Abstract
Osteoarthritis (OA) is a debilitating joint disease resulting from chronic joint inflammation and erosion of articular cartilage. A promising biological treatment for OA is intra-articular administration of platelet-rich plasma (PRP). However, immediate bolus release of growth factors limits beneficial therapeutic effects of PRP, thus necessitating the demand for sustained release platforms. In this study, we evaluated the therapeutic value of PRP released from a polyethylene glycol (PEG) hydrogel on OA patient-derived articular chondrocytes.
Lyophilized PRP (PRGF) was encapsulated in PEG hydrogels at 10% w/v and hydrogel swelling, storage modulus and degradation and PRGF release kinetics were determined. PRGF releasate from the hydrogels was collected on day 1, 4 and 11. Encapsulation of PRGF at 10% w/v in PEG hydrogels had minimal effect on hydrogel properties. PRGF was released with an initial burst followed by sustained release until complete hydrogel degradation. Effect of PRGF releasates and bolus PRGF (1% w/v PRGF) on patient-derived cartilage explants or chondrocytes was assessed by chondrocyte proliferation (pico-green assay), gene expression for COL1A1, COL2A1, MMP13, COX2 and NFKB1 (real-time polymerase chain reaction), and measurement of nitric oxide concentration (Griess’ assay). Compared to bolus PRGF, PRGF releasates enhanced chondrocyte proliferation, suppressed the expression of genes like MMP13, NFKB1, COL1A1 and COL2A1 and reduced levels of nitric oxide. Taken together, these results indicate that release of PRGF from PEG hydrogels may improve the therapeutic efficacy of PRP and merits further investigation in an animal model of OA.
Keywords: Osteoarthritis, growth factors, cell proliferation, inflammatory arthritis, gene expression, releasates, platelet-rich plasma (PRP)
INTRODUCTION
Osteoarthritis (OA) is a debilitating joint disease caused by chronic inflammation and erosion of articular cartilage. It is the most common form of arthritis, affecting 32+ million Americans and totaling over $100 billion in socioeconomic costs.1; 2 Commonly used treatments for OA are non-steroidal anti-inflammatory drugs, local corticosteroid injections or viscosupplements: these treatment are either palliative or transient and are associated with deleterious side-effects.3 Currently there are no disease-modifying or progression-limiting OA drug; however, there are innovative therapeutic strategies under investigation including approaches for local delivery of bioactive factors.3 One approach with high potential and minimal side-effects is platelet-rich plasma (PRP) therapy.4–6 PRP is an autologous blood-derived product and a source of bioactive cytokines and growth factors involved in tissue repair and regeneration.4; 7 Many PRP growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), insulin derived growth factor (IGF), and fibroblast growth factor (FGF) are considered important for regulating chondrocyte viability and cell metabolism.4; 8; 9
Several clinical studies have demonstrated significant functional improvement and pain relief upon intra-articular PRP injection in the OA knees compared to traditionally used hyaluronic acid injection or placebo treatments.10–12 While PRP has shown efficacy in accelerating chondrocyte proliferation, matrix production, and resolving inflammation in-vitro, it has yielded mixed results in animal studies13; 14 and in the clinic.5 Studies have shown that upon activation of platelets, there is a bolus release with 70% of PRP growth factors released within 10 minutes of clotting, and nearly 100% within 1 hour.5 This burst release fails to maximize the cell-stimulating potential since factors are cleared before exerting a therapeutic effect, leading to variable therapeutic outcomes.15 Several in-vivo studies have demonstrated enhanced therapeutic benefit of localized sustained PRP release by using gelatin microspheres,13 lyophilized PRP powder,16 or alginate beads17 as compared to intra-articular PRP bolus injection. However, these approaches are not amenable to tailoring the release kinetics of the PRP biomolecules for maximal therapeutic impact.
Recently, we demonstrated sustained release of bioactive PRP growth factors encapsulated in polyethylene glycol (PEG) hydrogels.18 PEG is a U.S. Food and Drug Administration-approved polymer used for sustained protein delivery leading to conservation of protein bioactivity during encapsulation and release.19; 20 PEG hydrogels are extensively used due to their beneficial properties such as high solubility in water and organic solvents alike, hydrophilicity, biocompatibility, inertness and non-immunogenicity. Here, we examine the therapeutic effect of PRP released from PEG hydrogels on human chondrocytes and cartilage explants derived from OA patients. We hypothesize that PRP release will have a beneficial effect on chondrocytes in an in-vitro model of inflammatory arthritis.
MATERIALS AND METHODS
PRGF preparation
Pooled whole blood from 5 de-identified donors was centrifuged at 200g for 20 min, the buffy-coat and plasma were collected and centrifuged at 1000g for 20 min to obtain a platelet pellet, which was re-suspended in supernatant plasma.14 The concentrated PRP platelets were lysed by a freeze-thaw cycle: −80°C for 24 h, 37°C for 1 h, and −80°C for 24 h. To create a dry growth factors-rich powder (PRGF), PRP was lyophilized (Lyophilizer, VirTis Sentry 2.0, Warminster, PA) for 24 h and stored at −20°C.
PEG hydrogel fabrication and PRGF encapsulation
PEG hydrogels were prepared by Michael-type addition reaction between PEG-dithiol (PEG-diSH; Laysan Bio Inc., Arab, AL) and 4-arm PEG acrylate (PEGAc; Jenkem Technology, Piano, TX).21 To prepare a 10% w/v gel, PEG-diSH and PEGAc were combined in a 1:1 molar ratio of Ac:SH in 0.3 M triethanolamine (TEA), pH 7.4. PRGF was added at 10% w/v. The solution was placed between two parafilm-lined glass slides separated by 0.5 mm silicon spacers and incubated at 22°C for 1 h for gelation.
Hydrogel characterization
Hydrogel gelation time was measured by the inverted tube method.21 Initial gel mass, MG, was measured upon gelation. Gels were then incubated in PBS at 37°C overnight to obtain swollen mass, MS, then dried at 60°C for 24 h to obtain dry mass, MD. The swelling ratio, QM, was calculated as MS/MD. Hydrogel degradation was determined indirectly by measuring QM until degradation.21 For rheology, hydrogels were made into 20×0.5 mm discs, swollen overnight in PBS, placed between two 20 mm parallel plates (AR-2000ex rheometer, TA Instruments) and measured at a frequency of 1–10 rad/s and 1% strain.21
PRGF release from hydrogels
PRGF-loaded PEG hydrogels (0.8 cm diameter, 0.07 cm thickness) were immersed in 2 mL of PBS, placed and incubated with rocking at 37°C.18 Aliquots (1 mL) were collected and replenished with fresh PBS until gel degradation. Releasates were analyzed for total protein by Bradford protein dye reagent (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s instructions. The cumulative release of the protein was estimated by calculating the fraction of protein released at each time point (Mi) over the total protein released at complete hydrogel degradation (Minf) and plotting it against time (Mi/Minf vs time). Modified Fick’s law for short release times for slab geometry was used for calculating the effective diffusion coefficient, De, as reported by us previously.18; 22
Chondrocyte isolation and culture
Human chondrocytes were isolated from articular cartilage from patients undergoing total knee arthroplasty (N=6) (IRB Approval #201104119).23 Briefly, cartilage was sliced, collected in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (DMEM/F12; Gibco, Waltham, MA) and digested for 18 h with 0.025% collagenase P and 0.025% pronase (Roche, Indianapolis, IN) in DMEM/F12 using a spinner flask on an orbital shaker (37°C, 5% CO2 and 95% humidity). The digest was filtered through a nylon mesh (pore size 70 μm) and centrifuged at 1500 rpm for 5 min. The collected pellet was washed in DMEM/F12 twice and centrifuged at 1500 rpm for 5 min. Dissociated cells were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (Sigma-Aldrich) at 20,000 cells/cm2 and passaged once before experiments. We also used full-thickness cartilage explants for some experiments.
Treatment of chondrocytes with PRGF releasates
PEG hydrogels containing 10% w/v PRGF were prepared and incubated with rocking at 37°C in supplemented DMEM/F12. PRGF releasates were collected on day 1, 4 and 11 (complete gel degradation) and stored at −80°C. Chondrocytes were seeded at 15,000 cells/cm2, incubated overnight in supplemented DMEM/F12, and treated with 25 ng/mL human recombinant interleukin-1β (IL-1β; Sigma-Aldrich) for 24 h to simulate inflammation in-vitro.23; 24 Chondrocytes were then exposed to PRGF releasates, a bolus 1% w/v PRGF, or no PRGF for 72 h. Chondrocytes without IL-1β pre-treatment or PRGF treatment were used as controls.
Analysis of PRGF releasates
PRGF releasates were analyzed by enzyme-linked immunosorbent assay (ELISA) using kits for vascular growth factor (VEGF), PDGF-BB, endothelial derived growth factor (EGF) (Mini Kits, PeproTech, Rocky Hill, NJ), and TGF-β (Invitrogen) as per the manufacturer’s instructions. Growth factors properties are listed in Supplementary Table 1.
PRGF releasates’ effect on chondrocytes
RNA isolation.
Total RNA was prepared using TRIzol-reagent (Ambion, Waltham, MA) and purified by RNeasy spin columns (Qiagen, Hilden, Germany).25 Chloroform was added to lysed cells, shaken for 30 sec, incubated for 5 min at room temperature, and centrifuged at 13,000 rpm for 15 min at 4°C. The upper aqueous phase with RNA was collected, 70% RNase-free ethanol was added, and centrifuged at 8,000 rpm for 30 s. After decant flowed through, the remaining sample was added, and the procedure repeated. Contaminants were washed away by spinning 3 times with supplied buffers. RNA was eluted in 30 μl of RNase-free water. RNA quality and concentration were assessed using NanoDrop (Thermo Fisher Scientific).
Real-time polymerase chain reaction (PCR).
RNA samples were treated with DNase-I (Thermo Fisher Scientific) and then reverse transcribed with SuperScript II reverse transcriptase (Thermo Fischer Scientific) to synthesize first strand complementary DNA (cDNA) using random primers (Invitrogen). Transcript sequences for the housekeeping gene (GAPDH) and target genes (MMP13, COL1A1, COL2A1,) are shown in Chinzei et al.25 The primer sequence for NFKB1 is 5’-tgcactgtaactgctggacc-3’ (forward) and 5’-gccccttatacacgcctctg-3’ (reverse) (location 837–943; amplicon 126bp), and for COX2 is 5’-acaggcttccattgaccagagc-3’ (forward) and 5’-accatagagtgcttccaactctgc-3’ (reverse) (location 1399–1543; amplicon 144bp). Quantitative real-time PCR was carried with 20 μL reaction mixture consisting of 10 μL of SYBR Green PCR Master Mix (Applied Biosystems), 1.5 μl cDNA, and 200 nM primers on a 7500 Fast rtPCR System (Applied Biosystems). Samples were amplified by: activation at 95°C for 10 min (1 cycle), denaturation at 95°C for 15 sec (40 cycles) and annealing at 60°C for 1 min (40 cycles). Comparative gene expression was calculated by the 2−ΔΔCt approach.26
Cell proliferation.
DNA content was assessed by pico-green assay (Invitrogen) according to the manufacturer’s protocol. Chondrocytes were frozen at −80°C overnight, thawed for 1 h, then lysed using lysis buffer (10 mM Tris buffer, 1 mM EDTA, 0.2% Triton X-100, pH 8). Pico-green reagent was diluted 1:200 in Tris EDTA buffer, mixed with equal volume of samples and incubated in dark at room temperature for 5 min. Fluorescence (480/520 nm) was measured using a spectrophotometer (SpectraMax i3, Molecular Devices, Sunnyvale, CA). DNA concentration was determined from a standard curve prepared using calf-thymus DNA.
Nitric oxide (NO).
A colorimetric assay (Promega, Mannheim, Germany) based upon the Griess’ reagent system (1% sulphanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% phosphoric acid)27 was used to quantify NO in samples according to the supplier’s protocol. Sample and Griess’ reagent was mixed and incubated for 5 min and absorption (540 nm) measured by a plate reader. 0.1 M sodium nitrite was used to generate a standard curve.
Cartilage explants preparation, culture, and conditioning
Cartilage explants (N=5) were cultured as described.8; 23 Explants were maintained in supplemented DMEM/F12 media for 48 h, treated with 25 ng/mL IL-1β for 24 h, and exposed for 72 h to PRGF releasates, bolus 1% w/v PRGF or media. Control explants were not IL-1β pre-treated. Post-PRGF exposure explants were weighed to obtain wet weight, then lyophilized to obtain dry weight. Dried explants were digested in digest buffer with 0.25 mg/mL papain for 24 h at 60°C and analyzed for sulphated glycosaminoglycan (sGAG) using dimethylene blue (DMMB) assay as per the manufacturer’s protocol.28 Digested explant samples were diluted 60 times in digest buffer. The DMMB dye (0.32 mg/mL) was mixed with the sample at 10:1 ratio and absorbance (535 nm) measured using a spectrophotometer (SpectraMax i3, Molecular Devices). sGAG concentration was determined using a standard curve prepared with chondroitin-4-sulfate (Sigma-Aldrich).
Statistical Analysis
Each experiment was conducted using 5–6 replicates per condition in at least 2 independent experiments. The results are presented as mean ± standard deviation. Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparison or Kruskal–Wallis with Dunn’s multiple comparison tests using GraphPad Prism. P<0.05 was considered statistically significant.
RESULTS
Effect of PRGF encapsulation on PEG hydrogel properties
We first evaluated the effect of PRGF encapsulation (10% w/v) on PEG hydrogel properties: gelation time, QM, mesh size, degradation and storage modulus (G’) (Table 1). Gelation time decreased upon PRGF encapsulation (p=0.041). We tested gels swollen for 24 h in PBS or cell medium with 1% FBS (used for chondrocytes in further experiments) to determine whether the incubation medium affected swelling. PRGF encapsulation resulted in decreased gel swelling in PBS (p=0.027), but not in cell medium (p=0.08). Hydrogel mesh size and G’ with or without PRGF did not show significant difference when incubated in PBS or cell medium. QM, an indirect measure of degradation, increased until complete degradation at day 11 independently of PRGF encapsulation (Supplementary Figure 1).
Table 1:
Properties of PEG hydrogels with and without encapsulated PRGF in two different incubation media
| Medium | PRGF | Mesh size (nm) | Qm | Gelation time (min) | Degradation time (d) | G’ (kPa) |
|---|---|---|---|---|---|---|
| PBS | + | 7.67 ± 0.04 | 19 ± 0.7 | 35 ± 3 | 10–11 | 3.08 ± 0.06 |
| − | 9.46 ± 0.047 | 22.8 ± 1.8 | 43 ± 4 | 9–10 | 3.40 ± 0.09 | |
| Media | + | 6.88 ± 0.10 | 17.3 ± 0.8 | N/A | 8–10 | 3.61 ± 0.07 |
| − | 7.25 ± 0.25 | 19.1 ± 1.1 | N/A | 9–10 | 3.52 ± 0.14 |
All values are shown as mean ± SD.
PRGF release from PEG hydrogels
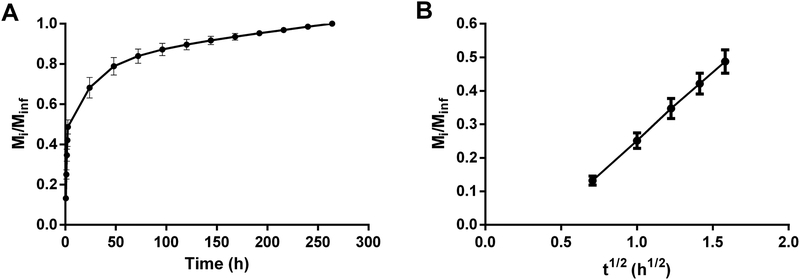
PRGF was released from PEG hydrogels until degradation, but showed a burst release, where ~40% proteins were released in the first 2.5 h (Figure 1A, B). A total of ~80% proteins were released in the first 6 days and complete release was seen at 250 h, coinciding with hydrogel degradation. A fractional release was linear for short diffusion times of Mi/Minf < 0.6 (Figure 1B), where Mi is the PRGF mass released at time i and Minf is the mass released at degradation. The effective diffusivity, De, for PRGF was calculated as 3.30 ± 0.05 × 10−8 cm2/s.
Figure 1. Diffusion of PRGF from PEG hydrogels.
A) Cumulative release of PRGF from PEG hydrogels in PBS. B) Fractional release of PRGF for Mi/Minf < 0.6. The vertical axes denote the fraction of PRGF released from PEG hydrogels.
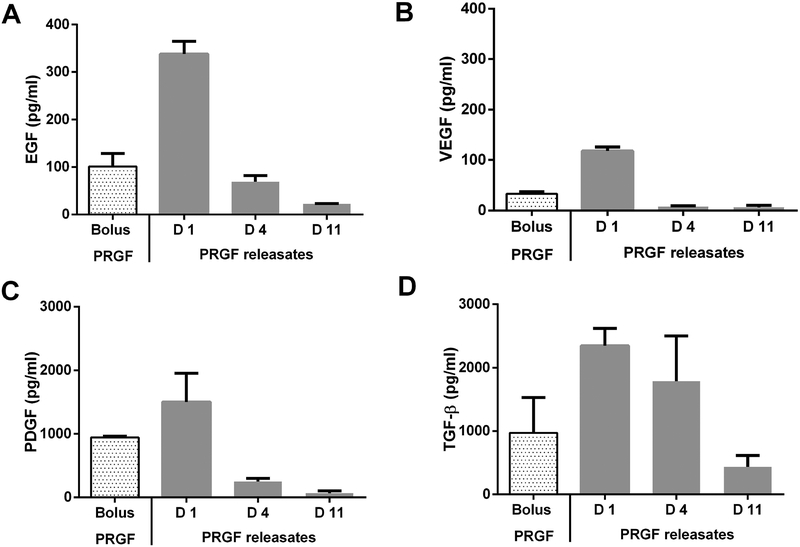
We analyzed the release of specific PRGF proteins - EGF, PDGF-2, VEGF and TGF-β (Supplementary Table 1), in 1% FBS medium via ELISA, using bolus 1% w/v PRGF as control (Figure 2). We analyzed day-1, day-4, and day-11 (degradation) releasate fractions. The growth factors were chosen because of their varying sizes, abundance in PRGF29 and implication in OA treatment.4 Corroborating bulk release experiments, all growth factors were released continually until hydrogel degradation but showed initial burst. Approximately 90% VEGF, 80% PDGF-BB and EGF, and 50% TGF-β were released in the day-1 fraction. Additional ~40% TGF-β, 13–16% PDGF-BB and EGF, and ~6 % VEGF were released in the day-4 fraction. The remaining growth factors were released in the day-11 fraction. VEGF was released the fastest and TGF-β the slowest.
Figure 2.
ELISA analysis of A) EGF, B) VEGF, C), PDGF, and D) TGF-β present in either bolus PRGF (1% w/v PRGF in culture medium) or PRGF releasates collected on day 1, 4 and 11 (complete gel degradation) from PRGF-PEG hydrogels.
Effect of PRGF releasates on chondrocyte proliferation and NO synthesis
Chondrocytes were treated with IL-1β to induce inflammation and to simulate an arthritic condition. IL-1β dose and treatment time were chosen from preliminary experiments, where we tested different doses (25, 50 and 75 ng/mL) and cell density (15,000, 60,000, and 120,000 cells/cm2) to induce chondrocytes death (Supplementary Figure 2). IL-1β decreased chondrocyte number only for the lowest cell density at all doses of IL-1β after 24 h incubation. The lowest IL-1β dose of 25 ng/mL and lowest cell density of 15,000 cells/cm2 were chosen for all experiments. To test if IL-1β pre-treatment induced adequate inflammation and de-differentiation of cultured chondrocytes, we evaluated the expression of some chondrocyte-specific and pro-inflammatory markers used in the study before IL-1β treatment and 72 h after IL-1β treatment (cells were treated with IL-1β for 24 h only) (Supplementary Figure 3). Our data indicates that treatment with IL-1β lead to significant down-regulation of chondrocyte specific markers, such as COL2A1 and up-regulation of pro-inflammatory markers, such as MMP-13, NF-κB and COX-2, when compared to either pre-differentiation chondrocytes or chondrocytes not treated with IL-1β (control). Additionally, we confirmed the biocompatibility of PEG hydrogels only releasates (no PRGF) by showing no adverse effect on chondrocyte proliferation (Supplementary Figure 4).
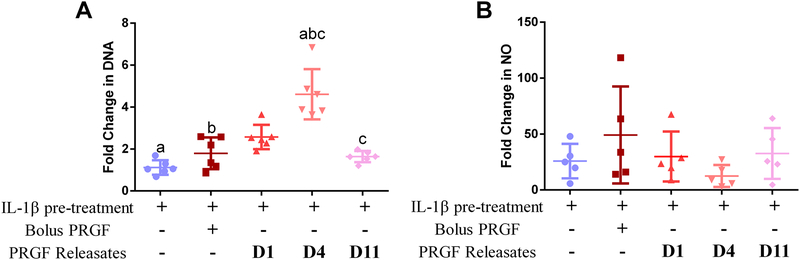
Both bolus 1% PRGF and PRGF releasates showed increased DNA content (cell proliferation) compared to IL-1β pre-treated chondrocytes (Figure 3A). Day-4 releasates showed significant increase in chondrocyte proliferation compared to IL-1β pre-treated (p=0.003), bolus PRGF and day-11 PRGF releasates samples. The results indicate a rescue effect of PRGF releasates on chondrocytes, especially day-4 releasate.
Figure 3.
Effect of PRGF on chondrocyte proliferation and NO synthesis. A) Fold change in DNA content of chondrocytes post-treatment. DNA was measured using a pico-green assay. B) Fold change in NO in chondrocytes post-treatment All chondrocytes were treated with either bolus PRGF (1% w/v) or PRGF releasates collected from PEG hydrogels on day 1, 4, and 11 (complete gel degradation). All samples except control (no IL-1β pre-treatment of PRGF treatment) were pre-treated with IL-1β (25 ng/mL) for 24 h and then were either given no treatment or treated with bolus PRGF or PRGF releasates. Data is averaged from 6 patient samples and normalized to control chondrocytes. Same lower-case alphabets denote statistical significance (p<0.05) between indicated groups.
Bolus PRGF did not inhibit IL-1β-induced NO synthesis in chondrocytes (Figure 3B). All PRGF releasates-treated chondrocytes showed decreased NO synthesis, especially day-4 releasate, which decreased the NO synthesis by 13.4-fold but were not significantly different from chondrocytes with IL-1β pre-treatment or bolus PRGF group.
Effect of PRGF releasates on chondrocyte-specific gene expression
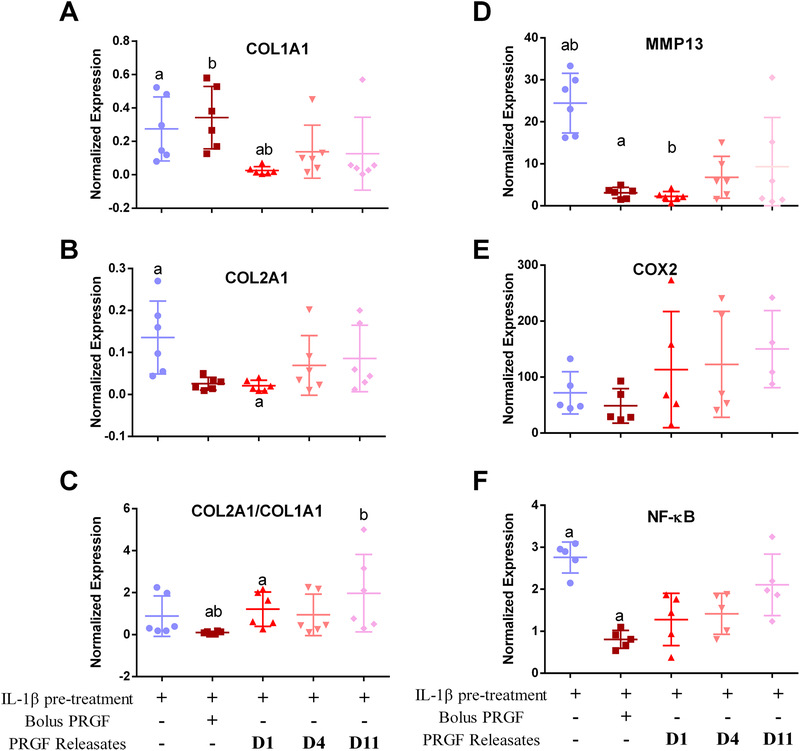
Expression of COL1A1 and COL2A1 was suppressed by IL-1β pre-treatment compared to no pre-treatment. Treatment with bolus PRGF showed increased COL1A1 expression (Figure 4A), but not COL2A1 (Figure 4B) when compared to IL-1β pre-treated chondrocytes. COL1A1 expression was significantly suppressed when treated with day-1 PRGF releasates compared to IL-1β pre-treated and bolus PRGF treatment groups. Treatment with all PRGF releasates suppressed COL2A1 expression. COL2A1 expression was significantly less when treated with day-1 releasate compared to IL-1β pre-treated chondrocytes (p=0.0185) but was not different from bolus PRGF. COL2/COL1 ratio (Figure 4C) revealed a significant improvement when chondrocytes were treated with day-1 and day-11 PRGF releasates than bolus PRGF (p=0.020, p=0.004 respectively). Expression of MMP13 was enhanced 24-fold (p<0.0001) by IL-1β pre-treatment, but PRGF decreased IL-1β-induced MMP13 expression. Bolus PRGF and day-1 PRGF releasate showed the highest suppression of MMP13 expression. Day-4 PRGF releasate was less effective and decreased MMP-13 expression by 17.7-fold when compared to IL-1β pre-treated samples. Day-11 PRGF releasate did not significantly decrease MMP13 expression compared to IL-1β pre-treated samples (Figure 4D). Inflammatory genes NFKB1 and COX2 were up-regulated upon IL-1β pre-treatment 2.7-fold and 72-fold respectively (Figure 4E–F). Expression of COX2 was enhanced by IL-1β pre-treatment; treatment with either bolus or PRGF releasates did not suppress IL-1β-enhanced COX-2 expression (Figure 4E). IL-1β-enhanced expression of NFκB1 was suppressed by 1.9-fold (p=0.003) upon treatment with bolus PRGF and by 1.4-fold (p=0.087) upon treatment with day-1 PRGF releasate. Treatment with day-4 PRGF releasate also suppressed NFKB1 expression but not significantly (Figure 4F).
Figure 4.
Relative changes in chondrocyte transcript expression for A) COL1A1, B) COL2A1, C) ratio of COL2A1/COL1A1, D) MMP13, E) COX2 and F) NFKB1, post-treatment with either bolus PRGF (1% w/v) or PRGF releasates collected from PEG hydrogels on day 1, 4, and 11 (complete gel degradation). All samples except control (no IL-1β pre-treatment of PRGF treatment) were pre-treated with IL-1β (25 ng/mL) for 24 h and then were either given no treatment or treated with bolus PRGF or PRGF releasates. Data is averaged from 5 to 6 patient samples and normalized to control chondrocytes. Similar lower-case letters denote statistical significance (p<0.05).treatment) were pre-treated with IL-1β (25 ng/mL) for 24 h and then were either given no treatment or treated with bolus PRGF or PRGF releasates. Data is averaged from 6 patient samples and normalized to control chondrocytes. Similar lower-case letters denote statistical significance (p<0.05).
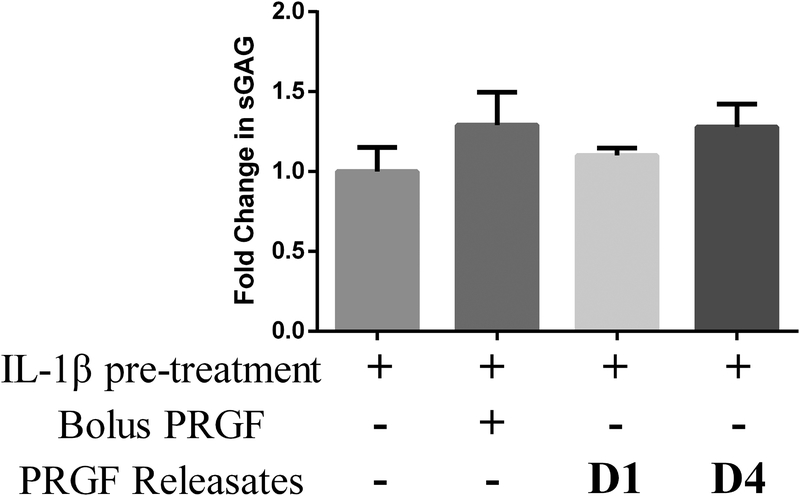
Effect of PRGF releasates on sGAG synthesis in cartilage explants
We tested the effect of bolus PRGF and PRGF releasate fractions on sGAG synthesis in IL-1β pre-treated cartilage explants (Figure 5). IL-1β treatment did not significantly inhibit sGAG synthesis in explants. Bolus PRGF and PRGF releasate day-4 showed a moderate 1.2-fold increase in sGAG synthesis compared to explants pre-treated with IL-1β. Explants maintained in PRGF day-1 releasate did not show change in sGAG synthesis. PRGF day-11 releasates were not tested as they had shown minimal benefit in earlier tests.
Figure 5.
Fold change in sGAG content in cartilage explants after treatment with either bolus PRGF (1% w/v) or PRGF releasates collected from PEG hydrogels on day 1 and 4. All data is averaged from 5 patient samples. sGAG was determined using DMMB assay. sGAG content of each explant was normalized to explant dry weight. A fold change for each condition was calculated with respect to a control explant maintained in 1% FBS media (no IL-1β pre-treatment of PRGF treatment).
DISCUSSION
Diverse strategies have been adopted to overcome the variability in PRP therapy outcomes. Several studies show that sustained PRP release improves therapy outcomes compared to bolus PRP.13; 15; 30 Others have customized PRP preparations for specific application by removing or neutralizing PRP components. For example, delivery of PRP post-neutralizing TGF-β1 enhances healing and reduces fibrosis upon skeletal muscle injury.31 These studies indicate that preparations, which can sustain release and tailor PRP components can enhance therapy efficacy.
We previously designed biodegradable PEG hydrogels for encapsulation and sustained release of lyophilized PRP (PRGF).18 Here, our objective was to determine whether PRGF release from hydrogels can stimulate human OA chondrocyte survival and function compared to bolus PRP. PEG hydrogels are advantageous as delivery vehicles because: i) bioactive molecules can be encapsulated during hydrogel preparation, while preserving activity,20 and ii) release can be modulated by changing polymer composition, molecular weight or concentration.20; 21 Moreover, previous studies by our group and others have shown no detectable reaction between free acrylates and free thiols, which may be present on encapsulated proteins.18; 32; 33 We first analyzed the effect of PRGF encapsulation on hydrogel properties. PRGF led to a slight decrease in gelation time and swelling, possibly due to the high PRGF concentration (10% w/v), leading to a lower water uptake by the gels. Presence of 10% PRGF increased the viscosity of the hydrogel precursor solution and, thus, could result in decreased gelation time. Further, high protein content of 10% w/v could also affect water uptake of the hydrogels due to the displacement of water molecules from the hydrogel pores. The minimal effect on properties indicates the potential of using PEG hydrogels as carriers for high concentrations of multicomponent mixture like PRGF.
Next, we investigated PRGF release from the PEG hydrogels. Similar to our previous study,18 we saw initial burst followed by a sustained PRGF release over 10 days. ELISA analysis of specific PRGF growth factors showed that VEGF (27 kDa) was released the fastest, followed by EGF (6.4 kDa), PDGF-BB (27 kDa), and TGF-β (12.5 kDa). The release kinetics of the growth factors did not correlate with their molecular weights (MW); we anticipated higher MW proteins to release slower. The release behavior could be partly attributed to higher initial concentration of EGF, PDGF-BB and TGF-β in our PRGF preparation, compared to VEGF. Release can also be governed by phenomena such as protein-protein interactions, non-specific protein-polymer interactions, or molecular crowding due to high protein concentration. For example, electrostatic interactions between positively charged TGF-β and negatively charged albumins (abundant in PRGF), could slow down TGF-β release. Further, TGF-β can be present as a dimer (25 kDa) or associated with latency-associated peptide, which can increase its molecular weight and delay release. Lastly, encapsulation of PRGF at 10% w/v inside the hydrogels can lead to clotting, resulting in entrapment of proteins inside the clot. PRP-only gels formed after clotting have been tested for sustained delivery, but they offer little control over gel properties and result in high burst release.34
Next, we tested the bioactivity of PRGF releasates on cultured human chondrocytes derived from OA patients. De-differentiated chondrocytes were pre-treated with IL-1β, a pro-inflammatory cytokine, which primarily participates in the activation cascade of OA through an apoptotic pathway and is known to induce acute chondrocyte degeneration and to inhibit extracellular matrix formation35. Cell treatment with IL-1β is reported as a reliable model to recapitulate the inflammatory mechanisms involved in OA8; 23 and induce de-differentiation in chondrocytes.24; 36; 37 The IL-1β dose was chosen based on the literature23; 38 as well as preliminary data. As anticipated,24 upon treating chondrocytes with bolus PRGF or PRGF releasates, we observed increased proliferation as measured by increased DNA content, most notably with day-4 releasates. The higher proliferation upon treatment with the day-4 releasate could be due to selective depletion of PRGF components, which impact chondrocytes negatively (e.g. VEGF). VEGF has been implicated in promoting OA progression39 and VEGF treatment has been shown to induce death of articular chondrocytes in-vitro40 and in-vivo.41 Note that most of the EGF and VEGF were released by day-1, while PDGF-BB and TGF-β were continuously released until hydrogel degradation. Reports have shown that PDGF-BB down-regulates NF-κB42 and that both PDGF-BB and TGF-β increase chondrocyte proliferation.42; 43 Moreover TGF-β is known to potentiate the effects of PDGF-BB.44 Thus, it is possible that the effects of PDGF-BB or TGF-β were subdued in the presence of VEGF in bolus PRGF as well as the day-1 fraction, but not in the day-4 PRGF fraction.
We next assessed secretion of NO by chondrocytes upon PRGF treatment. NO production is upregulated in OA joints; it induces apoptosis in chondrocytes45 and production of MMPs,46 and inhibits collagen47 and GAG synthesis.48 It has been shown that treatment with TGF-β or inhibition of NF-κB suppresses IL-1β-induced NO synthesis in immortalized murine chondrocytes.49 In our study, IL-1β induced an increase in NO production in chondrocytes, but treatment with PRGF did not suppress NO production. Only day-4 PRGF releasate showed considerable, but insignificant suppression of IL-1β induced NO production. Lack of inhibition of NO production could be due to the presence of multiple growth factors in PRGF, which may affect several regulatory pathways and interfere with the action of other growth factors and cytokines. In day-4 PRG relesates there is a selective loss of growth factors like VEGF, which has been shown to increase NO production in immortalized chondrocytes39 and retention of TGF-β, which is known to suppress NO production.49 Note that NO levels in each of the 6 patient samples varied considerably, making it difficult to obtain statistical differences amongst the various conditions.
We also measured the capacity of PRGF and PRGF releasates to counteract the effect of IL-1β on the expression of genes involved in matrix formation and degradation. IL-1β activates several catabolic factors like MMP-13 and signaling cascades, including NF-κB.23 We saw an up-regulation of MMP-13, COX-2 and NF-κB upon IL-1β pre-treatment in chondrocytes. Treatment with bolus PRGF or PRGF releasates showed an anti-inflammatory effect by suppressing a major catabolic (MMP-13) and a major pro-inflammatory (NF-κB) mediator. Over-expression of MMP-13 is involved in the breakdown of cartilage components, specifically, collagen type II50 and its suppression has been used as a therapeutic strategy for halting OA progression.51 Likewise, activation of NF-κB further promotes inflammation and drives the etiology of OA.4 Several NF-κB antagonists have shown beneficial effects on cultured chondrocytes and OA disease etiology.52 Similar results for NF-κB and MMP-13 suppression using PRGF are reported in earlier studies4 and were attributed to the presence of growth factors like PDGF-BB and TGF-β.4; 8; 24; 43; 53 Our results corroborate these findings and show that PRGF releasates had either similar or higher therapeutic efficacy in inhibiting the major catabolic and pro-inflammatory factors driving OA etiology. Bolus PRGF or PRGF releasates did not inhibit IL-1β induced COX-2 expression or rescue the suppression of collagen synthesizing genes COL1A1 and COL2A1. However, the ratio of COL2/COL1 was significantly improved in PRGF relesates treatment groups indicating an overall anti-inflammatory PRGF effect. These results further suggest that PRGF releastes contribute to stabilizing the re-differentiated state of chondrocytes.
Day-4 PRGF releasate had a minor positive effect on sGAG synthesis, but no other PRGF treatments did, which could be due to the short duration of the explant treatment with IL-1β (24 h) and the longer incubation time post-treatment (72 h), which could have brought back the sGAG levels back to normal.
Lastly, our discussion focused on the four major growth factors that we analyzed in the PRGF preparation. However, PRGF is a complex mixture of cytokines and growth factors, many of which could interact with chondrocytes. Some of the other PRGF components, which have been shown to have an effect on chondrocytes, include hepatocyte growth factor (HGF) and IGF.4 Corroborating previous studies, we observed a trend of decreasing NF-κB activity with either bolus PRGF or PRGF releasates. This effect of PRGF has been attributed to the presence of HGF in PRP, which enhances the expression of endogenous NF-κB inhibitor and thus decreases NF-κB expression.54 Similarly, IGF and PDGF (discussed above) also inhibit IL-1β-induced expression of NF-κB in chondrocytes.55 Interestingly, in our study only bolus PRGF but not PRGF releasates, showed statistically significant decrease in NF-κB activity post IL-1β treatment. This might be an indication that loss of one or more of such growth factors, which alone or in combination play a role in the suppression NF-κB expression, can reduce the potency of PRGF releasates. Some other factors present in PRP, which have been investigated for their role in OA and chondrocytes in particular, are connective tissue growth factor (CTGF) and metalloproteinase inhibitor. CTGF is known to affect collagen deposition and cartilage regeneration. Although CTGF is found in PRGF at levels 20-fold greater than any other growth factor, rapid degradation by MMP13 is believed to be one reason it doesn’t have a regenerative effect in OA. Lastly, PRGF is a major source of several MMP inhibitors such as α−2-macroglobulin and the aggrecanases ADAMTS4 and ADAMTS5. However, the very low concentrations of these inhibitors in PRGF are believed to reduce the biological efficacy of PRGF treatment.4
One study limitation is that we used non-autologous PRGF pooled from 5 human donors. This was done to reduce the variability in PRP preparations from different donors; however, an autologous PRP would have a higher translation value. Second, we saw a high PRGF burst release from the hydrogels. Modulating hydrogel mesh size or using strategies to control the release of specific growth factors can decrease the initial burst. Further, in this study we used chondrocytes cultured in 2D for simplicity in order to compare hydrogel released PRGF to bolus PRGF. However, evaluation of the delivery system on chondrocytes cultured in 3D conditions might yield more accurate results of the therapeutic efficacy of sustained PRGF release. Lastly, bolus PRGF and PRGF releasates showed variable results of proliferation and gene expression, which can be due to interference of mixture of multiple growth factors and cytokines with each other; some of them beneficial, while others inhibitory or deleterious to specific tissue repair processes. Thus, studies with targeted removal of specific growth factors from PRP will be critical to improve PRP therapy efficacy.
Conclusion
We compared the effect of bolus PRGF and PRGF releasate fractions (from a PEG hydrogel) in an IL-1β-induced inflammatory model of OA in human chondrocytes. We established that PRGF releasates collected at different time points were at least as potent as bolus PRGF in inducing chondrocyte proliferation and suppressing expression of anti-inflammatory genes. PEG hydrogels were suitable carriers for the efficient encapsulation of PRGF and could release PRGF in a sustained manner upon initial burst release. The developed approach holds clinical potential as a delivery device for PRGF or other multicomponent mixtures for the treatment of OA. Further studies are required to understand the role of specific PRGF components on chondrocyte function and to customize the release of those PRGF components for the treatment of OA.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge with thanks Dr. Ryan Nunley (Orthopaedic Surgery, Washington University) for providing cartilage samples for this study. This study was supported by the Institute of Clinical & Translation Science funding (CTRF108 to Drs. Rai and Zustiak) provided by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) grant UL1TR002345. Dr. Rai is also supported through Pathway to Independence Award (R00-AR064837) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NCATS or NIAMS.
REFERENCES
- 1.Sandell LJ. 2012. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol 8:77–89. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG. 2006. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 54:226–229. [DOI] [PubMed] [Google Scholar]
- 3.Simon TM, Jackson DW. 2018. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med Arthrosc 26:31–39. [DOI] [PubMed] [Google Scholar]
- 4.Andia I, Maffulli N. 2013. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 9:721–730. [DOI] [PubMed] [Google Scholar]
- 5.Foster TE, Puskas BL, Mandelbaum BR, et al. 2009. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37:2259–2272. [DOI] [PubMed] [Google Scholar]
- 6.Meheux CJ, McCulloch PC, Lintner DM, et al. 2016. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy 32:495–505. [DOI] [PubMed] [Google Scholar]
- 7.Lai LP, Stitik TP, Foye PM, et al. 2015. Use of Platelet-Rich Plasma in Intra-Articular Knee Injections for Osteoarthritis: A Systematic Review. Pm&R 7:637–648. [DOI] [PubMed] [Google Scholar]
- 8.van Buul GM, Koevoet WL, Kops N, et al. 2011. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med 39:2362–2370. [DOI] [PubMed] [Google Scholar]
- 9.Sakata R, Reddi AH. 2016. Platelet-Rich Plasma Modulates Actions on Articular Cartilage Lubrication and Regeneration. Tissue Eng Part B Rev 22:408–419. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez M, Fiz N, Azofra J, et al. 2012. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 28:1070–1078. [DOI] [PubMed] [Google Scholar]
- 11.Campbell KA, Saltzman BM, Mascarenhas R, et al. 2015. Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared With Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthroscopy 31:2213–2221. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Dhillon MS, Aggarwal S, et al. 2013. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis a prospective, double-blind, randomized trial. Am J Sports Med 41:356–364. [DOI] [PubMed] [Google Scholar]
- 13.Saito M, Takahashi KA, Arai Y, et al. 2009. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol 27:201–207. [PubMed] [Google Scholar]
- 14.Duan X, Sandell LJ, Chinzei N, et al. 2017. Therapeutic efficacy of intra-articular hyaluronan derivative and platelet-rich plasma in mice following axial tibial loading. PLoS One 12:e0175682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu HH, Vo JM, Chin HS, et al. 2008. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A 86:1128–1136. [DOI] [PubMed] [Google Scholar]
- 16.Pietramaggiori G, Scherer SS, Mathews JC, et al. 2008. Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen 16:218–225. [DOI] [PubMed] [Google Scholar]
- 17.Man Y, Wang P, Guo Y, et al. 2012. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials 33:8802–8811. [DOI] [PubMed] [Google Scholar]
- 18.Jain E, Sheth S, Dunn A, et al. 2017. Sustained release of multicomponent platelet-rich plasma proteins from hydrolytically degradable PEG hydrogels. Journal of Biomedical Materials Research Part A 105:3304–3314. [DOI] [PubMed] [Google Scholar]
- 19.Bal T, Kepsutlu B, Kizilel S. 2014. Characterization of protein release from poly(ethylene glycol) hydrogels with crosslink density gradients. J Biomed Mater Res A 102:487–495. [DOI] [PubMed] [Google Scholar]
- 20.Elbert DL, Hubbell JA. 2001. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules 2:430–441. [DOI] [PubMed] [Google Scholar]
- 21.Jain E, Hill L, Canning E, et al. 2017. Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker. Journal of Materials Chemistry B 5:2679–2691. [DOI] [PubMed] [Google Scholar]
- 22.Zustiak SP, Leach JB. 2011. Characterization of protein release from hydrolytically degradable poly(ethylene glycol) hydrogels. Biotechnol Bioeng 108:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandell LJ, Xing X, Franz C, et al. 2008. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthr Cartilage 16:1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CC, Chen WH, Zao B, et al. 2011. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials 32:5847–5854. [DOI] [PubMed] [Google Scholar]
- 25.Chinzei N, Brophy RH, Duan X, et al. 2018. Molecular influence of anterior cruciate ligament tear remnants on chondrocytes: a biologic connection between injury and osteoarthritis. Osteoarthr Cartilage 26:588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- 27.Green LC, Wagner DA, Glogowski J, et al. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138. [DOI] [PubMed] [Google Scholar]
- 28.Zheng CH, Levenston ME. 2015. Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. Eur Cell Mater 29:224–236; discussion 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weibrich G, Kleis WK, Hafner G, et al. 2002. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. Journal of cranio-maxillo-facial surgery 30:97–102. [DOI] [PubMed] [Google Scholar]
- 30.Sell SA, Ericksen JJ, Reis TW, et al. 2011. A case report on the use of sustained release platelet-rich plasma for the treatment of chronic pressure ulcers. J Spinal Cord Med 34:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Hicks JJ, Wang L, et al. 2016. Customized platelet-rich plasma with transforming growth factor beta1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 87:147–156. [DOI] [PubMed] [Google Scholar]
- 32.Zustiak SP, Boukari H, Leach JB. 2010. Solute diffusion and interactions in cross-linked poly(ethylene glycol) hydrogels studied by Fluorescence Correlation Spectroscopy. Soft Matter 6:3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elbert DL, Pratt AB, Lutolf MP, et al. 2001. Protein delivery from materials formed by self-selective conjugate addition reactions. Journal of Controlled Release 76:11–25. [DOI] [PubMed] [Google Scholar]
- 34.Carmona JU, Rios DL, Lopez C, et al. 2016. In vitro effects of platelet-rich gel supernatants on histology and chondrocyte apoptosis scores, hyaluronan release and gene expression of equine cartilage explants challenged with lipopolysaccharide. BMC Vet Res 12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Armada MJ, Carames B, Lires-Dean M, et al. 2006. Cytokines, tumor necrosis factor-alpha and interleukin-1beta, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr Cartilage 14:660–669. [DOI] [PubMed] [Google Scholar]
- 36.Chiou CS, Wu CM, Dubey NK, et al. 2018. Mechanistic insight into hyaluronic acid and platelet-rich plasma-mediated anti-inflammatory and anti-apoptotic activities in osteoarthritic mice. Aging (Albany NY) 10:4152–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen WH, Lo WC, Hsu WC, et al. 2014. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials 35:9599–9607. [DOI] [PubMed] [Google Scholar]
- 38.Rai MF, Graeve T, Twardziok S, et al. 2011. Evidence for Regulated Interleukin-4 Expression in Chondrocyte-Scaffolds under In Vitro Inflammatory Conditions. Plos One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton JL, Nagao M, Levine BR, et al. 2016. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J Bone Miner Res 31:911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XY, Hao YR, Wang Z, et al. 2012. The effect of vascular endothelial growth factor on aggrecan and type II collagen expression in rat articular chondrocytes. Rheumatol Int 32:3359–3364. [DOI] [PubMed] [Google Scholar]
- 41.Ludin A, Sela JJ, Schroeder A, et al. 2013. Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthr Cartilage 21:491–497. [DOI] [PubMed] [Google Scholar]
- 42.Montaseri A, Busch F, Mobasheri A, et al. 2011. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 6:e28663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandl A, Angele P, Roll C, et al. 2010. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J Orthop Res 28:354–360. [DOI] [PubMed] [Google Scholar]
- 44.Hiraki Y, Inoue H, Hirai R, et al. 1988. Effect of transforming growth factor beta on cell proliferation and glycosaminoglycan synthesis by rabbit growth-plate chondrocytes in culture. Biochim Biophys Acta 969:91–99. [DOI] [PubMed] [Google Scholar]
- 45.Blanco FJ, Ochs RL, Schwarz H, et al. 1995. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol 146:75–85. [PMC free article] [PubMed] [Google Scholar]
- 46.Murrell GA, Jang D, Williams RJ. 1995. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun 206:15–21. [DOI] [PubMed] [Google Scholar]
- 47.Cao M, Westerhausen-Larson A, Niyibizi C, et al. 1997. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J 324 (Pt 1):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarvinen TA, Moilanen T, Jarvinen TL, et al. 1995. Nitric oxide mediates interleukin-1 induced inhibition of glycosaminoglycan synthesis in rat articular cartilage. Mediators Inflamm 4:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vuolteenaho K, Moilanen T, Jalonen U, et al. 2005. TGFbeta inhibits IL-1 -induced iNOS expression and NO production in immortalized chondrocytes. Inflamm Res 54:420–427. [DOI] [PubMed] [Google Scholar]
- 50.Poole A, Kobayashi M, Yasuda T, et al. 2002. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Annals of the rheumatic diseases 61:ii78–ii81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malemud CJ. 2004. Cytokines as therapeutic targets for osteoarthritis. BioDrugs 18:23–35. [DOI] [PubMed] [Google Scholar]
- 52.Rigoglou S, Papavassiliou AG. 2013. The NF-κB signalling pathway in osteoarthritis. The international journal of biochemistry & cell biology 45:2580–2584. [DOI] [PubMed] [Google Scholar]
- 53.Xie X, Zhang C, Tuan RS. 2014. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther 16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendinelli P, Matteucci E, Dogliotti G, et al. 2010. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-kappaB inhibition via HGF. J Cell Physiol 225:757–766. [DOI] [PubMed] [Google Scholar]
- 55.Andia I, Sanchez M, Maffulli N. 2010. Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther 10:1415–1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.