Abstract
Aim
This investigation tested the construct validity of the first standardized assessment tool, the BaByVFSS Impairment Profile, (BaByVFSSImP©), developed for the quantification of swallowing observations made from videofluoroscopic swallow studies (VFSS) in bottle-fed babies.
Method
Construct validity of the measures was tested using descriptive methods and confirmatory factor analysis (CFA) of swallowing scores obtained from a cohort of bottle-fed babies (median age 3 months 1 day, interquartile range: 1 month 4 days – 7 months 4 days) sequentially referred for VFSS based on clinical signs, symptoms or risk factors associated with dysphagia and/or aspiration. Main outcome measures were emergence of functional domains derived from swallowing component impairment scores.
Results
CFA resulted in 21 significant components (factor loadings ≥ 0.5) grouping into five functional domains labeled for common contribution to overall swallowing function. The tool was organized into the BaByVFSSImP. Clinical relevance was explored using correlational analyses between domain scores, maximum penetration/aspiration scores, feeding status, and caregiver burden.
Interpretation
Quantification of physiologic swallowing impairment captured by BaByVFSSImP holds promise for identification of physiologically based targets for intervention, clinical decisions regarding enteral feeding, and tracking the trajectory of swallowing impairment throughout development in young children.
Keywords: Infant, VFSS, MBS, Reliability, Dysphagia, Deglutition, Deglutition Disorders
INTRODUCTION
The frequency of swallowing disorders (dysphagia) is increasing in young children secondary to advances in medical and life sustaining measures that improve the survival of infants born premature, with low-birthweight and with complex medical conditions. (1–4) The highest prevalence rates are reported for children with cerebral palsy and other neurodevelopmental disorders. (5–7) Significant morbidities associated with dysphagia include respiratory and nutritional compromise, the development of chronic feeding problems, and stressful child and caregiver interactions. (8–11) In addition, childhood dysphagia may signal the development of future speech and language delays (12, 13), poorer developmental outcomes (14), and the adult onset of disease and serious health conditions. (15–17) Early detection and prompt interventions are imperative to reduce the consequences of dysphagia and have resulted in the increased use of the Videofluoroscopic Swallowing Studies (VFSS) in children.
The VFSS is the primary method used to evaluate feeding difficulties and swallowing disorders in bottle-fed babies. To date, VFSS procedures and the interpretation of images have not kept pace with the advances in our understanding of the swallowing mechanism. Despite efforts by clinicians to adopt best practices based on the existing evidence, VFSS procedures in children are variable and largely determined by the skills of the examining clinician, institutional wisdom, and information derived from experience with adults. (18) Correspondingly, there are no broadly applied standardized approaches to characterize the type and severity of the swallowing impairments in the bottle-fed babies assessed with videofluoroscopic imaging.
Herein, we describe the development of a standardized VFSS assessment tool, the BaByVFSS Impairment Profile (BaByVFSSImP©), for the quantification of a range of physiologic swallowing impairments in bottle-fed babies. The current investigation builds upon the years of experience from the adult Modified Barium Swallow Impairment Profile (MBSImP™) (19) now field tested by over 7,000 clinicians in 25 countries. (19, 20) Similar to the MBSImP, the BaByVFSSImP goes beyond observations of aspiration and residue and aims to capture impairment in swallowing physiology. Although many components of swallowing are similar to those tested on the adult tool, several components and their scoring schema were modified based on the varying dynamics of swallowing in bottle-fed babies.
Our previous study demonstrated validation through expert consensus and rater-reliability for 24 components of swallowing function hypothesized as critical to swallowing in bottle-fed babies. (21) One component on the adult tool, esophageal clearance, was eliminated in the current project. This component was not consistently assessed during VFSS to adhere to practice mandates to keep radiation exposure “as low as reasonably achievable (ALARA)” by not unnecessarily repeating examinations of the esophagus. (22, 23) ACR-SPR Guidelines for examination of the esophagus and upper gastrointestinal tract specify that fluoroscopic studies be “optimally performed” while children are lying down for visualization of the nasopharynx to the gastric fundus. (24, 25) The primary purposes of the present study were to: 1) establish the construct validity of 23 physiologic components of swallowing and establish their functional groupings hereby referenced as ‘domains’, 2) organize the components and domains into a clinically logical tool (BaByVFSSImP), and 3) assess the clinical relevance of the BaByVFSSImP through association of domain scores with airway protection (Penetration-Aspiration Scale [PAS]) and specific external indicators of well-being, including feeding recommendations and caregiver burden. (26)
METHOD
Participants
Caregivers provided written informed consent for in- and out-patient bottle-fed babies referred for clinically indicated VFSS at Johns Hopkins University School of Medicine (JHH) and the Medical University of South Carolina (MUSC) between 2012 and 2016. Institutional Review Board approval was obtained at both sites. Babies were referred by primary physicians or subspecialists based on signs/symptoms of dysphagia or aspiration, or because they were considered high risk for dysphagia and aspiration based on their medical history. (27) Exclusion criteria included babies whose caregivers were non-English speaking and without adequate interpreter services, in foster care, under the care of the department of social services or wards of the state, whose caregivers refused to consent, or children who were not accompanied by an adult qualified to provide consent for study enrollment.
Data Collection
Caregivers provided demographic information and completed surveys about the health and medical status and feeding/swallowing histories as per standard clinical procedures. They also completed the Feeding/Swallowing Impact Survey (FSIS), a validated instrument designed to measure the impact of children’s feeding/swallowing difficulties on caregivers. (2, 8, 28) Feeding status prior to each VFSS and the level recommended by the examining speech-language pathologist (SLP) after each VFSS were recorded. Eight possible levels of feeding status were identified from clinical experience and the literature, and rank-ordered on a scale from 0 to 7, with 0 representing the lowest score (no restrictions) to 7 being the highest or most severe restrictions. (Figure 1)
FIGURE 1.
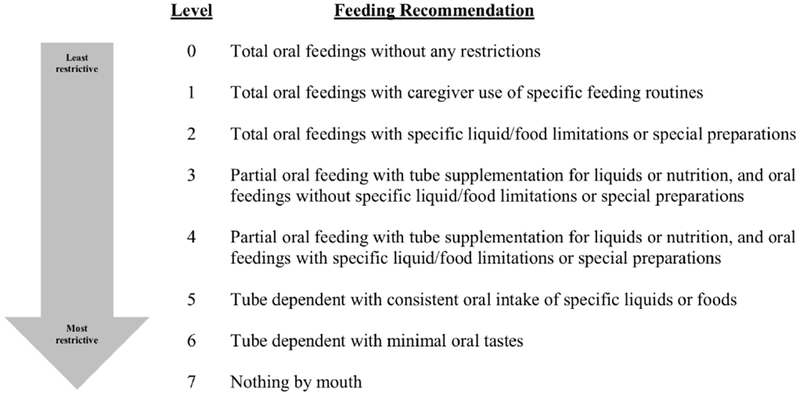
Feeding Level Recommendations
Standardization of Radiation Imaging
Dose and image quality of the fluoroscopic equipment were standardized by pediatric radiologists at each site. The lowest level of magnification (approximately 6.7 FOV) needed for visualization of space between the laryngeal surface of the epiglottis and arytenoids was used. The fluoroscopy acquisition rate was continuous, and images were recorded at the standard resolution of 30 frames/sec in digital format.
Radiologic Protocol
Each VFSS took place in a standard radiology fluoroscopy suite and was conducted jointly by a pediatric SLP and pediatric radiologist. Babies were positioned in their typical or optimal feeding position (head midline and neck in a neutral position, or pre-determined “best” position) in a Multiple Application Multiple Articulation seating system (MAMA Systems Inc., Oconomowoc, Wisconsin, USA), a specialized chair for pediatric VFSS. The visualization field included the lips anteriorly, nasal cavity superiorly, cervical spinal column posteriorly and the pharyngoesophageal segment inferiorly.
Commercially available standardized preparations of room temperature liquid barium (Varibar® thin and nectar) were presented as clinically indicated. Contrast was delivered by nipples and bottles that were chosen and adapted according to the clinical characteristics and needs of the baby. Fluoroscopic images were acquired during the first series of suck-swallow sequences and then randomly during periods of subsequent sequences--a measure used to limit exposure to radiation. (18, 29, 30) Outside the scope of this study, the SLP proceeded with clinically indicated compensatory strategies (e.g., bolus or nipple modifications or positional changes) for optimization of airway protection and bolus clearance. Consistent with the MBSImP scoring, therapeutic modifications were recorded for clinical purposes but not included in the scored observations. (31, 32)
Data Capture, Storage and Sharing
VFSS data were recorded using digital video imaging (Digital Swallowing Workstation™ [DSW], Model 7200 KayPENTAX, Lincoln Park, New Jersey, USA) for signal acquisition, digital storage, and retrieval of the swallowing data. Each VFSS exam was downloaded to computer storage media, saved on the DSW, de-identified, converted from native format to universal digital video format (.mpg) for scoring, and shared between the two institutions using the password protected Research Electronic Data Capture (REDCap) database system.
Component Scoring
Seven raters were ASHA (American Speech-Language Hearing Association) certified SLPs with 2-28 years (median: 7 years) of experience, were like-trained and achieved ≥ 80% scoring reliability as requisite for study participation. (21) SLP rater training included didactic sessions led by MPIs, independent practice and testing as previously detailed in a prior publication. (21) Five of the raters had experience with pediatric dysphagia, which included conducting and interpreting VFSS examinations in bottle-fed infants and young children. Two of the raters had worked exclusively with adult dysphagic patients and completed training with the MBSImP approach. (21)
Raters who were blinded to the clinical information and all identifiers, scored the exams using slow motion and frame by frame playback. Components were scored using a rank ordered numeric scale (highest number representing the worst performance) ranging from 3-5 possible scores per component with each representing a distinguishable observation and ambiguity eliminated as much as possible based on results from the previous reliability study. (21) Consistent with our clinical scoring approach for quantifying observations of swallowing impairment in adults (19, 32, 33) and our reliability testing in bottle-fed children (21), clinicians reviewed all images from each swallow study to identify the most impaired components. The most impaired component was assigned the highest (i.e., the most severe) score and termed the Overall Impression Score (OI). Raters also scored the maximum Penetration/Aspiration Score (PASmax) across all swallows in the series. (26) (Table 1)
Table 1.
Domains with Components and Score Variants
| Domain | Component | Range of Possible Scores |
|---|---|---|
| Lingual Motion/Pharyngeal Swallow Initiation | Initiation of Nutritive Sucks | 0 - 2 |
| Number of Sucks to Form Bolus | 1 - 7 | |
| Nutritive Suck Rhythmicity/Organization | 0 - 2 | |
| Suck/Swallow Bolus Control | 0 - 2 | |
| Bolus Location at Initiation of Pharyngeal Swallow | 0 - 3 | |
| Timing of Initiation of Pharyngeal Swallow | 0 - 2 | |
| Palatal Pharyngeal Approximation | Palatal-Pharyngeal Approximation/Palatal Integrity | 0 - 3 |
| Location of Bolus at Time of Palatal-Pharyngeal Approximation | 0 - 2 | |
| Airway Invasion/Laryngeal Closure | Early Laryngeal Vestibular Closure | 0 - 3 |
| Late Laryngeal Vestibular Closure | 0 - 3 | |
| Timing of Airway Entry | 0 - 4 | |
| Amount of Penetration | 0 - 2 | |
| Frequency of Penetration | 0 - 3 | |
| Aspiration | Amount of Aspiration | 0 - 2 |
| Frequency of Aspiration | 0 - 3 | |
| Pharyngeal Transport and Clearance | Epiglottic Movement | 0 - 2 |
| Tongue Base Retraction | 0 - 4 | |
| Pharyngeal Stripping Wave | 0 - 2 | |
| Valleculae Residue | 0 - 4 | |
| Pyriform Residue | 0 - 4 | |
| Pharyngoesophageal Segment (Upper Esophageal Sphincter) | 0 - 3 |
Statistical Analysis
Construct Validity
Data available for all 300 babies were included in computing a polychoric matrix because the scores were not multivariate normal. Only the thin liquids swallows were analyzed in this investigation. Components with missing data on greater than half of the sample were not included in the matrix. The correlation coefficients from the matrix were input into a Confirmatory Factor Analysis (CFA). The authors proposed a priori that the 23 swallowing components would aggregate into 5 factors or ‘domains’ based on their purportedly close association and shared roles in swallowing function as described in the literature. Minimal threshold to demonstrate loading of a component onto a factor (domain) was established as a loading ≥ 0.5. The hypothesized factors (or domains) to explain the desired latent construct, swallowing impairment, included: Nutritive Sucking/Oral Containment and Clearance, Pharyngeal Swallow Initiation, Pharyngeal Containment and Clearance, Airway Protection, and Esophageal Entry and Clearance.
Exploration of Clinical Relevance
Factor scores could not be calculated for each infant because the polychoric correlation matrix was used to generate the factor loadings rather than standardized scores. Therefore, we summed the component scores that loaded on a particular domain for each individual to derive domain scores. We examined the relationship of summative domain scores with: PASmax, recommendations for oral feeding intake status following the VFSS, and FSIS. Given the ordinal nature of the score data, Spearman Rank Order correlation was used to measure the strength and direction of association between the ranked variables.
Demographic and clinical characteristics were summarized using medians and interquartile ranges (IQRs) or counts and percentages and compared across sites using Wilcoxon rank-sum tests or Fisher’s exact tests as appropriate. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). All tests were two-sided and significance was set at p<0.05.
RESULTS
A total of 300 consecutive in- and out-patients referred for a VFSS met criteria for initial analysis. There were 121 females and 179 males with a median age of 3 months 1 day (interquartile range [IQR]: 1 month 4 days – 7 months 4 days), and a relatively equivalent sex distribution between the two sites (Table 2). One site enrolled a significantly greater number of Hispanic and non-white participants, and children from lower income families. Children presented with one or more diagnostic conditions with equal representation at both centers, except for a significantly higher number of children with pulmonary diagnoses from one site and more cardiac diagnoses at the other site.
Table 2.
Demographic and clinical characteristics of all infants and stratified by institution (N=300)
| Characteristic | All | JHH | MUSC | P value |
|---|---|---|---|---|
| Sex, N (%) | 0.35 | |||
| Male | 179 (59.7) | 85 (56.7) | 94 (62.7) | |
| Female | 121 (40.3) | 65 (43.3) | 56 (37.3) | |
| Ethnicity, N (%) | <0.001 | |||
| Hispanic | 28 (9.3) | 19 (12.7) | 9 (6.0) | |
| Not Hispanic | 255 (85.0) | 130 (86.7) | 125 (83.3) | |
| Unknown | 17 (5.7) | 1 (0.7) | 16 (10.7) | |
| Race, N (%) | 0.03 | |||
| African American | 78 (26.0) | 30 (20.0) | 48 (32.0) | |
| Asian | 6 (2.0) | 5 (3.3) | 1 (0.7) | |
| Caucasian | 176 (58.7) | 94 (62.7) | 82 (54.7) | |
| More than one race | 38 (12.7) | 21 (14.0) | 17 (11.3) | |
| Unknown | 2 (0.7) | 0 (0) | 2 (1.3) | |
| Household income ($, by zip code), median (IQR) | 53,324 (42,465-71,560) | 69,682 (52,493-85,588) | 45,003 (35,642-54,350) | <0.001 |
| Age at clinic visit (months), median (IQR) | 3.0 (1.1-7.2) | 4.8 (1.7-9.1) | 2.2 (1.0-5.1) | <0.001 |
| Preterm (<37 weeks), N (%) | 123 (41.0) | 64 (42.7) | 59 (39.3) | 0.64 |
| Adjusted age for preterm birth (months), median (IQR) | 1.0 (0-6.0) | 3.0 (0.0-7.0) | 1.0 (0.0-4.0) | <0.001 |
| Weight for age percentile, median (IQR) | 23.1 (3.3-64.1) | 28.1 (4.5-65.4) | 18.5 (2.4-54.1) | 0.11 |
| Height for age percentile, median (IQR) | 12.9 (0.2-51.8) | 20.7 (2.4-61.6) | 6.1 (0.1-42.0) | <0.001 |
| BMI for age percentile, median (IQR) | 46.8 (12.4-88.2) | 45.0 (11.8-85.9) | 57.5 (12.8-89.8) | 0.45 |
| Weight for height percentile, median (IQR) | 55.6 (12.4-90.2) | 44.8 (12.8-85.2) | 68.8 (11.9-93.7) | 0.07 |
| Weight for height percentile < 5% (failure to thrive), N (%) | 41 (15.4) | 17 (12.1) | 24 (19.2) | 0.13 |
| Feeding tube, N (%) | 142 (47.3) | 61 (40.7) | 81 (54.0) | 0.03 |
| Barium (ml), median (IQR) |
15 (7-27) | 20 (12-30) | 9 (5-20) | <0.001 |
| Diagnostic conditions, N (%) | ||||
| GI/Digestive/Nutritional | 183 (61.0) | 99 (66.0) | 84 (56.0) | 0.10 |
| Developmental Delays/Behavioral | 48 (16.0) | 30 (20.0) | 18 (12.0) | 0.08 |
| Pulmonary | 176 (58.7) | 113 (75.3) | 63 (42.0) | <0.001 |
| Nervous/Neuromuscular | 31 (10.3) | 19 (12.7) | 12 (8.0) | 0.25 |
| Anatomic/Structural | 102 (34.0) | 62 (41.3) | 40 (26.7) | 0.01 |
| Known Genetic/Syndromic/Metabolic | 58 (19.3) | 33 (22.0) | 25 (16.7) | 0.31 |
| Environmental Exposures/Social | 28 (9.3) | 18 (12.0) | 10 (6.7) | 0.16 |
| Cardiac | 98 (32.7) | 26 (17.3) | 72 (48.0) | <0.001 |
| Allergy/Immune/Systemic Processes | 11 (3.7) | 8 (5.3) | 3 (2.0) | 0.22 |
JHH John’s Hopkins Hospital, MUSC Medical University of South Carolina, IQR interquartile ranges
Fluoroscopy Time and Barium Dose
The mean (SD) fluoroscopy time across both sites was 106 (51) seconds. The median consumption of thin liquid barium by participants during the swallowing series was 15 mL, with a maximum consumption of 80 mL.
Component Scores and Functional Domains
From the 300 examinations included in computing the polychoric matrix, the effective sample size was reduced to 241 based on the component that had the least amount of non-missing data (Initiation of Nutritive Sucks component). The score for the Oral Residue component was missing in 52% of the sample and therefore, was not included in the factor analysis. Twenty-one of the 22 scored components factor loaded at ≥ 0.5 onto the five factors shown in Table 3. The Lip Closure component failed to load onto any component. Of interest, the Late Laryngeal Vestibular Closure component loaded onto two factors (henceforth, domains). It is grouped with the Airway Invasion/Laryngeal Closure domain because of its more robust association with other components loading in this domain.
Table 3.
Confirmatory Factor Analyses for 22 Swallowing Components
| Thin Component | Airway Invasion/Laryngeal Closure | Pharyngeal Transport and Clearance | Lingual Motion/Pharyngeal Swallow Initiation | Aspiration | Palatal Pharyngeal Approximation |
|---|---|---|---|---|---|
| Amount of Penetration | 0.981 | 0.113 | 0.076 | 0.107 | 0.083 |
| Frequency of Penetration | 0.893 | 0.156 | −0.007 | 0.020 | −0.006 |
| Early Laryngeal Vestibular Closure | 0.853 | 0.058 | −0.027 | 0.333 | 0.063 |
| Timing of Airway Entry | 0.742 | 0.272 | −0.007 | 0.419 | 0.022 |
| Late Laryngeal Vestibular Closurea | 0.636 | 0.072 | 0.009 | 0.565 | 0.113 |
| Pyriform Residue | 0.011 | 0.808 | 0.148 | 0.157 | 0.083 |
| Epiglottic Movement | 0.029 | 0.767 | 0.105 | −0.052 | 0.147 |
| Valleculae Residue | 0.058 | 0.708 | −0.017 | 0.176 | 0.160 |
| Tongue Base Retraction | 0.166 | 0.680 | 0.085 | 0.088 | 0.206 |
| Pharyngoesophageal Segment (Upper Esophageal Sphincter) | 0.104 | 0.673 | 0.065 | 0.104 | 0.014 |
| Pharyngeal Stripping Wave | 0.221 | 0.574 | 0.148 | 0.037 | 0.155 |
| Suck/Swallow Bolus Control | 0.108 | −0.210 | 0.930 | −0.118 | 0.019 |
| Bolus Location at Initiation of Pharyngeal Swallow | 0.333 | 0.020 | 0.717 | −0.098 | 0.027 |
| Initiation of Nutritive Sucks | −0.044 | 0.169 | 0.716 | 0.089 | 0.128 |
| Timing of Initiation of Pharyngeal Swallow | −0.055 | 0.201 | 0.696 | −0.166 | −0.073 |
| Nutritive Suck Rhythmicity/Organization | −0.118 | 0.145 | 0.643 | 0.054 | 0.043 |
| Number of Sucks to Form Bolus | 0.027 | 0.123 | 0.552 | −0.034 | 0.020 |
| Lip Closure | −0.040 | 0.005 | 0.354 | 0.151 | 0.153 |
| Amount of Aspiration | 0.307 | 0.191 | −0.026 | 0.938 | 0.055 |
| Frequency of Aspiration | 0.338 | 0.235 | −0.058 | 0.822 | 0.042 |
| Location of Bolus at Time of Palatal-Pharyngeal Approximation | 0.076 | 0.352 | 0.136 | 0.079 | 0.887 |
| Palatal-Pharyngeal Approximation/Palatal Integrity | 0.102 | 0.398 | 0.127 | 0.046 | 0.886 |
Factor loadings ≥0.5 are highlighted in bold
Late Laryngeal Vestibular Closure loaded on Airway Invasion/Laryngeal Closure and Aspiration domains. This component remains in the Airway Invasion/Laryngeal Closure domain because of its more robust association with other components loading in this domain.
The five-factor solution was confirmed from the CFA, however, specific components comprising the domains differed from those that were hypothesized. (Table 3) As such, the domains were renamed to reflect the latent construct or functional domain that the observed variables explained: Lingual Motion/Pharyngeal Swallow Initiation, Palatal Pharyngeal Approximation, Airway Invasion/Laryngeal Closure, Aspiration, and Pharyngeal Transport and Clearance. The resulting BaByVFSSImP was comprised of 21 components of swallowing impairment.
Domain Score Associations with Airway Invasion (PASmax), Feeding Recommendations and Impact on Caregiver (FSIS)
In accordance with the theory that measures more proximal (physiology) to the intended function (swallowing) and those more distal to it (quality of life) are expected to be related but not in a robust manner (33), moderate or clinically modest correlations were set at approximately 0.20 for associations between the domain scores and the external indicators of well-being (i.e., feeding status/recommendations and caregiver impact). (34, 35). Strong positive correlations were found between Airway Invasion/Laryngeal Closure domain (rS=0.66) and Aspiration domain (rS =0.88) with PASmax scores. Pharyngeal Transport and Clearance (rS=0.21) showed a modest relationship with PASmax. Clinically modest correlations were also found between feeding recommendations (made by the examining clinicians following the VFSS and blinded to scores) and Aspiration domain (rS=0.34), and Pharyngeal Transport and Clearance domain (rS=0.22). There were no clinically significant relationships between domain scores and subscales on the FSIS. (Table 4)
Table 4.
Association of Domain Summative Scores, PASmax, VFSS intake recommendations and FSIS
Spearman correlation of VFSS domain scores with feeding and quality of life measures
| Domaina | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Lingual Motion/Pharyngeal Swallow Initiation | Palatal Pharyngeal Approximation | Airway Invasion/Laryngeal Closure | Aspiration | Pharyngeal Transport and Clearance | |||||
| rS | P value | rS | P value | rS | P value | rS | P value | rS | P value | |
| FS-IS: | ||||||||||
| Limits subscale | 0.13 | 0.04 | −0.06 | 0.29 | −0.04 | 0.47 | −0.04 | 0.47 | −0.08 | 0.15 |
| Prevents subscale | 0.12 | 0.06 | −0.08 | 0.15 | −0.08 | 0.15 | −0.12 | 0.04 | −0.05 | 0.43 |
| Worry subscale | 0.09 | 0.15 | 0.01 | 0.85 | −0.01 | 0.92 | −0.05 | 0.36 | 0.10 | 0.10 |
| Feeding subscale | 0.10 | 0.14 | −0.01 | 0.90 | 0.03 | 0.61 | −0.04 | 0.50 | 0.02 | 0.71 |
| Worry breathing item | 0.08 | 0.23 | 0.09 | 0.14 | 0.04 | 0.49 | 0.02 | 0.68 | 0.12 | 0.04 |
| Feeding recs: | ||||||||||
| Post VFSS | −0.13 | 0.046 | 0.19 | 0.001 | 0.14 | 0.02 | 0.34 | <0.001 | 0.22 | <0.001 |
| Pre VFSS | −0.04 | 0.56 | 0.11 | 0.05 | −0.02 | 0.76 | 0.18 | 0.002 | 0.13 | 0.02 |
| Change (Post – Pre) | 0.06 | 0.33 | −0.02 | 0.69 | −0.11 | 0.06 | −0.05 | 0.42 | −0.01 | 0.80 |
| PAS(max) | −0.10 | 0.14 | 0.11 | 0.06 | 0.66 | <0.001 | 0.88 | <0.001 | 0.21 | <0.001 |
FS-IS Feeding/Swallowing Impact Survey, VFSS Video Fluoroscopic Swallow Study, PAS Penetration Aspiration Scale
CFA component factor groups were reordered and labeled in order of swallow physiology as above: Lingual Motion/Pharyngeal Swallow Initiation, Palatal Pharyngeal Approximation, Airway Invasion/Laryngeal Closure, Aspiration, and Pharyngeal Transport and Clearance
DISCUSSION
This study represents the first known prospective study focused on the quantitative assessment of swallowing observations from VFSS in bottle-fed babies directed toward minimizing unnecessary variation in current clinical practice. The BaByVFSSImP was developed to promote valid and reproducible examination results between clinicians and clinical settings to facilitate consistent communication among providers and to guide interventions that appropriately target swallowing impairment across the continuum of care. As such, the clinical contributions of this work are highly significant. Our previous work established the content validity of the tool through expert consensus and confirmed the reliability of the measures when scored by like-trained SLPs. (21) This investigation is an extension of that work and set out to test the construct validity of the tool and explore the clinical relevance of the measures.
Through the use of conventional methods of validity testing, 21 components of swallowing grouped into 5 functional domains. One component was eliminated by the CFA (Lip Closure) as it did not load on any factor (domain) because it either measured something different from the other factors or it was not within the imaging field on VFSS recordings. Modification of the VFSS to include lip closure should be dependent upon information necessary for clinical decision-making. Even though oral residue was shown to be a reliable observation on our original reliability study (21), scores for this component were missing on more than half of the VFSS samples rated in this investigation. Interviews with raters indicated this component was very difficult to discern because the rapid sequential swallowing characteristic of bottle-feeding and/or the presence of the nipple within the baby’s mouth obscured the raters’ view of oral residual between swallows. It should be noted that the results of the factor analysis are limited to the cohort under investigation, and the omitted components may have clinical relevance in a larger set of patients. We will evaluate the clinical relevance of these two components as additional clinicians are trained and data are acquired across multiple sites.
The grouping of the physiologic swallowing components into functional domains demonstrates that when one of the components in a domain is impaired, it was highly likely that the other components in that domain are also impaired. Although a hypothesized five-factor solution emerged from the CFA, the combination of components loading onto factors was different from those originally proposed. As such, the domains were re-labeled and logically reordered to reflect the swallowing function accomplished collectively by the components contained therein.
The modest to strong associations of the BaByVFSSImP domain scores with PASmax scores point to the relevance of the airway closure and pharyngeal clearance components within those domains and airway invasion. However, the lack of redundancy between physiologic scores and penetration/aspiration measures speak to the notion that one observation is not necessary nor sufficient to explain the other. (33) Clinicians not only need to identify the aspiration event during a VFSS, but as importantly determine its physiologic source that should become the target of feeding/swallowing intervention.
Three of the BaByVFSSImP summative domain scores showed clinically significant associations with feeding recommendations made by the examining clinicians who were blinded to the scores of the rating SLPs. This result is not surprising because mechanisms of pharyngeal clearance and airway invasion likely impact the feeding recommendation and support the inclusion of the items in the domain. On the other hand, the BaByVFSSImP summative scores are not sufficiently robust to test the association with swallowing impairment and caregiver burden. Future studies will test the associations of individual component scores, and other comorbidities that may be better able to detect the impact of swallowing impairments on caregivers
Study Limitations
While the distribution of the component scores demonstrated a fairly good range, they were predominately non-normal and may support further data reduction by collapsing the range of scores for some components. That is, some score variants for a given component rarely occurred and therefore may not sufficiently contribute meaningful information regarding impairment of that particular component. Further field testing in larger numbers of patients and subsequent refinements are predicted as has been seen in the adult model of the MBSImP. Also, larger numbers of patients will be necessary to identify meaningful cut-points that classify patients into severity levels based on a combination of scores and functional measures.
In addition, the current investigation included only thin liquid boluses. Components may load differently onto the factors (domains) when thicker liquids are compared to the thin liquids results reported herein. Future planned investigations will explore the differences in component scores between thin and thick liquid boluses during bottle-feeding.
Finally, heterogeneity of the patient sample is both a limitation and a strength. Our intention was to develop a tool for assessing swallowing physiology regardless of patient diagnosis for clinical translation with all bottle-fed babies, however, the patterns of impairment profiles are likely to differ by patient diagnosis. (36)
Conclusion
In summary, the BaByVFSSImP is a novel tool that promotes standardized assessment of physiologic swallowing impairment using visual observations of VFSS recordings in bottle-fed babies. The content and construct validity of the tool have been established. The tool holds promise for the identification of physiologically based treatments for dysphagia intervention, aiding in guiding clinical decision-making regarding enteral feeding, and tracking the trajectory of swallowing disorders through development in young children. The quantification of physiologic swallowing impairment afforded by this novel instrument has potential to serve as a performance metric in clinical trials, identify phenotypic profiles of swallowing impairment, and to predict feeding and swallowing outcomes and associated communication-cognitive development.
Acknowledgements
The authors wish to thank the families for partaking in this project. Our gratitude is expressed for the contributions of the Speech Language Pathologists that were involved in this work including Kate Davidson, M.S., M. Cara Erskine, M. Ed., Jeannine Hoch, M.A., Sandra B. Martin, M.S., Heather Mcghee, M.S., Katlyn McGrattan, Ph.D., Keeley McKelvey, M.S., and Melissa Montiel, M.S. This work was supported by the National Institutes of Health Grants NIDCD 5R01DC011290-05 (ML-G and BM-H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Bonnie Martin-Harris, Department of Communication Sciences and Disorders, School of Communication, Department of Otolaryngology Head and Neck Surgery, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, 2240 Campus Drive, Evanston, IL 60208, USA; Department of Otolaryngology Head and Neck Surgery, Evelyn Trammell Institute for Voice and Swallowing, College of Medicine, Medical University of South Carolina, 135 Rutledge Avenue, MSC 550, Charleston, SC 29425, USA; Research Service, Edward Hines, Jr. VA Hospital, 5000 South 5th Avenue Hines, IL 60141.
Kathryn A. Carson, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, 2024 East Monument Street, Suite 2-500, Baltimore, MD 21287, USA; Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
Jeanne M. Pinto, Eudowood Division of Pediatric Respiratory Sciences, Department of Pediatrics, The Johns Hopkins University School of Medicine, David M. Rubenstein Building, Suite 3070, 200 North Wolfe Street, Baltimore, MD 21287, USA
Maureen A. Lefton-Greif, Eudowood Division of Pediatric Respiratory Sciences, Department of Pediatrics, The Johns Hopkins University School of Medicine, David M. Rubenstein Building, Suite 3017, 200 North Wolfe Street, Baltimore, MD 21287, USA; Department of Otolaryngology-Head and Neck Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA; Department of Physical Medicine and Rehabilitation, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
REFERENCES
- 1.Bhattacharyya N: The prevalence of pediatric voice and swallowing problems in the United States. The Laryngoscope 125: 746–750, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Horton J, Atwood C, Gnagi S, Teufel R, Clemmens C: Temporal Trends of Pediatric Dysphagia in Hospitalized Patients. Dysphagia, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman E, Rosner A: Preterm infants are more at-risk for feeding-swallowing difficulties in the first three years of life. Journal of Neonatal Nursing 24: 331–335, 2018. [Google Scholar]
- 4.Cerro N, Zeunert S, Simmer K, Daniels L: Eating behaviour of children 1.5–3.5 years born preterm: Parents’ perceptions. Journal of paediatrics and child health 38: 72–78, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Arvedson JC: Food for Thought on Pediatric Feeding and Swallowing. Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 17: 110–118, 2008. [Google Scholar]
- 6.Lefton-Greif MA: Pediatric dysphagia. Phys Med Rehabil Clin N Am 19: 837–851, ix, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Manikam R, Perman JA: Pediatric feeding disorders. JClinGastroenterol 30: 34–46, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Lefton-Greif MA, Okelo SO, Wright JM, Collaco JM, McGrath-Morrow SA, Eakin MN: Impact of children’s feeding/swallowing problems: validation of a new caregiver instrument. Dysphagia 29: 671–677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borowitz KC, Borowitz SM: Feeding Problems in Infants and Children: Assessment and Etiology. Pediatr Clin North Am 65: 59–72, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Field D, Garland M, Williams K: Correlates of specific childhood feeding problems. J Paediatr Child Health 39: 299–304, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Rommel N, De Meyer AM, Feenstra L, Veereman-Wauters G: The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr 37: 75–84, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Malas K, Trudeau N, Chagnon M, McFarland DH: Feeding-swallowing difficulties in children later diagnosed with language impairment. Dev Med Child Neurol 57: 872–879, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Adams-Chapman I, Bann C, Carter S, Stoll B, Network N: Language outcomes among ELBW infants in early childhood. Early Human Development 91: 373–389, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolthuis-Stigter MI, Da Costa SP, Bos AF, Krijnen WP, Van Der Schans CP, Luinge MR: Sucking behaviour in infants born preterm and developmental outcomes at primary school age. Dev Med Child Neurol, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Hayden LP, Hobbs BD, Cohen RT, Wise RA, Checkley W, Crapo JD, Hersh CP, Investigators CO: Childhood pneumonia increases risk for chronic obstructive pulmonary disease: the COPDGene study. Respiratory research 16: 115, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J: Early postnatal nutrition and programming of the preterm neonate. NutrRev 69: 76–82, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Joss-Moore LA, Lane RH: The developmental origins of adult disease. Curr Opin Pediatr 21: 230–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiorns MP, Ryan MM: Current practice in paediatric videofluoroscopy. Pediatr Radiol 36: 911–919, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Harris B, Humphries K, & Garand KLF : The Modified Barium Swallow Impairment Profile (MBSImP™)--Innovation, Dissemination and Implementation. Perspectives of the ASHA Special Interest Groups 2, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norther Speech Services (NSS): Modified barium swallow impairment profile. . https://www.mbsimp.com 2017.
- 21.Lefton-Greif MA, McGrattan KE, Carson KA, Pinto JM, Wright JM, Martin-Harris B: First Steps Towards Development of an Instrument for the Reproducible Quantification of Oropharyngeal Swallow Physiology in Bottle-Fed Children. Dysphagia 33: 76–82, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss K: ALARA in pediatric fluoroscopy. J Am Coll Radiol 4: 931–933, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Strauss K, Kaste S: ALARA in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—a white paper executive summary. J Am Coll Radiol 3: 686–688, 2006. [DOI] [PubMed] [Google Scholar]
- 24.National Guideline Clearinghouse (NGC): ACR-SPR practice guideline for the performance of contrast esophagrams and upper gastrointestinal examinations in infants and children. American College of Radiology (ACR): Agency for Healthcare Research and Quality (AHRQ), 2015. [Google Scholar]
- 25.Fordham L: Imaging of the esophagus in children. Radiol Clin North Am 43: 283–302, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL: A penetration-aspiration scale. Dysphagia 11: 93–98, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Lefton-Greif MA: Pediatric dysphagia. PhysMed RehabilClin N Am 19: 837–851, ix, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Fracchia MS, Diercks G, Yamasaki A, Hersh C, Hardy S, Hartnick M, Hartnick C: Assessment of the feeding Swallowing Impact Survey as a quality of life measure in children with laryngeal cleft before and after repair. Int J Pediatr Otorhinolaryngol 99: 73–77, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Arvedson JC, Lefton-Greif MA: Pediatric Videofluoroscopic Swallow Studies: A Professional Manual with Caregiver Guidelines. San Antonio: Communication Skill Builders/Psychological Corporation, 1998. [Google Scholar]
- 30.Newman LA, Keckley C, Petersen MC, Hamner A: Swallowing function and medical diagnoses in infants suspected of Dysphagia. Pediatrics 108: E106, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Harris B: MBSImP™ Web Based Learning Module. Northern Speech Services, 2017. [Google Scholar]
- 32.Martin-Harris B: The MBSImP Guide.: Northern Speech Services, 2018. [Google Scholar]
- 33.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, … & Blair J: MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia 23: 392–405, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner MH, Curbow B, Legro MW: The proximal-distal continuum of multiple health outcome measures: the case of cataract surgery. Medical care 33: As236–244, 1995. [PubMed] [Google Scholar]
- 35.McHorney CA, Martin-Harris B, Robbins J, Rosenbek J: Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia 21: 141–148, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Garand KLF, Armeson KE, Hill EG, Martin-Harris B: Identification of Phenotypic Patterns of Dysphagia: A Proof of Concept Study. American journal of speech-language pathology 27: 988–995, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


