Abstract
Purpose:
To compare motion tracking by two modern methods (fat navigators - FatNavs and Moiré phase tracking - MPT) as well as their performance for retrospective correction of very high resolution acquisitions.
Methods:
A direct comparison of FatNavs and MPT motion parameters was performed for several deliberate motion patterns to estimate the agreement between methods. In addition, two different navigator resolution were applied.
0.5 mm isotropic MP2RAGE images with simultaneous MPT and FatNavs tracking were acquired in nine cooperative subjects with no intentional motion. Retrospective motion corrections based on both tracking modalities were compared qualitatively and quantitatively. The FatNavs impact on quantitative T1 maps was also investigated.
Results:
Both methods showed good agreement within a 0.3 mm/° margin in subjects that moved very little. Higher resolution FatNavs (2mm) showed overall better agreement with MPT than 4mm resolution ones, except for fast and large motion.
The retrospective motion corrections based on MPT or FatNavs were at par in 33 cases out of 36, and visibly improved image quality compared to the uncorrected images. In separate fringe cases, both methods suffered from their respective potential shortcomings: unreliable marker attachment for MPT and poor temporal resolution for FatNavs.
The magnetization transfer induced by the navigator RF pulses had a visible impact on the T1 values distribution, with a shift of the gray and white matter peaks of 12 ms at most.
Conclusion:
This work confirms both FatNavs and MPT as excellent retrospective motion correction methods for very high resolution imaging of cooperative subjects.
Keywords: MPT, FatNavs, Motion Correction, Brain
Introduction
Along with the increasing availability of ultra-high field MRI, in vivo, sub-millimeter imaging can be achieved at reasonable SNR and scan time. However, subject motion remains a major challenge1 because with higher imaging resolution and correspondingly longer scan durations, both the sensitivity to subject motion and the likelihood of motion occurring increase. At sub-millimeter resolution unintentional subject motion is on the order of the imaging resolution, thus, even small-scale motion such as slow head drifts and breathing can degrade the image quality2,3. Several solutions to address motion have been proposed4, especially in the case of brain imaging, where bulk motion can be reasonably well modelled as rigid.
Moiré phase tracking (MPT) is an optical method to track subject motion with a single in-bore camera and a single marker (attached to the subject) in six degrees-of-freedom. Using the motion estimates provided by this external hardware, the MR imaging volume’s position and orientation can be updated during scanning, thus, correcting motion prospectively. This technique allowed the highest resolution whole brain in vivo data acquisition3, with up to 250 µm and 150 µm isotropic resolution for anatomical5 and vascular6 data respectively. Due to its accuracy and short latency, MPT is often regarded as a gold standard7.
Another approach to motion compensation is to use MR navigators8,9. Among these, fat selective navigators (FatNavs) were proposed10 and showed11 successful application to retrospective motion correction of very high-resolution protocols for T1/T2/T2* imaging with up to 350 µm isotropic resolution. The main advantages of FatNavs are that the fat signal in the head is sparse, allowing very high acceleration for the navigators themselves, that they require no additional hardware and that they have only minimal impact on the water signal.
A hybrid hardware/MR based-method, dubbed ‘field probes’, was recently compared to MPT12 and showed good agreement, but direct comparison to modern navigator methods is lacking. In this work, we compared MPT and FatNavs motion estimates, as well as their application to retrospective motion correction of very-high resolution acquisitions. To this end, motion estimates of different FatNav protocols were compared to simultaneously recorded MPT estimates for various intentionally performed motion patterns of different motion amplitude and speed. Subsequently, unintentional motion in high resolution MP2RAGE was corrected retrospectively on the basis of the FatNavs or MPT estimates in a cohort of compliant volunteers. Results were analyzed qualitatively and quantitatively to compare FatNavs to MPT. Finally, the bias of FatNav magnetization transfer effects on T1 mapping was analyzed in a single subject experiment. More generally, this work explores relevant advantages and disadvantages of current navigator-based and hardware-based methods for retrospective motion correction.
Methods
All experiments were performed on a 7T whole-body MRI scanner (Siemens Healthineers, Erlangen, Germany) using a 32-channel RF head coil (Nova Medical, Wilmington, Massachusetts, USA).
All eleven subjects were healthy and compliant volunteers who are regularly scanned at 7T. Furthermore, all volunteers gave written consent prior to participation in this study, which was approved by the local ethics committee. Two experiments were performed to (1) compare the motion estimates of MPT against FatNavs and (2) to analyse the image reconstruction quality using estimates from both methods for retrospective motion correction.
Moiré phase tracking:
MPT (Metria Innovation, Milwaukee, Wisconsin, USA) consist of an in-bore camera, a single 15× 15 mm2 marker, and a tracking PC. The marker is attached to the subjects’ teeth of the upper jaw via custom-made mouthpieces (based on individual dental impressions). Tracking in six degrees of freedom with this single-marker, single-camera setup is realized by lithographically printing layers on the transparent marker to generate Moiré patterns. Under rotation these patterns change and by fitting sinusoidal functions to the gray levels along the pattern the out-of-plane-rotation can be estimated. Standard photogrammetric techniques are used to estimate the remaining four degrees of freedom. Tracking is performed with 86 frames per second and the precision of the motion estimates was previously reported as 0.01mm and 0.01 degree7. A detailed description and validation of the motion correction system can be found elsewhere3,7. Finally, motion estimates acquired by MPT need to be transformed from the camera to the scanner coordinate system, using a process called cross-calibration.
FatNavs:
FatNavs aim to acquire the fat signal of the head, mainly the subcutaneous fat, and exploit the signal sparsity (in space) to highly accelerate the whole image acquisition using parallel imaging13. The excitation consists of a binomial pulse, and a 1–2-1 implementation scheme is sufficient at 7T to almost exclusively measure fat and leave the brain signal mostly undetectable in the navigator image. Different implementations have been proposed, including 2D10, 3D collapsed14 and full 3D11 versions. The full 3D version was used in this study and shall be called FatNavs for simplicity. Approximately 1.5 s are required to acquire a 2mm isotropic navigator. As with almost all navigators, dedicated scan-time in the imaging sequence is required for the navigator acquisition, making inversion recovery based sequences such as MP2RAGE natural candidates due to their inherent dead-time. If no dead-time is present in the imaging sequence, alternatives can be considered at the price of addition scan-time15. Unlike MPT, FatNavs do not require any additional hardware or cross-calibration. Due to the computational load of reconstructing each accelerated FatNav and the additional latency this would incur for real-time correction, motion correction is typically applied retrospectively for 3D FatNavs. Finally, even with perfect fat selectivity, the navigator acquisition does have an impact on the brain signal, mainly due to magnetization transfer (MT) effects. These effects can be reduced by using a low excitation flip angle as the short T1 of fat allows for sufficient navigator signal, but depending on the total duration of each section of dead-time, the influence of the MT effect may still be directly observed.
Experiment 1: Motion estimates for intentional motion patterns
During the first experiment FatNavs were acquired successively while monitoring the subject’s motion using the MPT setup. This allowed direct comparison of rigid motion parameters with maximal scan-time efficiency as only navigators were acquired without any parent imaging sequence. The volunteer was asked to perform predefined motion patterns during the acquisitions. These six patterns were: rest (no intentional motion, TA=10 min), coughing (TA=30 s, single intentional coughing after approx. 15s of scanning, repeated three times), foot motion (dorsal plantar flexion, TA=60s), swallowing (TA=60s, swallowing twice during scanning), deep breathing (TA=60s), drawing a figure eight with the nose (TA=2min 30s), once slowly and once faster. For each motion pattern two different FatNavs protocols were acquired back-to-back, namely a 2 mm protocol (TE/TR 1.68/3.8 ms, TA=1.65 s) and a 4mm one (TE/TR 1.43/3.4 ms, TA=0.37 s), leading to motion-estimate frequencies of 0.6 Hz and 2.7 Hz respectively. Other parameters were identical for both scans: 1950 Hz/pixel readout bandwidth, 7° FA, 4×4 under-sampling and ¾ partial Fourier in both phase encoding directions (left-right and anterior-posterior). The auto-calibration signal needed for the FatNavs GRAPPA reconstruction was acquired before each scan (~4 s) without intentional motion. These protocols were chosen as they proved efficient in previous work11,15 and allow exploration of the tradeoff between spatial vs temporal resolution. MPT and navigator data were synchronized by an optical trigger sent at every FatNav and stored in the MPT log files.
The FatNavs were co-registered using SPM 13 without any region masking or down-weighting, and the time closest to acquisition of the k-space center of each navigator was defined as their measurement time-point. The method used to quantify differences between modalities was as follows. Similarly to a retrospective motion correction approach, the acquired FatNavs motion parameters were interpolated linearly to the MPT measurements time-points, in a range restricted to values between the first and last FatNavs measurements, resulting in motion estimates . With the associated N MPT estimates denoted by , the RMSE of the difference was then defined as
| (1) |
This definition amounts to estimate the normalized integral power of the difference of the FatNavs-based motion parameters compared to MPT, and was computed for each of the six motion parameters. The motion parameters ranges during FatNavs acquisition were defined as the difference between maximum and minimum MPT estimates, Translation and rotation ranges were then defined as the mean of the three associated motion parameters ranges, and shall serve as basis for motion amplitude comparison. The rate of motion was estimated as follows. The root mean squared of the temporal derivative of the MPT motion parameters were computed, then combined into the translation (or rotation) rate by taking the mean of the three associated parameters. For the coughing pattern, all computations were restricted to a 5 seconds window centered at the cough peak.
Experiment 2: Retrospective motion correction of unintentional motion using MPT and FatNavs
The second experiment, performed on nine volunteers, consisted of acquiring two 0.5 mm isotropic MP2RAGE16 scans (each MP2RAGE scan acquires two inversion images and generates a combined uniform contrast which aims to maximize the gray and white matter contrast). According to common practice, tight padding was used to limit subject motion and all volunteers were asked to stay still during scanning. No further motion instructions were given and volunteers swallowed and breathed at their own native pace. These measures should reproduce conditions of neuroscientific studies conducted without motion correction. Hence, the potential of both tracking modalities for high resolution imaging could be extrapolated to other studies with compliant, experienced subjects. MP2RAGE sequence parameters were: TI1/TI2/TR 800/2700/6000 ms, FA1/FA2 7/5 °, two-fold acceleration in anterior-posterior direction and ¾ partial Fourier in left-right direction. The total acquisition time of a scan was 23 min 34 s.
FatNavs were measured directly after the second inversion readout train11, using the 2mm protocol from the first experiment, but with a 3° nominal flip angle. Another difference to the first experiment had to be made for the sequence timing to be feasible by the hardware. In order to fit the FatNavs in the available dead-time, the center of the excitation passband of the 1–2-1 binomial pulse was put at −7 ppm, instead of −3.3. ppm which would be fat-centered. This leads to a roughly 50% shorter excitation duration but also makes the nominal flip angle higher than the effective fat excitation. However, the short T1 of fat and lower value of the pulse’s passband at the water frequency allow for very sharp fat images. Again the GRAPPA calibration signal for the navigators was acquired at the very beginning of the scan. The temporal resolution of the navigator was 6s.
The MP2RAGE sequence is an excellent candidate to measure T1 maps at 7T17. In order to investigate the impact of the FatNavs on the quantitative T1 maps computed from the uniform contrast of the MP2RAGE, single scans without navigators, with 2mm FatNavs, and with 4mm FatNavs were consecutively acquired on one additional volunteer. The T1 histograms were computed after brain masking and no motion correction was performed in order to remove bias from the correction method. Due to the lack of B1 maps, a global B1 efficiency for all three scans was estimated on the navigator free acquisition to center the white matter T1 peak around previously reported values16. The histogram of the scan without FatNavs was statistically compared to both others using a Kolmogorov–Smirnov test. The peak values of each scan were estimated by fitting the histograms with a sum of two Gaussians. Direct visual comparison of T1 maps was also performed.
The retrospective motion correction followed the same reconstruction pipeline after interpolation of the chosen motion data (MPT or FatNavs) to the times of measurement of all readout-events. This was done as follows: the motion estimates for each k-space readout event were linearly interpolated from the neighboring acquired time points (i.e. every 6 s for FatNavs and ~0.011 s for MPT). No temporal filtering was applied at any step. Motion correction consisted of multiplication by a pure phase factor (for translations) of the k-space data followed by a nuFFT18 operator (for rotations), and was applied channel-wise. A similar reconstruction pipeline is freely available online19. Each measurement was reconstructed without motion correction, with FatNav motion estimates, and with MPT motion estimates, thus, three datasets were created.
Qualitatively the uniform contrast image of the raw (no motion correction) and motion corrected images (FatNavs based or MPT based) were directly compared visually. Quantitatively, the normalized gradient squared of the images was computed, as it was shown to be an excellent metric candidate for autofocusing-based motion correction20. Increase of the metric generally correlates with better image quality / lower artefacts. Both inversion images were considered as their contrast is unbounded (unlike the uniform contrast image).
The MPT system is most commonly used with real-time updates of the scanner coordinate system. Within this study we chose to disable the real-time update feature so that motion-estimates from the MPT could be directly compared with those from the FatNavs within the same scan without additional bias. The difference to prospective correction with MPT is expected to be small (for small-scale head motion) as the spatially non-selective excitation used in the whole-head MP2RAGE protocol should be largely insensitive to through-slab motion and incoherent spin-history artefacts.
Results
Direct motion estimates experiment
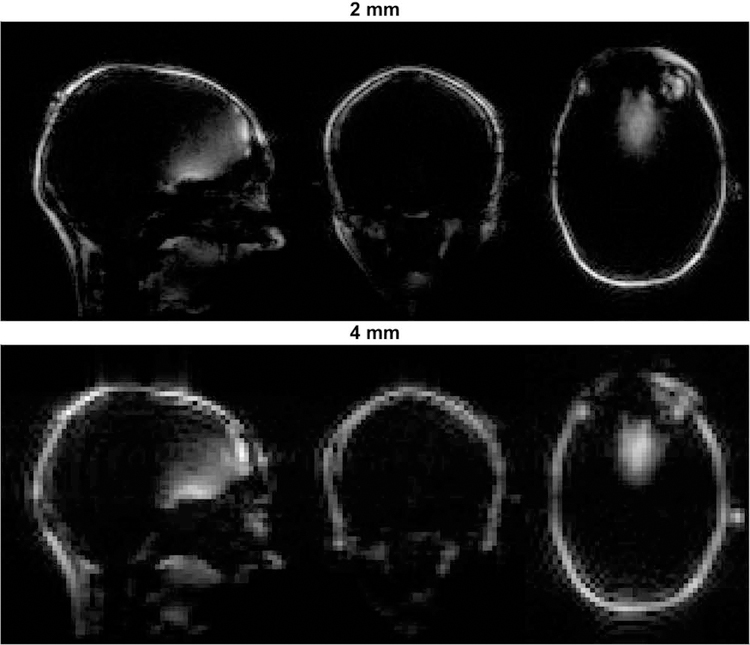
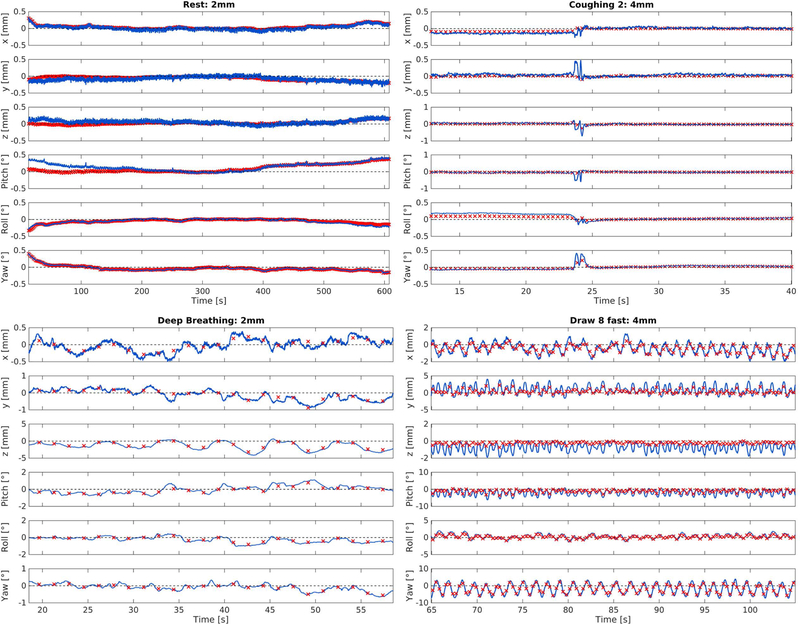
An example of the acquired FatNavs is presented in Figure 1. Figure 2 presents example time-courses of the motion parameters extracted from both tracking methods in different motion regimes. Generally, visual inspection reveals similar trends for MPT and FatNavs, especially for slower motion. The scale of the estimates varied between modalities, potentially explaining the offset visible at the beginning of the 2mm rest scan. As expected, in the case of faster motion such as coughing and swallowing, the navigators failed to track accurately the subject pose. Larger differences were observed for the figure eight patterns.
Figure 1:
Example volumes of both FatNav protocols acquired in the first experiment.
Figure 2:
Representative time-courses of both slow motion (left column) and faster motion (right column) acquired in the first experiment. Red crosses represent FatNavs and blue line represents MPT estimates. Temporal resolution of estimates: 1.5 / 0.37 s for 2mm / 4 mm FatNavs and 0.012 s for MPT.
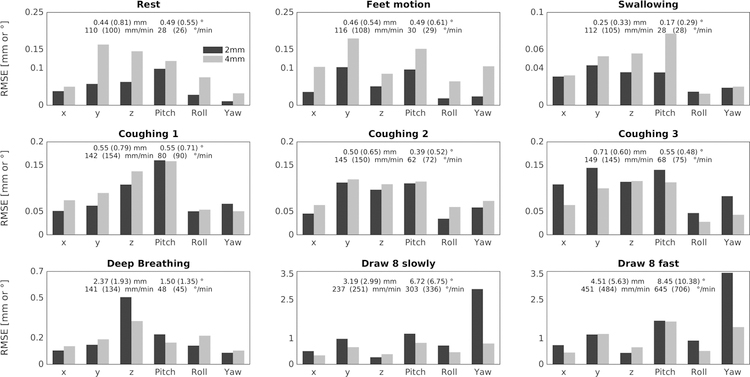
The RMSE values between the MPT and the FatNavs motion parameters are presented in Figure 3 as well as the translation and rotation ranges and rates during the scans. The RMSE were less than 0.3 mm (and °) except for deep breathing (<0.35 for 2mm FatNavs and <0.5 for the 4mm FatNavs) and figure eight pattern (≥ ~1). Lower spatial resolution (4mm) FatNavs performed worse than the 2mm navigators for smaller motion range patterns(rest, feet and swallowing). They show similar performance for deep breathing and coughing, presumably because of a tradeoff between the temporal and spatial resolutions of the FatNavs. The figure eight pattern was better captured by the 4mm FatNavs. The continuous, large motion of this pattern and its higher motion rate compared to the other patterns are expected to be the primary sources of this difference, as the 4mm FatNavs have a higher temporal resolution than the 2mm Fatnavs, effectively rendering the subject pose closer to being constant during a navigator acquisition. The temporal interpolation during metric computation also cannot be expected to properly capture the true pose change between navigator measurements (i.e. every 1.65 s for the 2mm navigators).
Figure 3:
RMSE between the FatNavs and the MPT estimates for all the motion patterns acquired during the first experiment. The translation and rotation ranges and rates are indicated in the plots. The values in brackets are for the 4mm FatNavs scans. 2mm FatNavs outperform 4mm FatNavs for slow motion patterns, and inversely for faster patterns.
Retrospective motion correction comparison for unintentional motion
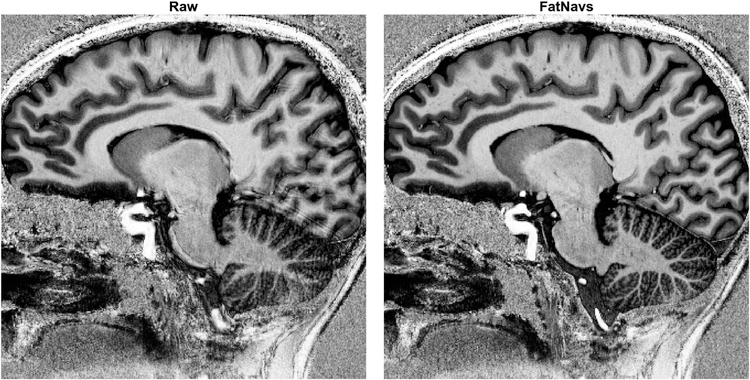
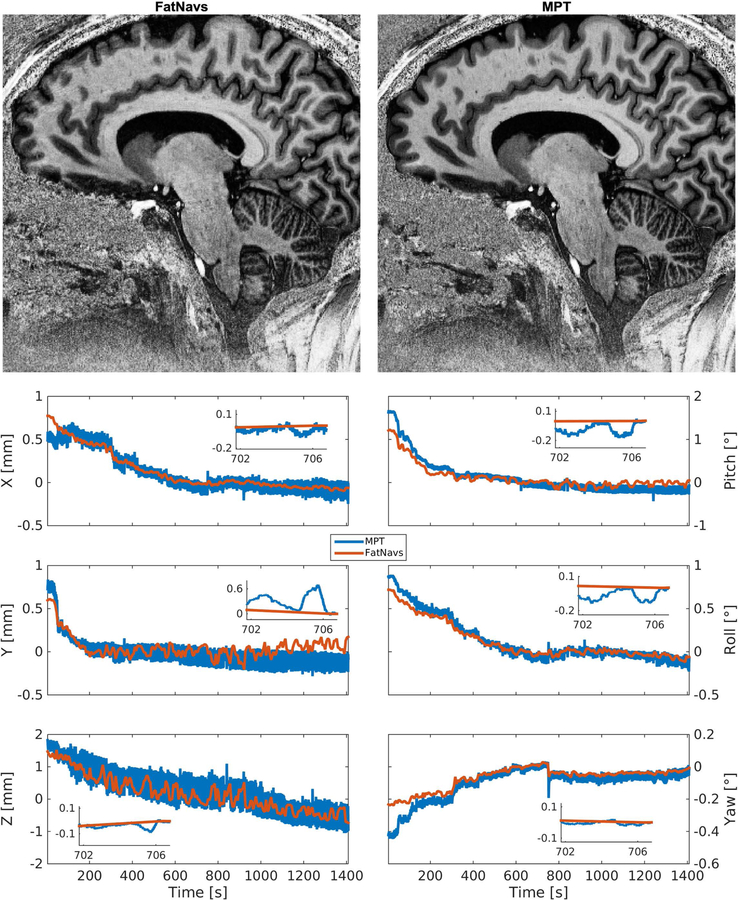
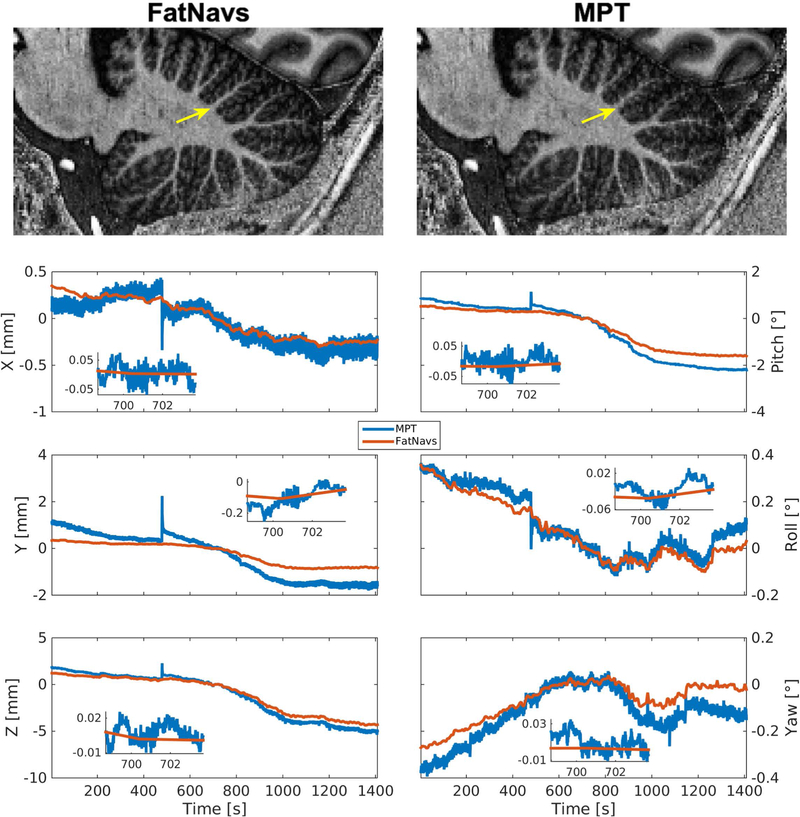
Visual inspection showed in 33 out of 36 cases motion corrected images were sharper and had overall reduced blurring and ringing artifacts compared to the uncorrected versions. Figure 4 shows an example of the improvements of FatNavs-based reconstruction compared to the raw reconstruction. Neither of the correction types (i.e. FatNavs or MPT based) showed consistently superior quality compared to the other across volunteers. We noted three cases where the corrected images were slightly worse than the raw reconstruction. These were: both scans of volunteer 8 for FatNavs and the second scan of volunteer 2 for the MPT (slight additional blurring). It was asserted that volunteer 8 had a high natural breathing depth during the scan and has a high BMI. Figure 5 shows both motion corrections for volunteer 8 (first scan). The oscillating motion during acquisition was entirely missed by the FatNavs due to the limited temporal resolution but captured by the MPT. The ringing, notably above the cerebellum and in the upper frontal cortex, is nicely suppressed in the MPT correction but was still present in the FatNavs correction. The FatNavs correction was however still sharper than the raw reconstruction. Supporting Information Figure S1 shows the three reconstructions side-by-side for the interested reader. By contrast, the FatNavs-based correction proved superior to the MPT correction for volunteer 2, see Figure 6. The motion parameters of MPT and FatNavs for this volunteer showed a similar trend but were different in amplitudes (scan 1) and abrupt motion in the MPT estimates occurred compared to FatNavs (scan 2). Supporting Information Figure S2 also shows the raw reconstruction for a more complete visual impression of this fringe case.
Figure 4:
Example views of the raw and motion corrected reconstruction using the 2mm FatNavs motion information (volunteer 2 first scan). The MPT corrected image also showed considerable improvement, but was inferior to the FatNavs one in this case, see Figure 6.
Figure 5:
Case of superior MPT correction compared to FatNavs (Volunteer 8 first scan). Notably, ringing artefact above the cerebellum and blurring in the frontal cortex present in the FatNavs correction are nicely suppressed by MPT.
Figure 6:
Case of superior FatNavs correction compared to MPT (volunteer 2 first scan), as can be seen by the overall better delineation of structures within the cerebellum. The yellow arrow indicates such a difference.
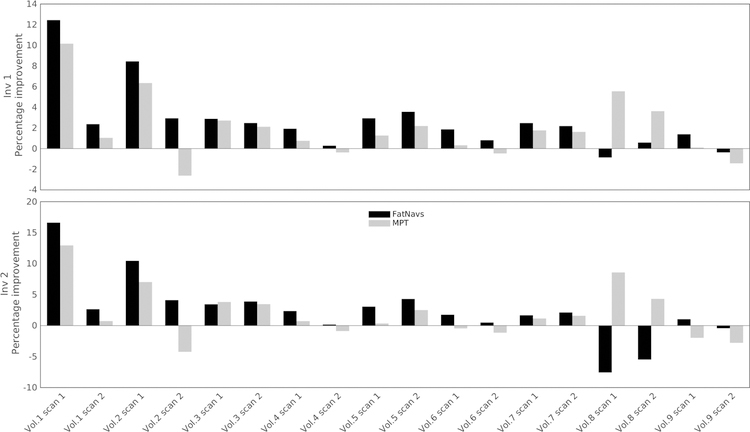
The normalized gradient metric confirmed the visual observation previously described, and is presented in Figure 7. However, it is our observation that changes of less than 2% did not correspond to visually perceived image degradation. Disagreement between the metrics of the first and second inversion images occurred once for FatNavs (volunteer 8 scan 2) and twice for MPT (volunteer 6 scan 1, volunteer 9 scan 1). The FatNavs case may be linked to inconsistent interpolation of the motion between FatNavs compared to true motion, as the low temporal resolution of the navigators did not capture the breathing-induced motion. In the MPT cases, the values are small and no difference on the images could actually be found, corroborating the metric limits mentioned above.
Figure 7:
Normalized gradient squared metric for both motion tracking modalities, and both inversion images produced by MP2RAGE. Graphed is the relative change of the metric compared to the raw reconstruction, in percent.
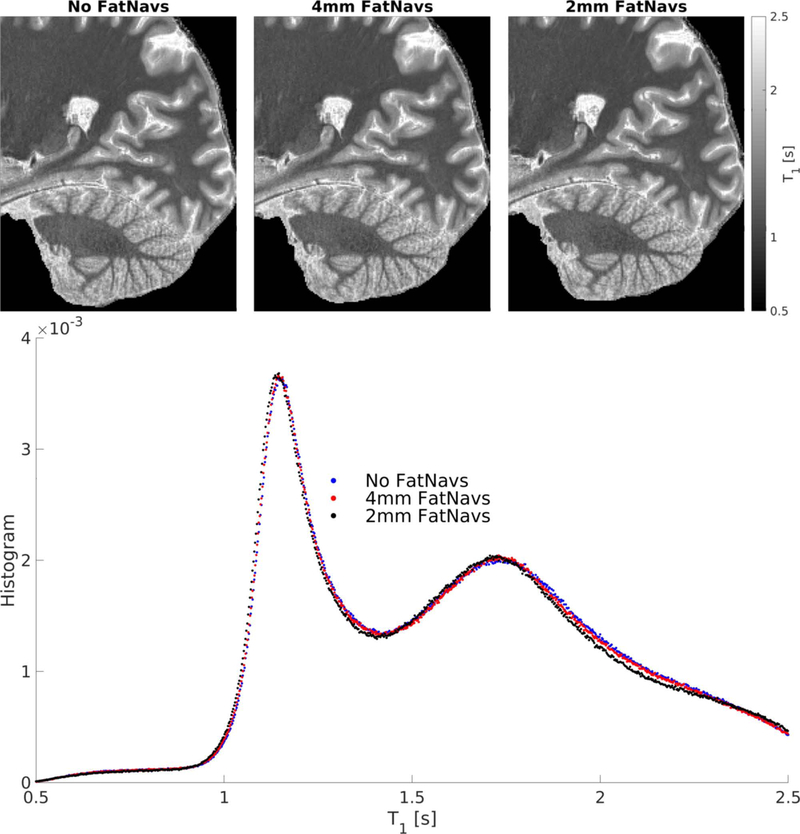
Slice images and histograms of the T1 values extracted from the single subject experiment without, with 4mm, and 2mm FatNavs inclusion are presented in Figure 8. While T1 maps were visually very similar, the histogram analysis shows the 4mm FatNavs T1 values to be significantly closer to the original protocol (without navigator) than with 2mm FatNavs inclusion, but slight bias can be still noted. This is corroborated by the Kolmogorov–Smirnov test statistics which were 0.0035 and 0.0122 for the 4mm and 2mm FatNavs respectively. The peaks of the fitted Gaussians were always centered at lower T1 values than in the navigator free scan. For white matter, the offset was 1.7 ms and 7.7 ms for the 4mm and 2mm FatNavs scans respectively. For gray matter it was 2.8 ms and 11.5 ms. It is interesting to note that the T1 bias differed approximately by a factor 4 between both navigated scans, which matches the ratio of the number of RF pulses between the 2mm and 4mm FatNavs. The same approximate ratio can be seen between the K-S statistics. As expected, the lower number of RF pulses of the 4mm FatNavs, combined with the longer relaxation period before the next inversion pulse, lowered the impact of the navigator inclusion on the T1 maps of the water signal compared to the 2mm navigator. While some brain signal was visible in the frontal area of the navigator for the direct comparison experiment (see Figure 1), it was not the case for the MP2RAGE experiments as the passband of the navigator excitation pulse was twice farther from water, making magnetization transfer the dominating source of disruption of the water signal. The approximate ad hoc B1 correction seems reasonable enough as the T1 values from the grey matter peak are in the range of reported values in the literature.
Figure 8:
Top: slices of the T1 maps obtained for three high-resolution imaging protocols: without navigators, with 4mm or with 2mm FatNavs. Bottom: the histograms of T1 values obtained after brain masking. The two clear peaks correspond to white-matter and grey-matter voxels.
Discussion
Experiment 1: direct comparison of intentional motion patterns
Overall fair agreement was found between tracking modalities, and the reported accuracies confirm that FatNavs as suitable for slow, small scale motion tracking, such as head drifts. Higher resolution navigators (2mm) were closer to MPT estimates than 4mm navigators, as had been expected. An exception to this observation occurred for large continuous motion of the figure eight pattern, where we attribute the superior accuracy of the registration for 4mm FatNavs to their four-times higher temporal resolution.
The reported RMSE values for coughing and swallowing may be slightly biased by the non-continuous nature of these motion patterns, as it is probable that the short rest periods outside of the motion events lower the final metric value.
Clearly, implementing FatNavs for motion correction is subjected to strict constraints by the imaging sequence, so the results presented here are to be understood as indication of FatNavs robustness against such motion patterns rather than FatNavs ability to correct for them, especially because any practical implementation of the FatNavs has a much lower temporal resolution than in this experiment. However, we believe the results obtained confirm them as valid candidates to motion-correct sub-millimeter imaging protocols in cooperative subjects who are expected to move slowly and on a small-scale.
Experiment 2: Comparison of retrospective correction of unintentional motion
Motion correction based on either modality virtually always improved the quality of the reconstructions. The delineation of high-resolution structures and sharpness obtained were similar across scans and motion tracking modalities. Globally, both methods showed similar performance when correcting for motion retrospectively within the studied cohort of compliant subjects without intentional motion. The presented results confirm that for cooperative subjects that mostly move slowly and with small to moderate amplitude, FatNavs has a retrospective motion correction ability equivalent to MPT, but requires much less effort experimentally as no custom-made mouth-piece and cameras are required. On the other hand, more agitated subject motion such as deep breathing is not fully recoverable by the FatNavs but are by MPT when the mouthpiece is robustly fixated, which represent the vast majority of cases in our experience (ten out of eleven subjects in the present work).
Previously reported potential shortcomings of both methods were observed. While conclusive evidence is not available, the results of volunteer 2, which had sub-par MPT performance, can presumably be explained by imperfect marker fixation to the upper jaw as both scans were impaired and similar problems did not occur for any other volunteer. Volunteer 8 demonstrated the limits of the FatNavs approach, as deep, unintentional breathing motion could not be adequately tracked. On the other-hand, MPT was naturally sensitive to these effects and allowed for their correction.
In this experiment, all volunteers swallowed and breathed at their own pace. In the first experiment intentional swallowing could not be fully resolved by FatNavs due to their limited temporal resolution. Due to the further reduced temporal resolution in this MP2RAGE experiment, FatNav based motion correction might contain residual motion degradation due to swallowing or other fast motion such as coughing. However, image quality for both motion correction modalities was comparable for most observed unintentional motion patterns, indicating in accordance to previous work2 that slow continuous drifts have a more profound impact on image quality than sparse, small-scale motion patterns such as swallowing within a cohort of young healthy adult volunteers.
A further effect potentially confounding FatNavs registration is gradient non-linearity. While the individual navigator images are definitely subject to distortions, the motion range is usually limited in practice thanks to head padding, and is low enough for the navigator distortion not to change significantly with motion. This leads to equivalent motion estimates when using un-warped images (data not shown).
The normalized gradient metric analysis corroborates our findings. Small metric variation did not represent a truly perceivable visual image change, but can potentially be due to removal of smaller scale and less coherent artefacts, unlike typical blurring or ringing suppression. Such changes are more difficult to pinpoint on the images. The absolute value of the change is, in our opinion, difficult to interpret, as not only different artifacts levels, but also different artifacts types, such as ringing or blurring, impact the metric in different ways. Still the larger variation definitely correlated to more prominent motion artefacts as expected from the literature. We do not expect a quantitative extrapolation of our findings beyond the studied cohort of healthy and compliant volunteers, however the FatNavs would be expected to underperform in cases where continuous significant motion is present, such as tremor-prone subjects, as they would lack the necessary temporal resolution, unlike MPT. For high resolution neuroscientific research conducted with experienced subjects and tight head padding image degradation due to unintentional motion could be prevented by MPT and FatNavs with comparable performance.
The T1 histograms showed the expected magnetization transfer impact induced by FatNavs acquisition. However, magnetization transfer is not taken into account in the computation of the MP2RAGE T1 maps by definition, and therefore these maps are susceptible to the specifics of the implementation of the sequence, such as the inversion pulse used (as always for T1 mapping). These small deviations compared to T1 values of a protocol without FatNavs should be kept in mind for any quantitative use of the data, especially if comparing navigated and non-navigated images, but a detailed study of the exact quantification of the FatNavs MT impact remains outside of the scope this study.
Prospective motion correction, as typically done with MPT, could theoretically reduce the artifacts level further, and especially so for accelerated protocols. Also bypassing the nuFFT based reconstruction theoretically allows for sharper effective resolution because of the absence of local Nyquist criteria violation and interpolation. Nevertheless, the high quality of the MPT reconstruction for volunteer 8, with continuously varying motion during the scans, leaves us confident in the validity of the presented retrospective corrections. We also take the same results to validate our implicit assumption of sufficiently accurate MPT cross-calibration for any residual errors to be neglected.
Implementation of FatNavs into a prospectively motion corrected acquisition might also be useful in some cases. In cases such as the example of volunteer 2 in this work, where we suspect the superiority of the FatNavs correction was due to poor marker attachment, the difference of motion parameters between MPT and FatNavs could be exploited to automatically detect potentially unreliable data from the MPT. Depending on how frequently such irregularities are shown to occur, future work could investigate the utility of additional retrospective FatNav-based correction to account for the offset – as well as whether the MPT marker attachment itself can be made even more reliable.
Conclusions
We directly compared the motion estimates of two established brain motion correction techniques, and showed that in a retrospective motion correction framework, both methods are roughly equivalent (up to the studied resolution and motion patterns) within the tested, healthy and compliant subject cohort. This work confirms the FatNavs as a solid alternative to MPT to prevent image degradation induced by small-scale, unintentional motion for high resolution protocols. Combining results from both experiments, we recommend to tune the navigator protocol depending not only on the imaging sequence parameters, such as resolution and amount of dead-time available, but also on its purpose, especially so for quantitative studies as the presence of additional RF pulses for the navigator will always have some influence on the main imaging sequence.
Supplementary Material
Supporting Information Figure S1: Zoomed-in view of the three reconstructions for volunteer 8 first scan. While the FatNavs image is sharper than the raw reconstruction, ringing is also more visible.
Supporting Information Figure S2: Zoomed-in view of the three reconstructions for volunteer 2 second scan. The MPT-corrected image is arguably of slightly lesser quality than the raw reconstruction (this was the only such case observed).
Acknowledgments
This work was supported in part by the NIH, grant number 1R01-DA021146, the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations, as well as the Swiss National Foundation through Grant 205321_153564.
References
- 1.Andre JB, Bresnahan BW, Mossa-Basha M, Hoff MN, Patrick Smith C, Anzai Y, Cohen WA. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. Journal of the American College of Radiology 2015;12(7):689–695. [DOI] [PubMed] [Google Scholar]
- 2.Herbst M, MacLaren J, Lovell-Smith C, Sostheim R, Egger K, Harloff A, Korvink J, Hennig J, Zaitsev M. Reproduction of motion artifacts for performance analysis of prospective motion correction in MRI. Magnetic Resonance in Medicine 2014;71(1):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stucht D, Danishad KA, Schulze P, Godenschweger F, Zaitsev M, Speck O. Highest Resolution In Vivo Human Brain MRI Using Prospective Motion Correction. PloS one 2015;10(7):e0133921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. Journal of Magnetic Resonance Imaging 2015;42(4):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lüsebrink F, Sciarra A, Mattern H, Yakupov R, Speck O. T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm. Scientific Data 2017;4:170032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattern H, Sciarra A, Godenschweger F, Stucht D, Lüsebrink F, Rose G, Speck O. Prospective motion correction enables highest resolution time-of-flight angiography at 7T. Magnetic Resonance in Medicine 2017;00:1–11. [DOI] [PMC free article] [PubMed]
- 7.Maclaren J, Armstrong BSR, Barrows RT, Danishad KA, Ernst T, Foster CL, Gumus K, Herbst M, Kadashevich IY, Kusik TP, et al. Measurement and Correction of Microscopic Head Motion during Magnetic Resonance Imaging of the Brain. PLoS ONE 2012;7(11):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van Der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine 2012;68(2):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Der Kouwe AJW, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magnetic Resonance in Medicine 2006;56(5):1019–1032. [DOI] [PubMed] [Google Scholar]
- 10.Skare S, Hartwig A, Mårtensson M, Avventi E, Engström M. Properties of a 2D fat navigator for prospective image domain correction of nodding motion in brain MRI. Magnetic resonance in medicine 2015;73(3):1110–9. [DOI] [PubMed] [Google Scholar]
- 11.Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magnetic Resonance in Medicine 2015;00:1–10. [DOI] [PubMed] [Google Scholar]
- 12.Eschelbach M, Aghaeifar A, Bause J, Handwerker J, Anders J, Engel E-M, Thielscher A, Scheffler K. Comparison of Prospective Head Motion Correction with NMR Field Probes and an Optical Tracking System. Magnetic Resonance in Medicine 2018;(October 2017):1–11. [DOI] [PubMed]
- 13.Gallichan D, Marques JP. Optimizing the acceleration and resolution of three-dimensional fat image navigators for high-resolution motion correction at 7T. Magnetic Resonance in Medicine 2017;77(2):547–558. [DOI] [PubMed] [Google Scholar]
- 14.Engström M, Mårtensson M, Avventi E, Norbeck O, Skare S. Collapsed fat navigators for brain 3D rigid body motion. Magnetic Resonance Imaging 2015;33(8):984–991. [DOI] [PubMed] [Google Scholar]
- 15.Gretsch F, Marques JP, Gallichan D. Investigating the accuracy of FatNav-derived estimates of temporal B0 changes and their application to retrospective correction of high-resolution 3D GRE of the human brain at 7T. Magnetic Resonance in Medicine 2018;80(2):585–597. [DOI] [PubMed] [Google Scholar]
- 16.Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 2010;49(2):1271–1281. [DOI] [PubMed] [Google Scholar]
- 17.Marques JP, Gruetter R. New Developments and Applications of the MP2RAGE Sequence - Focusing the Contrast and High Spatial Resolution R1Mapping. PLoS ONE 2013;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fessler JA, Sutton BP. Nonuniform fast fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing 2003;51(2):560–574. [Google Scholar]
- 19.Gallichan D retroMoCoBox: https://github.com/dgallichan/retroMoCoBox.
- 20.McGee KP, Manduca A, Felmlee JP, Riederer SJ, Ehman RL. Image metric-based correction (Autocorrection) of motion effects: Analysis of image metrics. Journal of Magnetic Resonance Imaging 2000;11(2):174–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1: Zoomed-in view of the three reconstructions for volunteer 8 first scan. While the FatNavs image is sharper than the raw reconstruction, ringing is also more visible.
Supporting Information Figure S2: Zoomed-in view of the three reconstructions for volunteer 2 second scan. The MPT-corrected image is arguably of slightly lesser quality than the raw reconstruction (this was the only such case observed).