Abstract
Objectives:
To investigate the determinants of bone mineral density through screening healthy children using a non-invasive quantitative ultrasound measurement device.
Methods:
A descriptive cross-sectional study carried out at King AbdulAziz University Hospital, Jeddah, Kingdom of Saudi Arabia. between May 2018 and January 2019 through interviewing, examining, and screening healthy children visiting general paediatric. Total sample size encompassed 450 children. The inclusion criteria were healthy children between the ages of 2 and 20 years. Exclusion criteria were previous pathological fractures, chronic medical diseases, or long-term medications. Data entry and analysis was conducted using Statistical Package for Social Sciences version 24 (IBM Corp, Armonk, NY, USA). Chi-square tests were used to determine the association between categorical variables, with calculated p<0.05 considered significant. With one-way Anova testing to study the relationship between categorical variables and continuous variables.
Results:
A significant association with bone mineral density (BMD) was found during first 2 years with height (p=0.015), vitamin D supplementation (p=0.03), and breastfeeding (p=0.025). A directly proportional relationship with BMD was found with pubertal status, physical activity, diet, sun exposure, and calcium supplement intake.
Conclusion:
This is a novel study in the investigation of the dietary, lifestyle and demographic determinants of bone mineral density in the healthy middle-eastern child otherwise unaffected by chronic medical or metabolic disease or exposed to long term medications that could have affected bone metabolism.
Bone is considered a highly specialised type of connective tissue with a primary function to provide mechanical support to the human body. In addition, it exercises a regulatory effect on mineral homeostasis in the bloodstream, gaining it its constant dynamic state between bone resorption and bone mineralisation.1 Starting from birth, bone mineralisation progressively increases throughout childhood until around 30 years of age, which is when the maximum value of bone density is reached, and therefore is defined as the peak bone mass.2 Bone density is the average concentration of mineral content relative to surface area (bone unit), and is largely constituted by hydroxyapatite crystals of calcium and phosphate. Factors that define bone mineral density (BMD) are either modifiable or non-modifiable, the latter demonstrating characteristics that do not allow for intervention such as age, gender, and genetic predispositions.3 However, modifiable factors can be investigated, understood, and therefore manipulated in order to create the ideal environment that allows for the attainment of optimal peak BMD. These factors are mainly in the aspects of nutrition, physical activity, endocrine status, and general state of health.3 Several approached approaches have been uncovered for measuring bone density, one of which is through a quantitative ultrasound (QUS). Quantitative ultrasound operates through evaluating speed of sound (SOS), and is therefore factored by velocity. This is carried out through analyzing the duration for the ultrasound waves to propagate through bone tissue. Important variables determining SOS include but are not limited to bone architecture, soft tissue thickness, and anthropometric findings such as height velocity and pubertal status.4 Sites for measuring the SOS are the calcaneus, tibial shaft, patella, and distal radius. A major advantage that holds QUS in superiority over other methods of measuring bone density, in part, is its lack of harmful ionising radiation. Others include its ease of use, portability, cost-effectiveness, capability to provide additional insight on bone structure, and prompt analysis, with results available within a matter of minutes. The disadvantage is that it is operator dependent. A wide technological diversity exists between devices and the difficulty to compare obtained results with those of x-ray-based densitometric techniques.5 Unfortunately, in Saudi Arabia, the genetic background, climate, customs, and traditions cultivate a propensity towards an unhealthy diet, a more sedentary lifestyle with decreased physical activity, and avoidance of sun exposure with a higher incidence of vitamin D deficiency. All of which result in a nation-wide predilection towards decreased bone mineral density, increased risk of pathological fracture, and development of osteoporosis.6 The objective of this study was to investigate the determinants of BMD through screening healthy children attending general ambulatory paediatric clinics.
Methods
This is a descriptive, cross-sectional study carried out between May 2018 and January 2019 in Jeddah, Kingdom of Saudi Arabia. Individual patient consent and an ethical approval was obtained from the Research Ethical Committee at King AbdulAziz University prior to the commencement of this study. Considering the involvement of human subjects, we declare this study to be following the Helsinki declaration. Written informed consents were obtained from the parents or guardians of the children. Verbal assents were also obtained from the children at the time of the study. A special room was prepared in the paediatric clinics to interview the patients in private with their legal guardians. Children were randomly selected in accordance with the inclusion criteria and guardians informed about the study. Those who accepted to participate were recruited to the study room. Data was then collected using a standardised questionnaire that the co-authors drafted. Following that, anthropometric measures were taken and the BMD assessed using quantitative ultrasound. Results were given in Z scores and were explained to the patients legal guardians. No data collectors were recruited, all steps were carried out through by the co-authors. A total of 745 children were seen, of which 295 were excluded in accordance with the exclusion criteria. A final sample of 450 participants were investigated. The characteristics of subjects are provided in Table 1.
Table 1.
Descriptive statistics and characteristic of patients (N=450).

The inclusion criterion was of all healthy children between 2 and 20 years old. Subjects were excluded if they were on any long-term medications like steroids, antiepileptics or immunosuppressives, suffered prior fractures, had metabolic disorders that could affect vitamin D or calcium metabolism, had chronic medical disease such as but not limited to eating disorders, growth hormone deficiency, inflammatory bowel disease, thyroid disorders, diabetes, and congenital adrenal hyperplasia. Additionally, children who were not able to provide consistent SOS results through the QUS screening were excluded.
The aim of this study was to investigate environmental and lifestyle factors, predictors, and determinants of bone mineral density in healthy children visiting general paediatric clinics through quantitative ultrasound bone screening.
Children were grouped into separate categories according to various variables such as age, gender, pubertal stage, diet, physical activity, sun exposure, and supplement intake. Bone mineral density was assessed in all children with the mean then being calculated for each group and reported with respect to each category. Children were considered to be prepubertal if breast development was at tanner stage 1 or age was less than 8 years for female and a testicular volume less than 4ml or age less than 9 years in male. Dairy product intake and milk consumption was considered insufficient if it was less than 2-3 times per week. Children who were exercising for a minimum of 30 minutes duration at a frequency of 3 times or more per week were considered active. Sun exposure was considered sufficient if children spent more than 30 minutes outdoors during daytime. Supplemental intake was considered if doses of 500mg/day calcium and vitamin D 400-800units/day were within the recommended daily dose.
Literature search for similar study was through typing relative medical subject headings terminology in PubMed search engine while filtering search results to the minimum timeline of within the past 5 years duration in order to obtain to recent references.
Quantitative ultrasound for the measurement of bone mineral density
The evaluation of SOS at either right or left distal radii for each subject was carried through using the Sunlight Omnisense 7000S device (Sunlight Technologies, Rehovot). The QUS constitutes a hand-held ultrasound probe emitting sound at a pulse of a particular frequency that is refracted through a certain angle from soft tissue to bone. A proportion of this returns from the bone at the same angle that is then received by the device. Therefore, the duration through which the ultrasound waves propagate through the bone tissue from signal emission to its detection at the transducer is measured as a value termed speed of sound (SOS) and consequently was attributed the unit of meters/second. There are 3 different types of QUS devices with respect to the form of ultrasound transmission employed: 1) trabecular sound transmission, 2) cortical transverse transmission, and 3) cortical axial transmission. The one used in this study operates through trabecular sound transmission. Measurements were taken from a site predefined by the manufacturer, which was the midpoint between the edge of the olecranon and the distal point of the third phalanx. The predefined diagnostic threshold with which this device operates is through an internal algorithm that attempts to obtain a mean value through 3 cycles that aims to detect a consistent SOS value that is then converted to a Z score using the manufacturer’s data bank for age and gender matched SOS values for the right (SOS-R) and left (SOS-L) radii. Should the SOS values prove inconsistent, the algorithm fails to produce a report and the process would have to be repeated. As the device did not provide the speed of sound values directly, and rather converted them through an internal algorithm to a Z score matching age and gender, the range used to distinguish osteoporotic from normal subjects was the standardised reference used to interpret Z scores in the paediatric population. Children were considered to screen positively for osteoporosis if their Z score was -2 or below. All measurements were carried out by the same trained operator blinded to the patients data. Prior to each use, the device was standardized against a phantom control supplied by the company.
Statistical analysis
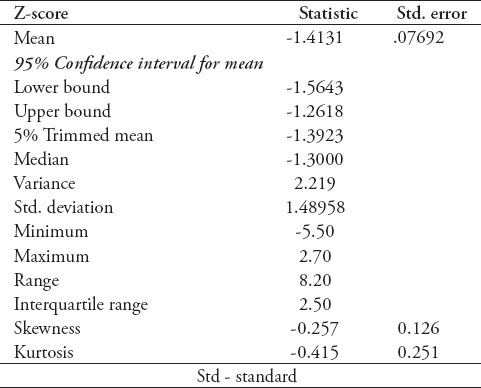
Data entry and statistical analysis was performed by Statistical Package for Social Sciences version 24 (IBM Corp, Armonk, NY, USA). Categorical variables were expressed either in frequency or in proportion. Continuous variables were expressed in range, mean, and standard deviation. One-way ANOVA testing was carried to study the relationship between categorical variables and continuous variables, and was used to determine the presence or absence of a significant association, with calculated p<0.05 was interpreted to be significant. Significant results were further presented in linear graphs. The relationship between vitamin D supplementation and Z-Score could not be assessed through one-way ANOVA test, and therefore was investigated through Chi-square test, yielding a significant p=0.03 after modifying Z score results into a categorical variable rather than a continuous variable. ANCOVA test was done to test for co-variance between variables that showed significant association with Z scores. The value of coefficient of variations are given for the measurement of BMD in Table 2. Ninety-five percent confidence interval for the upper bound was -1.2618 and for the lower bound was -1.5643. This was added to the methods section under statistical analysis.
Table 2.
Coefficient of variations for the measurement of bone mineral density (N=450).
Results
Gender distribution was almost equal with females at a slight predominance of 52.6%. The mean age of children was 8.75 years. The mean bone density measured for all population was -1.4. A significant association on one-way ANOVA with bone mineral density was found with only 3 variables, which were height (Figure 1, p=0.015), current vitamin D supplementation (Figure 2, p=0.03), and type of feeding during the first 2 years of life (Figure 3, p=0.025). All values were of a positive association. On ANCOVA testing, the type of feeding in the first 2 years of life showed significant p-values of 0.03 and 0.026 after accounting for vitamin D supplements and height respectively, and height as well showed significant p-values of 0.028 and 0.032 after accounting for type of feeding in the first 2 years of life and vitamin D supplements respectively. On the other hand, current vitamin D supplementation showed no significance in ANCOVA test with it providing a significant p-value in Chi-square test only.
Figure 1.

Estimated marginal means of Z score plotted against height.
Figure 2.
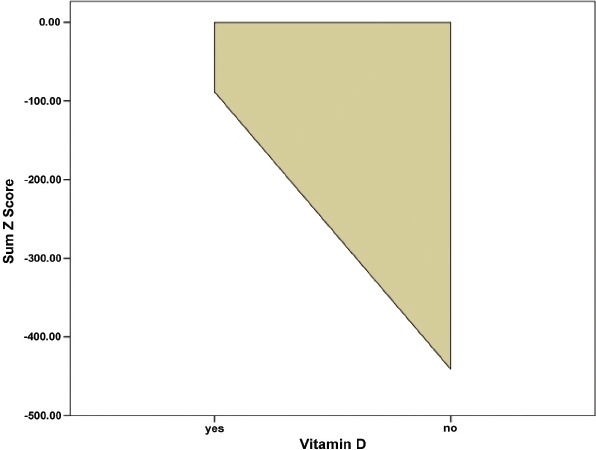
Mean of Z score plotted against children who were on vitamin D supplementation versus those who were not.
Figure 3.

Mean of Z score for each group of children who were exclusively breastfed, who were exclusively formula fed, and who were on combined feeding of breast and formula milk.
Correlating weight with Z score yielded p=0.097 which is a not significant result. Bone mineral density was shown to be higher in male than in female, with the mean Z score being -1.30 in the male gender population group and -1.50 in the female gender population group. Furthermore, a direct proportional relationship was demonstrated between the mean BMD and several risk factors studied, including pubertal status, physical activity, diet, sun exposure, calcium supplement intake, and type of feeding during infancy.
For pubertal status, children were grouped into 3 categories according to tanner staging. Bone mineral density was measured for all children with the mean BMD calculated for each group. The mean BMD was found to be higher with a directly proportionate trend in the groups with an advanced tanner stage when compared to the prepubertal group. Mean BMD in children at tanner stage one had a mean BMD of -1.38, tanner stage 2-3 had a mean BMD of -1.12, and tanner stage 3-4 had a mean BMD of -1.06.
When comparing the physically active group with the sedentary group, children who were physically active were found to have a mean BMD of -1.40 while those who led a more sedentary lifestyle had a mean BMD of -1.68. Diet also played a role in BMD acquisition. Children who consumed dairy products daily had a higher mean BMD when compared to those who never consumed dairy products, with relative mean BMDs of -1.30 when compared to -1.95. Drinking milk alone proved a similar relationship where children who drank milk on a daily basis had a mean BMD of -1.35 while those never drank milk were more closer to the osteoporotic range with mean BMD of -1.79.
In terms of sun exposure, a slight difference in BMD was noted between children who were exposed to sun for less than 15 minutes when compared to those exposed to sun for longer than 30 minutes with relative mean BMDs of -1.45 and -1.30. A similar modest relationship was seen between calcium supplemental intake and mean BMD, with a mean Z score of -1.17 in those who were on regular calcium supplements when compared to those who were not, -1.42.
Finally, children who were exclusively breastfed for the first 6 months and then given formula milk had the highest mean BMD of -1.27 when compared to groups of children who were either exclusively breast fed for the first 2 years had mean BMD of -1.35 or exclusively formula fed for the first 2 years with the mean BMD of -1.80.
Discussion
At the point of peak bone mass, osteoblastic bone formation is equally balanced by osteoclastic bone resorption. Shortly thereafter, the dynamic is tipped towards predominant bone resorption with a variable period of time ahead until a sufficient amount of bone density is lost and the considerable risk of fracture then surfaces.8 Therefore, the more bone is formed throughout childhood and adolescence, the higher the peak bone mass, the longer the duration preceding the risk of osteoporosis. In this study we outline the determinants of bone mass in healthy children alongside the factors which compromise it in order to promote favourable customs and rituals that encourage bone density and increase awareness to the predictors of unsatisfactory bone health.
The bulk of peak bone mass is formed during adolescence following the onset of puberty in attribution to the sudden acceleration in the formation of bone density as a result of pubertal changes. During puberty, levels of growth hormone and insulin growth factor (IGF-1) increase dramatically, mediating a positive effect on bone turnover through stimulating osteoblast proliferation and differentiation. This is witnessed particularly in females where pubertal levels of oestrogen not only directly increase bone density but modulate bone remodelling through osteoclastic resorption by suppressing bone turn over at the endocortical surface, leading to an increase in cortical thickness.9 Furthermore, the release of potent prepubertal androgens into the circulation contribute to the accretion of bone strength by increasing muscularity.10 This duration is therefore considered critical to the child’s prevention of future osteoporosis and its associated morbidity as the net balance between bone resorption and bone mineralisation is temporarily in favour of bone formation.11 The majority of our studied population were prepubertal, with 66.5% assessed through expert physical examination to be at tanner stage 1. This is acceptable considering that the studied population included children at an age as young as 2 years, with the mean age being 8 years old. All children were screened for a low BMD, they were then divided into 5 separate groups according to tanner stage with the mean BMD calculated for each group. Our findings show that children who were at a tanner stage 2 and above were found to have a higher mean of BMD than those who were still at stage 1, confirming the positive effect of the pubertal spurt on bone mineralisation.
This is one example of the several factors that promote bone mineralisation. By favoring the activation of osteoblasts, bone mineralisation is mediated through recruiting calcium and phosphate from the circulation, increasing bone density and therefore, bone mass. Another example is physical activity with respect to weight. The response exhibited by skeletal tissue towards mechanical loading and stress is through a parallel increase in density and strength in order to achieve durability.12 Vivanco-Muñoz et al12 investigated the significance of physical activity as a prognostic factor for BMD in children showed a clear positive correlation with an increment relating to the number of hours and the intensity of exercise. Furthermore, physical activity during childhood and adolescence appears to be an important predictor of peak bone mass accounting for up to 17% of the variance in BMD between individuals in their late twenties.8 It is evident and therefore logically acceptable that a higher BMD is correlated to a higher lean body mass, which is acquired through regular weight bearing exercise and is physiologically higher in the male gender. The effect of weight on BMD is thought to be mainly exerted by creating a load on weight-bearing bones.13 Therefore, underweight children are more likely to attain a lower BMD. Fortunately, 92.4% of our total sample were found to be physically active, with the BMD mean higher in physically active children than those who led a more sedentary lifestyle. However, regarding weight, our study concluded that it was not significant to BMD at a p=0.097. Predetermined examples that are not subject to intervention include age and gender. As previously mentioned, younger age is characterized by predominant bone formation and unbalanced osteoblastic activity, while older age is associated with predominant bone mass loss as a result of primary osteoclast-mediated bone remodelling and resorption. As our study only included children and therefore did not compare to other age groups such as adults and the elderly, age did not prove significant to BMD. Gender distribution was approximately proportionate with females constituting 52.6% of the sample. Physiologically, it is known that in the adult population, the male gender have a higher BMD than females, which we have proved to be the case even in children, as in our study, females were found to have a lower BMD mean comparatively to the males.
On the other hand, factors which promote the resorptive state do so through their effect on bone osteoclasts, favouring the dissolution of hydroxyapatite crystals in bone and releasing calcium and phosphate minerals into the blood, resulting in a lower bone density. An insufficient diet and an inadequate exposure to sun both lead to nutritional deficiencies in serum calcium and vitamin D, which in turn upregulates the production and release of PTH that parallels its effect on osteoclast activity, compromising the ongoing process of bone formation. Parathyroid hormone (PTH) acts indirectly on the mesenchymal cells that lead to the differentiation of osteoblasts, increasing the circulation of Interleukin-6 released by mature osteoblasts, which by turn mediate the differentiation of preosteoclasts into their mature form. This indirect mechanism of differentiation is necessary due to the lacking of osteoclasts for direct receptors to the action of parathyroid hormone.14 In terms of dietary sufficiency, only 37.4% of the sample were on regular vitamin D supplementation and a mere 3.7% who were taking supplemental calcium. Vitamin D supplementation didn’t show correlation with BMD on one-way ANOVA or ANCOVA testing, but proved to be of significance to BMD using Chi-square testing with a p-value of 0.03. Furthermore, children who were on vitamin D supplementation had a higher BMD than those who were not. Calcium showed a positive correlation with BMD, as children who were taking supplements had a higher mean BMD than those who did not. However, 70.2% of total children were regularly drinking milk and 84.2% were consuming dairy products in the form of cheese and yogurt at a minimum of 3 times per week. Furthermore, BMD was found to be directly proportional to the frequency of milk consumption and dairy product intake, with higher means achieved in groups who were on a daily diet including milk and a variety of dairy products. Unfortunately, only 16.6% of the children were getting sufficient sun exposure, when our study outlined that duration of sun exposure is directly proportional to increase in mean bone mineral density. Additionally, a lower BMD was found in children who complained of bone pain when compared to those who did not. Those who were not complaining of bone pain had an average BMD of -1.4, while those who suffered from bone pain demonstrated a mean BMD of -2.0.
A systematic review of the literature including over 600 studies investigating the effect of breast feeding on BMD in children yielded the conclusion that being breastfed has a positive effect on bone density, greater effect in the long term, particularly during adolescence, which is the phase during which approximately 90% of the peak bone mass is attained.15 It also outlined that in the studies showing a higher BMD with breastmilk, children that were formula-fed had a lower bone mass in comparison. History of feeding in the first 2 years of life was sought from parents, and children were grouped into 3 categories accordingly: 1) those who were exclusively breast fed, 2) those who were exclusively formula fed, and 2) those who were fed a combination of both, breast and formula milk. The highest mean of BMD was found in those who were on a combination of both breast and formula milk, and the lowest in those who were exclusively formula fed. Although breast milk contains lower levels of calcium, vitamin D, and phosphorus, our findings can be explained through previous conclusions reporting a greater bioavailability and absorption of these nutrients from breast milk than other types of milk. It has also been postulated that early introduction to breast milk can lead to alterations in the programming of bone cells that lead to a greater bone mass in later life.15 Exclusive breastfeeding beyond the first 6 months of life without introduction of a supplementary diet that is nutritionally sufficient and adequate will compromise normal growth and bone development as the infants needs will increase and the breast milk will no longer be enough.
This study provides a novel establishment of the first normative data of BMD determinants in healthy children within the Gulf and Middle-East region, which will add value to existing literature through referencing and use by future research to build up on.
Study limitations
Paucity in similar studies investigating the determinants of BMD using QUS screening in a healthy population of children within the gulf and middle-east region to be used comparatively with our data. Paucity in similar studies overall that investigate bone mineral density using QUS screening in a healthy population of children worldwide that are recent.
In conclusion, height, vitamin D supplements, and combined feeding of breast and formula milk in the first 2 years of life are significant factors in the determination of bone mineral density. Furthermore, lifestyle factors such as diet, physical activity, sun exposure, and calcium supplements play a role in the development of BMD and can be modified to an optimal level to allow for healthy BMD development. Other factors seen to affect BMD without proving significance were gender and pubertal status. This is the only study in Saudi Arabia and the middle-east to study the risk factors promoting a lower bone mineral density in a healthy population of Saudi children using non-invasive QUS. Therefore, our recommendation that future studies should be directed towards investigating risk factors of low BMD in a healthy population of Saudi children in order to establish a true understanding of the determinants of BMD.
Acknowledgment
The authors would like to acknowledge and thank the Deanship of Scientific Research at King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia for their technical and financial support.
Footnotes
Statistics
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to verify the reported results. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important information about effect size. References for the design of the study and statistical methods should be to standard works when possible (with pages stated). Define statistical terms, abbreviations, and most symbols. Specify the computer software used.
References
- 1.Ma NS, Gordon CM. Pediatric osteoporosis:where are we now? J Pediatr. 2012;161:983–990. doi: 10.1016/j.jpeds.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Stagi S, Cavalli L, Lurato C, Seminara S, Brandi ML, de Martino M. Bone health in children and adolescents:the available imaging techniques. Clin Cases Miner Bone Metab. 2013;10:166–171. [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrach LK, Sills IN. Clinical report-bone densitometry in children and adolescents. Pediatrics. 2011;127:189–194. doi: 10.1542/peds.2010-2961. [DOI] [PubMed] [Google Scholar]
- 4.Specker BL, Schoenau E. Quantitative bone analysis in children:current methods and recommendations. J Pediatr. 2005;146:726–731. doi: 10.1016/j.jpeds.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children:technical characteristics, performance, and clinical application. Pediatr Res. 2008;63:220–228. doi: 10.1203/PDR.0b013e318163a286. [DOI] [PubMed] [Google Scholar]
- 6.Jeddi M, Roosta MJ, Dabbaghmanesh MH, Omrani GR, Ayatollahi SM, Bagheri Z, et al. Normative data and percentile curves of bone mineral density in healthy Iranian children aged 9-18 years. Arch Osteoporos. 2013;8:114. doi: 10.1007/s11657-012-0114-z. [DOI] [PubMed] [Google Scholar]
- 7.Khan KM, Sarafoglou K, Somani A, Frohnert B, Miller BS. Can ultrasound be used to estimate bone mineral density in children with growth problems? Acta Paediatr. (e407) 2013;102 doi: 10.1111/apa.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies JH, Evans BAJ, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90:373–378. doi: 10.1136/adc.2004.053553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Nicholson PHF, Suuriniemi M, Lyytikainen A, Helkala E, Alen M, et al. Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab. 2004;89:1698–1703. doi: 10.1210/jc.2003-031113. [DOI] [PubMed] [Google Scholar]
- 10.Remer T, Boye KR, Hartmann M, Neu CM, Schoenau E, Manz F, et al. Adrenarche and bone modeling and remodeling at the proximal radius:Weak androgens make stronger cortical bone in healthy children. J Bone Miner Res. 2003;18:1539–1546. doi: 10.1359/jbmr.2003.18.8.1539. [DOI] [PubMed] [Google Scholar]
- 11.Ali GY, Abdelbary EE, Albuali WH, AboelFetoh NM, AlGohary EH. Bone mineral density and amp;bone mineral content in Saudi children, risk factors and early detection of their affection using dual-emission X-ray absorptiometry (DEXA) scan. Egyptian Pediatric Association Gazette. 2017;65:65–71. [Google Scholar]
- 12.Vivanco-Muñoz N, Reyes-Sánchez M, Lazcano E, Díaz R, Antúnez O, Clark P. Physical activity is a prognostic factor for bone mineral density in Mexican children. Bol Med Hosp Infant Mex. 2012;69:38–43. [Google Scholar]
- 13.Boot AM, De Ridder MAJ, Pols HAP, Krenning EP, De Muinck Keizer-Schrama SMPF. Bone mineral density in children and adolescents:relation to puberty, calcium intake, and physical activity. J Clin Endocrinol Metab. 1997;82:57–62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- 14.Kroll MH. Parathyroid hormone temporal effects on bone formation and resorption. Bull Math Biol. 2000;62:163–188. doi: 10.1006/bulm.1999.0146. [DOI] [PubMed] [Google Scholar]
- 15.Muniz LC, Menezes AMB, Buffarini R, Wehrmeister FC, Assunção MCF. Effect of breastfeeding on bone mass from childhood to adulthood:A systematic review of the literature. Int Breastfeed J. 2015;10:31. doi: 10.1186/s13006-015-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


