Abstract
We assessed whether adding magnetic resonance (MR)-based features to a base model of clinically accessible participant characteristics (i.e., serological, radiographic, demographic, symptoms, and physical function) improved classification of adults who developed accelerated radiographic knee osteoarthritis (AKOA) or not over the subsequent 4 years. We conducted a case-control study using radiographs from baseline and the first four annual visits of the Osteoarthritis Initiative to define groups. Eligible individuals had no radiographic KOA in either knee at baseline (Kellgren-Lawrence grade (KL) <2). We classified 2 groups matched on sex: 1) AKOA: at least one knee developed advanced-stage KOA (KL=3 or 4) within 48 months and 2) did not develop AKOA within 48 months. The MR-based features were assessments of bone, effusion/synovitis, tendons, ligaments, cartilage, and menisci. All characteristics and MR-based features were from the baseline visit. Classification and regression tree analyses were performed to determine classification rules and identify statistically important variables. The CART models with and without MR features each explained approximately 40% of the variability. Adding MR-based features to the model yielded modest improvements in specificity (0.90 versus 0.82) but lower sensitivity (0.62 versus and 0.70) than the base model. There was consistent evidence that serum glucose, effusion-synovitis volume, and cruciate ligament degeneration are statistically important variables in classifying individuals who will develop AKOA. We found common MR-based measures failed to dramatically improve classification. These findings also show a complex interplay among participant characteristics and a need to identify novel characteristics to improve classification.
Keywords: effusion, synovitis, cruciate ligaments, glucose
INTRODUCTION
Radiographic knee osteoarthritis (KOA) is typically considered a slowly progressive disorder. However, at least 1 in 5 cases of incident radiographic KOA develop an accelerated form of radiographic KOA (AKOA), progressing from no radiographic KOA to advanced-stage radiographic KOA within 4 years and often within 12 months.1–3 Adults with AKOA experience greater pain and functional disability than those with a typical onset of radiographic KOA.2; 4 Since the rate of disease onset offers only a short opportunity to intervene it would be helpful to classify people at-risk for AKOA to create an opportunity to develop and deliver prevention strategies. We previously classified individuals using serum, radiographic, and demographic/anthropometric (age and body mass index) participant characteristics and explained 31% of the variability with high specificity but low sensitivity.5 These results suggest that further analyses are needed with additional baseline characteristics to better discriminate between individuals that will and will not develop AKOA.
Prodromal symptoms, functional disability, and magnetic resonance (MR) imaging-based knee assessments may help classify adults at risk for AKOA. Adults who develop AKOA often report greater prodromal symptoms and experience greater functional disability (e.g., slower walking speed) than those with typical radiographic KOA up to 3 years prior to radiographic onset.2 More recently, magnetic resonance (MR)-based measurements have also been identified as early signs of AKOA (e.g., effusion-synovitis, meniscal pathology).6–10
The purpose of this study was to utilize classification and regression tree (CART) models to 1) assess the ability of MR features from the baseline visit to classify participants who will and will not develop AKOA in the subsequent 4 years, and 2) determine whether adding baseline MR features to a model that already includes clinically accessible measures of symptoms, function, radiographic features, clinical findings, and demographic characteristics from the baseline visit improves classification of adults who will develop AKOA over the subsequent 4 years.
METHODS
We conducted a case-control study using data and images from baseline and the first 4 annual visits in the Osteoarthritis Initiative (OAI), a longitudinal multicenter observational cohort study of adults with or at risk of developing symptomatic KOA. Almost 4,800 participants (ages 45–79 years) were enrolled at 4 clinical sites in the United States between February 2004 and May 2006.11 The protocol and detailed descriptions of the eligibility criteria are available at the OAI website.11 Institutional review boards at each of the 4 clinical sites and the OAI coordinating center approved the study. All participants provided informed consent.
Case and Control Definitions
Group assignment (AKOA, typical radiographic KOA, and no radiographic KOA) was determined using annual radiographs from the baseline to the 48-month OAI visits. We selected this definition instead of 5 other radiographic criteria for AKOA based on a previous analysis that determined this was an optimal definition of AKOA.3 This definition is consistent with our original attempt to classify adults at risk for AKOA.5 Eligible participants had Kellgren-Lawrence (KL) grade<2 at baseline in both knees. Cases were participants with AKOA, defined as the development of advanced-stage radiographic KOA (KL grade 3 or 4) by the 48-month visit. Typical radiographic KOA was defined as an increase in radiographic severity (KL grade) by month 48 (excluding those meeting the definition for accelerated KOA). No radiographic KOA was defined as no increase in the KL grade by month 48. Adults with typical and no radiographic KOA were controls.
We matched typical and no radiographic KOA participants by sex to the AKOA group (cases, n=54) in a 1:1:1 matching scheme. For the purposes of this study, typical and no radiographic KOA participants were combined into one group to explore whether participant characteristics or combinations of characteristics were able to classify adults who would develop AKOA. The knee that first met the criteria for AKOA or typical radiographic KOA was included in the analysis. Participants with no radiographic KOA were side-matched to the AKOA knee.
To determine eligibility and group assignment we relied on bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs. Central readers scored the KL grade of each knee (KL0 to KL4), with KL4 indicating the worst radiographic KOA severity. Good intra-rater reliability was observed for KL grade (weighted κ = 0.70 to 0.78).11 The protocol and data are publicly available at the OAI website (Files: kXR_SQ_BU # # _SAS; versions 0.6, 1.6, 3.5, 5.5, 6.3).11
Clinically Accessible Variables
Measures from the baseline visit that were clinically-accessible were chosen as candidate variables: radiographic assessments, demographic characteristics (age, sex, and body mass index), biospecimen measurements, symptoms (pain, function, quality of life, swelling and tenderness), and walking speed. These are described more fully in the following sections and in Supplement Table 1.
In addition to the measures in the previously published CART model (base model),5 we included clinically accessible pain, function, clinical knee exam, and quality of life measures in our base + symptoms + function model. All data are publicly available (File: allclinical00, version 0.2.2).11
Radiographic Assessments
We used bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs to assess two radiographic variables: femorotibial angle (FTA) and coronal tibial slope.
Femorotibial angle was measured to assess static knee alignment. We used the publicly available data on the OAI website (file: KXR_FTA_Duryea00, version 0.2).11 In brief, a customized software tool defined the tibial axis based on the central point of the knee and the center of the tibial diaphysis at approximately 10 cm distal to the tibial plateau. The femoral axis was perpendicular to a line tangent to the distal ends of the medial and lateral femoral condyles. The software tool calculated the angle between the tibial and femoral axis with negative values indicating knees with more varus alignment. Femorotibial angle was adjusted to match hip-knee-ankle angles as described in Iranpour-Boroujeni et al: adjustment for males = (0.91*femorotibial angle) + 2.9; adjustment for females = (0.97*femorotibial angle) + 4.8.12 Intra-reader and inter-reader ICCs were >0.95.11
Coronal tibial slope was assessed by one reader (JBD) using an EFilm Workstation 3.4 (Merge Healthcare, Chicago, IL).13 The reader identified the longitudinal tibial axis and drew a line connecting the peak points of the medial and lateral aspects of the tibial plateau. He then moved the longitudinal axis to the lateral aspect of the tibial plateau and drew a line perpendicular to the longitudinal axis. A positive coronal tibial slope indicated that the peak lateral aspect of the tibial plateau was proximal to the peak medial aspect. The intra-reader reliability was good (ICC3,1=0.87, n=15 knees).14
Demographic Characteristics
We included publicly available age, sex, and body mass index, which study staff collected at baseline per a standard protocol (Files: enrollees, version 22; allclinical00, version 02.2.).11
Biospecimens
Study staff performed blood draws at the baseline visit. Participants fasted for at least 8 hours prior to the blood draw. Blood samples were centrifuged and aliquoted into cryovials. Samples were stored in a −70°C freezer prior to shipping to Fisher Bioservices (Rockville, MD) for long-term storage. Protocols for the biospecimens collection and processing are available on the OAI website.11 Fisher Bioservices shipped the serum samples to Temple University School of Medicine in September 2015. One investigator (MA) performed all assays in duplicate. We used commercially available enzyme-linked immunosorbent assay kits to assess high-sensitivity C-reactive protein (Novex by Life Technology, Carlsbad, CA) and glycated serum protein (MyBiosource, San Diego, CA). The Abcam Glucose Assay Kits were used to assess serum glucose (after deproteinization). Further details about the biospecimen analysis for this study were previously described.15
Symptoms
The Western Ontario and McMaster (WOMAC) Universities Osteoarthritis Pain Scale (3.1 Likert version) was used to assess self-reported knee-specific pain within the last 7 days separately for each knee (range 0–20, with higher scores indicating higher pain).16 The scale is based on 5 questions about pain, each scored from 0 (no pain) to 4 (extreme pain). (The WOMAC Function Scale (3.1 Likert version) function scores range from 0–68, with higher scores indicating greater functional impairment. The scale is based on 17 questions, each scored from 0 (no impairment) to 4 (extreme impairment).
Separately, participants were asked whether “during the past twelve months, have you had pain, aching or stiffness, in or around your right knee, on most days for at least one month? By most days, we mean more than half the days in one month.” Possible responses were yes or no. A similar question was asked about the left knee.
Participants also self-reported impact of KOA on quality of life via the Knee Injury and Osteoarthritis Outcome Score (KOOS) quality of life subscale.17 Possible scores range from 0–100, with higher scores indicating better quality of life.
Bilateral clinical knee exams were performed at baseline to determine the presence of crepitus, tenderness and/or swelling. Specifically, we included patella-femoral crepitus, lateral and medial joint line tenderness, patella tenderness, knee swelling evidenced by a positive bulge sign or patellar tap test, and patellar/quadriceps tenderness/tendonitis.
Physical Function
Habitual walking speed was assessed via 2 timed 20-meter walks. The time needed to complete the 20-meters was converted to walking speed (i.e. meters/second [m/s]) and averaged across the two trials. All data are publicly available (File: allclinical00, version 0.2.2).11
MR-based Features Added to the CART
Magnetic Resonance Imaging Acquisition
Baseline MR images were acquired with one of four identical Siemens (Erlangen, Germany) Trio 3-Tesla MR systems at each clinical site using the OAI MR imaging protocol.11; 18 Bone marrow lesion (BML) volume and effusion-synovitis volume quantitative measurements were performed using a sagittal intermediate-weighted, turbo spin echo, fat-suppressed MR sequence with the following parameters: field of view=160mm, slice thickness=3mm, skip=0mm, flip angle=180 degrees, echo time=30ms, recovery time=3200ms, 313×448 matrix, x resolution=0.357mm, y resolution=0.511mm, and total slice number=37. Articular cartilage was quantified using a 3-dimensional dual-echo steady-state sequence with the following parameters: field of view=140mm, slice thickness=0.7mm, skip=0mm, flip angle=25 degrees, echo time=4.7ms, recovery time=16.3ms, 307×384 matrix, x resolution=0.365mm, y resolution=0.456mm, and total slice number=160. Musculoskeletal radiologists assessed semi-quantitative or dichotomous MR-based features using all MR sequences for each visit.
Bone Marrow Lesion Volume
One reader (ACS), unaware of group assignment, measured tibiofemoral BML volume with a semi-automated segmentation software.19; 20 The only manual step required the reader to identify crude boundaries of the tibia and femur in each slice of the MR sequence. The boundary furthest from the articular surfaces was marked just prior to the epiphyseal line or at the edge of the bone and soft tissue. The program then automatically identified the precise bone boundaries and performed a thresholding and curve evolution process twice to segment areas of high signal intensity, which may represent a BML. We eliminated false-positive regions by operationally defining a BML based on 2 criteria: 1) the distance between a BML to the articular surface should be <10 mm, and 2) a BML needed to span more than one MR image. The study principal investigator reviewed all measurements. Our reader demonstrated excellent intra-reader reliability (ICC3,1=0.91). Total tibiofemoral BML volume was used in the analysis.
Effusion-Synovitis Volume
We used a semi-automated segmentation software to measure knee effusion-synovitis, which reflects effusion and synovitis volume.6 Two readers (JBD and FA) used the software to mark the first and last MR slice that included bone, the proximal border of the patella, and the apex of the fibular head. The software then automatically segmented effusion-synovitis between these limits based on an existing threshold. The senior reader (JBD) then manually adjusted the threshold to change the effusion-synovitis boundaries and removed areas of high-signal intensity that were not effusion-synovitis (e.g., subchondral cysts, blood vessels). The senior reader demonstrated excellent intra-reader reliability (ICC3,1=0.96). Whole knee effusion-synovitis volume was used in the analysis.
Cartilage Damage Index
To measure tibiofemoral cartilage we used the validated cartilage damage index (CDI).21; 22 One reader (JED) manually marked the bone-cartilage boundary on specific knee slices that are automatically selected based on the presence of predefined informative locations.21; 22 The reader then measured cartilage thickness at predefined 36 informative locations, which the software automatically located. The software then computed the CDI for the medial femur, lateral femur, medial tibia, and lateral tibia by summing the products of cartilage thickness, cartilage length (anterior-posterior), and voxel size from 9 informative locations in each region. The study principal investigator reviewed all measurements. Our reader demonstrated excellent intra-reader reliability (ICC3,1=0.86 to 0.99). CDI in the medial and lateral compartments of the tibial and femoral were used in the analysis.
Semi-quantitative Features
Two musculoskeletal radiologists (RW:255 cases, JM:120 cases) performed the semi-quantitative MR readings. Readers had good agreement on the presence of each pathology among 25 cases: prevalence-adjusted and bias-adjusted kappa were 0.41 to 0.75 except for the posterior horn of the medial meniscus where the prevalence-adjusted and bias-adjusted kappa was fair at 0.25 (50% agreement).
The radiologists assessed the integrity of anterior/posterior cruciate ligaments, medial/lateral collateral ligaments, extensor mechanism, and gastrocnemius proximal tendons by noting if the structures appeared normal or degenerative. Degenerative tissue was defined as the presence of abnormal intrinsic high-signal intensity within the substance of the ligaments or tendon without discrete tear. Degenerative cruciate ligament pathology combined the presence of anterior or posterior cruciate ligament degenerative pathology. Degenerative collateral ligament pathology combined the presence of medial or lateral collateral ligament degenerative pathology.
The radiologists scored infrapatellar fat pad signal intensity alteration using the MR Imaging Osteoarthritis Knee Score grading system (i.e., normal, mild, moderate, and severe).23 Infrapatellar fat pad signal intensity was recoded as absence (i.e., normal) or presence (i.e., mild, moderate, and severe).
The radiologists scored medial and lateral meniscus extrusion using the MR Imaging Osteoarthritis Knee Score grading system (i.e., Grade 0: <2 mm, Grade 1: 2 to 2.9 mm, Grade 2: 3 to 5 mm, and Grade 3: >5mm).23 Meniscal extrusion was recoded as absence (i.e., Grade 0) or presence (i.e., ≥ Grade 1).
The radiologists used the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine meniscal tear classification, which was modified for MR imaging24, to assess the body, posterior/anterior horn of each meniscus as: normal, degeneration, horizontal, flap horizontal, vertical longitudinal, radial, morphologic deformity, maceration, complex, or vertical flap tear. Meniscal pathology was recoded as absence (i.e., normal or degeneration without tear) and presence (i.e., horizontal, flap horizontal, vertical longitudinal, radial, morphologic deformity, maceration, complex, or vertical flap tear). The medial/lateral menisci were considered pathologic if pathology was present in any of the three regions (body or anterior/posterior horn). Additionally, we determined the number of pathologic meniscal regions with meniscal pathology, which could range from 0–6 (i.e., medial/lateral and anterior/body/posterior horn).
The radiologists were also asked to record the presence of any other miscellaneous pathology: attrition, acute ligamentous or tendinous injuries, subchondral insufficiency fractures, and any other incidental findings. Attrition was assessed from 0 (normal) to 3 (severe), based on the perceived degree of deviation from a normal contour for the medial and lateral femur and tibia. We defined the presence of attrition as a score of 1 or more. We defined acute ligamentous or tendinous injury as per routine clinical practice, using the presence/absence of focal fiber disruption and intrinsic ligamentous or subjacent soft tissue edema to detect the presence of injury. We defined subchondral insufficiency fracture as a linear low signal in the subchondral bone on a fat suppressed image and subjacent edema. Since each of these pathologies were rare, we combined them into a single outcome variable: presence or absence of miscellaneous pathology.
Statistical Analysis
We performed classification and regression tree (CART) analyses to determine classification rules and important variables for: Model 1) a model with MR features only, Model 2) a previously published base model with clinically accessible variables (i.e., serological, radiographic, demographic)5 that now also includes self-reported symptoms, clinical exam findings, and physical function, and Model 3) a combined model comprised of the clinically accessible variables and MR features.
CART is a non-parametric method that has some advantages over other statistical techniques for classification by allowing for complex interactions and non-linear associations. This method identifies the most discriminating characteristic, with its associated cut point, to differentiate the two groups (e.g. accelerated KOA or no accelerated KOA). After the initial split based on the cut point, CART iteratively finds the next best characteristic with their corresponding associated cut point. CART analysis identifies the most important characteristics and is easily interpretable. The identification of statistically important variables is obtained by creating multiple trees via cross-validation, and observing which variables appear in most of the trees.25
Pruning and 10-fold cross-validation were performed to avoid overfitting and to identify the statistically most important variables. Sensitivity and specificity were calculated for all models. All analyses were performed using the rpart function in R (version 3.4.4, The R Foundation for Statistical Computing).
RESULTS
Fifty-four cases (AKOA) and 108 participants in the control group (54 adults with typical radiographic KOA and 54 adults with no radiographic KOA) met the inclusion criteria at baseline. This cohort has been described previously.5 In brief, the sample was predominantly female (63%), with mean age 59 years (SD=8), and body mass index 28 kg/m2 (SD=5;Table 1).
Table 1:
Baseline Characteristics of Individuals with and without Incident Accelerated Knee Osteoarthritis (AKOA)
| Variables mean(SD) except where noted | AKOA n=54 | No AKOA n=108 |
|---|---|---|
| Age (years) | 61.8 (8.6) | 58.1 (7.8) |
| BMI (kg/m2) | 28.9 (4.7) | 27.6 (4.7) |
| Female (n (%) | 34 (63.0) | 68 (63.0) |
| WOMAC pain (median [p25, p75]) | 0 [2, 4] | 0.0 [1, 3] |
| WOMAC function (median [p25, p75]) | 0 [4, 15] | 0.0 [2, 7] |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
p25, p75: 25th and 75th percentiles
Model 1: Model with MR Features Only
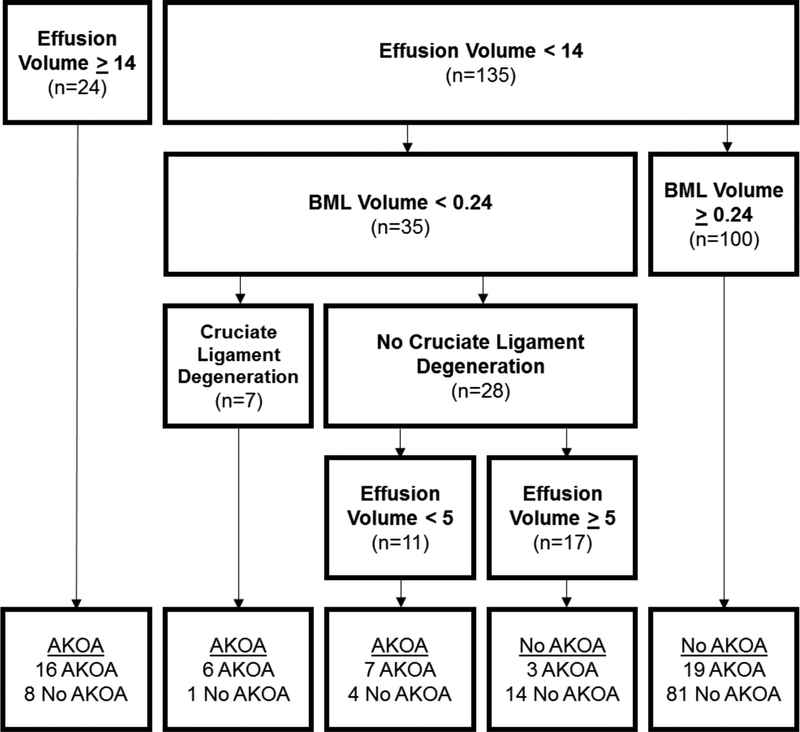
Figure 1 displays the CART model with only MR features to see which features are associated with development of AKOA. Effusion-synovitis volume, BML volume, and presence of cruciate ligament degeneration were the three most statistically important variables (in order of importance). Individuals with a higher effusion-synovitis volume (≥14 cc) were likely to develop AKOA over the subsequent 4 years. Participants with a lower effusion-synovitis volume but larger BML volume (≥0.24 cc) were less likely to develop AKOA. In contrast, adults with lower effusion-synovitis and BML volumes and cruciate ligament degeneration were more likely to develop AKOA. This model explained 31% of the variability, with specificity of 0.88 and sensitivity of 0.57.
Figure 1: Classification and Regression Tree: MR Features Only.
Abbreviations: Effusion=effusion-synovitis, BML=bone marrow lesion, AKOA=accelerated radiographic knee osteoarthritis
Units of measure: effusion-synovitis volume = cubic centimeters, bone marrow lesion volume = cubic centimeters
Model 2: Model with Clinically Accessible Variables (Base Model + Symptoms + Function)
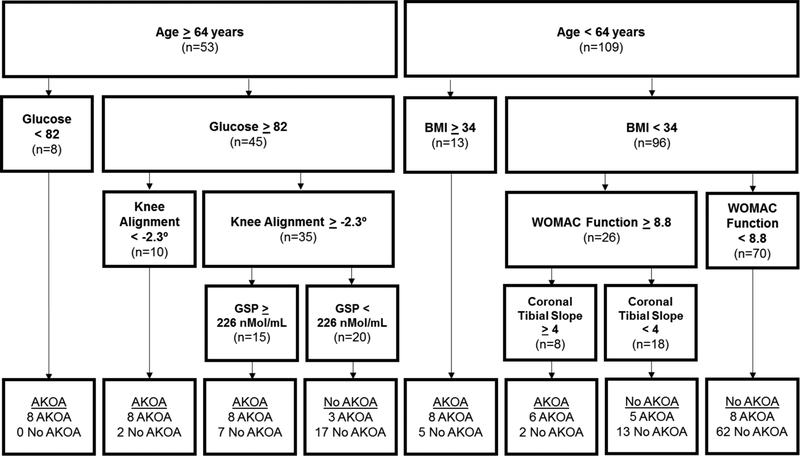
Figure 2 presents the base model based on clinically accessible variables.7 Baseline serum glucose, age, and static alignment were the three most statistically important variables (in order of importance). The first 3 cuts were based on age, serum glucose, and BMI. Most individuals <64 years did not develop AKOA over the subsequent 4 years, except for those with body mass index ≥34 kg/m2 and those with body mass index <34 kg/m2 but more impaired WOMAC function (≥8.8) and coronal tibia slope≥4 degrees (a more proximal lateral plateau than medial). Conversely, all of the adults 64 or older with glucose<82 developed AKOA. This model explained 41% of the variability and had specificity of 0.82 and sensitivity of 0.70.
Figure 2: Classification and Regression Tree: Clinically Accessible Variables (Base Model + Symptoms + Function).
Abbreviations: BMI=body mass index, AKOA=accelerated radiographic knee osteoarthritis
Units of measure: age = years, glucose = milligrams/deciliter, body mass index = kilograms/meters2, glycated serum protein = nanomoles/milliliter, coronal tibial slope = degrees
Model 3: Clinically Accessible Variables (Base Model + Symptoms + Function) + MR Features
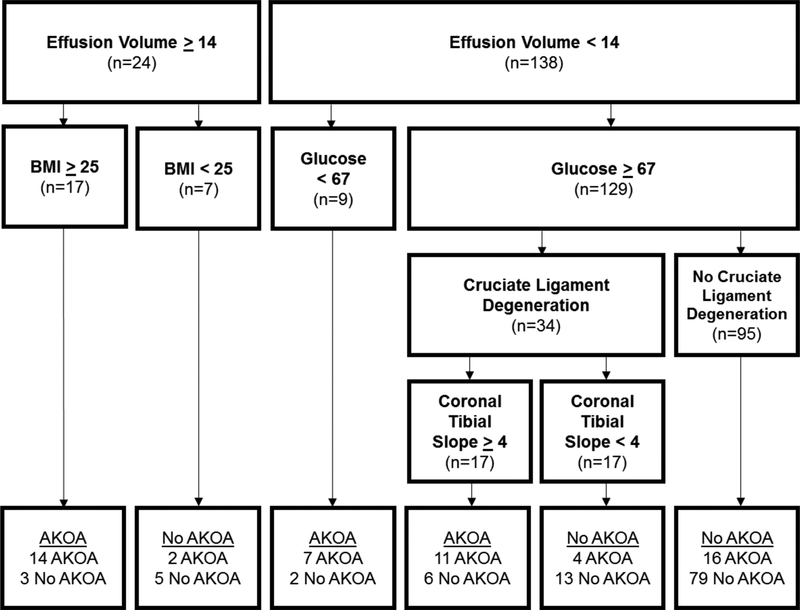
Figure 3 displays the CART with all candidate variables placed in the model. Effusion-synovitis volume, serum glucose, and the presence of cruciate ligament degeneration were the most statistically important variables (in order of importance). Overweight or obese individuals with higher effusion-synovitis volume (≥14 cc) were more likely to AKOA over the subsequent 4 years. Participants with lower effusion-synovitis volume (<14 cc) and glucose <67 were also likely to develop AKOA. The presence of cruciate ligament degeneration and coronal tibial slope played a role in classification of individuals with lower effusion-synovitis volume and glucose≥67. This model explained 39% of the variance and had specificity of 0.90 and sensitivity of 0.62.
Figure 3: Classification and Regression Tree: Clinically Accessible Variables (Base Model + Symptoms + Function) + MR Features.
Abbreviations: Effusion=effusion-synovitis, BMI=body mass index, AKOA=accelerated radiographic knee osteoarthritis
Units of measure: effusion-synovitis volume = cubic centimeters, glucose = milligrams/deciliter, body mass index = kilograms/meters2, coronal tibial slope = degrees
DISCUSSION
We expanded on prior work to classify adults at risk for AKOA, which we defined based on radiographic progression, by adding new baseline clinically accessible measures (i.e. clinical exam findings, symptoms, and physical function), and MR measures to a CART. Our primary goal was to assess whether adding MR measures in isolation or combined with our clinically accessible variables could improve the classification of individuals at risk for developing AKOA within the subsequent four years. Variables that consistently appeared in the models, and that may enable classification of individuals at risk of developing AKOA, were effusion-synovitis volume, presence of cruciate ligament degeneration, and serum glucose. The addition of an array of MR variables to the clinically accessible measures failed to improve model fit (percent of variance explained ~40% for both models).
Based our prior analyses2; 4, we hypothesized that the addition of baseline pain, function and quality of life measures would improve the classification of adults who will develop AKOA in the subsequent 4 years. While the model with these measures showed some improvement in terms of the percent of variability explained (41% vs 31% in previously published model5), WOMAC function was the only symptom, function, or quality of life variable that was selected by the model. We also hypothesized that the addition of MR measures would improve classification of adults who will develop AKOA, based on prior analyses.2; 6–9 However, the model with clinically accessible variables and MR features explained slightly less variability than the model with clinically accessible variables only (39% vs 41%). The full model also had lower sensitivity (0.62 vs 0.70) but higher specificity (0.90 vs 0.82) compared to the model with clinically accessible variables.
This lack of substantial improvement in classification after adding variables such as pain that have been shown to be associated with development of AKOA may be explained by the fact that CART’s strength is in classification rather than strength of association.26 There may be other variables that are more relevant in classification than pain. Additionally, the interactions may identify subsets of people that are more at risk based on their combined characteristics, rather than pain alone.
In spite of the improvements in the percent of variability explained by the models with symptoms and MR, 60% of the variance remains unexplained. The cost of obtaining self-reported function, pain, and quality of life is minimal and clinicians and researchers may consider including them when assessing risk for development of AKOA. MR measurement costs, on the other hand, can be quite substantial. It is not clear that there is a sufficient increase in precision of the classification to warrant the additional cost.
Within our models, both effusion-synovitis volume and cruciate ligament degeneration are consistently identified as statistically important MR-based features for the classification of AKOA. This complements prior work where we observed effusion-synovitis volume was greater among adults who developed AKOA up to 2 years prior to radiographic onset compared with adults with typical or no radiographic KOA.6 Furthermore, these results agree with prior results that cruciate ligament degeneration is more common among adults who developed AKOA up to 2 years prior to radiographic onset compared with adults with typical or no radiographic KOA.10 These MR-based findings may be some of the earliest structural manifestations of AKOA.
Despite previous findings that fasting serum glucose was not significantly associated with either prevalent or incident radiographic KOA27–30, we identified fasting serum glucose as a potentially statistically important variable for classifying people who will develop AKOA. While this finding is surprising, it may be explained by CART’s ability to detect possible interactions with small sample sizes, whereas standard statistical analyses would not be powered to detect interactions. For example, the CART results suggest that glucose may be an important factor among older adults, but not among younger adults. It may be useful to explore the association between fasting glucose levels and development of AKOA among adults 64 years and older.
CART is useful as an exploratory method to help identify possible informative participant characteristics and interactions that are not possible with regression in studies with limited sample size. The CART results suggest that there may be interactions between age, BMI, and glucose in the base model. There may also be interactions between effusion-synovitis volume and BMI, as well as effusion-synovitis volume and glucose. Future studies sufficiently powered to detect interactions will be important in improving classification of people at risk of developing AKOA.
While this study has identified potentially informative participant characteristics and possible interactions, we acknowledge that there are limitations. We have not yet replicated this model in an external dataset. Although external validation is a critical future project, we are still in the development stage of model development. Our best model still has a significant amount of unexplained variation and we feel this needs to be addressed by identification and inclusion of novel participant characteristics before proceeding to a standard development and validation model. Despite this limitation, we believe this study is an important step towards classifying adults who will develop AKOA.
In conclusion, acquisition of MR images for common structural features to identify individuals at risk of developing AKOA in the subsequent four years may not be justified due to the high cost of obtaining them and the minimal effect on classification compared to the base model. We also found that serum glucose, effusion-synovitis volume, and cruciate ligament degeneration are the most statistically important variables from these models in the classification of individuals at risk for AKOA in the next four years. However, all models continue to have ~60% of the variability unexplained, indicating a need for future research to identify novel participant characteristics that will improve the classification.
Supplementary Material
ACKNOWLEDGEMENTS
These analyses were financially supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01-AR065977. The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. Dr. Lo is supported by K23 AR062127, an NIH/NIAMS funded mentored award, providing support for design and conduct of the study, analysis, and interpretation of the data. This work is supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13–413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. Dr. Harkey was supported by the National Institutes of Health (grant no. 5 TL1 TR 1454–3). Dr. MacKay acknowledges the support of the National Institute for Health Research Cambridge Biomedical Research Centre. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication. The authors have no other conflicts of interest regarding this work. We would like to thank Alina Stout and Fatimah Al Eid for their assistance in reading the images.
REFERENCES
- 1.Driban JB, Eaton CB, Lo GH, et al. 2014. Knee Injuries Are Associated with Accelerated Knee Osteoarthritis Progression: data from the Osteoarthritis Initiative Arthritis Care Res (Hoboken) 66:1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driban JB, Price LL, Eaton CB, et al. 2016. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol 35:1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driban JB, Stout AC, Lo GH, et al. 2016. Best performing definition of accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis 8:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis J, Eaton CB, Lo GH, et al. 2017. Knee symptoms among adults at risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol 36:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driban JB, McAlindon TE, Amin M, et al. 2018. Risk factors can classify individuals who develop accelerated knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res 36:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JE, Ward RJ, MacKay JW, et al. 2019. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis Rheumatology (Oxford) 58:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driban JB, Davis JE, Lu B, et al. 2019. Accelerated Knee Osteoarthritis is Characterized by Destabilizing Meniscal Tears and Preradiographic Structural Disease Burden. Arthritis Rheumatol 71:1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driban JB, Ward RJ, Eaton CB, et al. 2015. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: Data from the Osteoarthritis Initiative. Clin Anat 28:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harkey MS, Davis JE, Lu B, et al. 2019. Diffuse tibiofemoral cartilage change prior to the development of accelerated knee osteoarthritis: Data from the osteoarthritis initiative. Clin Anat 32:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JE, Harkey MS, Ward RJ, et al. 2019. Accelerated Knee Osteoarthritis Is Associated with Pre-Radiographic Degeneration of the Extensor Mechanism and Cruciate Ligaments: Data from the Osteoarthritis Initiative. BMC Musculoskelet Disord 20:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://data-archive.nimh.nih.gov/oai/
- 12.Iranpour-Boroujeni T, Li J, Lynch JA, et al. 2014. A new method to measure anatomic knee alignment for large studies of OA: data from the osteoarthritis initiative. Osteoarthritis Cartilage 22:1668–1674. [DOI] [PubMed] [Google Scholar]
- 13.Driban JB, Stout AC, Duryea J, et al. 2016. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord 17:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrout PE, Fleiss JL. 1979. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychological Bulletin 86:420–428. [DOI] [PubMed] [Google Scholar]
- 15.Driban JB, Eaton CB, Amin M, et al. 2017. Glucose homeostasis influences the risk of incident knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res 35:2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellamy N 2009. WOMAC Osteoarthritis Index User Guide IX; [Google Scholar]
- 17.Roos EMT-L S 2003. Knee Injury and Osteoarthritis Outcome Score (KOOS)--validation and comparion to the WOMAC in total knee replacement. Health and quality of life outcomes 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterfy CG, Schneider E, Nevitt M. 2008. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 16:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driban JB, Price L, Lo GH, et al. 2013. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker--longitudinal relationships with pain and structural changes: data from the Osteoarthritis Initiative. Arthritis Res Ther 15:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang J, Driban JB, Destenaves G, et al. 2013. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the osteoarthritis initiative. BMC Musculoskelet Disord 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Driban JB, Price LL, et al. 2015. Development of a Rapid Cartilage Damage Quantification Method for the Lateral Tibiofemoral Compartment Using Magnetic Resonance Images: Data from the Osteoarthritis Initiative. Biomed Res Int 2015:634275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, B. DJ, Price LL, et al. 2014. Development of a rapid knee cartilage damage quantification method using magnetic resonance imaging. BMC Musculoskelet Disord 15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter DJ, Guermazi A, Lo GH, et al. 2011. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson AF, Irrgang JJ, Dunn W, et al. 2011. Interobserver reliability of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification of meniscal tears. Am J Sports Med 39:926–932. [DOI] [PubMed] [Google Scholar]
- 25.James G, Witten D, Hastie T, et al. 2017. An Introduction to Statistical Learning: with Applications in R. New York, New York: Springer; [Google Scholar]
- 26.Altman R, Asch E, Bloch DB, G., et al. 1986. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum 29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 27.Dawson LP, Fairley JL, Papandony MC, et al. 2018. Is abnormal glucose tolerance or diabetes a risk factor for knee, hip, or hand osteoarthritis? A systematic review. Semin Arthritis Rheum 48:176–189. [DOI] [PubMed] [Google Scholar]
- 28.Engstrom G, Gerhardsson de Verdier M, Rollof J, et al. 2009. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 17:168–173. [DOI] [PubMed] [Google Scholar]
- 29.Garessus ED, de Mutsert R, Visser AW, et al. 2016. No association between impaired glucose metabolism and osteoarthritis. Osteoarthritis Cartilage 24:1541–1547. [DOI] [PubMed] [Google Scholar]
- 30.Rogers-Soeder TS, Lane NE, Walimbe M, et al. 2018. Association of diabetes mellitus and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis Arthritis Care Res (Hoboken). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.