Abstract
Purpose:
B1+ and T1 corrections and dynamic multi-coil shimming approaches were proposed to improve the fidelity of high isotropic resolution Generalized slice dithered enhanced resolution (gSlider) diffusion imaging.
Methods:
An extended reconstruction incorporating B1+ inhomogeneity and T1 recovery information was developed to mitigate slab-boundary artifacts in short-TR gSlider acquisitions. Slab-by-slab dynamic B0 shimming using a multi-coil integrated ΔB0/Rx shim-array, and high in-plane acceleration (Rinplane=4) achieved with virtual-coil GRAPPA were also incorporated into a 1 mm isotropic resolution gSlider acquisition/reconstruction framework to achieve a significant reduction in geometric distortion compared to single-shot EPI.
Results:
The slab-boundary artifacts were alleviated by the proposed B1+ and T1 corrections compared to the standard gSlider reconstruction pipeline for short-TR acquisitions. Dynamic shimming provided >50% reduction in geometric distortion compared to conventional global 2nd order shimming. 1 mm isotropic resolution diffusion data show that the typically problematic temporal and frontal lobes of the brain can be imaged with high geometric fidelity using dynamic shimming.
Conclusions:
The proposed B1+ and T1 corrections and local-field control substantially improved the fidelity of high isotropic resolution diffusion imaging, with reduced slab-boundary artifacts and geometric distortion compared to conventional gSlider acquisition and reconstruction. This enabled high-fidelity whole-brain 1 mm isotropic diffusion imaging with 64 diffusion-directions in 20 minutes using a 3T clinical scanner.
Keywords: gSlider, B1+ inhomogeneity, shim array, diffusion-weighted imaging
Introduction
High-resolution diffusion-weighted imaging (DWI) with echo-planar imaging (EPI) acquisition technique is a powerful tool for many neuroscientific and clinical applications. Single-shot EPI (SS-EPI) (1) is one of the most commonly used methods in DWI due to its rapid encoding ability. However, the SS-EPI readout does not lend itself to high in-plane resolution imaging due to severe geometric distortion and T2*-related blurring artifacts, which are difficult to correct in post-processing, particularly where voxel pile-up occurs (2). Multi-shot EPI (3–5) is a promising approach to improve the geometric fidelity of DWI and achieve high in-plane resolution with low distortion and T2* blurring. However, due to the prolonged scan time, shot-to-shot phase variations and potential patient motion, multi-shot EPI continues to be a challenge in DWI. To mitigate these problems, previous studies combined multi-shot approaches with parallel imaging (6,7), sparse or low-rank models (8–10), joint reconstruction (11–15) and simultaneous multi-slice (SMS) (16–18) to accelerate the acquisition and correct for shot-to-shot phase variations.
Another approach to mitigate distortion is to generate a compensating B0 magnetic field to counteract the off-resonance effects. Conventional scanners are equipped with 1st order (the linear gradients) and static 2nd order spherical harmonic shim coils, which generate a spatial magnetic profile to compensate the B0 field over the target volume (19,20). Local multi-coil (MC) shimming using small shim coils patterned around the imaged object has been introduced as a convenient way to provide higher-order B0 shimming without the need to substantially modify the MRI scanner (21–24). Compared to 2nd order shimming, MC shim arrays have been shown to provide improved B0 homogeneity to improve EPI acquisitions for structural and fMRI applications (25,26), and magnetic resonance spectroscopic imaging (MRSI) acquisition for spectroscopy studies (27). The ability of MC arrays to rapidly switch their shim currents without causing image artifacts allows the B0 shim to be optimized on a slice-by-slice basis, providing further gains in B0 homogeneity and mitigation of EPI geometric distortion (21).
Improving SNR efficiency is critical for achieving high isotropic resolution DWI. Three dimensional multi-slab DWI has emerged as a promising strategy to enhance the SNR in such acquisitions (28–32). However, shot-to-shot phase variations and slab-boundary artifacts are key challenges for efficient sampling of whole-brain high-resolution DWI with this technique (33), where a number of effective techniques has been developed to mitigate these issues (30,33,34). Another promising approach for high-SNR efficiency, high-resolution DWI is the Generalized SLIce Dithered Enhanced Resolution (gSlider) method. gSlider is a simultaneous multi-slab (SMSb) acquisition technique with self-navigated RF slab-encoding, which has been demonstrated for motion-robust, high-resolution DWI (35,36). Here, the simultaneous multi-slabs are acquired together using the blipped-CAIPI acquisition scheme (37) and separated through parallel imaging. With gSlider, a large number of slices can be acquired together per EPI-encoding (e.g. 10 simultaneous slices using gSlider RF-encoding of 5x and Multiband factor of 2x) to achieve a short-TR for high resolution volumetric DWI and provide high SNR-efficiency. The encoding within each slab is then performed through sequential RF slab-encoding acquisitions and combined using super-resolution reconstruction to create high-resolution slices. The gSlider RF-encodings are designed to provide high signal in each of the RF-encoded acquisitions for robust estimation and removal of shot-to-shot phase corruptions, which removes the need for the additional lengthy navigator module per TR in conventional 3D multi-slab EPI acquisition. With such RF-encoding, there is a small reduction in the orthogonally of the encoding bases (i.e. compare to kz encoding), which translate to a minor reduction in the SNR gain from to for 5x encoding (35). The gSlider method is also robust to bulk motion using designed RF-encoding basis and spatially varying regularizations (36). With this approach, the effects of subject motion can be estimated and mitigated every TR/2 without motion navigator. As such, the motion sensitivity timeframe is less than 2 seconds rather than multiple tens of seconds in 3D multi-slab EPI. Finally, with this approach, the ratio of slab thickness relative to slice resolution can be kept small (e.g. 5 for gSlider 5x) which allows for sharp slab selective excitation with reduced slab boundary issues from partial excitation compared to typical 3D multi-slab DWI, and remove the need for slab-oversampling. This has enabled high-quality submillimeter gSlider DWI without slab boundary correction at TR ~4.5s or above (35,36,38). However, at shorter TRs slab boundary artifacts can still remain an issue, with partial T1 recovery in adjacent slabs causing striping artifacts. Therefore, a correction approach similar to nonlinear inversion for slab profile encoding (NPEN) (33) is needed to achieve high-quality reconstruction.
In this work, we developed approaches to improve the fidelity of gSlider: first by making it robust to slab-boundary artifacts, and second by reducing geometric distortion from B0 inhomogeneity. To mitigate slab-boundary artifacts in short TR acquisitions, dictionary-based B1+ inhomogeneity and T1 recovery corrections were incorporated into gSlider reconstruction. To mitigate image distortions, a 32-channel integrated ΔB0/Rx array (AC/DC coil) (21) was utilized for slab-by-slab shimming, to reduce B0 inhomogeneity by >50% as compared to static 2nd order shimming. This dual-purpose coil array provides both high spatial-order B0 field control as well as good parallel imaging capability. This is then combined with high in-plane acceleration (Rinplane=4) and virtual conjugate coil (VCC-) GRAPPA reconstruction (11,39), to achieve 8–11 fold total geometric distortion reduction in single-shot gSlider-EPI. We demonstrate that the proposed method can achieve high-fidelity whole-brain 1 mm isotropic DWI with 64 diffusion-directions in ~20 minutes on a clinical 3T scanner.
Methods
Pulse sequence design and slab-optimized dynamic shimming
Figure 1(a) shows the sequence diagram of gSlider, where two external triggers are added in each TR to enable slab-by-slab B0 shimming with the AC/DC coil (Fig 1b). To avoid poor performance in whole-brain fat suppression from large out-of-slab B0 inhomogeneity, the slab-by-slab shimming was turned off during fat saturation. In each TR, an additional ky blip was added to shift k-space and create more unique source points for improved virtual conjugate-coil GRAPPA reconstruction at high accelerations in DWI as outlined in (11,40).
Figure 1.
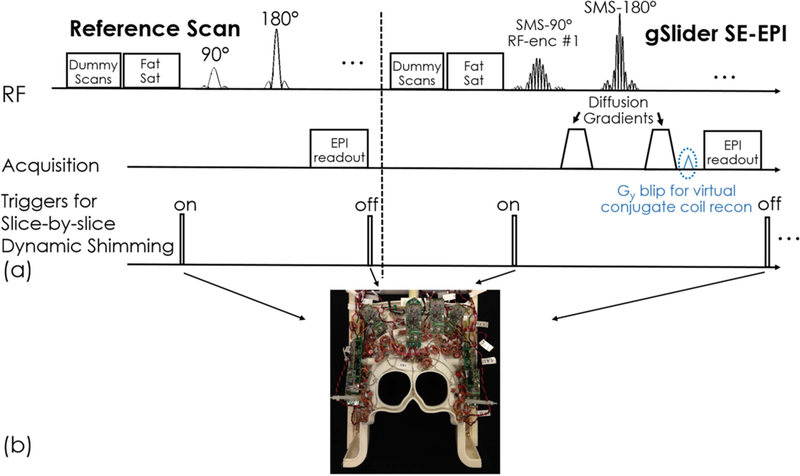
(a) Sequence diagram of gSlider acquisition and slab-by-slab triggers. (b) The 32-channel combined RF receive and B0 shim array (“AC/DC” coil) with independent control of shim currents in each loop in the array.
To generate a sharp slab-encoding performance, the Shinnar-Le Roux (SLR) algorithm (41) was used to design the gSlider excitation RF pulses. The time-bandwidth product of the 90o gSlider RF excitation pulses was 12, with a pulse duration of 11 ms. Figure 2(a) and (b) show the waveforms of five gSlider-encoding RF pulses that were used and their corresponding Bloch simulated slab profiles. After slab-encoding, a standard SLR spin-echo (SE) refocusing pulse was applied without gSlider-encoding. The time-bandwidth product of refocusing pulse was 8 and the duration of the refocusing pulse was 7.3 ms. The blue lines in Fig. 2(b) show the sub-slab profiles of gSlider-encoding after the SE refocusing pulse. To reduce the peak power of the RF pulses, a VERSE method (42) was applied to both gSlider excitation RF pulses and SE refocusing pulse.
Figure 2.
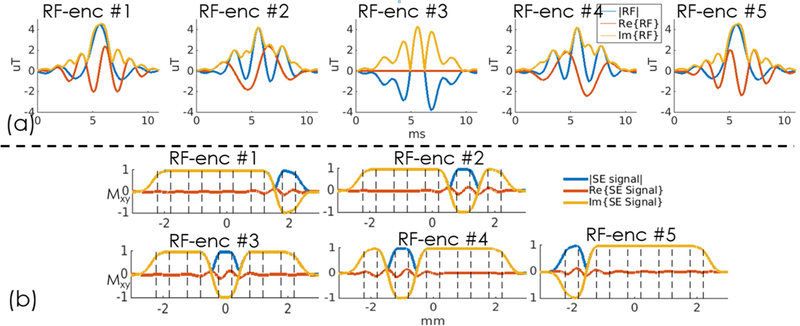
(a) Five RF-encoded gSlider pulses and (b) their corresponding Bloch simulated slab profiles.
To implement slab-optimized shimming, a low-resolution B0 field-map with conventional global-shims applied was acquired using a vendor-provided two-TE gradient echo field mapping sequence. The field maps were registered to the thin-slab gSlider images and then masked using FSL BET and phase unwrapped using FSL PRELUDE (43). The optimal DC shim currents in each channel of the AC/DC coil were then computed on a slab-wise basis using a previously-acquired calibration B0 map basis set for the array. The details of the constrained optimization algorithm can be found in (21). For SMSb acquisition, the shims were jointly optimized over the two simultaneously-acquired slabs. To match the geometric distortions between slab-collapsed EPI data and fully-sampled reference data, the slab ordering of shimming and the currents used in the individual slab of reference data are the same as the corresponding slab group of the SMSb data. To avoid artifacts, the GRAPPA and SMS calibration scan data for each slab were shimmed with the same MC shim fields as the DWI acquisition.
B1+ and T1 corrections for robust gSlider reconstruction
To eliminate shot-to-shot background phase variations in the acquired diffusion data, real-valued diffusion processing (44) was applied. gSlider reconstruction was then performed to obtain high slice-resolution data, using a forward model based on the Bloch simulated slab profiles of the gSlider encodings. Pseudoinverse with Tikhonov regularization was used:
| [1] |
where b (matrix size: (Nslab·Nrf-encoding)×1) is the concatenation of acquired thin-slab data at a given in-plane spatial location, X (matrix size: (Nslice×1) is the corresponding super-resolution reconstruction, A (matrix size: (Nslab·Nrf-encoding)×(Nslab·Nrf-encoding)) is the RF-encoding matrix that contains the sub-slab profiles simulated from the Bloch equations and λ is a Tikhonov regularization parameter. In our previous work (35), the same slab profiles (Mxy) of the RF-encodings were used for the reconstruction at all spatial locations contained within the A encoding-matrix. However, this does not account for potential spatial variations in Mxy due to B1+ inhomogeneity. Furthermore, the initial longitude magnetization (Mz) in the Bloch simulation was set to 1, which ignored incomplete T1 recovery. This can be particularly problematic in the adjacently partial excitation regions of a non-ideal RF excitation after TR/2 slab-interleaved acquisition at short TRs. These imperfections could cause slab-boundary artifacts due to B1+ variations.
To mitigate slab-boundary artifacts, RF-encoding imperfections due to B1+ inhomogeneity and incomplete T1 recovery were estimated and incorporated into the RF-encoding matrix A of the gSlider reconstruction in Eq.[1]. Figure 3(a) shows the flowchart of B1+ inhomogeneity correction, where RF-encoding profiles at a range of discretized B1+ values ([0.70:0.05:1.30], ±30% B1+ variations) are simulated by using Bloch equation, which enabled the creation of a dictionary of RF-encoding matrices with different B1+ variations. The proposed B1+ correction method is a 3D ‘voxel-by-voxel’ correction scheme based on a 3D discretized B1+ map. For each spatial location, the corresponding RF-encoding matrix from the dictionary was selected based on the corresponding B1+ value (shown in Fig. 3c) and thereby spatial varying B1+ inhomogeneity is corrected by voxel-by-voxel correction.
Figure 3.
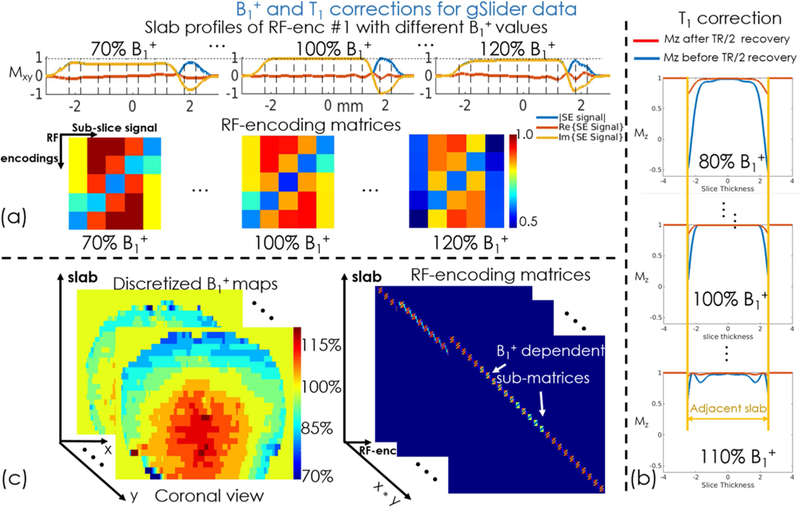
(a) Slab profiles (Mxy) of a RF-encoded gSlider pulse with B1+ inhomogeneity and (b) Longitude Magnetizations (Mz) of the adjacent slab before and after TR/2 recovery. (c) The flowchart of B1+ correction using the pre-scan B1+ maps.
For incomplete T1 recovery, non-ideal slab-profiles of RF-encodings can cause partial excitations in adjacent slabs which are not fully-recovered in slab-interleaved acquisitions with short TRs. This effect was also modeled by adding partial recovered initial longitude magnetizations Mz into the Bloch simulation of the RF-encodings (assuming average T1=1000 ms in the brain), thereby incorporating them into the encoding matrix. Figure 3(b) shows the partial Mz-recovery from adjacent slabs excitations before and after TR/2 longitudinal-relaxation at various B1+ excitation levels.
Data acquisition
All in vivo measurements were performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) with a custom 32-channel AC/DC receive array with added B0 shim capability (21). To assess the improvements provided by slab-optimized shimming, gSlider-EPI with 5 slab-encodings and the corresponding B0 field maps were acquired. The imaging parameters for gSlider data were: FOV 220×220×170 mm3, 34 thin-slabs (5 mm slab-encoding), TR/TE=5100/77 ms, and echo spacing=0.93 ms. To accentuate changes in geometric distortion, data were acquired using both Anterior-to-Posterior (AP) and Posterior-to-Anterior (PA) phase-encodings at different in-plane accelerations (Rinplane=1 and Rinplane =4), with and without slab-optimized shimming. The B0 field maps were acquired using two-echo GRE with 2.5mm slice-thickness and 100% gap. The slice resolution including gap matches the 5 mm gSlider slab-encoding. To validate the shim performance of SMSb imaging, the same gSlider data were acquired with a multi-band (MB) factor of 2 and compared with non-SMSb gSlider data. A matching T2 turbo spin-echo (T2-TSE) data was also acquired as a distortion-free reference.
Whole-brain 1 mm isotropic resolution diffusion imaging data were also acquired with gSlider-EPI and dynamic MC shimming. The protocol used: FOV 220×220×170 mm3, Rinplane× MB×gSlider =4×2×5, 34 thin-slabs (5 mm slab-encoding), b = 1000 s/mm2 with 64 diffusion-directions and 4 interleaved b =0 s/mm2, TR/TE =3500/86 ms. The total acquisition time is ~20 minutes.
To correct the B1+ effects in gSlider data, an FOV-matched B1+ map was obtained by using a Turbo-FLASH scan with pre-conditioning RF pulses (45). The in-plane resolution is 3.4×3.4 mm2 with 2.5 mm slice thickness and 100% gap. The slice resolution including gap matched the 5 mm gSlider slab-encoding.
Reconstruction and post-processing
To enable higher in-plane acceleration compared to conventional slice- and in-plane-GRAPPA reconstruction and further reduce the geometric distortions, virtual conjugate-coil GRAPPA with phase-matching was used to achieve high fidelity reconstruction for high acceleration factors. To achieve faster GRAPPA reconstruction, SVD coil compression (46) was applied to compress the 32 channel coil to 20 channels. After GRAPPA reconstruction, the five RF-encoded volumes of each diffusion-direction were then combined to create thin-slice data, using gSlider reconstruction with and without the proposed modified RF-encoding matrix. The RF-encoding matrix was generated using the SLR RF-pulse design and Bloch simulation toolbox (47) (https://vuiis.vumc.org/~grissowa/software.html). The virtual conjugate-coil GRAPPA and gSlider reconstruction algorithms were implemented in MATLAB R2014a (The MathWorks, Inc., Natick, MA). The reconstructed data were then corrected for motion and eddy-current distortion using the “eddy_correct” function from the FMRIB Software Library (43) (FSL, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Diffusion tensor model was fitted using FSL’s “dtifit” function to obtain the fractional anisotropy (FA) maps and the primary eigenvectors.
Results
Figure 4 shows the results of B1+ and T1 corrections in gSlider reconstruction at TR of 3.5s. The slab-boundary artifacts shown in the sagittal and coronal views of a diffusion-weighted volume are well mitigated by incorporating T1 correction into the B1+ corrected processing compared to the standard gSlider reconstruction without corrections and with B1+ correction only, which demonstrates the utility of the proposed B1+ and T1 corrections in gSlider reconstruction in a short-TR acquisition.
Figure 4.

gSlider diffusion-weighted image results without correction, with B1+ correction only, and with B1+ & T1 corrections. The white arrows highlight the further mitigation of striping artifacts by using T1 correction. To demonstrate the efficiency of the proposed B1+ & T1 correction methods, the diffusion weighted gSlider data were acquired in axial orientation, with resolution of 1.5×1.5×1.0 mm3.
Figure 5 shows compares image distortion with and without dynamic slab-optimized MC B0 shimming. The green arrows highlight the B0 distortion that was alleviated, with slab-by-slab shimming achieving >50% reduction of standard deviation (std) in ΔB0 across the slab when compared to baseline global 2nd order shimming. Dynamic MC shimming was then combined with in-plane acceleration (Rinplane=4) to achieve an 8–11x total reduction in ΔB0 distortion (depending on the slab), yielding images with outlines (red outlines in Fig. 5) closely matching that of the reference T2-TSE images.
Figure 5.
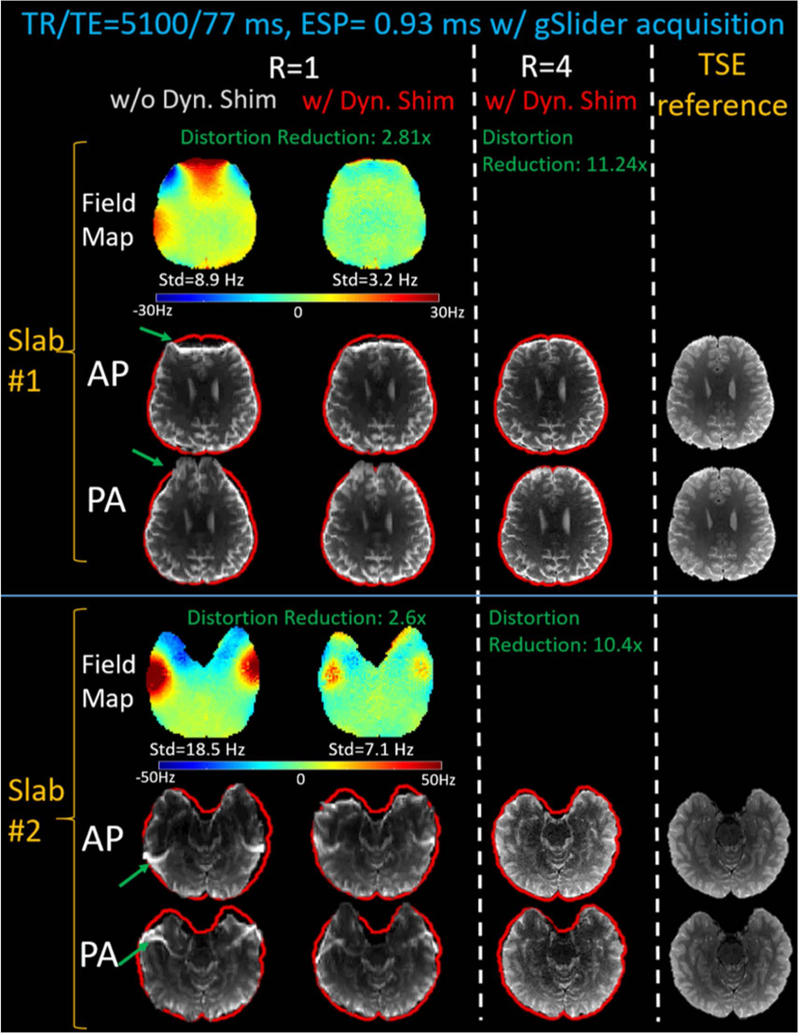
The comparisons of the B0 field maps and MB-1 EPI distortions with Anterior-to-Posterior (AP) and Posterior-to-Anterior (PA) phase-encodings, with and without dynamic multi-coil B0 shimming. The shimming is then combined with Rinplane=4 acceleration to achieve ~10-fold reduction in EPI distortion, bringing the AP and PA images into closer alignment. The resulting low-distortion EPI slices resemble the distortion-free T2-TSE reference slices (see red brain mask outline).
Figure 6 shows the B0 field maps obtained from global 2nd order shim, MB-2 and MB-1 dynamic MC shimming. Compared to MB-1 slab-optimized shimming, the MB-2 case applies shims simultaneously to two distant slabs, providing similar standard deviations of ΔB0 variations to the MB-1 case, while the out-of-slab regions were unconstrained and allowed to have a poor shim.
Figure 6.
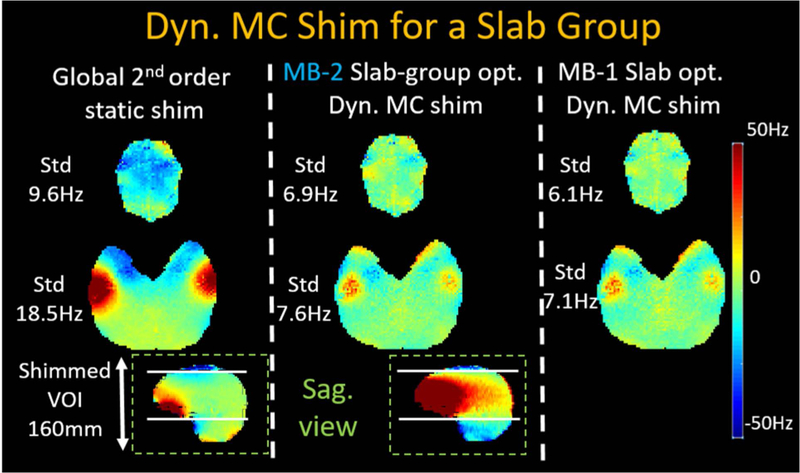
Compared to baseline 2nd-order spherical harmonic global shimming, dynamic MC shimming for MB-2 acquisitions jointly shims two slabs at the same time, while out-of-slab regions are unconstrained and are allowed to have poor shims. The shim results of MB-2 are performance closely to MB-1 slab-optimized shimming.
Figure 7 shows the dynamic MC shimming results of two representative slabs for MB-1 and MB-2 with A-P and P-A phase encoding directions. Compared to the reference images, the contours of both the MB-1 and MB-2 images closely match those of the T2-TSE images, which demonstrates that the dynamic MC shimming of MB-2 achieved a similar performance to MB-1 slab-optimized dynamic MC shimming. This demonstrates that the 32ch AC/DC coil has enough degrees of freedom to simultaneously control the B0 field in two spatially-separated slices at the same time with minimal loss of performance.
Figure 7.
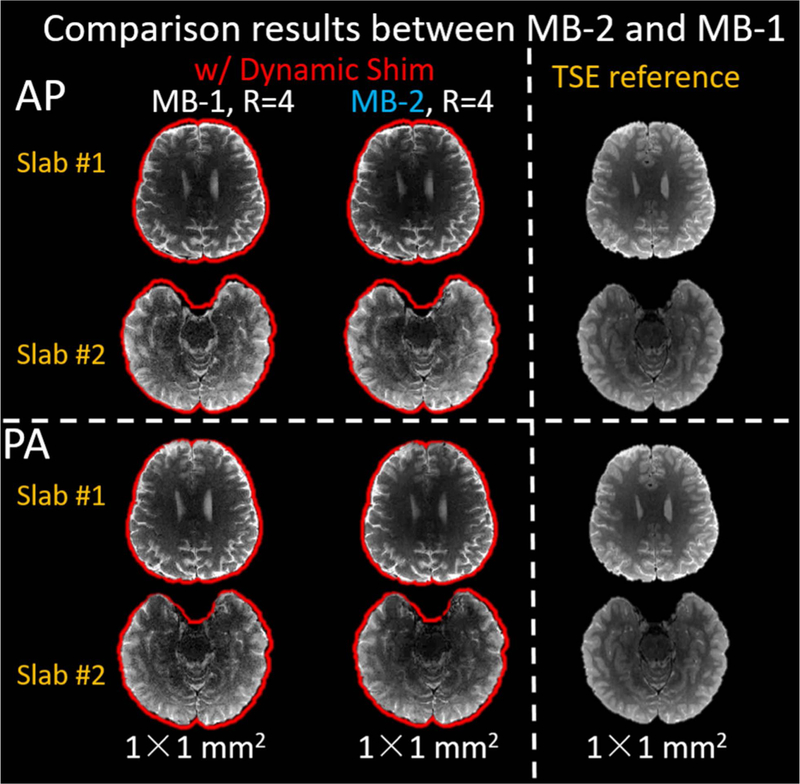
Comparison between MB-2 and MB-1. The MB-2 shimming performance closely resembles that of the MB-1 slab-optimized shimming. The red outlines show the contours of T2-TSE reference brain mask.
Figure 8 shows the averaged DW images from 64 diffusion-encoding directions (Fig. 8a) and directionally-encoded color FA maps (Fig. 8b) of the 1mm isotropic gSlider diffusion data. High-quality results are shown in Fig. 8(c), with minimal geometric distortions in the typically problematic temporal and frontal lobes. The high-quality results depict the primary eigenvectors from DTI with high fidelity (Fig. 8c) which are beneficial for mapping structural connectivity using diffusion tractography and mapping cortical diffusion patterns in these problematic regions.
Figure 8.

(a) Averaged diffusion-weighted images and (b) directionally-encoded color fractional anisotropy (FA) maps of the 1 mm isotropic diffusion-weighted data with 64 directions obtained in ~20-minutes using the proposed correction and dynamic shimming framework. (c) The primary eigenvectors from diffusion tensor imaging in typically problematic temporal and frontal lobes of the brain were more accurately depicted by synergistically combining dynamic B0 shimming with parallel imaging acceleration. The primary eigenvectors were color-encoded (red: left-right, green: anterior-posterior, blue: superior-inferior) and overlaid on FA maps.
Discussion
In this work, we developed synergistic approaches to improve gSlider acquisitions where i) reconstruction with B1+ and T1 corrections effectively mitigate slab-boundary artifacts in short-TR acquisitions, and ii) dynamic MC B0 shimming and high in-plane acceleration achieve an 8–11-fold reduction in B0 distortion. The results demonstrate that the proposed corrections and local-field control can together achieve high-quality, high-fidelity DWI with 1 mm isotropic resolution in 20 minutes on a 3T clinical scanner.
To minimize the slab-boundary artifacts, there is a trade-off between the design of slab-thickness of the RF refocusing pulse and the sensitivity of gSlider to B1+ inhomogeneity. Using a slightly thicker refocusing slab than the target slab thickness can improve the refocusing performance at the edges of the slab, since the refocusing slab’s transition bands are moved away from the target slab region. This is particularly useful in the presence of B1+ inhomogeneity, where the refocusing performance at the edge of the refocusing slab can degrade significantly (much more than in the excitation pulse). This thicker refocusing approach was employed in our previous work to provide robustness to B1+ inhomogeneity, where the gSlider acquisition was performed with a TR of ~4.5s. However, the partial excitations in adjacent slabs induced by this broader refocusing pulse can cause striping artifacts for short TR acquisitions due to the partial recovery. For this work, we employed matching excitation and refocusing slab thickness to avoid large T1 recovery issue while correcting for the increased B1+ inhomogeneity effect through a modified reconstruction. The proposed method corrects both B1+ inhomogeneity and partial T1 recovery jointly by using a dictionary of RF-encoding matrices, which has been demonstrated to improve the fidelity of gSlider data for short-TR acquisitions.
In this work, a TR of 3.5s was chosen for the acquisition to balance the trade-off between SNR efficiency vs. spin-history and motion sensitivity issues. With our whole-brain 1mm isotropic gSlider acquisition, the TR can be further shortened to 2.5s to provide higher SNR efficiency (48) and shorter total acquisition time. However, at such TR, with a shorter T1 recovery period, slab boundary artifacts and motion sensitivity will be more severe, requiring the development of more advanced correction approach such as in (33). Employing a TR of 3.5s markedly reduced these issues, while still achieving an SNR-efficiently level that is at 80%−95% of the optimal value for white matter and gray matter tissues (48).
There are some limitations in the proposed B1+ and T1 corrections. First, in the Bloch simulation and dictionary generation, the continuous B1+ variations were discretized and sorted into several discretized bins, which may not reflect the accurate slab-profile at a given position. However, since the spatial B1+ values vary slowly and smoothly, the discretization of B1+ maps should not affect the B1+ correction unduly. Second, for T1 correction, the T1 value used in the study is 1000ms, which is taken to be the average value across the gray- and white-matter tissues of the brain (49). However, this assumption does not accurately reflect the complicated biochemical environment in the brain, leading to residual slab-boundary artifacts due to partial volume effects caused by high-T1 compartments such as blood and cerebrospinal fluid. Nevertheless, these residual artifacts have minimal impact on diffusion-weighted images because the fluid is almost completely attenuated by the diffusion encodings.
Slab-by-slab dynamic shim updating was used for gSlider acquisition. With a 32-channel custom-built AC/DC coil, the B0 inhomogeneity was reduced by >50%, thereby significantly mitigating the geometric distortion in EPI slices. Combined with parallel imaging with high in-plane acceleration (Rinplane=4), the geometric distortion was further reduced. Consequently, the resultant images closely matched the TSE reference across all brain regions. Furthermore, we extended our slab-by-slab shimming scheme to slab-group-by-slab-group shimming for SMSb acquisitions that shim two distant slabs simultaneously. Compared to post-processing based EPI distortion correction methods (50–52) which require additional data (e.g. reversed phase-encoding images) and processing time, our proposed dynamic MC shimming method only needs a fast low-resolution B0 scan for computing the optimal shim currents and reduce the geometric distortion during the acquisition. Furthermore, for high-resolution DWI, the dynamic MC shimming method reduces geometric distortion at its source, whereas the post-processing methods have limited ability to accurately correct for severe voxel pile-up.
The level of distortion reduction from our approach at 10–12x is similar to that of current state-of-the-art multi-shot EPI approaches, such as readout-segmented EPI (5). However, for high resolution imaging, there will still be some remaining distortion even with such approach. For example, for the 1mm resolution case (the FOV is 220 mm and the echo spacing is 0.93 ms), the distortion level at large susceptibility regions at e.g. 50Hz off-resonance, would be 0.91 voxel with dynamic shim 2.8x reduction and in-plane 4x acceleration (the distorted voxels in this region can be calculated as: 50Hz/[1000/(220*0.93ms)*(2.8*4)] =0.91 voxel). In applications where geometric fidelity is of high priority, our acquisition method can also be combined with the dual AP and PA acquisition scheme, along with post-processing correction, such as the topup approach (2). In such case, the distortion level to be corrected would be much smaller with our method, allowing the topup correction to work robustly.
Dynamic MC shimming is not only applicable to gSlider acquisitions, but can also be easily applied to single- and multi-shot diffusion acquisitions such as single-shot SMS-EPI (37), multi-shot EPI acquisitions (3,5), which can enable high-resolution, high-quality functional MRI and DWI with low geometric distortion and short acquisition times.
Conclusion
We proposed B1+ and T1 corrections with the dynamic MC B0 shimming strategy to improve the fidelity of high-resolution gSlider-EPI acquisitions with low geometric distortion. In vivo studies demonstrated that the proposed methods enabled markedly improved image quality with reduced slab-boundary artifacts for short-TR acquisition. The more than 2x geometric distortion reduction can be achieved by dynamic multi-coil shimming. Combined with parallel imaging with high in-plane acceleration (Rinplane=4), the geometric distortion was further reduced, which should improve the quality of diffusion data that can be applied to many clinical and neuroscience applications.
Acknowledgement
We thank Dr. Merlin Fair for the careful proofreading. This work was supported in part by NIH research grants: R01EB020613, R01MH116173, P41EB015896, R00EB021349, R01EB016695, U01EB025162, U01EB026996, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, and S10RR023043.
References
- 1.Mansfield P Multi-planar image formation using NMR spin echoes. J. Phys. C Solid State Phys 1977;10:L55–L58 doi: 10.1088/0022-3719/10/3/004. [DOI] [Google Scholar]
- 2.Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003;20:870–888 doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen N kuei, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41–47 doi: 10.1016/j.neuroimage.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong HK, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn. Reson. Med 2013;69:793–802 doi: 10.1002/mrm.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn. Reson. Med 2009;62:468–475 doi: 10.1002/mrm.22024. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Ma X, Truong TK, Song AW, Guo H. Phase-updated regularized SENSE for navigator-free multishot diffusion imaging. Magn. Reson. Med 2017;78:172–181 doi: 10.1002/mrm.26361. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Huang F, Ma X, Xie S, Guo H. Self-feeding MUSE: A robust method for high resolution diffusion imaging using interleaved EPI. Neuroimage 2015;105:552–560 doi: 10.1016/j.neuroimage.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Liao C, Chen Y, Cao X, et al. Efficient parallel reconstruction for high resolution multishot spiral diffusion data with low rank constraint. Magn. Reson. Med 2017;77:1359–1366 doi: 10.1002/mrm.26199. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Levine EG, Tian Q, et al. Motion-robust reconstruction of multishot diffusion-weighted images without phase estimation through locally low-rank regularization. Magn. Reson. Med 2018. doi: 10.1002/mrm.27488. [DOI] [PMC free article] [PubMed]
- 10.Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS). Magn. Reson. Med 2017;78:494–507 doi: 10.1002/mrm.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao C, Manhard MK, Bilgic B, et al. Phase-matched virtual coil reconstruction for highly accelerated diffusion echo-planar imaging. Neuroimage 2019;194:291–302 doi: 10.1016/j.neuroimage.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Haldar JP, Wedeen VJ, Nezamzadeh M, et al. Improved diffusion imaging through SNR-enhancing joint reconstruction. Magn. Reson. Med 2013;69:277–289 doi: 10.1002/mrm.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilgic B, Kim TH, Liao C, et al. Improving parallel imaging by jointly reconstructing multi-contrast data. Magn. Reson. Med 2018;80:619–632 doi: 10.1002/mrm.27076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilgic B, Chatnuntawech I, Manhard MK, et al. Highly accelerated multishot echo planar imaging through synergistic machine learning and joint reconstruction. Magn. Reson. Med 2019: 10.1002/mrm.27813. [DOI] [PMC free article] [PubMed]
- 15.Manhard MK, Bilgic B, Liao C, et al. Accelerated whole‐brain perfusion imaging using a simultaneous multislice spin‐echo and gradient‐echo sequence with joint virtual coil reconstruction. Magn. Reson. Med 2019: 10.1002/mrm.27784. [DOI] [PMC free article] [PubMed]
- 16.Setsompop K, Cohen-Adad J, Gagoski BA, et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage 2012;63:569–580 doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson 2013;229:90–100 doi: 10.1016/j.jmr.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai E, Ma X, Zhang Z, Yuan C, Guo H. Simultaneous multislice accelerated interleaved EPI DWI using generalized blipped-CAIPI acquisition and 3D K-space reconstruction. Magn. Reson. Med 2017;77:1593–1605 doi: 10.1002/mrm.26249. [DOI] [PubMed] [Google Scholar]
- 19.Roméo F, Hoult DI. Magnet field profiling: Analysis and correcting coil design. Magn. Reson. Med 1984;1:44–65 doi: 10.1002/mrm.1910010107. [DOI] [PubMed] [Google Scholar]
- 20.Stockmann JP, Wald LL. In vivo B0 field shimming methods for MRI at 7 T. Neuroimage 2018;168:71–87 doi: 10.1016/J.NEUROIMAGE.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockmann JP, Witzel T, Keil B, et al. A 32-channel combined RF and B0shim array for 3T brain imaging. Magn. Reson. Med 2016;75:441–451 doi: 10.1002/mrm.25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han H, Song AW, Truong T-K. Integrated parallel reception, excitation, and shimming (iPRES). Magn. Reson. Med 2013;70:241–247 doi: 10.1002/mrm.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong T-K, Darnell D, Song AW. Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain. Neuroimage 2014;103:235–240 doi: 10.1016/J.NEUROIMAGE.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juchem C, Nixon TW, McIntyre S, Boer VO, Rothman DL, de Graaf RA. Dynamic multi-coil shimming of the human brain at 7 T. J. Magn. Reson 2011;212:280–288 doi: 10.1016/J.JMR.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juchem C, Umesh Rudrapatna S, Nixon TW, de Graaf RA. Dynamic multi-coil technique (DYNAMITE) shimming for echo-planar imaging of the human brain at 7 Tesla. Neuroimage 2015;105:462–472 doi: 10.1016/J.NEUROIMAGE.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T, Lee Y, Zhao T, Hetherington HP, Pan JW. Gradient-echo EPI using a high-degree shim insert coil at 7 T: Implications for BOLD fMRI. Magn. Reson. Med 2017;78:1734–1745 doi: 10.1002/mrm.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boer VO, Klomp DWJ, Juchem C, Luijten PR, de Graaf RA. Multislice 1H MRSI of the human brain at 7 T using dynamic B0 and B1 shimming. Magn. Reson. Med 2012;68:662–670 doi: 10.1002/mrm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang HC, Hui ES, Chiu PW, Liu X, Chen NK. Phase correction for three-dimensional (3D) diffusion-weighted interleaved EPI using 3D multiplexed sensitivity encoding and reconstruction (3D-MUSER). Magn. Reson. Med 2018;79:2702–2712 doi: 10.1002/mrm.26944. [DOI] [PubMed] [Google Scholar]
- 29.Bruce IP, Chang H-C, Petty C, Chen N-K, Song AW. 3D-MB-MUSE: A robust 3D multi-slab, multi-band and multi-shot reconstruction approach for ultrahigh resolution diffusion MRI. Neuroimage 2017;159:46–56 doi: 10.1016/J.NEUROIMAGE.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Poser BA, Douaud G, et al. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. Neuroimage 2016;143:1–14 doi: 10.1016/J.NEUROIMAGE.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frost R, Miller KL, Tijssen RHN, Porter DA, Jezzard P. 3D Multi-slab diffusion-weighted readout-segmented EPI with real-time cardiac-reordered k-space acquisition. Magn. Reson. Med 2014;72:1565–1579 doi: 10.1002/mrm.25062. [DOI] [PubMed] [Google Scholar]
- 32.Engström M, Skare S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magn. Reson. Med 2013;70:1507–1514 doi: 10.1002/mrm.24594. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Koopmans PJ, Frost R, Miller KL. Reducing slab boundary artifacts in three-dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN). Magn. Reson. Med 2016;76:1183–1195 doi: 10.1002/mrm.26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van AT, Aksoy M, Holdsworth SJ, Kopeinigg D, Vos SB, Bammer R. Slab profile encoding (PEN) for minimizing slab boundary artifact in three-dimensional diffusion-weighted multislab acquisition. Magn. Reson. Med 2015;73:605–613 doi: 10.1002/mrm.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setsompop K, Fan Q, Stockmann J, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med 2018;79:141–151 doi: 10.1002/mrm.26653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Bilgic B, Dong Z, et al. Motion-robust sub-millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magn. Reson. Med 2018;80:1891–1906 doi: 10.1002/mrm.27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med 2012;67:1210–1224 doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Witzel T, Grissom WA, Setsompop K. Minimum peak power root- flipped gSlider-SMS RF pulses for high-resolution in vivo diffusion imaging In: ISMRM; Honolulu; 2017. p. 0523. [Google Scholar]
- 39.Blaimer M, Gutberlet M, Kellman P, Breuer FA, Köstler H, Griswold MA. Virtual coil concept for improved parallel MRI employing conjugate symmetric signals. Magn. Reson. Med 2009;61:93–102 doi: 10.1002/mrm.21652. [DOI] [PubMed] [Google Scholar]
- 40.Blaimer M, Jakob PM, Breuer FA. Regularization method for phase-constrained parallel MRI. Magn. Reson. Med 2014;72:166–171 doi: 10.1002/mrm.24896. [DOI] [PubMed] [Google Scholar]
- 41.Pauly J, Nishimura D, Macovski A, Le Roux P. Parameter Relations for the Shinnar-Le Roux Selective Excitation Pulse Design Algorithm. IEEE Trans. Med. Imaging 1991;10:53–65 doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 42.Hargreaves BA, Cunningham CH, Nishimura DG, Conolly SM. Variable-rate selective excitation for rapid MRI sequences. Magn. Reson. Med 2004;52:590–597 doi: 10.1002/mrm.20168. [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790 doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Eichner C, Cauley SF, Cohen-Adad J, et al. Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. Neuroimage 2015;122:373–384 doi: 10.1016/j.neuroimage.2015.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn. Reson. Med 2010;64: 10.1002/mrm.22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. Magn. Reson. Med 2007;57:1131–1139 doi: 10.1002/mrm.21237. [DOI] [PubMed] [Google Scholar]
- 47.Grissom WA, McKinnon GC, Vogel MW. Nonuniform and multidimensional Shinnar-Le Roux RF pulse design method. Magn. Reson. Med 2012;68:690–702 doi: 10.1002/mrm.23269. [DOI] [PubMed] [Google Scholar]
- 48.Engström M, Mårtensson M, Avventi E, Skare S. On the signal-to-noise ratio efficiency and slab-banding artifacts in three-dimensional multislab diffusion-weighted echo-planar imaging. Magn. Reson. Med 2015;73:718–725 doi: 10.1002/mrm.25182. [DOI] [PubMed] [Google Scholar]
- 49.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med 2005;54:507–512 doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 50.Skare S, Ashburner J, Andersson JLR. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870–888 doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 51.Niazy RK, De Stefano N, Beckmann CF, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219 doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 52.Bhushan C, Haldar JP, Choi S, Joshi AA, Shattuck DW, Leahy RM. Co-registration and distortion correction of diffusion and anatomical images based on inverse contrast normalization. Neuroimage 2015;115:269–280 doi: 10.1016/j.neuroimage.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


