Abstract
Optimal management of spontaneous intracerebral hemorrhage (ICH) remains one of the highly debated areas in the field of neurosurgery. Earlier studies comparing open surgical intervention with best medical management failed to show a clear benefit. More recent experience with minimally invasive techniques has shown greater promise. Well-designed phase II trials have confirmed the safety and preliminary treatment effect of thrombolytic aspiration and clearance of spontaneous ICH and associated intraventricular obstructive hemorrhage. Those trials are reviewed, including respective protocols and technical nuances, and lessons learned regarding patient selection, the concept of hemorrhage stabilization, optimization of the surgical procedure, and thrombolytic dosing decisions. These concepts have been incorporated in the design of ongoing definite phase III randomized trials (MISTIE and CLEAR) funded by the National Institutes of Health. These are presented including the role of surgical leadership in the training and monitoring of the surgical task and quality assurance. The impact of these techniques on neurosurgical practice is discussed.
Keywords: Intracerebral hemorrhage, Intracranial hemorrhage, Intraventricular hemorrhage, Minimally invasive surgery, Thrombolysis
The spontaneous, nontraumatic rupture of blood vessels in the brain parenchyma, in the absence of any underlying structural vascular lesion, can lead to the accumulation of the blood within the brain substance. This is known as spontaneous intracerebral hemorrhage (ICH) or primary intracerebral hemorrhage (ICH) (Figure 1). Worldwide clinical management of spontaneous ICH varies significantly along the spectrum of this illness and lacks a consensus-based standard of care.
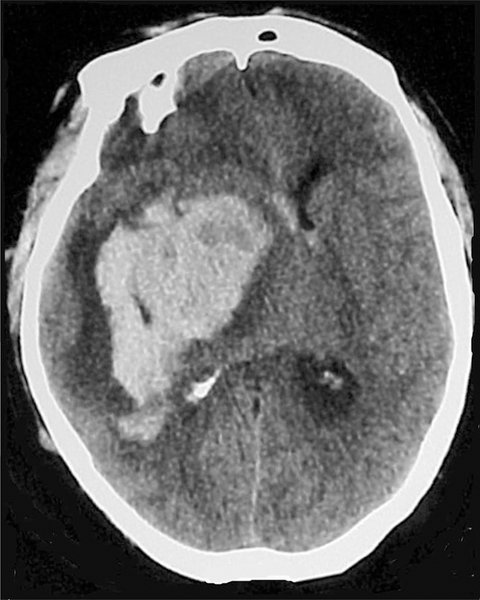
FIGURE 1.
CT of head demonstrating large ICH with surrounding edema and significant midline shift. ICH, intracerebral hemorrhage.
EPIDEMIOLOGY AND SCOPE OF THE PROBLEM
Only 10% to 15% of all strokes are hemorrhagic in nature; however, 30-day mortality rates associated with this devastating illness range from 35% to 52%, with half of those deaths occurring in the first 2 days.1–6 Several factors influence the extremely poor outcome associated with spontaneous ICH, such as the level of consciousness at presentation, volume of parenchymal hemorrhage, volume of intraventricular hemorrhage (IVH), and the extent of cerebral damage. Critical care treatment alone, aiming at intracranial pressure control and multisystem support, is often ineffective in these patients and does not prevent death or disability.
The outcome of patients who have this devastating illness depends on age, severity of ICH, presenting Glasgow Coma Scale score, and extent of IVH. Volume of ICH has consistently been shown to be a powerful predictor of poor outcome.7–13
IVH is a frequent complication of spontaneous ICH. The extension of ICH into the ventricles has been consistently shown to be an independent predictor of poor outcome.14–19 Of all the \recent studies that have attempted to grade the extent of IVH in relation to patient outcome, 14, 18–20 the “Graeb score,” which takes into account the extent of involvement of the respective ventricles and associated ventriculomegaly, has been extensively validated in outcomes studies.18,21,22 Using new data emerging from the CLEAR IVH trial, Morgan et al23 developed and validated a modification of the original Graeb scale to facilitate rapid assessment of IVH over time, and found that it is a reliable measure with prognostic validity suitable for rapid use in clinical practice and in research.
BEST MEDICAL MANAGEMENT AND OPEN SURGICAL INTERVENTION
Modern stroke care systems have contributed to the recognition of symptoms, rapid transport, and earlier imaging and diagnosis of hemorrhagic stroke, including spontaneous ICH. Advances in acute resuscitation and critical care support have been deployed in this disease, aimed at preventing hematoma growth (blood pressure control and reversal of coagulopathy), optimizing brain perfusion (including control of intracranial pressure), and multisystem homeostasis (including the management of secondary sequelae such as aspiration and deep vein thrombosis). These interventions are outside the scope of this review and are summarized in evidence-based guidelines by the American Stroke Association.24 There is a broad consensus about the role of surgery in cases with cerebellar hematoma greater than 3 cm in diameter, and possibly in younger patients with larger spontaneous ICH and impending herniation.24 But there remains wide controversy about the potential role of surgery in the majority of spontaneous ICH cases where the hematoma has stabilized, and the patients progress nevertheless to a poor outcome with high rates of mortality and disability. Despite modern protocolized management, the outcome in this disease remains sobering, especially in cases with larger ICH volume.13
Several clinical trials comparing surgical intervention with best medical management, published between 1961 and 2004, failed to show clear benefit of surgical evacuation of hematoma over best medical management.25–34 A meta-analysis of all 12 trials of surgical interventions in the setting of ICH published before 2006 provided an odds ratio of 0.85 (confidence interval, 0.71–1.02) in favor of surgical treatment.35 Based on the encouraging results from these earlier studies, a larger international multicenter prospectively randomized clinical trial, Surgical Treatment of ICH (STICH), was designed to compare early surgery with initial conservative treatment for patients with ICH. The trial enrolled 1033 patients from 83 centers in 27 countries who were randomly assigned to early surgery (503) or initial conservative treatment (530). Of 468 patients randomly assigned to early surgery, 26% had a favorable outcome at 6 months, compared with 24% of 496 patients randomly assigned to initial conservative treatment. The STICH trial failed to demonstrate the superiority of surgical treatment of ICH over medical management. However, subgroup analysis showed that in a subset of patients with lobar hematoma and no IVH, with initial conservative treatment, 37% achieved a favorable outcome, whereas 49% of patients achieved a favorable outcome with early surgery (P = .08).34 However, STICH lacked sufficient power to address this subgroup finding.
Thus, the STICH II trial was designed to test the role of craniotomy vs best medical therapy for lobar spontaneous ICH 10 to 100 mL in volume and no associated IVH, with 307 of 601 cases randomly assigned to early surgery.36 The results of that trial were recently published and demonstrated no difference in the intention-to-treat analysis of primary outcome (extended Glascow Outcome Score) at 6 months. An absolute difference of primary outcome dichotomy of 3.7% favored surgery, and other trends of a secondary shift in good disability scale strata and mortality also favored surgery with a 6% effect, trending but not reaching statistical significance.37
MINIMALLY INVASIVE SURGICAL INTERVENTIONS
The era of minimally invasive surgical intervention for ICH evacuation started in the late 1980s when Auer et al26 published a controlled randomized study of endoscopic evacuation vs medical treatment in 100 patients with spontaneous ICH, and showed that surgical patients with smaller hematomas made a significantly better functional recovery than did patients of the medically treated group (30% vs 70%, P < .05), and patients with larger hematomas showed significantly lower mortality rates after operation than the medically treated group. Early attempts at simple clot aspiration or other means of mechanical clot evacuation failed to accomplish satisfactory volume reduction of ICH.38,39 A Chinese study by Doi et al40 first reported directly instilling urokinase after stereotactic aspiration to liquefy the hematoma. Since the publication of this study, several reports have reported the usefulness of ICH volume reduction by using urokinase thrombolysis.41–45 Especially in elderly and debilitated patients, minimally invasive surgical techniques have the potential to substantially decrease hematoma volume while avoiding the morbidity of a major craniotomy procedure. A prospectively controlled Chinese study by Zhou et al46 comparing minimally invasive stereotactic clot removal and thrombolysis with open conventional craniotomy showed that patients treated with minimally invasive surgery showed obvious amelioration in Glasgow Coma Scale score compared with that of open procedure. Incidence of postoperative complication in the minimally invasive group was much lower than the open group (32.3% and 80.7%, respectively, P = .001). This trial reinforced the idea that minimally invasive approaches might be superior to open surgery, especially with further optimization of the technique.
Based on multiple small studies, 2 larger-scale trials have been undertaken and will be discussed in detail. Minimally Invasive Surgery and Thrombolytic evacuation in ICH (MISTIE) trial was a phase II trial that targeted cases with larger ICHs without obstructive IVH, and aimed at calibrating the surgical task, confirming safety and effectiveness, and defining an estimate of treatment effect to guide the design of a more definitive phase II trial. The second trial, Clot Lysis Evaluating Accelerated Resolution (CLEAR), targeted intraventricular thrombolysis in the setting of IVH. The CLEAR IVH phase II trial was completed, and it motivated an ongoing definitive phase III trial. Both trials used recombinant tissue plasminogen activator (rtPA) (Genen-tech) as the thrombolytic agent. Table describes the inclusion and exclusion criteria of both trials.
TABLE.
Inclusion and Exclusion Criteria for CLEAR III and MISTIE III Studiesa
| CLEAR III |
| Phase III, prospective randomized, case-controlled, open-label, 500-subject clinical trial of minimally invasive surgery plus rtPA in the treatment of large ICH (> 30 mL). Subjects randomly assigned to receive a surgical procedure of minimally invasive image-guided hematoma aspiration plus 1 mg of rtPA instillation every 8 hours through a hematoma catheter (with reopening of the catheter for drainage 1 hour after each dose), until clot volume <10 mL or 12 doses, plus best critical care management, vs best critical care management alone. Primary outcome parameter per intention to treat, powered to show increase in mRS 0–3 “good outcome” strata at 1 year postprocedure. Funded by the NIH (NINDS) 2013–2018. |
| Inclusion criteria |
| Large supratentorial spontaneous ICH ≥30 mL diagnosed by using CT, CTA, etc, with a GCS ≤14 or a NIHSS ≥6. Deep and lobar ICH is allowed. Associated IVH is allowed and may receive a separate EVD (but thrombolysis is used in the ICH catheter). |
| Clot stability, 6-hour clot size equal to the most previous clot size (within 5 mL) as determined by additional CT scans at least 6 hours apart. |
| Symptoms less than 24 hours before diagnostic CT scan. |
| Intention to initiate surgery between 12 and 72 hours after diagnostic CT. First dose can be given within 76 hours after diagnostic CT. |
| SBP <180 mm Hg sustained for 6 hours recorded closest to the time of randomization. |
| Historical Rankin score of 0 or 1. |
| Age ≥18 and ≤80. |
| Exclusion criteria |
| Infratentorial hemorrhage. |
| Thalamic bleeds with apparent midbrain extension with third nerve palsy or dilated and nonreactive pupils. Other (supranuclear) gaze abnormalities are not exclusions. Patients with a posterior fossa ICH or cerebellar hematomas are ineligible. |
| Irreversible impaired brainstem function (bilateral fixed, dilated pupils and extensor motor posturing), GCS ≤4. |
| Ruptured aneurysm, AVM, vascular anomaly, Moyamoya disease diagnosed with radiographic imaging. |
| Patients with unstable mass or evolving intracranial compartment syndrome. |
| Platelet count <100,000, INR >1.4, or an elevated prothrombin time (PT) or activated partial thromboplastin time (aPTT), any irreversible coagulopathy, bleeding disorder, or known clotting disorder. |
| Inability to sustain INR ≤1.4 using short- and long-active procoagulants (such as, but not limited to, NovoSeven, FFP, and/or vitamin K). |
| Subjects requiring long-term anticoagulation are excluded. |
| Use of Dabigatran before symptom onset. |
| Pregnancy |
| Allergy/sensitivity to rtPA. |
| Previous enrollment in the study or any other interventional or surgical ICH studies. |
| Subjects who are not expected to survive to the day 365 visit because of comorbidities and/or have DNR/DNI status before randomization are excluded. |
| Complicating medical comorbidities. |
| Any other condition that the investigator believes would pose a significant hazard to the subject if the investigational therapy were initiated. |
| Active drug or alcohol use or dependence. |
| In the investigator’s opinion, the patient is unstable and would benefit from a specific intervention rather than supportive care plus or minus MIS + rtPA removal of the ICH. |
| Inability or unwillingness of the subject or legal guardian/representative to give written informed consent. |
| CLEAR III |
| Phase III, prospective randomized, case-controlled double-blinded clinical trial to compare EVD plus rtPA vs placebo in the management and treatment of 500 subjects with small ICHs and large IVHs defined as ICH <30 mL and obstruction of the third or fourth ventricles by intraventricular blood clot. All patients to receive EVD for obstructive IVH, with best critical care management. Dose of rtPA is 1 mg every 8 hours through the EVD (vs saline placebo), with 1 hour of clamping of EVD after each dose. More than one EVD allowed and recommended for casted ventricles with mass effect and shift. Primary outcome parameter per intention to treat, powered to show increase in mRS 0–3 “good outcome” strata at 6 months postprocedure. Funded by the NIH (NINDS) 2009–2014 (360 cases enrolled through summer 2013). |
| Inclusion criteria |
| Age 18–80 |
| Symptom onset less than 24 hours before diagnostic CT scan |
| Spontaneous ICH less than or equal to 30 mL or primary IVH |
| IVH obstructing third and/or fourth ventricles |
| ICH and IVH clot stability at 6 hours or more after EVD placement |
| Catheter tract bleeding stability 12 hours or more after EVD placement |
| EVD placed per standard medical care |
| SBP less than 200 mm Hg sustained for 6 hours before drug administration |
| Able to randomize within 72 hours of diagnostic CT scan |
| Historical Rankin score of 0 or 1 |
| Exclusion criteria |
| Suspected or untreated ruptured cerebral aneurysm, SAH, AVM, or tumor Presence of a choroid plexus vascular malformation or Moyamoya |
| Clotting disorders (except when demonstrably corrected), platelet count less than 100 000, INR greater than 1.4 |
| Pregnancy |
| Infratentorial hemorrhage |
| ICH/IVH enlargement that cannot be stabilized in the treatment time window |
| Ongoing internal bleeding or superficial or surface bleeding |
| Previous enrollment in the study or planned or simultaneous participation (between screening and day 30) in another interventional medical investigation or clinical trial. |
| Any other condition that the investigator believes would pose a significant hazard to the subject if the investigational therapy were initiated Inability or unwillingness of the subject or legal guardian/representative to give written informed consent |
ICH, intracerebral hemorrhage; mRS, modified Rankin Score; NIH, National Institutes of Health; NINDS, National Institute of Neurological Disorders and Stroke; rtPA, recombinant tissue plasminogen activator; ICH, intracerebral hemorrhage; CTA, computed tomographic angiography; GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; IVH, intraventricular hemorrhage; EVD, external ventricular drain; SBP, systolic blood pressure; AVM, arteriovenous malformation; INR, international normalized ratio; FFP, fresh frozen plasma; DNR/DNI, do not resuscitate/do not intubate; SAH, subarachnoid hemorrhage.
MINIMALLY INVASIVE SURGERY AND THROMBOLYSIS FOR ICH EVACUATION (MISTIE)
Phase II MISTIE (www.mistietrial.com), a rigorous trial sponsored by the National Institutes of Health (NIH), explored the safety, efficacy, technique, and dose optimization of image-guided catheter aspiration and delivery of rtPA in patients with ICH volume ≥20 mL after demonstrated clot stability for 6 hours. These patients were treated with either 0.3 mg or 1 mg of rtPA every 8 hours for up to 9 doses to either final volume of <10 mL or 72 hours. The trial was a 2-arm study designed to observe the differences between patients randomly assigned to best-practice neurocritical care medical management with or without minimally invasive surgery plus rtPA. A total of 60 enrolled patients completed this phase of the study (each stage of dose finding will enroll 20 patients with a 3:1 minimally invasive surgery + rtPA to medical management randomization scheme). Preliminary results of MISTIE were presented at the International Stroke Conference in February 2013 and are summarized at the Web site (http://my.americanheart.org/professional/Sessions/InternationalStrokeConference/Archive/ISC-2013-Late-Breaking-Science-Oral-Abstracts_UCM_447814_Article.jsp).
Protocol and Technical Nuances
The safety of MISTIE procedure most likely depends on the overarching principle of clot stability. Cases were only enrolled in the trial after demonstrating clot stability for >6 hours, and with strict correction of any coagulopathy or platelet dysfunction. Any catheter track bleed or ICH expansion automatically triggered dosage cessation in MISTIE and additional stability for more than 12 hours. Catheters were not moved or repositioned, and the brain was not instrumented within 24 hours of rtPA dose nor in the setting of any coagulopathy or thrombocytopenia. Brain surface bleeds, at the catheter entry site, were particularly vulnerable to expansion and required judicious monitoring and stabilization before the use of a thrombolytic. Etiology screening is essential to the exclusion of cases with ruptured aneurysm or arteriovenous malformation (AVM) from the trial. Most practitioners would agree that an unsecured ruptured aneurysm or vascular malformation would contraindicate brain thrombolysis, except in rare compassionate and justified situations. Hence, cases in MISTIE are routinely screened by computed tomographic (CT) angiography, magnetic resonance imaging, and, in selected cases, catheter angiography, to ensure that true spontaneous ICHs are subjected to brain instrumentation and thrombolysis.
Another valuable and early lesson learned from the MISTIE trial was that the clot resolution rate for surgical patients highly correlated with catheter placement. To objectively study the catheter placement, the trial focused on 3 criteria: catheter target engaging the center of the clot, catheter trajectory parallel to the long axis of the clot, and catheter perforations spanning the center of the clot (catheter tip at the deepest third of the ICH). By the use of a scoring rubric based on these 3 criteria, CT scans of all surgical patients were graded for catheter placement efficiency and overall clot resolution through the treatment period. The study reported an appreciable trend toward optimal catheter placement leading to greater clot resolution, and better clinical outcome (Figure 2).47 Because a poor catheter placement score was associated with higher residual clot volume and suboptimal clot resolution, careful planning of the surgical trajectory for catheter placement, deployed in the final tier of the trial, resulted in improved performance by all participating surgeons. These and other technical nuances have been described in detail by our team48 and in the trial protocol (www.mistetrial.com).
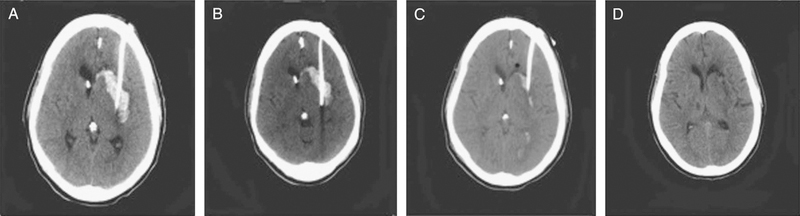
FIGURE 2.
CT of head demonstrating an optimal placement of a catheter along the long axis of the ICH and progressive resolution of the clot from A to D, with progressive thrombolytic dose. ICH, intracerebral hemorrhage.
Another very important issue addressed by MISTIE was the effect of perihematoma edema. Several studies have shown that perihematoma edema can worsen outcomes after ICH; thus, hematoma evacuation should reduce volume and favorably impact outcome. To address this issue, Mould et al conducted a semiautomated, computerized volumetric analysis on CT scan of patients enrolled in the MISTIE trial and showed significantly lower edema volume at the end of treatment in the surgical cohort. A total of 79 surgical and 39 medical patients were analyzed. Mean hematoma volume at the end of treatment was 19.6 ± 14.5 cm3 for the surgical cohort and 40.7 ± 13.9 cm3 for the medical cohort (P < .001), with significantly lower edema volume at the end of treatment in the surgical cohort: 27.7 ± 13.3 cm3 than the medical cohort: 41.7 ± 14.6 cm3 (P < .001). There was a graded effect of clot removal on perihematoma edema when patients with >65%, 20% to 65%, and <20% ICH removed were analyzed (P < .001) and positive correlation between perihema- toma edema reduction and the percentage of ICH removed was identified (ρ = 0.658; P < .001). Comparison within the surgical cohort of patients who underwent surgical aspiration and rtPA, with patients that underwent surgical aspiration only, showed that perihematoma edema was not exacerbated by rtPA.49
Outcome and Effectiveness
Preliminary analysis of the 90 patients enrolled in the study and followed for 180 days, and the 48 patients followed for 365 days, showed that patients responded favorably to the surgery plus rtPA compared with just medical management. On average, 20% of the clot was removed through surgical aspiration alone; after treatment with rtPA, the average clot size was reduced by nearly 50% of starting volume, whereas patients randomly assigned to medical management showed only a 6% reduction of clot. Recorded adverse events were within safety limits, including 30-day mortality, 8%; symptomatic rebleeding, 8%; and bacterial meningitis or ventriculitis, 0%.47 With the latest presentation of trial results in 2013, the safety and feasibility of ICH clot removal were confirmed, and there was a significant therapeutic advantage, with a greater proportion of treated cases in the better outcome strata, especially among cases with greater hematoma evacuation. Long-term outcome in terms of modified Rankin Score (mRS) at 180 days and 365 days showed a trend toward an increase in the number of people in the mRS 0 to 3 category compared with mRS 4 and above. This trial for the first time showed that the procedure is successfully helping people avoid a long-term dependence state and is actually increasing the functional independence of patients who have this devastating illness. Length-of-stay and cost-of-treatment analyses showed a significant reduction in both categories. In essence, MISTIE validated the proof of concept, feasibility of ICH volume reduction, and confirmed the safety of the technique in comparison with randomized controls with third-party adjudication. It also shows that this procedure has the potential to reduce the societal and economic burden of this disease. Based on these findings, a phase III trial was recently funded by the NIH to be launched in 2013, with sufficient power to delineate the treatment effect, which was seen in the phase II trial.
CLOT LYSIS EVALUATING ACCELERATED RESOLUTION (CLEAR) OF IVH
Thrombolytic therapy aimed at enhancing IVH clearance has evolved with the aim of preventing the deleterious effects of IVH on the surrounding brain. In fact, the administration of thrombolytics through an external ventricular drain (EVD) has been shown to be safe and effective in both animal studies50–52 and in small clinical case series.53–57
A systematic review of all published retrospective case series between 1990 and 1998 compared the outcome of conservative treatment, EVD alone and EVD in combination with fibrinolysis in the setting of severe IVH due to subarachnoid hemorrhage or spontaneous ICH, and showed that the fatality rate for conservative treatment was 78%, the fatality rate for was EVD 58%, and the fatality rate for EVD with fibrinolytic agents was 6%; the poor outcome rate for conservative treatment was 90%, EVD 89%, and EVD with fibrinolytic agents 34%.58 A randomized, double-blind, controlled, multicenter, pilot study assessing the safety and efficacy of intraventricular thrombolysis showed that intraventricular thrombolysis with urokinase speeds the resolution of blood clots in comparison with treatment with ventricular drainage alone (4.69 vs 8.48 days).59 This led to the conception of CLEAR IVH (Parts A and B), a phase II trial funded by the US Food and Drug Administration, which evaluated the safety and efficacy of using multiple injections of low-dose rtPA to accelerate the lysis and evacuation of IVH,60 and led to the subsequent design and launching of an ongoing phase III trial funded by the NIH (www.cleariii.com).
Protocol and Technical Nuances
As with MISTIE, an overarching principle in the CLEAR trials has been the concept of hemorrhage stability. Cases are not enrolled in the study unless there has been demonstrated stability of the ICH and IVH volume for >6 hours after placement of the EVD. Any new catheter tract hemorrhages or demonstrated expansion of ICH or IVH trigger an additional stability of >12 hours before instillation of the thrombolytic. Surface bleeds at the site of the EVD cortical entry are particularly closely watched and stabilized. Cases where more than 2 passes are needed for the placement of EVD mandate >24 hours of stability before dosing. Catheters are not manipulated (removed or repositioned), nor new catheters placed, in the setting of coagulopathy or platelet dysfunction, or within 24 hours of the administration of thrombolytic in the EVD. With these precautions, symptomatic bleeds have been very uncommon in CLEAR III, despite the use of thrombolytics (<2% within 30 days of EVD placement), with close prospective systematic monitoring.
As with MISTIE, etiology screening is essential to the exclusion of cases with ruptured aneurysm or AVM from the trial. An ongoing registry in CLEAR III has identified many cases of AVM or fistula, ruptured aneurysm, mycotic aneurysm, and Moyamoya disease that precluded enrollment in the trial. Many such cases included “typical bleed location” and history of hypertension in elderly patients that may not have otherwise warranted an etiology screen in clinical practice.
Sometimes, very large IVH and complete casting of 1 or both ventricles requires the cannulation of both ventricles to optimally control intracranial pressure and enhance blood clearance. 61,62 To assess the hypothesis that EVD laterality may influence the clearance of blood from the ventricular system with and without thrombolytic agent, authors of the CLEAR IVH trial assessed EVD location in 100 patients (Figure 3). They showed that laterality of catheter correlated with clearance rates, clearance of IVH over the first 3 days was significantly greater in the thrombolytic group than in placebo regardless of catheter laterality, and, when thrombolytic was administered, there was a trend toward more rapid clearance of total IVH through an EVD placed on the side of dominant IVH compared with an EVD on the side with less blood, which was not the case when placebo was administered. Clearance of third and fourth ventricular blood was unrelated to EVD laterality.63
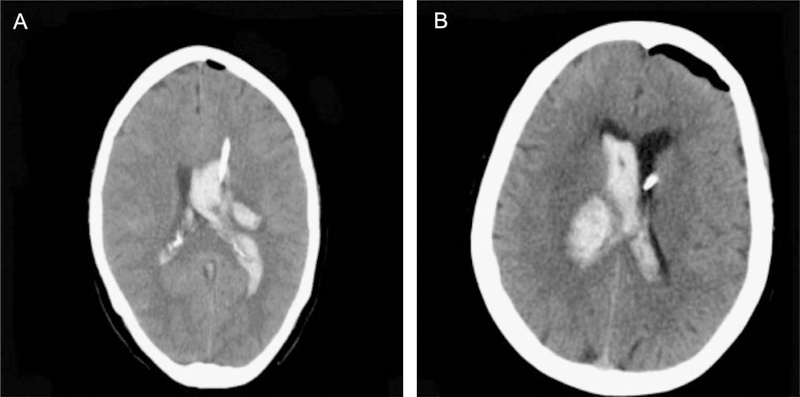
FIGURE 3.
CT of head demonstrating large IVH with casting of lateral ventricles. A shows presence of an EVD catheter in the dominant IVH clot, and B shows presence of EVD catheter in the ventricle contralateral to the dominant IVH clot. IVH, intraventricular hemorrhage; EVD, external ventricular drain.
Based on these results, the CLEAR III trial protocol currently includes guidelines for the placement of the initial catheter in the contralateral ventricle to the dominant IVH, for optimizing intracranial pressure control, and a second catheter is placed in the ipsilateral ventricle for cases with casting, trapping mass effect, and/or shift due to dominant lateral ventricle IVH. In the first 300 cases enrolled to date in CLEAR III, about one-third of patients have been managed with more than a single EVD.
Outcome and Effectiveness
In the CLEAR IVH phase II trial, patients were randomly assigned to treatment with 0.3, 1.0, or 3.0 mg of rtPA, every 8 or 12 hours, vs placebo, in a prespecified dose escalation scheme. The median duration of dosing was 7.5 days for rtPA and 12 days for placebo. The reported mortality in the phase II trial was 18% with rtPA vs 23% in the placebo group; ventriculitis rate of 8% with rtPA vs 9% in the placebo group; and symptomatic bleeding of 23% with rtPA vs 5% in the placebo group. The study found that low-dose rtPA, with a 1.0-mg dose every 8 hours, has an acceptable safety profile compared with placebo and historical controls, and there was a significant beneficial effect of rtPA on the rate of clot resolution. The higher dose of 3.0 mg of rtPA resulted in more frequent catheter track hemorrhages. In effect, The CLEAR IVH phase II trial optimized the dose effect and estimated a favorable shift toward better outcome strata, motivating a more definitive phase III trial. The CLEAR III trial is currently underway for more definitive assessment of the potential benefits of thrombolysis on patient outcome.64 It aims to enroll 500 patients with obstructive IVH randomly assigned to receive EVD with administration every 8 hours of 1 mL of saline or 1 mg/mL rtPA. Safety monitoring with more than 300 CLEAR III patients enrolled to date has included a less than 2% rate of symptomatic bleeding within 30 days of the protocol, a dramatic improvement in comparison with CLEAR II with rigorous observation of clot stability and dosing lessons, and less than 2% adjudicated rate of bacterial meningitis/ventriculitis (unpublished data).
A subgroup analysis of the CLEAR IVH phase II trial assessed the effect of the dose of rtPA by region of the brain on the clearance of IVH. It found that rtPA accelerates the resolution of IVH in a dose-dependent manner, and the resolution is greatest in the midline ventricles and least in the posterolateral ventricles.65 Intraventricular rtPA had no impact on systemic coagulation, nor did it compound the effects of systemic anticoagulation for deep venous thrombosis prophylaxis.66
ROLE OF THE SURGICAL CENTER IN CLINICAL TRIALS
The 2 large trials in the setting of ICH and IVH highlighted several technical and protocol nuances that influence the safety and effectiveness of the surgical task, and ultimately the clinical outcome of patients enrolled in the trials. One of the most important criteria for the success and good outcome of patients enrolled in the surgical arm of the MISTIE trial is catheter location. For optimal placement, meticulous planning and execution is required, which is achieved most of the time with experience or experienced guidance. Similarly, catheter location and the possible need of dual catheters influence the safety and effectiveness of the CLEAR task. In both trials, strict stabilization of the hemorrhage or any new bleeding, thoughtful etiology screening to rule out vascular lesions as a cause of ICH/IVH, and the correction of any coagulopathy have contributed to the safety and effectiveness of the surgical task.
The 2 trials motivated the development of a surgeon-led process to educate, oversee, and evaluate surgical performance and protocol compliance at participating sites. This led to the creation of a trial Surgical Center, tasked with the monitoring, assurance, and enhancement of the safety and quality of the surgical task. This process needed to consider and overcome variations in local surgical practice and a myriad of surgical team structures and expertise throughout the world.
Every surgical complication is analyzed in a confidential “virtual” Mortality Morbidity peer review, including root-cause analysis and the identification of potential trends influencing outcome or quality. This information is shared with the various sites and with the trials’ Executive Committees and Data and Safety Monitoring Board. And every surgical case is finally subjected to a narrative analysis and assessment of protocol adherence. This evaluation is shared with the trial site, reinforcing positive practices, and identifies situations where targeted site education is needed, and, in rare cases, site sanctions or termination. Finally, a Surgical Center Report is presented quarterly to the trials’ Executive Committee, summarizing trialwide surgical performance and complications, and brings forth relevant concerns and questions, including any surgeon-motivated proposed changes to the trial protocol.
A surgical principal investigator is always “On Call,” and remotely available as each case is being screened for enrollment to review and approve case eligibility, and to approve the proposed catheter trajectory (MISTIE) and final catheter location (MISTIE and CLEAR) before initiating thrombolytic dose. The trial’s surgical expert “On Call” serves “as a senior partner in a neurosurgical practice,” answering any questions related to protocol steps or influencing the safety of execution of the surgical task, the prevention of bleeding, the choice and optimization of catheter trajectory and location, and decisions about dosing and catheter management during the course of the trial.
These processes of Surgical Center engagement are careful not to interfere with the local clinicians’ decision-making process, with direct knowledge about and responsibility for the patient’s care and safety. Surgeons are empowered with deviating from any protocol steps, if they feel that is in the best interest of the patient. Yet no individual surgeon’s experience can make up for lessons learned with hundreds of cases at multiple sites. These trials’ Surgical Center processes aim to introduce a dimension of peer review and quality assurance that is still lacking, with limited volume of experience with these procedures at individual sites. The Surgical Center experience with MISTIE and CLEAR provides a model for the expert evaluation and management of surgical performance with emerging technologies during phases of development and accreditation of practice standards.
CONCLUSION
The spontaneous ICH remains one of the deadliest and most disabling diseases, with a high cost burden to society. A wide range of practice patterns, vague practice standards, the lack of proven therapies, and persistent poor outcomes cloud its management. Therapeutic nihilism has been compounded by the failure of early clinical trials of surgical treatment in comparison with medical management. However, new emerging results of minimally invasive thrombolytic evacuation of ICH and IVH, in MISTIE and CLEAR, respectively, have been very encouraging. Ongoing clinical trials promise to change clinical practice by optimizing case selection and the surgical task and by integrating these novel tools in the therapeutic armamentarium.
ABBREVIATIONS:
- AVM
arteriovenous malformation
- EVD
external ventricular drain
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Score
- NIH
National Institutes of Health
- rtPA
recombinant tissue plasminogen activator
Footnotes
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Awad is co-Principal Investigator and Surgical co-Chairman of the MISTIE III and CLEAR III clinical trials.
REFERENCES
- 1.Chiewvit P, Danchaivijitr N, Nilanont Y, Poungvarin N Computed tomographic findings in non-traumatic hemorrhagic stroke. J Med Assoc Thai. 2009;92(1):73–86. [PubMed] [Google Scholar]
- 2.Badjatia N, Rosand J Intracerebral hemorrhage. Neurologist. 2005;11(6):311–324. [DOI] [PubMed] [Google Scholar]
- 3.Binz DD, Toussaint LG 3rd, Friedman JA Hemorrhagic complications of ventriculostomy placement: a meta-analysis. Neurocrit Care. 2009;10(2): 253–256. [DOI] [PubMed] [Google Scholar]
- 4.Ehtisham A, Taylor S, Bayless L, Klein MW, Janzen JM Placement of external ventricular drains and intracranial pressure monitors by neurointensivists. Neurocrit Care. 2009;10(2):24l–247. [DOI] [PubMed] [Google Scholar]
- 5.Murry KR, Rhoney DH, Coplin WM Urokinase in the treatment of intraventricular hemorrhage. Ann Pharmacother. 1998;32(2):256–258. [DOI] [PubMed] [Google Scholar]
- 6.Sudlow CL, Warlow CP Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28(3):491–499. [DOI] [PubMed] [Google Scholar]
- 7.Fayad PB, Awad IA Surgery for intracerebral hemorrhage. Neurology. 1998;51 (3 suppl 3):S69–S73. [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ, Hon C Surgery for primary intracerebral hemorrhage: is it safe and effective? A systematic review of case series and randomized trials. Stroke. 1997;28 (11):2126–2132. [DOI] [PubMed] [Google Scholar]
- 9.Prasad K, Browman G, Srivastava A, Menon G Surgery in primary supratentorial intracerebral hematoma: a meta-analysis of randomized trials. Acta Neurol Scand. 1997;95(2):103–110. [DOI] [PubMed] [Google Scholar]
- 10.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. [DOI] [PubMed] [Google Scholar]
- 11.Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC Early presentation of hemispheric intracerebral hemorrhage: prediction of outcome and guidelines for treatment allocation. Neurology. 1994;44(1):133–139. [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987–993. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe J, AlKhawam L, Du H, et al. Outcome predictors and spectrum of treatment eligibility with prospective protocolized management of intracerebral hemorrhage. Neurosurgery. 2009;64(3):436–445; discussion 445–446. [DOI] [PubMed] [Google Scholar]
- 14.Tuhrim S, Horowitz DR, Sacher M, Godbold JH Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27(3):617–621. [DOI] [PubMed] [Google Scholar]
- 15.St Louis EK, Wijdicks EF, Li H, Atkinson JD Predictors of poor outcome in patients with a spontaneous cerebellar hematoma. Can J Neurol Sci. 2000;27(1):32–36. [DOI] [PubMed] [Google Scholar]
- 16.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir O, Calisaneller T, Hasturk A, Aydemir F, Caner H, Altinors N Prognostic significance of third ventricle dilation in spontaneous intracerebral hemorrhage: a preliminary clinical study. Neurol Res. 2008;30(4):406–410. [DOI] [PubMed] [Google Scholar]
- 18.Hanley DF Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40(4):1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40(4):616–619. [DOI] [PubMed] [Google Scholar]
- 20.Roos YB, Hasan D, Vermeulen M Outcome in patients with large intraventricular haemorrhages: a volumetric study. J Neurol Neurosurg Psychiatry. 1995;58(5):622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143(1):91–96. [DOI] [PubMed] [Google Scholar]
- 22.Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med. 2009;37(3):969–974, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan TC, Dawson J, Spengler D, et al. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013;44(3):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKissock W, Richard A, Taylor J Primary intracerebral haemorrhage: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;278(7196):221–226. [Google Scholar]
- 26.Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neuro-surg. 1989;70(4):530–535. [DOI] [PubMed] [Google Scholar]
- 27.Juvela S, Heiskanen O, Poranen A, et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70(5):755–758. [DOI] [PubMed] [Google Scholar]
- 28.Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47(10):1103–1106. [DOI] [PubMed] [Google Scholar]
- 29.Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51(5):1359–1363. [DOI] [PubMed] [Google Scholar]
- 30.Zuccarello M, Brott T, Derex L, et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30(9):1833–1839. [DOI] [PubMed] [Google Scholar]
- 31.Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34(4):968–974. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen JP, Decq P, Brugieres P, et al. A technique for stereotactic aspiration of deep intracerebral hematomas under computed tomographic control using a new device. Neurosurgery. 1992;31(2):330–334; discussion 334–335. [DOI] [PubMed] [Google Scholar]
- 33.Hattori N, Katayama Y, Maya Y, Gatherer A Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101(3):417–420. [DOI] [PubMed] [Google Scholar]
- 34.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387–397. [DOI] [PubMed] [Google Scholar]
- 35.Prasad K, Mendelow AD, Gregson B Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev. 2008;(4):CD000200. [DOI] [PubMed] [Google Scholar]
- 36.Mendelow AD, Gregson BA, Mitchell PM, Murray GD, Rowan EN, Gholkar AR Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials. 2011; 12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backlund EO, von Holst H Controlled subtotal evacuation of intracerebral haematomas by stereotactic technique. Surg Neurol. 1978;9(2):99–101. [PubMed] [Google Scholar]
- 39.Kandel EI, Peresedov VV Stereotaxic evacuation of spontaneous intracerebral hematomas. J Neurosurg. 1985;62(2):206–213. [DOI] [PubMed] [Google Scholar]
- 40.Doi E, Moriwaki H, Komai N, Iwamoto M [Stereotactic evacuation of intracerebral hematomas]. Neurol Med Chir (Tokyo). 1982;22(6):461–467. [DOI] [PubMed] [Google Scholar]
- 41.Montes JM, Wong JH, Fayad PB, Awad IA Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: protocol and preliminary experience. Stroke. 2000;31(4):834–840. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto K, Hondo H CT-guided stereotaxic evacuation of hypertensive intracerebral hematomas. J Neurosurg. 1984;61(3):440–448. [DOI] [PubMed] [Google Scholar]
- 43.Miller DW, Barnett GH, Kormos DW, Steiner CP Stereotactically guided thrombolysis of deep cerebral hemorrhage: preliminary results. Cleve Clin J Med. 1993;60(4):321–324. [DOI] [PubMed] [Google Scholar]
- 44.Hondo H, Uno M, Sasaki K, et al. Computed tomography controlled aspiration surgery for hypertensive intracerebral hemorrhage. Experience of more than 400 cases. Stereotact Funct Neurosurg. 1990;54–55:432–437. [DOI] [PubMed] [Google Scholar]
- 45.Mohadjer M, Braus DF, Myers A, Scheremet R, Krauss JK CT-stereotactic fibrinolysis of spontaneous intracerebral hematomas. Neurosurg Rev. 1992;15(2):105–110. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Zhang Y, Liu L, et al. A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147–151. [DOI] [PubMed] [Google Scholar]
- 48.Awad IA, Dey M, Jaffe J Image-guided catheter evacuation and thrombolysis for intracerebral hematoma. Core Tech Oper Neurosurg Elsevier. 2011;244–252. [Google Scholar]
- 49.Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44(3):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang D, Sclabassi RJ, Horton JA Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19(4):553–572. [DOI] [PubMed] [Google Scholar]
- 51.Pang D, Sclabassi RJ, Horton JA Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19(4):547–552. [DOI] [PubMed] [Google Scholar]
- 52.Pang D, Sclabassi RJ, Horton JA Lysis of intraventricular blood clot with urokinase in a canine model: part 1. Canine intraventricular blood cast model. Neurosurgery. 1986;19(4):540–546. [DOI] [PubMed] [Google Scholar]
- 53.Shen PH, Matsuoka Y, Kawajiri K, et al. Treatment of intraventricular hemorrhage using urokinase. Neurol Med Chir (Tokyo). 1990;30(5):329–333. [DOI] [PubMed] [Google Scholar]
- 54.Huttner HB, Tognoni E, Bardutzky J, et al. Influence of intraventricular fibrinolytic therapy with rt-PA on the long-term outcome of treated patients with spontaneous basal ganglia hemorrhage: a case-control study. Eur JNeurol. 2008;15 (4):342–349. [DOI] [PubMed] [Google Scholar]
- 55.Vereecken KK, Van Havenbergh T, De Beuckelaar W, Parizel PM, Jorens PG Treatment of intraventricular hemorrhage with intraventricular administration of recombinant tissue plasminogen activator A clinical study of 18 cases. Clin Neurol Neurosurg. 2006;108(5):451–455. [DOI] [PubMed] [Google Scholar]
- 56.Kumar K, Demeria DD, Verma A Recombinant tissue plasminogen activator in the treatment of intraventricular hemorrhage secondary to periventricular arteriovenous malformation before surgery: case report. Neurosurgery. 2003;52 (4):964–968; discussion 968–969. [DOI] [PubMed] [Google Scholar]
- 57.Findlay JM, Weir BK, Stollery DE Lysis of intraventricular hematoma with tissue plasminogen activator. Case report. J Neurosurg. 1991;74(5):803–807. [DOI] [PubMed] [Google Scholar]
- 58.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247(2):117–121. [DOI] [PubMed] [Google Scholar]
- 59.Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54(3):577–583; discussion 583–584. [DOI] [PubMed] [Google Scholar]
- 60.Morgan T, Awad I, Keyl P, Lane K, Hanley D Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008;105:217–220. [DOI] [PubMed] [Google Scholar]
- 61.Hinson HE, Melnychuk E, Muschelli J, Hanley DF, Awad IA, Ziai WC Drainage efficiency with dual versus single catheters in severe intraventricular hemorrhage. Neurocrit Care. 2012;16(3):399–405. [DOI] [PubMed] [Google Scholar]
- 62.Staykov D, Huttner HB, Lunkenheimer J, et al. Single versus bilateral external ventricular drainage for intraventricular fibrinolysis in severe ventricular haemorrhage. J Neurol Neurosurg Psychiatry. 2010;81(1):105–108. [DOI] [PubMed] [Google Scholar]
- 63.Jaffe J, Melnychuk E, Muschelli J, et al. Ventricular catheter location and the clearance of intraventricular hemorrhage. Neurosurgery. 2012;70(5):1258–1263; discussion 1263–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naff N, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42(11):3009–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb AJ, Ullman NL, Mann S, Muschelli J, Awad IA, Hanley DF Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventric-ular thrombolytic: the clot lysis: evaluating accelerated resolution of IVH (CLEAR IVH) program. Stroke. 2012;43(6):1666–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrick DB, Ziai WC, Thompson CB, Lane K, McBee NA, Hanley DF Systemic hematologic status following intraventricular recombinant tissue-type plasminogen activator for intraventricular hemorrhage: the CLEAR IVH Study Group. Stroke. 2011;42(12):3631–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]