnosis given to healthy children who persist in walking on their toes after they should typically have achieved a heel‐toe gait. The literature discusses conservative and surgical interventions using a variety of treatment modalities. Young children and children without a limitation in ankle dorsiflexion (the upwards movement of the foot towards the shin of the leg) are commonly treated with conservative interventions. Older children who continue toe walking and present with limitations in ankle dorsiflexion are sometimes treated with surgical procedures. This systematic review is needed to evaluate the evidence for any intervention for the treatment of ITW. The conclusions of this review may support decision making by clinicians caring for children with ITW. It may also assist families when deciding on treatment options for their children with ITW. Many of the treatments employed have financial implications for parents or healthcare services. This review also aims to highlight any deficits in the current research base.
Objectives
To assess the effects of conservative and surgical interventions in children with ITW, specifically effects on gait normalisation, ankle range of motion, pain, frequency of recurrence, and any adverse effects.
Search methods
On 29 April 2019, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL Plus, and PEDro. We searched the following registers of clinical trials for ongoing and recently completed trials: the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP, apps.who.int/trialsearch), and ClinicalTrials.gov (clinicaltrials.gov). We searched conference proceedings and other grey literature in the BIOSIS databases and System for Information on Grey Literature in Europe (OpenGrey, opengrey.eu). We searched guidelines via the Turning Research Into Practice database (TRIP, tripdatabase.com) and National Guideline Clearinghouse (guideline.gov). We did not apply language restrictions.
Selection criteria
We considered randomised or quasi‐randomised trials for inclusion in the review if they involved participants diagnosed with ITW gait in the absence of a medical condition known to cause toe walking, or associated with toe walking. As there is no universally accepted age group for ITW, this review includes ITW at any age, who have been toe walking for more than six months, who can or cannot walk with a heel‐toe gait, and who may or may not have limited dorsiflexion of the ankle joint.
Data collection and analysis
We used standard Cochrane methodological procedures. The primary outcome was improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking). Secondary outcomes were active and passive range of motion of the ankle joint, pain, recurrence of ITW after treatment, and adverse events. We assessed the certainty of the evidence using the GRADE framework.
Main results
Four studies, comprising 104 participants, met the inclusion criteria. One study did not report data within the appropriate follow‐up timeframe and data from two studies were insufficient for analysis. The single study from which we extracted data had 47 participants and was a randomised, controlled, parallel‐group trial conducted in Sweden. It tested the hypothesis that combined treatment with serial casting and botulinum toxin type A (BTX) was more effective than serial casting alone in reducing ITW gait.
This study found that more participants treated with BTX improved (defined as toe walking less than 50% of the time, as reported by parents) (risk ratio (RR) 1.21, 95% confidence interval (CI) 0.57 to 2.55; 1 trial, 46 participants; very low‐certainty evidence). However, there was little or no difference between groups in passive ankle joint dorsiflexion range of movement on the right with the knee extended (mean difference (MD) ‐1.48º, 95% CI ‐4.13 to 1.16; 1 trial, 47 participants), on the right with the knee flexed (MD ‐0.04º, 95% CI ‐1.80 to 1.73; 1 trial, 46 participants), on the left with the knee flexed (MD 1.07, 95% CI ‐1.22 to 3.37), or on the left with the knee extended (MD 0.05, 95% CI ‐0.91 to 1.91). Nor was there a clear difference between the groups in recurrence of toe‐walking gait (assessed via severity of toe walking (graded 1 (mild), 2 (moderate), or 3 (severe)) on gait analysis, analysed as continuous data: MD 0.34 points, 95% CI ‐0.09 to 0.78; 46 participants). In principle, MDs greater than zero (i.e.) positive values) would favour BTX and casting and negative values would favour casting alone. We have not reported effects as better or worse because all results were from evidence of very low certainty. We downgraded the certainty of evidence because of study limitations (outcome assessment was not blinded) and imprecision. Outcomes of pain and active range of motion were not reported in the included study.
In terms of adverse events, calf pain was reported twice in the casting‐only group and three times in the BTX group. There were three minor skin problems in each group and one reported case of pain directly after BTX injection. The report did not state if calf pain and skin irritation were from the same or different participants. The study authors reported that adverse events did not alter treatment adherence.
Authors' conclusions
The certainty of evidence from one study, which compared serial casting with serial casting with BTX for ITW in children, was too low for conclusions to be drawn. A further three studies reported outcomes relating to BTX, footwear, exercises, and different types of orthoses as interventions, however the outcome data were too limited to assess their effects.
Plain language summary
Treatments for toe walking in children that is not associated with a medical condition
Background
It is estimated that up to five per cent of children are diagnosed with toe walking without a medical cause (known as idiopathic toe walking; ITW). We do not know why children have this walking style or what its long‐term impact might be. Children with ITW often present to health professionals with tight muscles at the back of their lower legs. This tightness is most commonly treated with stretches, plaster casts, or surgery.
Review question
We were interested in the effects of treatments for ITW in children. Cochrane authors collected relevant clinical trials to answer this question and assessed the evidence.
Date up to date
This Cochrane Review is current to 29 April 2019.
Study characteristics
Four trials met the inclusion criteria. They included a total of 104 people; however, three trials did not provide results that we could include in the review. (One trial studied different kinds of foot orthoses, which are in‐shoe devices that redistribute force and change gait), and two investigated the effects of adding botulinum toxin injections to various treatments such as stretching, exercises, splints, and footwear.) This review therefore only included the results of one trial, in which 47 children (aged between 5 and 14.5 years) received treatment with either plaster casts alone or plaster casts and injections of botulinum toxin A (BTX) into calf muscles. The study reported how much the children toe walked (based on their parents' observation), any change in ankle range of movement, and relapse (whether the children were still toe walking 12 months after treatment). The included study took place in Sweden and was not funded by anyone with a commercial interest in the results of the study.
Results
The evidence was too uncertain to determine whether or not there were differences in outcomes (amount of toe walking observed by parents, range of movement at the ankle, or recurrence of toe walking at 12 months) between children who received plaster casts and injections of BTX into calf muscles, compared to those who received plaster casts alone.
There were small numbers of adverse events in both groups, including calf pain and minor skin problems during treatment.
Conclusion
The available evidence is too uncertain to determine whether treatment with BTX injections and plaster casts are any more effective than just plaster casts in children with toe walking not associated with a medical condition. The limited evidence found in this review indicates a need for future research on treatments for this condition.
Summary of findings
Summary of findings for the main comparison. Botulinum toxin plus conservative treatment versus conservative treatment only.
| Casting + botulinum toxin A (BTX) compared with casting only for idiopathic toe walking (ITW) | |||||||
|
Population: children with ITW gait Settings: outpatient Intervention: casting + BTX Comparison: casting only | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk1 | Corresponding risk | ||||||
| Casting only | Casting + BTX | ||||||
|
Improvement (greater than 50% of time spent) heel‐toe walking Measured by parents' perception of toe walking frequency more than 50% of the time. Follow‐up: 12 months |
462 per 1000 | 558 per 1000 (263 to 1000) | RR 1.21 (0.57 to 2.55) | 46 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low | ‐ | |
| Active range of motion of the ankle joint | Not measured | ||||||
|
Change from baseline in passive ankle dorsiflexion range of movement Follow‐up: 12 months |
Right knee extended | The mean change in ankle range of movement in the casting‐only group was an increase of 6.48º | The mean change in passive ankle dorsiflexion range of movement in the casting + BTX group was on average 1.48º lower (less increase) than in the casting group (4.13º lower to 1.16º higher) | ‐ | 47 (1 RCT) | ⊕⊝⊝⊝a,b,d Very low | A increase in the range of movement is desirable. The minimum clinically important change has not been established. |
| Right knee flexed | The mean change in ankle range of motion in the casting‐only group was a decrease of 0.96º | The mean change in passive ankle dorsiflexion range of movement in the casting + BTX group was on average 0.04º lower (more decrease) than in the casting group (1.80º lower to 1.73º higher) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝a,b,d Very low | ||
| Left knee extended | The mean change in ankle range of motion in the casting‐only group was an increase of 5.93º | The mean change in passive ankle dorsiflexion range of movement in the casting + BTX group was on average 1.07º higher (more increase) than in the casting group (1.22º lower to 3.37º higher) | ‐ | 47 (1 RCT) | ⊕⊝⊝⊝a,b,d Very low | ||
| Left knee flexed | The mean change in ankle range of motion in the casting‐only group was 0.00º | The mean change in passive ankle dorsiflexion range of movement in the casting + BTX group was on average 0.50º higher (more increase) than in the casting group (0.91º lower to 1.91º higher) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝a,b,d Very low | ||
| Pain | Not measured | ||||||
|
Recurrence of ITW post‐treatment Assessed by improvement in toe walking based on Gait Analysis Classification An increase indicates improvement (an improvement indicated by a change in downgrading of the severe or 3, moderate or 2, and mild or 1 classification) Follow‐up: 12 months |
The mean change in ITW severity in the casting only group was 0.85 points | The mean change in ITW severity in the casting + BTX group was on average 0.34 points more than in the casting group (0.09 less to 0.78 more) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low | ‐ | |
| Adverse events | No discontinuation of treatment or fatal events were reported. The trial author provided individual participant data by personal communication and reported that there were no discontinuations of treatment. Minor adverse events were reported, such as skin irritation and mid‐calf pain related to the cast or casting process. | 47 (1 RCT) | ⊕⊝⊝⊝a,b,c Very low | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation | |||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||||
a Downgraded due to indirectness (for continuous outcomes the assumed risk is the control group). b Downgraded due to risk of bias (participants and outcome assessors were not blinded to the allocation). c Downgraded due to imprecision (the optimal information size has not been met). d Downgraded due to imprecision (the CI includes effects that may lead to different clinical decisions).
Background
Description of the condition
Idiopathic toe walking (ITW) is a diagnosis given to healthy children who persist in walking on their toes after they should typically have achieved a heel‐toe gait. The estimated prevalence of ITW is approximately five per cent of healthy children (Engström 2012), and the condition commonly affects boys more than girls (Bernhard 2005; Engelbert 2011).
Children progress through a number of stages when learning to walk. Toddlers initially walk with stilted leg shuffling, then cruise around furniture before starting to walk flat‐footed. As children mature, they walk with a heel‐toe gait (Hallemans 2003; Norlin 1981), which comprises three distinct phases: initial heel strike, midfoot contact, and toe‐off. During the development of heel‐toe gait, some children walk on their toes. However, toe walking is not a stage that all children will progress through, and heel strike is present in most children by the age of 18 months (Sutherland 1980). A toe‐walking gait is described by authors as a normal variant in motor development for healthy children up to the age of 18 months (Sala 1999; Sutherland 1980), two years (Fox 2006; Hemo 2006; Stricker 1998), five years (Westberry 2008), and even until seven years of age (Kalen 1986). Parents are encouraged to seek advice from health professionals when toe walking persists. However, a lack of consensus remains on when to seek advice.
Idiopathic toe walking is an exclusionary diagnosis given to otherwise healthy children who continue to toe walk longer than is normally expected. Many conditions are associated with a toe‐walking gait and exclusion of these often make the diagnosis of ITW challenging for health professionals (Engelbert 2011; Williams 2010). Toe walking may result from an inability to achieve heel strike due to an underlying neurological or neuromuscular impairment. Conditions commonly associated with a toe‐walking gait include cerebral palsy (Wren 2010), muscular dystrophy (Hyde 2000), and orthopaedic conditions, such as congenital talipes equinus (Caselli 1988). Toe walking is also commonly observed in children with autistic spectrum disorders (Barrow 2011; Ming 2007), intellectual disabilities, and developmental speech and language disorders (Accardo 1989; Accardo 1992).
Persistent toe walking in healthy children was first defined by Hall and colleagues as "congenital short tendo calcaneus" (Hall 1967). This definition was given because the population in this study demonstrated Achilles tendon tightness. This diagnosis was later changed to "habitual toe walking" (Griffin 1977), and subsequently the term "idiopathic toe walking" was introduced in 1980 (Conrad 1980). Most authors agree that ITW is a diagnosis of exclusion, made when children persist in walking on their toes with no signs of a neurological, orthopaedic, or psychological condition (Armand 2006; Brouwer 2000; Eastwood 1997; Engström 2010; Griffin 1977; Hall 1967; Hemo 2006; Hicks 1988; Hirsch 2004; Kalen 1986; Kogan 2001; McMulkin 2006; Papariello 1985; Pendharkar 2012; Shulman 1997; Solan 2010; Stott 2004; Williams 2010).
The aetiology of ITW continues to be investigated. Many hypotheses exist as to why healthy children continue to toe walk, including presence of a hereditary genetic disorder with an autosomal dominant pattern of inheritance with variable expression (Katz 1984), an increase in the proportion of type I muscle fibres (Eastwood 1997), sensory processing difficulties (Williams 2012), and the use of infant walkers (Engelbert 1999). Some authors describe children with ITW as having the ability to walk with a heel strike, but preferring to walk on their toes (Crenna 2005; Williams 2010). Children who have ITW‐gait often also have limited ankle dorsiflexion (Brouwer 2000; Hall 1967; Hicks 1988; Williams 2013a).
The consequences of persistent and untreated toe walking are unclear. Untreated toe walking may lead to a higher chance of tripping or falling (Caselli 1988), or have a social or cosmetic impact (Gormley 1997; Pendharkar 2008); however, these statements are not based on systematic observations. Many authors believe intervention for ITW should be undertaken to improve any restriction in ankle range of motion, and that if no treatment is undertaken, children with ITW are at a greater chance of developing more severe limitations in this range (Bernhard 2005; Brunt 2004; Gormley 1997; Hemo 2006; Sobel 1997). However, no clinical trials (i.e. parallel‐group randomised trials) have compared treatment to no treatment. This limits the evidence supporting treatments for this condition. One study recruited 80 children and reassessed them three to eight years after initial presentation. Of these, 48 children did not receive active intervention (but were observed over time, wore modified footwear, or performed gastrocnemius and soleus stretching exercises under the supervision of a parent or healthcare professional). The study concluded that persistent toe walking did not result in significant functional disturbance, foot deformities, or pain (Stricker 1998). Similarly, a small study involving 11 adolescents and adults found limited long‐term structural impact of toe walking, with a small percentage continuing to toe walk from 7 to 21 years after initial presentation (three participants, 27%) (Hirsch 2004). Another study also found spontaneous correction of ITW associated with age in 32 out of 41 children aged between three and eight years (Taussig 2001). This evidence has led authors to postulate that ITW is a benign condition that resolves spontaneously in most instances and causes a child (but not necessarily their parents) little concern while it lasts.
Description of the intervention
The literature discusses both conservative and surgical interventions for ITW. Young children and children without a limitation in ankle dorsiflexion are commonly treated with conservative interventions. Older children who continue toe walking and present with limitations in ankle dorsiflexion are sometimes treated with surgical procedures.
Many types of conservative inactive and active interventions have been investigated, including observation over time (Eastwood 2000; Eiff 2006; Stricker 1998); muscle stretching exercise programmes targeting gastrocnemius, soleus, or both (Stricker 1998); motor control intervention (Clark 2010); auditory feedback (Conrad 1980); footwear (Caselli 2002); ankle‐foot or foot‐only orthoses (Caselli 2002; Herrin 2016;Sala 1999; Stricker 1998); serial casting (Brouwer 2000; Eastwood 2000; Griffin 1977; Katz 1984; Stott 2004; Stricker 1998); different flooring surfaces (Fanchiang 2016); and botulinum toxin type A (BTX) (Engstrom 2013; Gormley 1997).
Surgical interventions include percutaneous Achilles tendon lengthening (Caselli 2002; Hemo 2006; Jahn 2009; Kogan 2001; McMulkin 2006; Stott 2004); open Achilles tendon lengthening via a Z‐lengthening or slide technique (Hemo 2006); Baker’s gastrocnemius‐soleus lengthening (Stott 2004); and the Vulpius procedure (gastrocnemius recession surgery) (Jahn 2009; McMulkin 2006). Most surgical interventions aim to achieve at least 10° of ankle dorsiflexion (McMulkin 2006).
How the intervention might work
Apart from simple observation, most interventions aim to lengthen the Achilles tendon, increase dorsiflexion of the ankle joint, and thereby facilitate a typical heel‐toe gait pattern. Other interventions have aimed to inhibit the gait pattern, facilitate heel contact, and challenge the sensory system of the child. A short description of the mechanisms behind interventions for ITW follows.
Observation (regular review) is used to monitor any developing limitation in ankle dorsiflexion (Eiff 2006), and identify the need for further active intervention.
Stretching exercises are often prescribed in the presence of reduced ankle dorsiflexion. The Cochrane Review 'Stretch for the treatment and prevention of contractures' describes the general theory of stretching and presents detailed evidence (Katalinic 2010).
Ankle‐foot orthoses, full‐length foot orthoses, footwear, and serial casts may prolong the time of a stretching intervention (according to the theory about stretching presented by Katalinic 2010). These devices may also physically inhibit the child from getting up on their tiptoes. These interventions are commonly used in children with good ankle dorsiflexion range of motion for gait re‐education, motor control, or both (Brouwer 2000).
Motor control intervention is based on the premise that a toe‐walking gait in children older than three years may be due to motor control deficiency (Clark 2010). The motor control intervention aims to facilitate an erect standing and walking posture, and to secure a ground reaction force relative to the ankle axis.
Augmented auditory feedback is a device that produces an auditory signal when the foot‐switch is closed on heel contact (Conrad 1980). The device aims to establish a normal muscle response and heel‐strike gait.
Footwear therapy utilises footwear with a rigid sole and straight last (Caselli 2002). This shoe design is thought to limit dorsiflexion at the metatarsal‐phalangeal joint and thereby prevent toe walking.
Different flooring surfaces have been utilised to challenge sensory processing abilities with different plantar tactile input (Fanchiang 2016).
Intramuscular BTX injection into gastrocnemius or soleus (or both) is used to reduce muscle tightness. When it is injected into the gastrocnemius or soleus muscles, it reduces the ability to develop plantarflexion torque, especially at the end of swing phase (Brunt 2004). BTX is commonly used in children with cerebral palsy who walk on their toes to reduce calf muscle tightness. The Cochrane Review 'Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy' describes this in detail (Ade‐Hall 2000).
Percutaneous and open Achilles tendon lengthening are surgical techniques to treat a fixed contracture of the Achilles tendon. The procedure is described in more detail in Moreau 1987. Percutaneous lengthening of the Achilles tendon is generally preferred over open lengthening, because less scar formation and less pain occur (Kogan 2001).
The Vulpius procedure for lengthening of the Achilles tendon consists of an incision of the gastrocnemius and, commonly, also the soleus fascia. The Baker procedure is a variation of the Vulpius procedure, where the gastrocnemius fascia is cut in a tongue‐and‐groove fashion instead of more transversely (Yngve 1996).
Why it is important to do this review
This systematic review is required to evaluate the evidence for any intervention for the treatment of ITW. The conclusions of this review may guide clinicians in tailoring care for children with ITW. As many of the treatments employed have financial implications for parents or healthcare services, this review also aims to highlight any deficits in the current research base.
Objectives
To assess the effects of conservative and surgical interventions in children with idiopathic toe walking (ITW), specifically effects on gait normalisation, ankle range of motion, pain, frequency of recurrence, and any adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (studies that use methods of allocation which are not strictly random, for example, hospital numbers or date of birth).
Types of participants
We considered for inclusion any study that involved participants diagnosed with ITW‐gait in the absence of a medical condition known to cause toe walking, or be associated with toe walking. As there is no universally accepted age group for ITW, we included children who had been diagnosed with ITW at any age, who had been toe walking for more than six months, who could and could not walk with a heel‐toe gait, and who may or may not have had limited dorsiflexion of the ankle joint (a definition based on Eastwood 1997; Hemo 2006; Hirsch 2004; Kalen 1986; Kogan 2001; McMulkin 2006; Sala 1999; Shulman 1997; Stricker 1998; Stott 2004; and Westberry 2008).
Types of interventions
We considered conservative and surgical treatment. We defined conservative treatment as watchful waiting, stretching exercises (guided either by a health professional within the clinical setting or implemented within the home environment), footwear, orthoses, serial casting (with or without botulinum toxin treatment), botulinum toxin treatment (with or without serial casting), external stimulation aimed at tactile input, and feedback devices in footwear. We operationally defined surgical interventions as all percutaneous and open procedures aimed at improving dorsiflexion of the ankle joint and regaining a typical heel‐toe gait pattern. We considered comparisons of any intervention versus another, or versus no intervention (also known as watchful waiting).
Types of outcome measures
We specified that we would report the following outcomes; however, we did not use them as criteria for selecting studies for the review.
Primary outcomes
Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking), preferably measured as the proportion of participants with improvement based on quantifiable judgements by the child, parent, or healthcare professional, or by kinematic or gait analysis. Time frame: six months.
Secondary outcomes
Active range of motion of the ankle joint, measured by goniometry, degree of change from baseline. Time frame: six months.
Passive range of motion of the ankle joint, measured by goniometry, degree of change from baseline. Time frame: six months.
Pain, measured by different scales, e.g. visual analogue scale (VAS). Time frame: six months (change).
Recurrence of ITW post‐treatment. Time frame: 12 months.
Adverse events (whereby 'any adverse events' are those which lead to discontinuation of treatment, and 'serious adverse events' are those which are fatal, life‐threatening, or require prolonged hospitalisation). Depending on the type of intervention, adverse events are likely to include injuries, rupture of the Achilles tendon, weakness of the lower limb muscles due to over‐correction or adverse effects of BTX, compartment syndrome, pressure wounds from serial casting, swelling, and infection.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web, 29 April 2019; Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web (29 April 2019; Appendix 2)
MEDLINE (1946 to 26 April 2019; Appendix 3)
Embase (1947 to 26 April 2019; Appendix 4)
CINAHL Plus (1937 to 26 April 2019; Appendix 5)
Web of Science meetings and proceedings (29 April 2019; Appendix 6)
Open Grey (Grey Literature in Europe opengrey.eu/) (29 April 2019; Appendix 7)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; Appendix 8)
World Health Organization International Clinical Trials Registry Platform (ICTRP, apps.who.int/trialsearch/; Appendix 9)
We also searched guidelines via the Turning Research into Practice database (TRIP, tripdatabase.com), and National Guideline Clearinghouse (guideline.gov). We did not apply language restrictions; however a RCT filter was placed on all search strategies where possible within the database.
Searching other resources
We checked reference lists of included studies and journals citing the article. We contacted trial authors to identify any additional published or unpublished data.
We contacted experts in the field and trial authors for ongoing trials. We applied no limitations in terms of publication status (i.e. published or unpublished) or language.
Data collection and analysis
Selection of studies
Two review authors (AC and MF) independently assessed all the titles and abstracts identified by the search. We obtained the full text of studies that possibly or definitely fulfilled inclusion criteria according to the abstract. We resolved disagreements through discussion and, when necessary, we consulted another review author (CW). We also searched the reference lists and citations of included articles to determine if any further articles met the inclusion criteria. All included studies were in English. We developed a PRISMA flow diagram to document the study selection process (Moher 2009).
We described reasons for exclusion of studies that appeared eligible, but failed to meet the inclusion criteria, in a Characteristics of excluded studies table.
Data extraction and management
Two review authors (AC and KG) carried out the data extraction independently. They resolved discrepancies through discussion and when necessary, consulted another review author (CW). The review authors used a standard data extraction form from Covidence. The form recorded information on the eligibility of the study in relation to the research question; methods; population; intervention; comparison interventions; outcomes; results; any adverse events; and miscellaneous information (such as funding and conflicts of interest). Where data were missing, we requested data from the author.
Assessment of risk of bias in included studies
Two review authors (AC and RE) independently assessed the risk of bias in included studies using the Cochrane 'Risk of bias' tool (Higgins 2011). They resolved disagreements through discussion and, when necessary, another review author assisted (CW). If necessary information was not available within the article, we contacted the trial authors. We reported the source of any such information in the review.
We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias.
We graded each potential source of bias as high, low or unclear risk, and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table for the included trials.
Assessment of bias in the review process
We conducted the review according to the published protocol (Williams 2016); minor deviations are outlined in Differences between protocol and review. We included four trials in this review. There was a deviation from the protocol to allow extraction of data from an alternative time point. This enabled us to extract data from Engstrom 2013, as this trial reported data at three and 12 months. We contacted the trial author and obtained individual participant data so that we could calculate the change from baseline for the required outcome measures, and calculate change from baseline to 12 months. We determined three months was not long enough for a true representation of treatment effect, and included only the 12‐month data.
Measures of treatment effect
We collected both dichotomous and continuous data. For continuous outcomes, we reported the mean difference (MD) and corresponding 95% confidence interval (CI). For dichotomous outcomes, we calculated a risk ratio (RR) with a 95% CI.
Unit of analysis issues
Randomisation within the included trial was done at the individual level. Trial outcomes were assessed per participant, not per foot. The trial reported outcomes per foot and these were reported within the results.
Dealing with missing data
We contacted trial authors to gather or clarify missing data.
Data synthesis
Meta‐analysis was not possible due to a lack of data. We presented the results in a narrative format. We planned to use Review Manager 5 for data analysis (RevMan 2014), but instead carried out statistical analysis using SPSS as data were provided in this format (IMB Corp. 2013).
'Summary of findings' and GRADE
We created a 'Summary of findings' table and presented the following outcomes.
Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking), preferably measured as the proportion of participants with improvement based on quantifiable judgements by the child, parent, or healthcare professional, or by kinematic or gait analysis. Time frame: six months.
Active range of motion of the ankle joint, measured by goniometry, degree of change from baseline. Time frame: six months.
Passive range of motion of the ankle joint, measured by goniometry, degree of change from baseline. Time frame: six months.
Pain, measured by different scales, e.g. visual analogue scale (VAS). Time frame: six months (change).
Recurrence of ITW post‐treatment. Time frame: 12 months.
Adverse events (whereby 'any adverse events' are those which lead to discontinuation of treatment, and 'serious adverse events' are those which are fatal, life‐threatening, or require prolonged hospitalisation). Depending on the type of intervention, adverse events are likely to include injuries, rupture of the Achilles tendon, weakness of the lower limb muscles due to over correction or adverse effects of BTX, compartment syndrome, pressure wounds from serial casting, swelling, and infection.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence (studies that contribute data for the prespecified outcomes) (Atkins 2004). We considered RCTs as high‐certainty evidence if the five factors above were not present to any serious degree, but downgraded the certainty to moderate, low, or very low according to the GRADE criteria. We downgraded evidence once if a consideration was serious and twice if very serious. We justified all decisions to downgrade or upgrade the certainty of evidence using footnotes. We used GRADEpro GDT software to create the table (GRADEpro GDT).
Additional methods
We documented methods that were described in the protocol, but were not required due to the absence of meta‐analysis, in Appendix 10.
Results
Description of studies
Results of the search
The initial search yielded 906 potentially relevant records prior to removal of duplicates. There were 513 references from MEDLINE, 198 from Embase, 55 from CINAHL, 10 from the Cochrane Neuromuscular Specialised Register, and 130 from the Cochrane Central Register of Controlled Trials (CENTRAL). Following removal of duplicates, we screened the titles or abstracts of 825 articles. Nineteen full‐text articles or abstracts met inclusion criteria. One abstract was a secondary reference for an included study (Engstrom 2013), and we matched a published article to a thesis (Fanchiang 2014). From these 19 studies, we included four trials, of which one had outcome data for extraction. We identified no further studies through handsearching. The PRISMA flow chart describes the study selection process (Figure 1).
1.
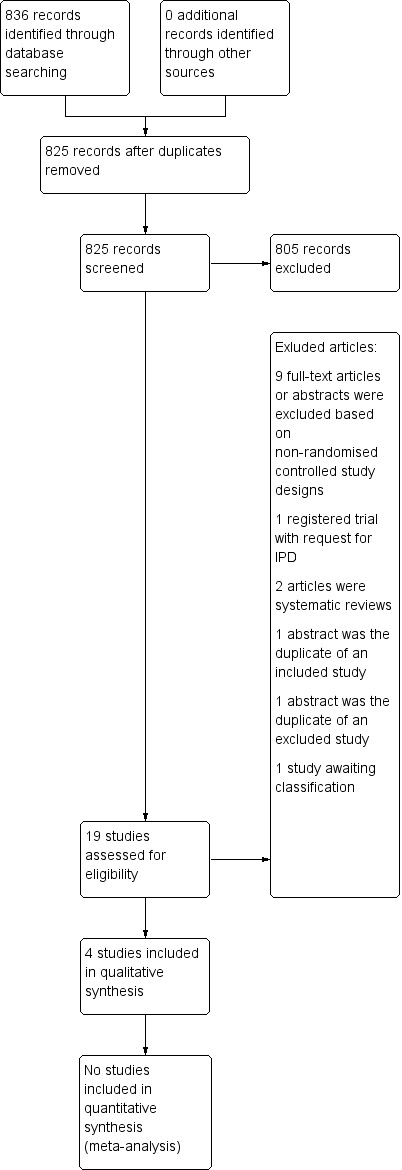
Study flow diagram.
Included studies
Four studies met the inclusion criteria (D'Apote 2012; Engstrom 2013; Herrin 2016; Satila 2016); however, only Engstrom 2013 provided usable outcome data. Herrin 2016 was a randomised, parallel‐group, controlled trial that compared the use of ankle‐foot orthoses to full‐length in‐shoe orthoses. The authors reported outcomes at six weeks, which was shorter than the six‐month minimum timeframe outlined in the review protocol. Satila 2016 was also a randomised, parallel‐group, controlled trial; it compared a group of conservative treatments (indoor shoes with firm heel cup and straps, night splints, home strengthening programme) to conservative treatments in addition to BTX injections. This trial met the outcome timeframe stipulated in our protocol; however, outcome data were only published in graphical format, and accurate numbers for the outcome were not extractable. D'Apote 2012 presented an abstract of a pilot randomised cross‐over trial comparing BTX only to BTX and ankle‐foot orthoses and exercises. The overall pilot trial results were presented in this abstract. This study met our specified time frames for outcome measurement; however, on contact with the study authors we found that the trial had been discontinued, and the authors were unable to provide any summary results for outcomes of interest. Outcome measurement data from these three studies were therefore too limited for further analysis or narrative reporting.
One randomised, controlled, parallel‐group trial underwent full data extraction and analysis (Engstrom 2013). The study was designed to test the hypothesis that combined treatment of casts and BTX was more effective than casts alone in reducing toe walking. It included an equivalent standard care programme for both groups. The children were prescribed a home exercise programme, including calf stretches. The study randomised 47 children to four weeks of treatment with below‐the‐knee walking casts, either as the sole treatment (casting group) or following BTX injection into the calf muscles (casting + BTX group).
The demographics of all four included trials are presented in Characteristics of included studies.
Interventions
Casting
All children in Engstrom 2013 had bilateral below‐the‐knee walking casts applied for four weeks. Casts were applied with the ankle in a neutral position. Each cast was applied by the same nurse who had additional training in casting.
Casting with the addition of botulinum toxin type A
The children allocated to the casting and BTX group underwent bilateral injections with BTX 12 units/kg body weight, under sedation. Each child had four injections in each calf: two into the proximal third of the lateral and medial gastrocnemius muscle belly, and two in the distal aspect of the gastrocnemius‐soleus complex. The cast was applied to the children in the casting group in a similar manner, by the same nurse. The casts were applied between one and two weeks after the injections.
Home exercise programme
Children in both groups were prescribed a stretching and heel‐walking programme by a physiotherapist, following cast removal. Children and parents received verbal and written instructions. The stretching dosage was five times per week; however, the length of each stretch was not reported (Engstrom 2013). The prescribed heel‐walking dosage was 50 steps per day. No monitoring or record of adherence was reported.
Excluded studies
We excluded 11 studies (see: Characteristics of excluded studies). Although all were clearly ineligible for the review, we listed them as excluded studies because of the paucity of RCT evidence. Of these studies, we excluded nine for not being RCTs (Alvarez 2007; Benedetti 2013; Brouwer 2000; Brunt 2004; Fanchiang 2014; Jadhav 2017; Jahn 2009; Michalitsis 2019; Thielemann 2019), and two for being systematic reviews (Eiff 2006; Gámez‐Iruela 2015).
The excluded studies reported interventional treatment modalities such as gait retraining, orthotic intervention, stretching, serial casting, BTX, and surgery.
Ongoing studies
A Brazilian study comparing different physiotherapy protocols is ongoing (RBR‐7QNFFG).
Studies awaiting classification
We have requested further information about a trial registry record, as we have found no associated publication and the status of the trial is unclear (NCT00175669).
Risk of bias in included studies
There were four included studies. We have provided explanations of 'Risk of bias' assessments in the Characteristics of included studies table (see also the 'Risk of bias' summary, Figure 2).
2.
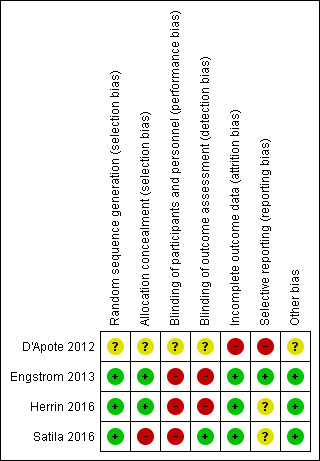
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Green (+) = low risk of bias; red (‐) = high risk of bias; yellow (?) = unclear risk of bias.
Allocation
We assessed Engstrom 2013 and Herrin 2016 as being at low risk of bias for random sequence generation and allocation concealment. We deemed Satila 2016 to have a high risk of bias for allocation concealment, but a low risk of bias for sequence generation. We assessed D'Apote 2012 as being at unclear risk of bias, as the report stated that it was a cross‐over RCT, but provided no information on how participants were randomised or allocated to treatment, or on the method of allocation concealment.
Blinding
We assessed Engstrom 2013 as having a high risk of bias for blinding of participants and personnel, and for blinding of outcome assessors. Although randomisation was undertaken prior to baseline examinations, the staff conducting the baseline examinations, and children and parents, were blinded to which group the child had been allocated until after the baseline examinations. Children occasionally revealed group allocation to the physical therapist. We deemed Herrin 2016 to have a high risk of bias for both blinding of participants and blinding of assessors, as all parties knew the children's allocations. We assessed Satila 2016 as having high risk of performance bias, since participants and personnel were aware of the participant groups; however, outcome assessors were blinded so we judged the study to be at low risk of detection bias. D'Apote 2012 was assessed as having unclear risk of bias as the article does not state if any blinding was applied to participants, personnel, or assessors.
Incomplete outcome data
We assessed Engstrom 2013, Herrin 2016, and Satila 2016 as having a low risk of bias for incomplete outcome data as attrition rates were low. We considered D'Apote 2012 to have a high risk of bias, as the trialists did not supply data for each data collection interval or each outcome measure.
Selective reporting
We assessed Engstrom 2013 as having a low risk of bias for selective reporting as all planned outcome data were reported within the study. We judged D'Apote 2012 to have a high risk of bias as data were not supplied for each data collection interval or each outcome measure. We assessed Herrin 2016 and Satila 2016 as having an unclear risk of bias as reporting of the outcomes of interest was incomplete, and we could not use the data in a meta‐analysis.
Other potential sources of bias
We assessed Engstrom 2013, Herrin 2016, and Satila 2016 to be at low risk of other potential sources of bias. The groups were similar at baseline, with no reported co‐interventions. We assessed D'Apote 2012 to be at high risk of other bias due to incomplete data collection.
Effects of interventions
See: Table 1
We intended to extract data for the degree of change from a pre‐intervention baseline to six months, for the outcome measures specified in our protocol. Three included studies did not report outcomes within the specified time frame, nor were data available for extraction (D'Apote 2012; Herrin 2016;Satila 2016). The only study providing data before the intervention (baseline) at a time point at, or greater than, six months following the intervention was Engstrom 2013.
Botulinum toxin plus conservative treatment versus conservative treatment only
Casts and botulinum toxin type A versus casts alone
One study (Engstrom 2013) compared a combination of casting (conservative treatment) and botulinum toxin type A to casting alone. See Table 1.
Primary outcome: improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking). Time frame: six months
The protocol of this review specified a six‐month time frame; Engstrom 2013 reported data at three months and 12 months. The review authors determined three months to be insufficient follow‐up for a true representation of a treatment effect. We contacted the author of Engstrom 2013 and obtained individual participant data to calculate the change from baseline at 12 months, and reported this outcome.
Table 2 indicates the number of children toe walking more than 50% of the time, according to parent perception.
1. Number of children toe walking greater than 50% of the time according to parent perception responses.
| Group | Baseline | Total | 12‐month review | Total | ||
| Less than or equal to 50% | 51% or more | Less than or equal to 50% | 51% or more | |||
| Casting | 3 | 23 | 26 | 18 | 8 | 26 |
| Casting + BTX | 2 | 18 | 20 | 18 | 1 | 19 |
Data from Engstrom 2013.
Parents were asked to quantify the amount of toe walking, rather than our primary outcome of heel‐toe walking. While there was improvement in parent perception of toe walking for less than 50% of the time at 12 months in both intervention groups, there was no clear difference (based on individual participant data) between the casting‐plus‐BTX group (n/N = 18/19) versus the casting‐alone group (n/N = 18/26): risk ratio (RR) 1.21, 95% confidence interval (CI) 0.57 to 2.55; 45 participants; very low‐certainty evidence. One participant in the casting‐plus‐BTX group did not have a 12‐month follow‐up.
Secondary outcomes
We report secondary outcomes from Engstrom 2013 at the 12‐month time point only.
Active range of motion of the ankle joint, measured by goniometry, degree change from baseline. Time frame: six months
This outcome was not reported.
Passive range of motion of the ankle joint in knee extension and knee flexion, measured by goniometry. Time frame: six months
We obtained individual participant data for passive dorsiflexion range of motion at the ankle joint and calculated the change in passive dorsiflexion from baseline to 12 months (Table 3). We used individual participant data to calculate the change in passive ankle joint range from baseline at 12 months. The study performed randomisation at the participant level and we therefore used whole participant data. Where there were left and right measures, we have reported both sides. There was little or no difference between groups in passive ankle joint dorsiflexion range of movement on the right with the knee extended (mean difference (MD) ‐1.48º, 95% CI ‐4.13 to 1.16; 1 trial, 47 participants; very low‐certainty evidence), on the right with the knee flexed (MD ‐0.04º, 95% CI ‐1.80 to 1.73; 1 trial, 46 participants; very low‐certainty evidence), on the left with the knee flexed (MD 1.07, 95% CI ‐1.22 to 3.37), or on the left with the knee extended (MD 0.05, 95% CI ‐0.91 to 1.91). In principle, MDs greater than zero (i.e. positive values) would favour BTX and casting, and negative values would favour casting alone. We have not reported effects as better or worse because of the very uncertain evidence. See Table 3.
2. Change in passive dorsiflexion from baseline to 12 months.
| Secondary outcomes | Pre‐treatment | 12 months post‐treatment | Change in ROM (between pre‐treatment and 12 months post‐treatment), mean (SD) | Difference in change in ROM between groups | |||||
|
Casting group Mean (SD) n/N |
BTX + casting group Mean (SD) n/N |
Casting group Mean (SD) n/N |
BTX + casting group Mean (SD) n/N |
Casting group Mean (SD) |
BTX + casting group Mean (SD) |
Mean difference (SE) | 95% CI | ||
| Passive dorsiflexion range of movement (knee extended) in degrees (right) | 4.45 (5.60) 27/27 |
5.75 (4.94) 20/20 |
10.93 (4.17) 27/27 |
10.75 (4.06) 20/20 |
6.48 (4.77) |
5.00 (3.97) |
‐1.48 (1.31) | ‐4.13 to 1.16 | |
| Passive dorsiflexion range of movement (knee flexed) in degrees (right) | 16.73 (5.65) 26/27 |
16.00 (5.03) 20/20 |
15.77 (6.11) 26/27 |
15.00 (4.86) 20/20 |
‐0.96 (2.46) |
‐1.00 (3.48) |
‐0.04 (0.88) | ‐1.80 to 1.73 | |
| Passive dorsiflexion range of movement (knee extended) in degrees (left) | 4.63 (7.06) 27/27 |
5.00 (4.59) 20/20 |
10.56 (4.23) 27/27 |
12.00 (2.99) 20/20 |
5.93 (4.39) | 7.00 (2.99) | 1.07 (1.14) | ‐1.22 to 3.37 | |
| Passive dorsiflexion range of movement (knee extended) in degrees (left) | 15.19 (5.19) 26/27 |
15.50 (4.84) 20/20 |
15.19 (5.19 26/27 |
16.00 (4.76) 20/20 |
0.00 (2.82) | 0.50 (1.54) | 0.50 (0.70) | ‐0.91 to 1.91 | |
| ROM: range of movement n: number of participants that data was collected from N: total number of participants in each cohort SD: standard deviation SE: standard error | |||||||||
Data from Engstrom 2013.
Pain, measured by different scales, e.g. visual analogue scale (VAS). Time frame: six months (change)
Pain was not measured in this study.
Recurrence of idiopathic toe walking post‐treatment. Time frame: 12 months
The study did not describe recurrence of ITW; however, it did describe a severity measure relating to amount of heel contact during gait (Engstrom 2013). We considered that this outcome was a pseudo measure of recurrence and reported the change‐from‐baseline severity of toe walking 12 months after treatment.
The study also used a system devised by Alvarez 2007 to classify the severity of ITW. This classification system has a high degree of sensitivity and uses both kinematic and kinetic variables to group ITW into mild, moderate, or severe categories. Alvarez 2007 described mild ITW as the first ankle rocker (heel versus forefoot contact) being present; moderate ITW as the early third ankle rocker (early heel rise) being present; and severe ITW as predominantly the first ankle plantar flexion moment during loading response (forefoot weight acceptance) being observed. Table 4 displays the number of participants in each severity grouping at baseline and at 12 months. There were no clear differences between the two treatment groups at pre‐treatment, three months or 12 months; however, both groups improved significantly between pre‐treatment and 12 months. Engström 2013 reported no change between the three‐month and 12‐month follow‐up.
3. Number of participants in each severity grouping at baseline and 12 months.
| Pre‐treatment (n/N) | 12 months post‐treatment (n/N) | |||||
| Group | Mild | Moderate | Severe | Mild | Moderate | Severe |
| Casting | 0/26 | 9/26 | 17/26 | 7/26 | 17/26 | 2/26 |
| Botox + casting | 0/21 | 4/21 | 17/21 | 8/20 | 10/20 | 2/20 |
| n: number in each severity class N: total number of participants in each cohort | ||||||
Data from Engstrom 2013.
In order to analyse the change in severity grouping based on gait analysis parameters for individual participants, the review authors treated the rating of severity as a continuous variable (mild = 1, moderate = 2, severe = 3). We calculated the change in severity for each participant from pre‐treatment to 12 months post‐treatment, with a positive change indicating improvement in toe walking and a negative change indicating a deterioration in toe‐walking status. We then performed a one‐way ANOVA to compare the change in severity between groups. The data in Table 5 are based on unpublished ordinal data that the author group analysed as continuous data. We found that the result favoured the casting‐and‐BTX group over the casting‐only group (MD 0.34, 95% CI ‐0.09 to 0.78; 46 participants; moderate‐certainty evidence; Table 5).
4. Change in severity after 12 months (re‐occurrence).
| Mean within participant change in severity after 12 months re‐occurrence) | ||
|
Mean (SD) n/N |
Mean difference (95% CI) | |
| Casting | 0.85 (0.61) 26/27 |
0.34 (‐0.09 to 0.78) |
| BTX and casting | 1.19 (0.87) 20/20 |
Data from Engstrom 2013,
Adverse events
The study reported no discontinuations of treatment or fatal events. The trial author provided individual participant data by personal communication and reported that there were no discontinuations of treatment. Minor adverse events were reported, such as skin irritation and mid‐calf pain related to the cast or casting process.
Botulinum toxin type A with footwear, night splints and strengthening exercises versus footwear, night splints and strengthening exercises
One trial (Satila 2016) compared BTX with night splints and strengthening exercises versus night splints and strengthening exercises alone.
Primary outcome: improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking). Time frame: six months
No individual data were available for extraction from the study, following contact with the authors. The publication reported that 16/16 (100%) of participants in the BTX group, versus 11/13 (85%) in the comparison group, ceased toe walking. This was measured using an investigator‐driven observation template.
Secondary outcomes
Active range of motion of the ankle joint, measured by goniometry, degree change from baseline. Time frame: six months
Active range of motion was included as an outcome measure; however, no results were published.
Passive range of motion of the ankle joint in knee extension and knee flexion, measured by goniometry. Time frame: six months
At 24 months, the passive range of motion of the ankle joint had declined in five out of 16 children in the BTX group, and five out of 14 children in the comparator group. The range of motion remained the same in two children in each group, and increased in nine children in the BTX group, and six children in the comparator group.
Pain, measured by different scales, e.g. visual analogue scale (VAS). Time frame: six months (change)
Pain was not measured in this study.
Recurrence of idiopathic toe walking post‐treatment. Time frame: 12 months
All children continued to toe walk at 24 months.
Adverse events
The study indicated no discontinuation of treatment or fatal events (Satila 2016). We emailed the author to obtain outcome data; however, this was not available for participants in either group.
Botulinum toxin type A plus stretching and exercises versus stretching and exercises alone
One study (D'Apote 2012) compared BTX plus stretching and exercises to stretching and exercises alone. We contacted the trial author but no data were available other than the published abstract (D'Apote 2012).
Most outcomes were not measured. Data were not available for any outcome other than for passive range of motion at the ankle joint, but results reported were not attributable to either treatment group.
Ankle‐foot orthosis versus foot orthosis with a carbon fibre foot plate
One study (Herrin 2016) compared an ankle‐foot orthosis to a foot orthosis with a carbon fibre foot plate. The study reported outcomes at six weeks, which much shorter than our prespecified time point of six months. No data were available for our specified measures of effectiveness.
Adverse events
The study indicated one dropout, which was not due to adverse events but to the participant being unavailable.
Discussion
Summary of main results
Four studies met the inclusion criteria, comprising of a total of 104 participants (Engstrom 2013;D'Apote 2012; Herrin 2016; Satila 2016). The trial by D'Apote and colleagues was an abstract that provided no meaningful data for inclusion in qualitative synthesis (D'Apote 2012). In the study by Herrin and colleagues, outcomes were measured at less than the minimum six‐month timeframe (Herrin 2016). We contacted the authors of Satila 2016, as the article only provided summary data in graphical form. The authors did not have any further summary or individual participant data available. Only Engstrom 2013 provided data suitable for extraction.
The study by Engstrom and colleagues was a randomised, controlled, parallel‐group trial that tested the hypothesis that combined treatment with serial casting and BTX injections was more effective than serial casting alone in reducing ITW gait (Engstrom 2013). Participants in both intervention groups improved after treatment in all reported study parameters. The evidence provided by this study was too uncertain to determine whether or not the administration of BTX injections prior to four weeks of casting treatment improved treatment outcomes when compared to serial casting alone (Engstrom 2013).
We extracted data on three outcome measures from Engstrom 2013. The study had 47 participants and described results for improvement of time spent toe walking, passive ankle joint range of motion, and recurrence of toe walking at baseline, three months, and 12 months. The protocol of this review required outcomes at baseline and six months for range of motion and parent report of time spent toe walking. The included study also reported the severity of the toe walking gait pattern, which the review authors determined as a pseudo measure for toe walking recurrence and best described by a return to the baseline severity of toe walking, 12 months after treatment. This outcome met the timeframe of the protocol.
Results from this analysis were too uncertain to determine whether the addition of BTX to serial casting had any effect on parent report of improvement in toe walking to under 50% of the time, or the change in passive ankle joint dorsiflexion range of movement at 12 months.
Overall completeness and applicability of evidence
There is a lack of well designed and reported studies on ITW that include a control or comparison group. Within this review, only two interventions have been reported, although there are a number of alternative interventions described within the literature. The reasons many studies explored the use of alternative interventions were not included. Home exercises or stretches were also included as standard care or an intervention; the trials provided no data on how these were introduced, what they consisted of, or if any functional measures were used to determine their effect. Similarly, the effect of the treatments on quality of life are unknown, and this may be considered in future trials.
Quality of the evidence
This review is limited by the small number of RCTs available. Four studies met the inclusion criteria, but only one study was suitable for data extraction. Its outcome measures and reported time frames required us to deviate from the published protocol. We downgraded the evidence to very‐low certainty, the reasons for this being indirectness (because for continuous outcomes the assumed risk was the control group), risk of bias (because participants and outcome assessors were not blinded to the allocation), and imprecision (because the optimal information size was not met and CI included effects that could lead to different clinical decisions).
Potential biases in the review process
We assessed Engstrom 2013, Herrin 2016, and Satila 2016 as being at low risk of bias for most domains other than blinding of outcome assessors and participants. We considered D'Apote 2012, which was published as an abstract, as having unclear or high risk of bias for all domains.
Bias may have been introduced at the protocol stage; this includes the decision‐making process when setting the boundaries of the inclusion and exclusion criteria, the outcome measures and time frames for extraction.
After the screening process, no study met our criteria for both outcome measures and time frame. We decided on a deviation from protocol. This deviation was that we changed the specified length of follow‐up time from six months to 12 months (see Differences between protocol and review); this did not affect the number of included articles. See Characteristics of excluded studies.
We emailed the authors of D'Apote 2012 and Satila 2016, but were not able to obtain additional data. These two articles reported the outcome measures and time frame outlined in the protocol. Recruitment for NCT00175669 was reported to be completed in 2011, according to the clinical trials registry entry. We have attempted to contact the trial authors but have not yet been able to identify an associated publication.
Agreements and disagreements with other studies or reviews
A systematic review by van Kuijk 2014 included 19 studies. The review did not have a registered protocol but investigated a similar question to this present review. It included non‐randomised study designs, whereas this Cochrane Review only included randomised control trials. van Kuijk 2014 did not include abstracts; therefore D'Apote 2012 was not included for analysis, whereas we did include that abstract in this Cochrane Review. Two of the studies included in the present review (Herrin 2016 and Satila 2016) have both been published since van Kuijk 2014 was published.
The protocol outlined within this Cochrane Review provided a framework for outcomes and timelines for extraction. Both reviews commented on passive range of motion as an outcome measure. The protocol for the present review stated that this should be calculated as the change from baseline and required individual participant data to be obtained.
Similarly to this Cochrane Review, van Kuijk 2014 also concluded there were limited evidence‐based treatments for ITW.
Another systematic review explores a similar research question, however it was published in Spanish and was not translated for this review (Gámez Iruela 2014). We believe there are no other systematic reviews with a similar research question.
Authors' conclusions
Implications for practice.
The treatment of restricted ankle dorsiflexion is a common method of intervention for children with an idiopathic toe walking (ITW) gait pattern. There is insufficient evidence from randomised controlled trials (RCTs) on the effect of interventions for ITW. One study provided outcome data on the effects of serial casting with or without botulinum toxin type A injection (BTX) prior to casting, however the evidence was too uncertain for any conclusions to be drawn. A further three studies reported outcomes relating to BTX, footwear, exercises, and different types of orthoses as interventions for ITW; however, outcome data were too limited to determine the effects of these interventions.
Implications for research.
The findings of this review indicate a need for future high‐quality RCTs of commonly used interventions for ITW. Future research in ITW should consider consistent and reliable outcome measures across both clinical and laboratory settings. Individual participant data should be reported or made available due to the variability in the presentation of this condition. In addition, follow‐up time frames with a minimum of six months are required to understand the long‐term impact of treatment.
Acknowledgements
The review authors would like to thank the editorial team of Cochrane Neuromuscular, particularly Ruth Brassington, and Angela Gunn for assisting in developing the search strategy. The review team would also like to thank Jan WH Custers for input into protocol development.
The review authors are grateful to the following peer reviewers for their time and comments: Paula Bray, Children's Hospital at Westmead, Sydney, Australia; Karl Landorf, La Trobe University, Melbourne, Australia; Tao Ding, University College London, UK.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS Web) search strategy
Search run on 29 April 2019
#1 toe or toes or "achilles tendon*" or "ankle joint*" AND INSEGMENT #2 tiptoe* or "tip toe*" AND INSEGMENT #3 #1 or #2 AND INSEGMENT #4 MeSH DESCRIPTOR Gait Disorders, Neurologic AND INSEGMENT #5 walking or gait or contracture or "equinus deformity" AND INSEGMENT #6 idiopathic or habitual or walk* or gait* or congenital or stiff* or equinus AND INSEGMENT #7 #4 or #5 or #6 AND INSEGMENT #8 #3 and #7 AND INSEGMENT #9 "toe walk*" AND ( habitual or idiopathic) AND INSEGMENT #10 #8 or #9 AND INSEGMENT #11 infan* or newborn* or "new born*" or baby* or babies or neonat* or perinat$ or postnat$ or child$ or schoolchild$ or kid or kids or toddler* AND INSEGMENT #12 adolesc* or teen* or boy* or girl* or minors* or underag* or "under ag*" or juvenil* or youth* AND INSEGMENT #13 puber* or pubescen* or prepubescen* or prepubert* or pediatric* or paediatric* or peadiatric* AND INSEGMENT #14 kindergar* or nursery or preschool* or "pre school*" or "primary school*" AND INSEGMENT #15 "elementary school*" or "secondary school*" or highschool* or "high school*" or schoolage* or "school age*" AND INSEGMENT #16 #11 or #12 or #13 or #14 or #15 AND INSEGMENT #17 #10 and #16 AND INSEGMENT #18 (#10 and #16) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Trials (CRS Web) search strategy
Search run on 29 April 2019 #1 toe or toes or "achilles tendon*" or "ankle joint*" AND CENTRAL:TARGET #2 tiptoe* or "tip toe*" AND CENTRAL:TARGET #3 #1 or #2 AND CENTRAL:TARGET #4 MeSH DESCRIPTOR Gait Disorders, Neurologic AND CENTRAL:TARGET #5 walking or gait or contracture or "equinus deformity" AND CENTRAL:TARGET #6 idiopathic or habitual or walk* or gait* or congenital or stiff* or equinus AND CENTRAL:TARGET #7 #4 or #5 or #6 AND CENTRAL:TARGET #8 #3 and #7 AND CENTRAL:TARGET #9 "toe walk*" AND ( habitual or idiopathic) AND CENTRAL:TARGET #10 #8 or #9 AND CENTRAL:TARGET #11 infan* or newborn* or "new born*" or baby* or babies or neonat* or perinat$ or postnat$ or child$ or schoolchild$ or kid or kids or toddler* AND CENTRAL:TARGET #12 adolesc* or teen* or boy* or girl* or minors* or underag* or "under ag*" or juvenil* or youth* AND CENTRAL:TARGET #13 puber* or pubescen* or prepubescen* or prepubert* or pediatric* or paediatric* or peadiatric* AND CENTRAL:TARGET #14 kindergar* or nursery or preschool* or "pre school*" or "primary school*" AND CENTRAL:TARGET #15 "elementary school*" or "secondary school*" or highschool* or "high school*" or schoolage* or "school age*" AND CENTRAL:TARGET #16 #11 or #12 or #13 or #14 or #15 AND CENTRAL:TARGET #17 #10 and #16 AND CENTRAL:TARGET #18 (#10 and #16) AND (CENTRAL:TARGET)
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to April 26, 2019> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (480941) 2 controlled clinical trial.pt. (93047) 3 randomized.ab. (441066) 4 placebo.ab. (197155) 5 drug therapy.fs. (2103249) 6 randomly.ab. (309676) 7 trial.ab. (461204) 8 groups.ab. (1905295) 9 or/1‐8 (4426560) 10 exp animals/ not humans.sh. (4574312) 11 9 not 10 (3828315) 12 toes/ (9914) 13 Achilles Tendon/ (7701) 14 Ankle Joint/ (14865) 15 (toe*1 or tiptoe*1 or tip toe*1 or achilles tendon* or ankle joint*).tw. (34284) 16 or/12‐15 (53027) 17 Walking/ (30113) 18 Gait/ (25646) 19 Gait Disorders, Neurologic/ (5968) 20 Contracture/ (7787) 21 Equinus Deformity/ (424) 22 congenital.fs. (73050) 23 (idiopathic or habitual or walk$ or gait$ or congenital or stiff$ or equinus).tw. (546459) 24 or/17‐23 (595946) 25 16 and 24 (10704) 26 toe walk*3.kh,ti. and (habitual or idiopathic).mp. (98) 27 or/25‐26 (10704) 28 (infan* or newborn*1 or new born*1 or baby* or babies or neonat* or perinat$ or postnat$ or child$ or schoolchild$ or kid or kids or toddler*).tw,sh. (3033890) 29 (adolesc* or teen* or boy* or girl* or minors* or underag* or under ag* or juvenil* or youth*).tw,sh. (2204744) 30 (puber* or pubescen* or prepubescen* or prepubert* or pediatric* or paediatric* or peadiatric*).tw. (361665) 31 (kindergar* or nursery or preschool* or pre school* or primary school*).tw,sh. (54115) 32 (elementary school* or secondary school* or highschool* or high school* or schoolage* or school age*).tw,sh. (65178) 33 or/28‐32 (4187312) 34 11 and 27 and 33 (562) 35 remove duplicates from 34 (561)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase Classic+Embase <1947 to 2019 April 26> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (59211) 2 double‐blind procedure.sh. (162202) 3 single‐blind procedure.sh. (34815) 4 randomized controlled trial.sh. (547905) 5 (random* or crossover* or cross over* or placebo* or (doubl* adj blind*) or allocat*).tw,ot. (1648249) 6 trial.ti. (275731) 7 or/1‐6 (1830848) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1877841) 9 animal/ or nonanimal/ or animal experiment/ (4243802) 10 9 not 8 (3543478) 11 7 not 10 (1681567) 12 limit 11 to (conference abstracts or embase) (1419541) 13 toe/ (14345) 14 achilles tendon/ (9911) 15 ankle/ (34648) 16 idiopathic toe walking/ (45) 17 (toe*1 or tiptoe*1 or tip toe*1).tw,kw. (30507) 18 (achilles tendon*1 or ankle joint*).tw. (20420) 19 or/13‐18 (83101) 20 walking/ (65743) 21 gait/ (51083) 22 neurologic disease/ (135577) 23 exp contracture/ (26178) 24 pes equinus/ (1569) 25 (idiopathic or habitual or walk* or gait* or congenital or stiff* or equinus).tw. (798025) 26 or/20‐25 (962862) 27 infant/ (663803) 28 exp infant/ (1101986) 29 exp newborn/ (590161) 30 exp school/ (371521) 31 juvenile/ (44569) 32 adolescent/ (1563747) 33 exp puberty/ (44993) 34 exp pediatrics/ (111056) 35 (infan* or newborn* or new born* or baby* or babies or neonat* or perinat* or postnat*).tw. (1069430) 36 (child* or kid or kids or toddler* or adoles* or teen* or boy* or girl* or minor* or underag* or under ag or juvenil* or youth*).tw. (2570249) 37 (puber* or pubescen* or prepubescen* prepubert* or pediatric* paediatric* or peadiatric*).tw. (55209) 38 (schools or nursery school* or preschool* or pre school* or primary school* or secondary school* or elementary school* or high school* or highschool* or school age*1 or schoolage* or school age*).tw. (197227) 39 or/27‐38 (4847810) 40 12 and 19 and 26 and 39 (232) 41 remove duplicates from 40 (228)
Appendix 5. CINAHL Plus (EBSCOhost) search strategy
Monday, April 29, 2019 7:53:47 AM S38 S36 AND S37 15 S37 EM 20180301‐ 511,869 S36 S18 AND S28 AND S34 Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase 68 S35 S18 AND S28 AND S34 342 S34 S29 OR S30 OR S31 OR S32 OR S33 1,076,658 S33 "elementary school*" or "secondary school*" or highschool* or "high school*" or schoolage* or "school age*" 35,683 S32 kindergar* or nursery or preschool* or "pre school*" or primary school* 191,640 S31 puber* or pubescen* or prepubescen* or prepubert* or pediatric* or paediatric* or peadiatric* 163,702 S30 adolesc* or teen* or boy* or girl* or minors* or underag* or "under ag*" or juvenil* or youth* 525,893 S29 infan* or "newborn*1" or "new born*1" or baby* or babies or neonat* or perinat$ or postnat$ or child$ or schoolchild$ or kid or kids or toddler* 765,143 S28 S26 OR S27 3,222 S27 ( TI "toe walk*" OR AB "toe walk*" ) AND ( TI (habitual or idiopathic) or AB (habitual or idiopathic) ) 70 S26 S21 AND S25 3,222 S25 S22 OR S23 OR S24 116,486 S24 TI ( idiopathic or habitual or walk* or gait* or congenital or stiff* or equinus ) OR AB ( idiopathic or habitual or walk* or gait* or congenital or stiff* or equinus ) 91,469 S23 walking or gait or contracture or "equinus deformity" 51,882 S22 (MH "Gait Disorders, Neurologic") 1,967 S21 S19 OR S20 14,702 S20 tiptoe* or "tip toe*" 83 S19 toe or toes or "achilles tendon*" or "ankle joint*" 14,661 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 1,218,982 S17 ABAB design* 119 S16 TI random* or AB random* 285,896 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 570,565 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 221,048 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 80,944 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 42,928 S11 PT ("clinical trial" or "systematic review") 165,886 S10 (MH "Factorial Design") 1,120 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 381,302 S8 (MH "Meta Analysis") 37,808 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 97 S6 (MH "Quasi‐Experimental Studies") 10,803 S5 (MH "Placebos") 11,210 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 41,200 S3 (MH "Clinical Trials+") 258,272 S2 (MH "Crossover Design") 17,442 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 91,098
Appendix 6. Web of Science meetings search strategy
TOPIC: (idiopathic toe walking) Refined by: DOCUMENT TYPES: ( MEETING ABSTRACT OR PROCEEDINGS PAPER ) Timespan: All years. Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.
Appendix 7. Open Grey search strategy
idiopathic toe walking
Appendix 8. ClinicalTrials.gov search strategy
Advanced search
Condition: idiopathic toe walking
Intervention:
Recruitment status: select ALL
Appendix 9. ICTRP search strategy
Advanced search
Condition: idiopathic toe walking
Intervention:
Recruitment status: select ALL
Appendix 10. Methods described in the protocol
In the protocol we described the following methods which, in the absence of meta‐analysis or suitable data, were not applicable.
Measures of treatment effect
Continuous outcomes: changes in amount of toe walking to heel‐toe gait for participants will comprise the main outcomes. If the same measurement instrument or scale is used across studies, we will use the mean difference (MD) to pool and compare post‐treatment mean scores, with the weight given to each study determined by the precision of the estimate of effect. If different instruments or scales are used across studies, we will calculate the standardised mean difference (SMD) with a 95% confidence interval (CI) to pool and compare post‐treatment mean scores.
As a rule of thumb for the interpretation of Cohen’s SMD, we will use the criteria discussed in Higgins 2011 in the Cochrane Handbook for Systematic Reviews of Interventions:
< 0.40: small effect;
0.40 to 0.70: moderate effect; and
> 0.70: large effect.
For dichotomous outcomes, we will calculate a risk ratio (RR) with a 95% CI.
Unit of analysis issues
We will include cluster‐randomised trials only when the intervention and control groups are totally comparable except for location and recruitment.
Where studies report multiple interventions, we will report only the interventions eligible for inclusion in the review. If a trial includes three groups that could be included in the same meta‐analysis, we will combine groups for a 2‐way analysis if combining groups makes clinical sense, or halve the comparison group.
Assessment of heterogeneity
We will assess heterogeneity by visual inspection of the forest plots, and the I² statistic. We will define significant heterogeneity as I² > 50% because this may represent substantial or considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If there are more than 10 trials in a single analysis, we will create funnel plots to assess publication bias; we will interpret this with caution.
Data synthesis
If meta‐analysis is possible, we will use a fixed‐effect analysis and perform a sensitivity analysis using a random‐effects model if there is evidence of significant statistical heterogeneity. When a meta‐analysis cannot be performed, we will describe the effects of interventions narratively in a qualitative synthesis.
Subgroup analysis and investigation of heterogeneity
Where possible, we will conduct subgroup analyses for the severity of contracture (range of joint motion of dorsiflexion of the ankle joint of more than 10°, 0° to 10°, and less than 0°). We will use ITW gait pattern as the outcome measure for subgroup analyses and use the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We will consider sensitivity analyses during the review process, and if required we will:
repeat the analysis, excluding unpublished studies (if there are any);
repeat the analysis, excluding studies at high risk of bias;
repeat the analysis if there is one or more very large study/studies, excluding them to look at how much they dominate the results;
repeat the analysis to assess the effects of imputing missing data.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
D'Apote 2012.
| Methods | Randomised, controlled, cross‐over trial | |
| Participants |
Number of participants: planned enrolment of 15 in each group. At the time of reporting, 9 children (sex unknown) were included in the intervention group. Baseline characteristics Average age: 7.6 years Participant inclusion criteria: not stated Participant exclusion criteria: not stated |
|
| Interventions | Group A: botulinum toxin A (6 U/kg for muscle) bilaterally in gastrocnemius + AFO + exercise Group B: botulinum toxin A |
|
| Outcomes |
Primary outcome Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking)
Secondary outcomes Passive ankle dorsiflexion
|
|
| Conflicts of interest | Not reported | |
| Funding | Not reported | |
| Notes | Location: Italy (assumed) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The article states it is a cross‐over RCT however there is nothing that states how the participants are randomised or how treatments were allocated. |
| Allocation concealment (selection bias) | Unclear risk | The article does not state the method of allocation concealment, therefore we cannot place a judgement on this. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The article does not state if any blinding was applied to either participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The article does not state if any blinding was applied. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The article reports data for all participants enrolled in the study at the time of publication with reference to further participants being recruited. |
| Selective reporting (reporting bias) | High risk | No data were available for extraction. |
| Other bias | Unclear risk | The article reported interim outcome data from an incomplete study. |
Engstrom 2013.
| Methods | Randomised controlled, parallel‐group trial | |
| Participants |
Number of participants: 47 Baseline characteristics Casting group
Casting + botulinum toxin A group
Overall
Participant inclusion criteria: parents’ perception, he/she walked at least 25% of the time on his/her toes and had done so for at least 3 months. No other explanation for the toe walking. No known congenital Achilles tendon contracture Participant exclusion criteria: previous treatment for idiopathic toe walking such as Achilles tendon surgery, casts, orthotics, and injection of botulinum toxin A. Plantar flexion contracture beyond 10° Pretreatment: all children were randomised to 4 weeks of treatment with below‐the‐knee walking casts either as the sole treatment (CA group) or following botulinum toxin‐A injection into the calf muscles (CA1BX group). There were no differences between groups with regard to age or sex distribution. |
|
| Interventions | Casting Casting + botulinum toxin A 12 U/kg body weight of Botox (Allergan, Irvine, California): 4 injections in each calf (2 in the proximal third of the lateral and medial gastrocnemius bellies and 2 distally in the gastrocnemius‐soleus complex). Injections performed with electromyogram amplifier guidance to ensure intramuscular position |
|
| Outcomes |
Primary outcome Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking)
Secondary outcomes Passive ankle dorsiflexion
Adverse effects
|
|
| Conflicts of interest | Nil declared | |
| Funding | Centre of Competence Blekinge County Council, Promobilia Foundation, Samariten Foundation, and Society for Childcare. The funding sources did not play a role in the investigation. | |
| Notes |
Location: Sweden Study recruited between 2005 and 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in randomly permuted blocks of four. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done with the use of sealed envelopes with randomly permuted blocks of four. A medical statistician prepared the envelopes and a nurse performed the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded at initial assessment; however, participants were not blinded to treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors at baseline were blinded to group allocation. Some children and parents revealed the intervention group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition from study. The study recruited 47 participants, with data obtained for most outcomes of interest from 45 participants. One participant dropped out. |
| Selective reporting (reporting bias) | Low risk | No study protocol was available, but the trial report lists the outcomes of interest in both the methods and the results section. All outcomes were reported or data were available from the author group. |
| Other bias | Low risk | Groups were similar at baseline; there were no cointerventions. |
Herrin 2016.
| Methods | Randomised, parallel‐group, controlled trial | |
| Participants |
Number of participants: 18 Baseline characteristics Ankle‐foot orthoses group
Foot orthoses group
Overall
Participant inclusion criteria: diagnosis of idiopathic toe walking, aged between 2 and 8 years, determined by physician as a candidate for orthotic intervention Participant exclusion criteria: diagnosis of any neurological conditions, previous treatment for Achilles tendon contractures (including serial casting and surgery), or presence of ankle equinus which will limit the ankle achieving 90º of ankle angle alignment. |
|
| Interventions | Ankle‐foot orthoses: polypropylene AFO with 90º plantarflexion stop, free dorsiflexion and full length toe plate Foot orthoses: custom foot orthoses attached to carbon fibre foot plates |
|
| Outcomes |
Primary outcome Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking)
|
|
| Conflicts of interest | No conflicts declared First author affiliated with a company providing orthoses services |
|
| Funding | Funding was obtained from the Orthotic and Prosthetic Education and Research Foundation (OPERF‐2011‐SGA‐1). The funding agency played no role in the study. | |
| Notes | Location: Georgia, USA: children with ITW were recruited from area physical therapists and physicians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was undertaken with a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of personnel or participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding of outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but the trial report lists the outcomes of interest in both the methods and the results section. Outcomes of interest are reported incompletely and therefore could not be entered into meta analysis. |
| Other bias | Low risk | No other bias was identified. |
Satila 2016.
| Methods | Randomised, parallel‐group, controlled trial | |
| Participants |
Number of participants: 30 Baseline characteristics Conservative treatment group
Botulinum toxin A group
Overall
Participant inclusion criteria: children who had toe walked for at least 6 months, were graded on a severity scale, between the ages of 2 and 10 years with no medical condition known to cause or be associated with toe walking gait. Children were required to have passive ankle dorsiflexion with an extended knee to 0 degrees or more. Participant exclusion criteria: previous treatment for idiopathic toe walking such as Achilles tendon surgery, casts, or injection of botulinum toxin A |
|
| Interventions | Conservative treatment: indoor shoes with firm heel cup and straps; night splints of soft cast worn a minimum of 5 nights per week; home strengthening program performed 5 days per week for a minimum of 10 minutes per day Convervative + botulinum toxin type A (BTX‐A): all conservative treatment interventions plus BTX‐A injections into gastrocnemius‐soleus complex, repeated at 6 month intervals if needed. BTX dosage of 3 U/kg per head of the gastrocnemius muscle and 2 U/kg into soleus while the child was under light sedation. |
|
| Outcomes |
Primary outcome Improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking)
Secondary outcomes Active ankle ROM
Passive ankle ROM
Adverse effects
|
|
| Conflicts of interest | No conflicts declared | |
| Funding | Medical research funds of Tampere University Hospital, Kanta‐Hamp and Paijat‐Ham Hospitals, and the ARVO and Lea Yippo Foundation, Finland | |
| Notes | Location: Finland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with the use of sealed envelopes prepared by a statistician, with randomly permuted blocks of 4. Grading on baseline assessment was taken into account in the randomisation process. |
| Allocation concealment (selection bias) | High risk | No concealment was documented. Younger children (2 to 6 years) versus older children (7 to 10 years) were enrolled in separate categories; children were subsequently paired on toe‐walking severity. Participant numbers were low, and sub‐grouping was considered to reduce concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition was reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but the trial report lists the outcomes of interest in both the methods and the results section. Outcomes of interest were not reported by treatment group. |
| Other bias | Low risk | There was no other bias. |
AFO: ankle‐foot orthosis EMG: electromyography ITW: idiopathic toe walking RCT: randomised controlled trial ROM: range of motion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2007 | Case study |
| Benedetti 2013 | Cohort study |
| Brouwer 2000 | Cohort study |
| Brunt 2004 | Cohort study |
| Eiff 2006 | Narrative literature review |
| Fanchiang 2014 | PhD thesis with case study |
| Gámez Iruela 2014 | Systematic review |
| Jadhav 2017 | Retrospective chart audit |
| Jahn 2009 | Non‐randomised pre‐post study |
| Michalitsis 2019 | Within‐subject randomised controlled trial |
| Thielemann 2019 | Non‐randomised pre‐post study with non‐ITW concurrent control group |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00175669.
| Methods | Randomized, parallel‐assignment, placebo‐controlled, double‐blind (participant, investigator) |
| Participants | Children with idiopathic toe walking (planned recruitment 32 participants), aged 5 to 15 years old Diagnostic criteria: toe walkers according to gait laboratory's definition (loss of first ankle rocker, early heel rise (prior to 30% of the cycle) and A1 kinematic data) Co‐operative with gait analysis and injection |
| Interventions |
Intervention group: botulinum A toxin injection into the gastrocnemius muscle and casting Control group: placebo and casting |
| Outcomes |
Primary outcome Gait analysis data ‐ specifically whether post‐injection gait analyses show a reduction in the features that were used to determine the severity of the subjects' toe walking problem Secondary outcome Ankle range of motion (dorsiflexion and plantarflexion) at every visit |
| Notes | Start date 2007, completion date November 2009. No results posted on clinicaltrials.gov |
Characteristics of ongoing studies [ordered by study ID]
RBR‐7QNFFG.
| Trial name or title | The effects of muscle strengthening of the plantar flexors on the gait pattern of children with idiopathic toe walking. |
| Methods | Randomized, blinded, controlled, two‐arm parallel‐group trial |
| Participants | Planned 30 participants with idiopathic toe walking, 5 years to 11 years old Exclusions: "structural ankle deformity in equine (inability to reach the ankle neutral position passively); with central or peripheral neurological impairment; who are unable to minimally understand the questions used during the evaluations; which have undergone surgery in the lower limbs or who have undergone the application of botulinum toxin in the last 12 months" |
| Interventions |
Intervention group: same protocol of conservative treatment, with the addition of the triceps sural muscle strengthening exercise. The protocol will also have a total duration of 8 weeks and will be applied twice a week. The duration of a single session will be approximately 1 hour and 15 minutes (N = 15 (planned)) Control group: conservative treatment (stretching exercises of the triceps muscle, muscle strengthening of the anterior tibial, sensory‐motor and gait phases training). Duration 8 weeks, applied twice a week. The duration of a single session will be approximately 1 hour (N = 15 (planned)) |
| Outcomes |
Primary outcome Active range of motion of ankle dorsiflexion, assessed by a two‐dimensional kinematic analysis of the sagittal plane. The kinematic evaluation will be performed using a video recording in the sagittal plane of the lower limbs. The most affected side (with a lower amplitude of dorsiflexion) will be used to compare the groups. Two‐dimensional kinematics will be performed at baseline and at the end of treatment (8 weeks). "A mean difference of 6.29 degrees of the amplitude of ankle dorsiflexion will be taken as the basis for establishing clinical significance. Such a difference is expected between the beginning and the end of the treatment and between groups, to affirm that the intervention group was superior to the control group." Secondary outcomes Static and dynamic balance, measured by the KTK test (baseline and at the end of treatment (8 weeks)) Passive range of motion, measured by the lunge‐test (baseline and at the end of treatment (8 weeks)) Ped4.0 quality of life questionnaire (baseline, at the end of treatment (8 weeks) and 24 weeks after the baseline (follow‐up)) |
| Starting date | Date of first enrolment: 2 February 2018 |
| Contact information | Vanessa Gonçalves Coutinho de Oliveira vanessagcoliveira@gmail.com Universidade Federal de São Paulo, Brazil |
| Notes | Primary sponsor: Universidade Federal de São Paulo Secondary sponsor: Instituto de Assistência ao Servidor Público Estadual de São Paulo UTN number: U1111‐1200‐9696 |
Differences between protocol and review
The wording of the primary outcome was changed from "improvement (majority or greater than 50%) of time spent toe walking, measured by either quantifiable judgement from the child, parent, or healthcare professional, or by kinetic or kinematic gait analysis. Time frame: six months" to "improvement in toe walking (defined as greater than 50% of time spent heel‐toe walking), preferably measured as the proportion of participants with improvement based on quantifiable judgements by the child, parent, or healthcare professional, or by kinematic or gait analysis. Time frame: six months." This change in wording did not alter the intent of the extraction.
We included Engstrom 2013, which reported outcomes at three and 12 months. We contacted the author and obtained individual participant data. This enabled calculation of the change from baseline at 12 months. We determined three months was not long enough for a true representation of treatment effect, but included the 12‐month data, which was different from our specified six‐month time point.
Meta‐analysis was not possible; additional methods related to meta‐analysis are in Appendix 10. We clarified methods for dealing with unit‐of‐analysis issues in multi‐arm trials, should there be any such trials in future updates.
We provided additional detail on the GRADE assessment process in the Data synthesis section.
We acquired raw data from study authors in SPSS formatting. Therefore, we calculated mean differences and risk ratios in SPSS (IMB Corp. 2013).
The protocol stated that the participant rather than the foot would be the unit of analysis. However, in conditions which are bilateral, there is likely a correlation between the outcomes of each limb. This has been demonstrated in other trials in which interventions were applied to both feet. “The independence assumption is one of these, and it would therefore seem prudent to advise against the pooling of limb data in most situations and to consider the unit of analysis to be people rather than limbs” (Menz 2004). Given ITW is considered a bilateral condition (Sala 1999), the review authors concluded that only data that could satisfy the assumption for independence would be included (Sutton 1997).
In future versions of this review we will aim to analyse studies of BTX + conservative treatments versus conservative treatments in a single analysis, if the data allow. If there are various conservative treatment subgroups we will endeavour to run their analysis by type.
We planned to search the metaRegister of Controlled Trials (mRCT, controlled‐trials.com/mrct) but it was no longer available.
Contributions of authors
Antoni Caserta screened titles and abstracts for eligibility, carried out data extraction, assessed the risk of bias of included trials, and analysed and interpreted data. He critically reviewed the initial manuscript for intellectual content (with a focus on paediatric podiatry), drafted the initial manuscript, and approved the final manuscript.
Verity Pacey analysed and interpreted data. She was involved in critically reviewing the initial manuscript for intellectual content (with a focus on paediatric physiotherapy), and approved the final manuscript.
Michael Fahey screened titles and abstracts for eligibility. He assisted in drafting the manuscript, critically reviewing the manuscript for intellectual content (with focus on paediatric neurology), and approved the final manuscript.
Kelly Gray assessed the risk of bias of the included trials and carried out data extraction. She critically reviewed the manuscript, and approved the final manuscript for review.
Raoul HH Engelbert was involved in conceptualisation. He assessed the risk of bias of the studies and critically reviewed the initial manuscript for intellectual content (with a focus on paediatric physiotherapy), assessed the risk of bias, and approved the final manuscript.
Cylie M Williams assisted in the selection of studies included within the review when Antoni Caserta and Michael Fahey did not reach consensus. She assisted in carrying out data extraction and assisted in assessing risk of bias when Kelly Gray and Raoul HH Engelbert did not reach consensus. She was involved in critically reviewing the manuscript and approving the final manuscript for review.
Declarations of interest
Cylie M Williams: has received funding from the NHMRC and other competitive funding bodies to undertake research and provide professional education about idiopathic toe walking. She has also published research relating to idiopathic toe walking which may feature within systematic reviews.
Verity Pacey: none known.
Antoni J Caserta: none known.
Kelly Gray: none known.
Raoul HH Engelbert: none known.
Michael Fahey: has served on a scientific advisory board and as a consultant for and received funding for travel from Actelion Pharmaceuticals Ltd and UCB, receives research support from NHMRC and the NIH (1R03HD058625‐01, CI); has held/holds stock in Sigma Pharmaceuticals; and has given expert testimony on behalf of the Therapeutic Goods Administration.
Edited (no change to conclusions)
References
References to studies included in this review
D'Apote 2012 {published data only}
- D’Apote G, Volini S, Fusaro I, Benedetti MG. Effects of focal treatment with botulinum toxin on the “idiopathic toe walking”: clinical and instrumental assessment. Gait & Posture 2012;35 Suppl 1:S6. [Google Scholar]
Engstrom 2013 {published data only}
- Engstrom P, Bartonek A, Tedroff K, Haglund‐Akerlind Y, Orefelt C, Gutierrez‐Farewik EM. Botulinum toxin a does not improve cast treatment for idiopathic toe‐walking ‐ a prospective randomized trial. Gait & Posture 2013;38:S2‐S3. [EMBASE: 71274290] [DOI] [PubMed] [Google Scholar]
- Engstrom P, Bartonek A, Tedroff K, Orefelt C, Haglund‐Akerlind Y, Gutierrez‐Farewik EM. Botulinum toxin A does not improve the results of cast treatment for idiopathic toe‐walking: a randomized controlled trial. Journal of Bone and Joint Surgery. American Volume 2013;95(5):400‐7. [DOI: 10.2106/JBJS.L.00889; PUBMED: 23467862] [DOI] [PubMed] [Google Scholar]
- NCT01590693. Botulinum toxin A for idiopathic toe‐walking [Botulinum toxin A does not improve cast treatment for idiopathic toe‐walking‐ a prospective randomized trial]. clinicaltrials.gov/show/NCT01590693 (first received 3 May 2012).
Herrin 2016 {published data only}
- Herrin, K, Geil, M. A comparison of orthoses in the treatment of idiopathic toe walking: a randomized controlled trial. Prosthetics and Orthotics International 2016;40(2):262‐9. [PUBMED: 25628380] [DOI] [PubMed] [Google Scholar]
Satila 2016 {published data only}
- Satila H, Beilmann A, Olsen P, Helander H, Eskelinen M, Huhtala H. Does botulinum toxin A treatment enhance the walking pattern in idiopathic toe‐walking?. Neuropediatrics 2016;47(3):162‐8. [PUBMED: 27089542] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2007 {published data only}
- Alvarez C, Vera M, Beauchamp R, Ward V, Black A. Classification of idiopathic toe walking based on gait analysis: development and application of the ITW severity classification. Gait & Posture 2007;26(3):428‐35. [DOI] [PubMed] [Google Scholar]
Benedetti 2013 {published data only}
- Benedetti MG, Fusaro I, Volini S, Gasparre G, Berti L. Clinical and instrumental evaluation of botulinum toxin effects on the "idiopathic toe walking": a pilot study. Gait & Posture 2013;38:S60‐1. [DOI: ] [Google Scholar]
Brouwer 2000 {published data only}
- Brouwer B, Davidson LK, Olney SJ. Serial casting in idiopathic toe‐walkers and children with spastic cerebral palsy. Journal of Pediatric Orthopedics 2000;20(2):221‐5. [PubMed] [Google Scholar]
Brunt 2004 {published data only}
- Brunt D, Woo R, Kim HD, Ko MS, Senesac C, Li S. Effect of botulinum toxin type A on gait of children who are idiopathic toe‐walkers. Journal of Surgical Orthopaedic Advances 2004;13(3):149‐55. [PubMed] [Google Scholar]
Eiff 2006 {published data only}
- Eiff MP Steiner E, Judkins DZ. What is the appropriate evaluation and treatment of children who are "toe walkers"?. Journal of Family Practice 2006;55(5):447‐50. [PubMed] [Google Scholar]
Fanchiang 2014 {published data only}
- Fanchiang HD. The effects of walking surface and vibration on the gait pattern and vibration perception threshold of typically developing children and children with idiopathic toe walking [Dissertation]. https://scholarworks.gsu.edu/kin_health_diss/11/. Atlanta, GA (USA): Georgia State University, 2014.
- Fanchiang HD, Geil MD, Wu J, Ajisafe T, Chen YP. The effects of walking surface on the gait pattern of children with idiopathic toe walking. Journal of Child Neurology 2016;31(7):858‐63. [PUBMED: 26733505] [DOI] [PubMed] [Google Scholar]
Gámez Iruela 2014 {published data only}
- Gámez‐Iruela J, Sedeño‐Vidal A, Fernández‐Herrera D. Effectiveness of the treatment in the approach to idiopathic toe walking. A systematic review [Efectividad del tratamiento en el abordaje de la marcha de puntillas idiopática]. Fisioterapia 2014;37(1):35‐40. [DOI: 10.1016/j.ft.2014.04.002] [DOI] [Google Scholar]
Jadhav 2017 {published data only}
- Jadhav S, Deshpande S, Gormley M. Treatment of paediatric idiopathic toe walking with and without botulinum toxin and ankle foot orthosis. Developmental Medicine and Child Neurology 2017;59(Suppl 3):81. [Google Scholar]
Jahn 2009 {published data only}
- Jahn J, Vasavada AN, McMulkin ML. Calf muscle‐tendon lengths before and after Tendo‐Achilles lengthenings and gastrocnemius lengthenings for equinus in cerebral palsy and idiopathic toe walking. Gait & Posture 2009;29(4):612‐7. [DOI] [PubMed] [Google Scholar]
Michalitsis 2019 {published data only}
- ACTRN12612000975897. Do external stimuli impact the gait of children with idiopathic toe walking? A study protocol for a within‐subject randomised control trial. http://www.anzctr.org.au/ACTRN12612000975897.aspx (first received 10 September 2012). [DOI] [PMC free article] [PubMed]
- Michalitsis J, Murphy AT, Rawicki B, Haines TP, Williams C. Full length foot orthoses have an immediate treatment effect and modify gait of children with idiopathic toe walking. Gait & Posture 2019;68:227‐31. [DOI] [PubMed] [Google Scholar]
- Murphy A, Williams C, Michalitsis J. The utilisation of full length orthotics to improve gait in children with idiopathic toe walking. Gait & Posture 2017;57(Suppl 1):221‐2. [Google Scholar]
Thielemann 2019 {published data only}
- Thielemann F, Rockstroh G, Mehrholz J, Druschel C. Serial ankle casts for patients with idiopathic toe walking: effects on functional gait parameters. Journal of Children's Orthopaedics 2019;13(2):147‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00175669 {published and unpublished data}
- NCT00175669. Trial of a botulinum A toxin (Botox) injection in the gastrocnemius muscle in children with idiopathic toe walking [Randomized controlled trial of a botulinum A toxin (Botox) injection in the gastrocnemius muscle in children with idiopathic toe walking]. clinicaltrials.gov/show/NCT00175669 (first received 15 September 2005).
References to ongoing studies
RBR‐7QNFFG {unpublished data only}
- RBR‐7QNFFG. The effects of muscle strengthening of the plantar flexors on the gait pattern of children with idiopathic toe walking [Efeito do fortalecimento dos músculos flexores plantares na marcha de crianças com pé equino idiopático: ensaio clínico controlado randomizado]. http://ensaiosclinicos.gov.br/rg/RBR‐7qnffg/ (first received 17 August 2017).
Additional references
Accardo 1989
- Accardo P, Whitman B. Toe walking. A marker for language disorders in the developmentally disabled. Clinical Pediatrics 1989;28(8):347‐50. [PUBMED: 2474401] [DOI] [PubMed] [Google Scholar]
Accardo 1992
- Accardo P, Morrow J, Heaney MS, Whitman B, Tomazic T. Toe walking and language development. Clinical Pediatrics 1992;31(3):158‐60. [PUBMED: 1547588] [DOI] [PubMed] [Google Scholar]
Ade‐Hall 2000
- Ade‐Hall RA, Moore AP. Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001408; PUBMED: 10796784] [DOI] [PubMed] [Google Scholar]
Armand 2006
- Armand S, Watelain E, Mercier M, Lensel G, Lepoutre FX. Identification and classification of toe‐walkers based on ankle kinematics, using a data‐mining method. Gait & Posture 2006;23(2):240‐8. [PUBMED: 16399521] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrow 2011
- Barrow WJ, Jaworski M, Accardo PJ. Persistent toe walking in autism. Journal of Child Neurology 2011;26(5):619‐21. [PUBMED: 21285033] [DOI] [PubMed] [Google Scholar]
Bernhard 2005
- Bernhard MK, Töpfer M, Vogler L, Merkenschlager A. Prevalence of toe‐walking in childhood. Neuropediatrics 2005;36(02):P116. [Google Scholar]
Caselli 1988
- Caselli MA, Rzonca EC, Lue BY. Habitual toe‐walking: evaluation and approach to treatment. Clinics in Podiatric Medicine and Surgery 1988;5(3):547‐59. [PUBMED: 3293753] [PubMed] [Google Scholar]
Caselli 2002
- Caselli MA. Habitual toe walking: learn to evaluate and treat this idiopathic childhood condition. Podiatry Management 2002;21(9):163‐74. [Google Scholar]
Clark 2010
- Clark E, Sweeney JK, Yocum A, McCoy SW. Effects of motor control intervention for children with idiopathic toe walking: a 5‐case series. Pediatric Physical Therapy 2010;22(4):417‐26. [PUBMED: 21068642] [DOI] [PubMed] [Google Scholar]
Conrad 1980
- Conrad L, Bleck EE. Augmented auditory feed back in the treatment of equinus gait in children. Developmental Medicine and Child Neurology 1980;22(6):713‐8. [PUBMED: 7450297] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, accessed before August 2019.
Crenna 2005
- Crenna P, Fedrizzi E, Andreucci E, Frigo C, Bono R. The heel‐contact gait pattern of habitual toe walkers. Gait & Posture 2005;21(3):311‐7. [PUBMED: 15760747] [DOI] [PubMed] [Google Scholar]
Eastwood 1997
- Eastwood DM, Dennett X, Shield LK, Dickens DR. Muscle abnormalities in idiopathic toe‐walkers. Journal of Pediatric Orthopedics. Part B 1997;6(3):215‐8. [PUBMED: 9260653] [DOI] [PubMed] [Google Scholar]
Eastwood 2000
- Eastwood DM, Menelaus MB, Dickens DR, Broughton NS, Cole WG. Idiopathic toe‐walking: does treatment alter the natural history?. Journal of Pediatric Orthopedics. Part B 2000;9(1):47‐9. [PUBMED: 10647110] [DOI] [PubMed] [Google Scholar]
Engelbert 1999
- Engelbert RH, Empelen R, Scheurer ND, Helders PJ, Nieuwenhuizen O. Influence of infant‐walkers on motor development: mimicking spastic diplegia?. European Journal of Paediatric Neurology 1999;3(6):273‐5. [PUBMED: 10595672] [DOI] [PubMed] [Google Scholar]
Engelbert 2011
- Engelbert R, Gorter JW, Uiterwaal C, Putte E, Helders P. Idiopathic toe‐walking in children, adolescents and young adults: a matter of local or generalised stiffness?. BMC Musculoskeletal Disorders 2011;12:61. [PUBMED: 21418634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engström 2010
- Engström P, Gutierrez‐Farewik EM, Bartonek A, Tedroff K, Orefelt C, Haglund‐Åkerlind Y. Does botulinum toxin A improve the walking pattern in children with idiopathic toe‐walking?. Journal of Children's Orthopaedics 2010;4(4):301‐8. [PUBMED: 21804891] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engström 2012
- Engström P, Tedroff K. The prevalence and course of idiopathic toe‐walking in 5‐year‐old children. Pediatrics 2012;130(2):279‐84. [PUBMED: 22826572] [DOI] [PubMed] [Google Scholar]
Engström 2013
- Engström P, Bartonek A, Tedroff K, Orefelt C, Haglund‐Åkerlind Y, Gutierrez‐Farewik EM. Botulinum toxin A does not improve the results of cast treatment for idiopathic toe‐walking. Journal of Bone and Joint Surgery 2013;95(5):400‐7. [PUBMED: 23467862] [DOI] [PubMed] [Google Scholar]
Fanchiang 2016
- Fanchiang HD, Geil MD, Wu J, Ajisafe T, Chen Y. The effects of walking surface on the gait pattern of children with idiopathic toe walking. Journal of Child Neurology 2016;31(7):858‐63. [DOI] [PubMed] [Google Scholar]
Fox 2006
- Fox A, Deakin S, Pettigrew G, Paton R. Serial casting in the treatment of idiopathic toe‐walkers and review of the literature. Acta Orthopaedica Belgica 2006;72(6):722‐30. [PUBMED: 17260610] [PubMed] [Google Scholar]
Gormley 1997
- Gormley ME, Herring GM, Gaebler‐Spira DJ. The use of botulinum toxin in children: a retrospective study of adverse reactions and treatment of idiopathic toe‐walking. European Journal of Neurology 1997;4 Suppl 2:827‐30. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 1 September 2016.
Griffin 1977
- Griffin PP, Wheelhouse WW, Shiavi R, Bass W. Habitual toe‐walkers. A clinical and electromyographic gait analysis. The Journal of Bone and Joint Surgery. American Volume 1977;59(1):97‐101. [PUBMED: 833184] [PubMed] [Google Scholar]
Gámez‐Iruela 2015
- Gámez‐Iruela J, Sedeño‐Vidal A, Fernández‐Herrera D. Effectiveness of the treatment in the approach to idiopathic toe walking. A systematic review [Efectividad del tratamiento en el abordaje de la marcha de puntillas idiopática. Revisión sistemática]. Fisioterapia 2015;37(1):35‐40. [DOI: ] [Google Scholar]
Hall 1967
- Hall JE, Salter RB, Bhalla SK. Congenital short tendo calcaneus. Journal of Bone and Joint Surgery. British Volume 1967;49(4):695‐7. [PUBMED: 6073187] [PubMed] [Google Scholar]
Hallemans 2003
- Hallemans A, D'Août K, Clercq D, Aerts P. Pressure distribution patterns under the feet of new walkers: the first two months of independent walking. Foot & Ankle International 2003;24(5):444‐53. [PUBMED: 12801204] [DOI] [PubMed] [Google Scholar]
Hemo 2006
- Hemo Y, Macdessi SJ, Pierce RA, Aiona MD, Sussman MD. Outcome of patients after Achilles tendon lengthening for treatment of idiopathic toe walking. Journal of Pediatric Orthopedics 2006;26(3):336‐40. [PUBMED: 16670545] [DOI] [PubMed] [Google Scholar]
Hicks 1988
- Hicks R, Durinick N, Gage JR. Differentiation of idiopathic toe‐walking and cerebral palsy. Journal of Pediatric Orthopedics 1988;8(2):160‐3. [PUBMED: 3350949] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hirsch 2004
- Hirsch G, Wagner B. The natural history of idiopathic toe‐walking: a long‐term follow‐up of fourteen conservatively treated children. Acta Paediatrica 2004;93(2):196‐9. [PUBMED: 15046273] [DOI] [PubMed] [Google Scholar]
Hyde 2000
- Hyde SA, FlŁytrup I, Glent S, Kroksmark AK, Salling B, Steffensen BF, et al. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscular Disorders 2000;10(4‐5):257‐63. [PUBMED: 10838252] [DOI] [PubMed] [Google Scholar]
IMB Corp. 2013 [Computer program]
- IBM Corp. IBM SPSS statistics for Windows. Version 22.0. Armonk, NY: IBM Corp, 2013.
Kalen 1986
- Kalen V, Adler N, Bleck EE. Electromyography of idiopathic toe walking. Journal of Pediatric Orthopedics 1986;6(1):31‐3. [PUBMED: 3941176] [DOI] [PubMed] [Google Scholar]
Katalinic 2010
- Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007455.pub2; PUBMED: 20824861] [DOI] [PubMed] [Google Scholar]
Katz 1984
- Katz MM, Mubarak SJ. Hereditary tendo Achillis contractures. Journal of Pediatric Orthopedics 1984;4(6):711‐4. [PUBMED: 6511899] [DOI] [PubMed] [Google Scholar]
Kogan 2001
- Kogan M, Smith J. Simplified approach to idiopathic toe‐walking. Journal of Pediatric Orthopedics 2001;21(6):790‐1. [PUBMED: 11675556] [PubMed] [Google Scholar]
McMulkin 2006
- McMulkin ML, Baird GO, Caskey PM, Ferguson RL. Comprehensive outcomes of surgically treated idiopathic toe walkers. Journal of Pediatric Orthopedics 2006;26(5):606‐11. [PUBMED: 16932099] [DOI] [PubMed] [Google Scholar]
Menz 2004
- Menz HB. Two feet, or one person? Problems associated with statistical analysis of paired data in foot and ankle medicine. The Foot 2004;14(1):2‐5. [Google Scholar]
Ming 2007
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain & Development 2007;29(9):565‐70. [PUBMED: 17467940] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moreau 1987
- Moreau MJ, Lake DM. Outpatient percutaneous heel cord lengthening in children. Journal of Pediatric Orthopedics 1987;7(3):253‐5. [PUBMED: 3584437] [DOI] [PubMed] [Google Scholar]
Norlin 1981
- Norlin R, Odenrick P, Sandlund B. Development of gait in the normal child. Journal of Pediatric Orthopedics 1981;1(3):261‐6. [PUBMED: 7334104] [DOI] [PubMed] [Google Scholar]
Papariello 1985
- Papariello SG, Skinner SR. Dynamic electromyography analysis of habitual toe‐walkers. Journal of Pediatric Orthopedics 1985;5(2):171‐5. [PUBMED: 3988918] [PubMed] [Google Scholar]
Pendharkar 2008
- Pendharkar G, Lai DT, Begg RK. Detecting idiopathic toe‐walking gait pattern from normal gait pattern using heel accelerometry data and support vector machines. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society 2008:4920‐3. [PUBMED: 19163820] [DOI] [PubMed]
Pendharkar 2012
- Pendharkar G, Percival P, Morgan D, Lai D. Automated method to distinguish toe walking strides from normal strides in the gait of idiopathic toe walking children from heel accelerometry data. Gait & Posture 2012;35(3):478‐82. [PUBMED: 22300731] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sala 1999
- Sala DA, Shulman LH, Kennedy RF, Grant AD, Chu ML. Idiopathic toe‐walking: a review. Developmental Medicine and Child Neurology 1999;41(12):846‐8. [PUBMED: 10619285] [DOI] [PubMed] [Google Scholar]
Shulman 1997
- Shulman LH, Sala DA, Chu ML, McCaul PR, Sandler BJ. Developmental implications of idiopathic toe walking. The Journal of Pediatrics 1997;130(4):541‐6. [PUBMED: 9108850] [DOI] [PubMed] [Google Scholar]
Sobel 1997
- Sobel E, Caselli MA, Velez Z. Effect of persistent toe walking on ankle equinus. Analysis of 60 idiopathic toe walkers. Journal of the American Podiatric Medical Association 1997;87(1):17‐22. [PUBMED: 9009544] [DOI] [PubMed] [Google Scholar]
Solan 2010
- Solan MC, Kohls‐Gatzoulis J, Stephens MM. Idiopathic toe walking and contractures of the triceps surae. Foot and Ankle Clinics 2010;15(2):297‐307. [PUBMED: 20534357] [DOI] [PubMed] [Google Scholar]
Stott 2004
- Stott NS, Walt SE, Lobb GA, Reynolds N, Nicol RO. Treatment for idiopathic toe‐walking: results at skeletal maturity. Journal of Pediatric Orthopedics 2004;24(1):63‐9. [PUBMED: 14676536] [PubMed] [Google Scholar]
Stricker 1998
- Stricker SJ, Angulo JC. Idiopathic toe walking: a comparison of treatment methods. Journal of Pediatric Orthopedics 1998;18(3):289‐93. [PUBMED: 9600550] [PubMed] [Google Scholar]
Sutherland 1980
- Sutherland DH, Olshen R, Cooper L, Woo SL. The development of mature gait. Journal of Bone and Joint Surgery. American Volume 1980;62(3):336‐53. [PUBMED: 7364807] [PubMed] [Google Scholar]
Sutton 1997
- Sutton AJ, Muir KR, Jones AC. Two knees or one person: data analysis strategies for paired joints or organs. Annals of the Rheumatic Diseases 1997;56(7):401‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taussig 2001
- Taussig G, Delouvée E. Idiopathic toe walker child. Diagnosis and spontaneous evolution [La marche en equin idiopathique de l'enfant. Diagnostic et evolution spontanee]. Annales de Réadaptation et de Medecine Physique 2001;44(6):333‐9. [PUBMED: 11587675] [DOI] [PubMed] [Google Scholar]
van Kuijk 2014
- Kuijk AA, Kosters R, Vugts M, Geurts A. Treatment for idiopathic toe walking: a systematic review of the literature. Journal of Rehabilitation Medicine 2014;46(10):945‐57. [DOI] [PubMed] [Google Scholar]
Westberry 2008
- Westberry DE, Davids JR, Davis RB, Morais Filho MC. Idiopathic toe walking: a kinematic and kinetic profile. Journal of Pediatric Orthopedics 2008;28(3):352‐8. [PUBMED: 18362803] [DOI] [PubMed] [Google Scholar]
Williams 2010
- Williams CM, Tinley P, Curtin M. The Toe Walking Tool: a novel method for assessing idiopathic toe walking children. Gait & Posture 2010;32(4):508‐11. [PUBMED: 20692159] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams CM, Tinley P, Curtin M, Nielsen S. Vibration perception thresholds in children with idiopathic toe walking gait. Journal of Child Neurology 2012;27(8):1017‐21. [PUBMED: 22433426] [DOI] [PubMed] [Google Scholar]
Williams 2013a
- Williams CM, Tinley PD, Curtin M, Nielsen S. Foot and ankle characteristics of children with an idiopathic toe‐walking gait. Journal of the American Podiatric Medicial Association 2013;103(5):374‐9. [PUBMED: 24072365] [DOI] [PubMed] [Google Scholar]
Wren 2010
- Wren TA, Cheatwood AP, Rethlefsen SA, Hara R, Perez FJ, Kay RM. Achilles tendon length and medial gastrocnemius architecture in children with cerebral palsy and equinus gait. Journal of Pediatric Orthopedics 2010;30(5):479‐84. [PUBMED: 20574267] [DOI] [PubMed] [Google Scholar]
Yngve 1996
- Yngve DA, Chambers C. Vulpius and Z‐lengthening. Journal of Pediatric Orthopedics 1996;16(6):759‐64. [PUBMED: 8906648] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Williams 2016
- Williams CM, Pacey V, Bakker PB, Caserta AJ, Gray K, Engelbert RHH. Interventions for idiopathic toe walking. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012363] [DOI] [PMC free article] [PubMed] [Google Scholar]


