Abstract
Although procrastination is a widespread phenomenon with significant influence on our personal and professional life, its genetic foundation is somewhat unknown. An important factor that influences our ability to tackle specific goals directly instead of putting them off is our ability to initiate cognitive, motivational and emotional control mechanisms, so-called metacontrol. These metacontrol mechanisms have been frequently related to dopaminergic signaling. To gain deeper insight into the genetic components of procrastination, we examined whether genetically induced differences in the dopaminergic system are associated with interindividual differences in trait-like procrastination, measured as decision-related action control (AOD). Analyzing the data of 278 healthy adults, we found a sex-dependent effect of TH genotype on AOD. Interestingly, only in women, T-allele carriers showed lower AOD values and were therefore more likely to procrastinate. Additionally, we investigated whether differences in the morphology and functional connectivity of the amygdala that were previously associated with AOD happen to be related to differences in the TH genotype and thus to differences in the dopaminergic system. However, there was no significant amygdala volume or connectivity difference between the TH genotype groups. Therefore, this study is the first to suggest that genetic, anatomical and functional differences affect trait-like procrastination independently.
Keywords: TH genotype, dopamine, action control, structural and functional MRI, sex differences
Introduction
‘Never put off until tomorrow what you can do today.’ Although we are probably all familiar with sayings like this, it can be difficult to actually stick to them. Especially when we are facing a demanding task, we happen to first rush into other, simpler activities, before we devote ourselves to the actual problem. This phenomenon is commonly known as procrastination. Thus, procrastination refers to the voluntary delay of activities that are needed to achieve certain goals (Ferrari, 2004; Klingsieck, 2013; Sirois & Giguère, 2018). Despite knowing that putting things off is often associated with negative outcomes, adversely affecting academic success (Blunt & Pychyl, 1998; Jaramillo & Spector, 2004; Schlüter et al., 2018b), as well as mental and physical health (Palfai, 2002; Palfai et al., 2002; Freund & Hennecke, 2015), we often struggle to tackle tasks directly. Successful goal achievement is highly dependent on the individuals’ ability to perform self-induced modulation of neural processes, including cognitive, motivational and emotional control mechanisms (Kuhl, 1984, 1994b; Goschke & Bolte, 2014). This leads to the constant context-sensitive evaluation of how much effort and control should be invested in a particular goal, a process often referred to as metacontrol (Goschke & Bolte, 2014; Hommel & Colzato, 2017; Hommel & Wiers, 2017). Former research has shown that there are substantial interindividual differences in these metacontrol processes (Kuhl, 1994b). While some individuals manage to control their motivation, emotion and cognition in an action-promoting way, others lack the necessary metacontrol skills to shield a specific goal from competing alternatives showing a chronic tendency to procrastinate (Kuhl, 1994b; Steel, 2007).
From a theoretical point of view, metacontrol is highly dependent on the processing of value-based or reward anticipation mechanisms (Shenhav et al., 2016; Hommel & Wiers, 2017; Beste et al., 2018) as well as on gating functions regulating the inflow of competing context information (Goschke & Bolte, 2014). Both value-based and gating mechanisms have been related to dopaminergic signaling (Braver & Cohen, 2000; Dreisbach et al., 2005; Müller et al., 2007; Tai et al., 2012; Howe et al., 2013; Schultz et al., 2017; Yee & Braver, 2018). Thus, interindividual differences in the functioning of the dopaminergic system may be central not only for differences in metacontrol but also for our understanding of interindividual differences in the tendency to procrastinate. Here, previous research suggests a rather complex connection between dopamine and goal-directed metacontrol (Goschke & Bolte, 2014). Depending on the target region, an increased dopamine base level can lead either to stabilization of working memory and thus to improved goal-directed behavior or to an increase in cognitive flexibility that might be accompanied by distraction and procrastination (Goschke & Bolte, 2014).
Regarding the stabilization of working memory, it is assumed that a release of dopamine affects the activity level of prefrontal cortex (PFC) neurons. Here the activity of already activated neurons representing goal-related information is enhanced, while inactive neurons are simultaneously inhibited, resulting in a better signal-to-noise ratio between goal-relevant and goal-irrelevant information (Durstewitz & Seamans, 2008). Thus, an increased dopamine level would promote goal-directed behavior in this case. Considering the aspect of cognitive flexibility, the gating function of dopamine has to be taken into account. Dopamine neurons in substantia nigra and ventral tegmental area (VTA) encode reward prediction, changing their firing rate as soon as an appetitive stimulus is present (D’Ardenne et al., 2012; Ott & Nieder, 2019). These reward-related bursts in neural activity are considered to adjust the amount of information entering the PFC, leading to an update in working memory (Ott & Nieder, 2019). Here, an increased dopamine base level could facilitate reward-related neural activity, lowering the updating threshold. While this promotes cognitive flexibility, it also leads to increased distractibility, which, depending on the context, hinders goal-directed behavior (Goschke & Bolte, 2014).
One means to approach this is to examine genetic factors modulating the dopaminergic system in relation to trait-like measures of procrastination. A critical factor that determines how much dopamine is available is tyrosine hydroxylase (TH) (Fillenz, 1993). TH is the rate-limiting enzyme for the biosynthesis of catecholamines, including dopamine and norepinephrine (Kobayashi & Nagatsu, 2005). Therefore, its transcriptional activity is tightly regulated. However, one single-nucleotide polymorphism (SNP) rs10770141 (C-824T) in the TH promoter region has functional consequences on its expression. Horiguchi et al. (2014) demonstrated that a plasmid with the rare T-allele at position −824 showed higher transcriptional activity than that with the C-allele in a transient transfection experiment using a luciferase gene as a reporter, thus implying that T-allele carriers may have higher TH activities and retain higher levels of catecholamines in the brain. In the current study, we therefore examine associations of this functional SNP with measures of interindividual differences relevant to procrastination that have been described in Kuhl’s (1992, 1994a) action control theory. According to the action control theory, individuals differ in their ability to control their cognition, motivation and emotion. Here a lack of control is considered to raise the susceptibility to distractions and thus increase the tendency to procrastinate (Sirois & Giguère, 2018). While individuals who can efficiently use metacontrol processes to achieve a particular goal are considered to be action oriented, individuals having difficulties in utilizing metacontrol processes to tackle a distinct goal classify as state oriented (Kuhl, 1992). Especially in demanding situations, action orientation is associated with an action-promoting mode of control, whereas state orientation is considered to be action preventing (Jostmann & Koole, 2010; Gropel et al., 2014).
The propensity to be action or state oriented at a given moment is thought to depend on the context in which action control is needed (Kuhl, 1994a). This leads to three scales of action control (see Table 1 and Methods) of which the second scale, namely, prospective and decision-related action orientation (AOD), is considered to describe interindividual differences in the tendency to procrastinate (Beswick & Mann, 1994; Kuhl, 1994b). Here, individuals high on action control are assumed to efficiently execute an intentional action, while state-oriented individuals are more likely to hesitate and delay the beginning of tasks without any good reason (Blunt & Pychyl, 1998). Thus, individuals that classify as state oriented in decision-related contexts (low AOD) are more likely to procrastinate when facing a demanding task. Similar to procrastination, low AOD values are negatively associated with occupational (Diefendorff et al., 2000; Landman et al., 2016) and academic performance (Blunt & Pychyl, 1998; Jaramillo & Spector, 2004; Schlüter et al., 2018b).
Table 1.
Sample Items of the ASC-90
| Scale | Sample question |
|---|---|
| AOF | When I am told that my work has been completely unsatisfactory:
|
| AOD | When I have to solve a difficult problem:
|
| AOP | When I am watching a really good movie:
|
Note. Sample items for each of the three ACS-90 scales adapted from Kuhl (1994a). Action-oriented answers are highlighted in bold.
Until recently, only little was known about the biological correlates of AOD. However, one of our latest studies showed that interindividual differences in AOD are associated with the anatomical architecture and functional network of the amygdala (Schlüter et al., 2018a). Our results demonstrated a significant negative correlation between AOD and amygdala volume. Furthermore, we showed that the functional resting-state connectivity between the amygdala and the dorsal anterior cingulate cortex (dACC) was significantly associated with AOD. Specifically, stronger functional connectivity was associated with higher AOD scores. Thus, larger amygdala volume and lower functional connectivity between the amygdala and the dACC are associated with being more prone to procrastination. The trait like tendency to procrastinate might therefore be due to a permanent imbalance between both brain regions, in favor of the amygdala, leading to an impairment in the top-down control that is needed to successfully carry out an intended action (Ochsner et al., 2002).
The study at hand aims to investigate the genetic mechanisms behind interindividual differences in AOD. Here, we assume that interindividual differences in the TH genotype and therefore differences in the amount of dopamine available in the brain are associated with interindividual differences in AOD. Considering that interindividual differences in AOD were associated with interindividual differences in the structure and functional resting-state connectivity of the amygdala (Schlüter et al., 2018a), we additionally aim to investigate whether the neural correlates of AOD are mediated by TH gene expression. Since both AOD (Schlüter et al., 2018b) and the effects of the TH gene expression (Sadahiro et al., 2010) are assumed to be sex dependent, sex was taken into account as a modulating factor.
Methods
Participants
We present results from a cohort of 278 neurologically and psychologically healthy subjects with a mean age of 24.05 (s.d. = 3.87) years (range, 18–37 years), including 143 males (mean age, 24.53 years; s.d. = 4.11 years) and 135 females (mean age, 23.54 years; s.d. = 3.54 years). A total of 99 out of the 135 participating women used hormonal contraceptives. The sample mainly comprised university students of different majors (mean years of education, 16.44 years; s.d. = 2.61 years), who received either a financial reward or course credits for their participation and all were of Caucasian descent. All participants matched the standard inclusion criteria for magnetic resonance imaging (MRI) examinations. Information on the state of health was part of the demographic questionnaire and was therefore self-reported by the subjects. Subjects who reported current or past neurological or psychological issues were not admitted to the study. The study protocol was approved by the local ethics committee of the Faculty of Psychology at Ruhr University Bochum. All participants had to give their written informed consent and were treated in accordance with the Declaration of Helsinki.
Genotyping
The analyzed SNP rs10770141 (C-824T) in the TH promoter region was selected due to its potential functional impact on the transcriptional activity, implying that the T-allele carriers may have higher TH activities and retain higher levels of catecholamines in the brain (Horiguchi et al., 2014). Genotyping was performed by : PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) techniques. Primers were designed with Primer Express 2.0 software (Applied Biosystems). All other details of the methodology and primer sequences are available upon request.
Acquisition and analysis of behavioral data
Procrastination
Interindividual differences in the trait-like tendency to procrastinate were captured within the framework of Kuhl’s (1992, 1994b) action control theory. More precisely, we used the Action Control Scale 90 (ACS-90) by Kuhl (German version: HAKEMP 90, 1990). The questionnaire records the participant’s degree of action control under different circumstances: (i) action orientation subsequent to failure (AOF), (ii) prospective and decision-related action orientation (AOD) and (iii) action orientation during (successful) performance of activities (AOP). Twelve items represent each of the subscales. With each item, the participant is confronted with a given situation and must choose one out of two possible behaviors. This answer is either coded as action or state oriented (see Table 1). The individual’s degree of action control was calculated by summation of the action-oriented responses of each scale, which leads to a total value between 0 and 12. High values in action control indicate action orientation and therefore advanced cognitive, motivational and emotional control mechanisms. In the context of this study, we are particularly interested in the individuals’ expression on the AOD scale, as it has the closest relation to trait-like procrastination. While individuals with high AOD scores tend to tackle tasks directly, low AOD scores are associated with unreasonable postponement of actions and hesitation (Kuhl, 1994a, 1994b).
Subsequently, we conducted an analysis of variances including sex as a between-subject factor to test whether the interindividual differences in trait-like procrastination tendency, namely, AOD, were associated with interindividual differences in TH genotype. For the sake of completeness, this variance analysis was also performed for the two remaining action control scales, AOF and AOP.
Imaging
Since our previous study indicated that interindividual differences in AOD are associated with interindividual differences in morphology and functional connectivity of the amygdala (Schlüter et al., 2018a), we aim to investigate whether the neural correlates of AOD are mediated by TH gene expression. The acquisition and analysis of imaging data are described in more detail below.
Acquisition of imaging data
All imaging data were obtained at the Bergmannsheil Hospital in Bochum, Germany, using a 3 T Philips Achieva scanner (Best, the Netherlands) with a 32-channel head coil.
To estimate the amygdala volume and to determine the anatomical landmarks needed for the connectivity analyses, a T1-weighted high-resolution anatomical image was acquired [Magnetization Prepared Rapid Gradient-Echo (MP-RAGE): repetition time (TR), 8.18 ms; echo time (TE), 3.7 ms; flip angle, 8°; 220 slices; matrix size, 240 × 240; resolution, 1 × 1 × 1 mm]. The acquisition of the anatomical image took about 6 min.
Afterward, we acquired a functional MRI resting-state image using echo planar imaging (EPI) (TR, 2000 ms; TE, 30 ms; flip angle, 90°; 37 slices; matrix size, 80 × 80; resolution, 3 × 3 × 3 mm) to analyze interindividual differences in the functional connectivity of the amygdala and the dACC. For the duration of the resting-state sequence, the participants were asked to lie still and keep their eyes closed. The acquisition time of the resting-state images was 7 min.
Analysis of imaging data
To reconstruct the cortical surfaces of the T1-weighted images, we used published surface-based methods in FreeSurfer (http://surfer.nmr.mgh.harvard.edu, version 5.3.0) (Fischl et al., 1999). The automated reconstruction steps included skull stripping and gray and white matter segmentation, as well as reconstruction and inflation of the cortical surface. These steps were performed for each participant individually. Subsequently, we conducted a slice by slice quality control of each segmentation. Any inaccuracies were corrected manually, if necessary. After the initial segmentation into gray and white matter, an automated gyral/sulcal based parcellation procedure in FreeSurfer was conducted to extract both the amygdala (Fischl et al., 2002) and the dACC (Desikan et al., 2006) as our regions of interest (ROIs). Subsequently, the gray matter volume of the amygdala was assessed (see Figure 1a). Moreover, six regions representing the four ventricles of the brain were extracted to serve as a reference for later BOLD signal analyses.
Fig. 1.
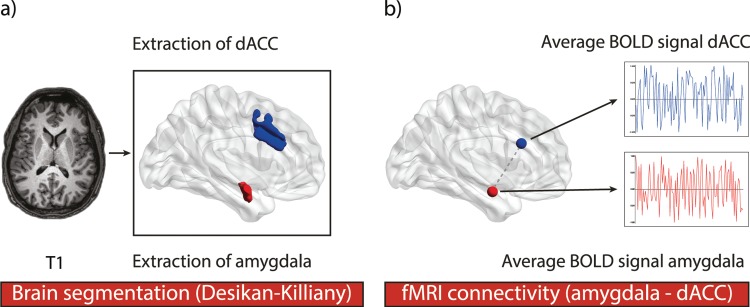
The methodological sequence for the extraction of the amygdala and the dACC as ROIs and the analysis of resting-state connectivity between both brain areas. a) After an initial segmentation into gray and white matter, the amygdala and the dACC were extracted as our ROIs according to the Desikan–Killiany atlas. Subsequently, the amygdala’s gray matter volume was computed. Second, the ROIs were linearly transformed into the native space of the resting-state images. b) Third, the functional connectivity between the amygdala and dACC was investigated. Thus, we obtained the correlation between the mean BOLD signals of the left and right dACC and the left or right amygdala, respectively. All correlation coefficients were subsequently transformed using Fisher’s r to z transformation. These z-transformed connectivity values were averaged to get the mean correlation of the amygdala and the dACC.
Finally, the previously extracted ROIs, as well as the ventricular regions, were linearly transformed into the native space of the resting-state images using mri_label2vol in Freesurfer. The analysis would then be continued in FMRIB Software Library (FSL) (www.fmrib.ox.ac.uk/fsl). Data from each participant were visually inspected to confirm that the transformation procedure was successful. After successful transformation FSL’s MELODIC toolbox was used to conduct the following preprocessing steps: discarding the first two volumes from each resting-state scan to allow for signal equilibration, motion and slice-timing correction, and high-pass temporal frequency filtering (0.005 Hz). To avoid introducing spurious correlations in neighboring voxels, we did not apply spatial smoothing.
To examine the functional resting-state connectivity between the amygdala and the dACC, we first calculated the mean resting-state time courses for both ROIs extracted from the automatic segmentation described above. Herto, the pre-processed time courses of corresponding voxels were averaged. Later, we computed partial correlations between the average time courses of the left and right amygdala and the left and right dACC while controlling for several nuisance variables (see Figure 1b). We regressed out the trajectories of all six motion parameters as well as the mean time courses averaged across all voxels representing white matter or cerebrospinal fluid, which were also obtained from the automatic Freesurfer segmentation mentioned above. Afterward, the correlation coefficients were transformed using Fisher’s r-to-z′ transformation. The z-transformed connectivity values were then averaged to get the mean correlation of the total amygdala and the total dACC.
Finally, we conducted an analysis of variances including sex as a between-subject factor to test whether the interindividual differences in amygdala volume and functional resting-state connectivity between the amygdala and the dACC were associated with interindividual differences in TH genotype. Additionally, we investigated whether we can confirm our previous findings regarding the neural correlates of AOD. For this purpose, we correlated the individual amygdala volume of our participants as well as their functional resting-state connectivity with their respective AOD scores. For the latter, the individual’s z′-transformed connectivity value was correlated with the individual’s AOD score. Both correlations were controlled for influences of sex and age. Since our previous study did not yield any significant neuronal correlates of AOF and AOP (Schlüter et al., 2018a), these two variables were not considered any further.
Results
Genotyping revealed 113 homozygous CC genotypes, 101 heterozygous CT genotypes and 62 homozygous TT genotypes. For two participants, the TH genotype could not be determined. The final sample was thus N = 276. For further statistical analysis, we combined the heterozygous genotype with the rare homozygous TT genotypes to ensure greater statistical power. This resulted in 113 CC genotypes being compared to 163 TT/CT genotypes. Sex-specific distribution of genotypes is depicted in Table 2.
Table 2.
Distribution of TH genotypes by Sex
| Sex | n | Genotype | |||
|---|---|---|---|---|---|
| CC | TT | CT | TT/CT | ||
| Male | 143 | 52 | 31 | 59 | 90 |
| Female | 135 | 61 | 31 | 42 | 73 |
Note. Table 2 depicts the distribution of the TH genotypes for males and females separately. For further statistical analysis, we combined the heterozygous genotype CT with the rare homozygous TT genotypes to ensure greater statistical power.
Based on previous studies, it is assumed that sex has a significant influence on both the tendency to procrastinate measured by AOD (Schlüter et al., 2018a; Schlüter et al., 2018b) and the effect of the TH genotype on personality traits (Sadahiro et al., 2010). We therefore took sex into account as a potential confounding variable. Regarding action control, our analysis yielded the expected sex differences. Here females (M = 7.26, SE = 0.26) tended to be significantly more action oriented than males did [M = 5.73, SE = 0.26; t(276) = −4.15, P < 0.001] when it comes to initiating intended actions (AOD, see Supplementary Figure 1b). This means that, while women tend to tackle a certain goal directly, men are more likely to procrastinate (Kuhl, 1994b). Regarding AOF, men showed significantly higher action control values (M = 6.95, SE = 0.26) when it comes to dealing with failures than women did [M = 5.46, SE = 0.25; t(276) = 4.15, P < 0.001; see Supplementary Figure 1a]. There were no significant sex differences for AOP [t(276) = −1.34, P = 0.183; see Supplementary Figure 1c]. With regard to the TH genotype groups, we did not find a significant association between sex and the genotype of the participant [χ2(3) = 3.35. P = 0.35; see Supplementary Figure 1d]. Moreover, there was no significant age difference between TH genotype groups (CCmean = 23.77 years, SE = 0.36; TT/CTmean = 24.21 years, SE = 0.30; P = 0.352], as well as no significant interaction between TH genotype and sex regarding age (males: CCmean = 24.40 years, SE = 0.41, TT/CTmean = 24.65 years, SE = 0.53); females: CCmean = 23.13 years, SE = 0.49, TT/CTmean = 23.86 years, SE = 0.45, P = 0.541). Also, there was no significant AOD difference between women using hormonal contraception (M = 7.53, SE = 0.29) and women not using hormonal contraception [M = 6.53, SE = 0.51; t(133) = −1.73, P = 0.085].
Subsequently, we analyzed whether there were significant AOD differences between the TH genotype groups CC and CT/TT. We conducted an analysis of variances including sex as a between-subject factor. The analysis yielded a significant main effect for both TH genotype [F(1,272 = 8.40, P = 0.004, ηp2 = 0.03] and sex [F(1,272 = 17.61, P = 0.000, ηp2 = 0.06] on AOD. Moreover, the interaction between TH genotype and sex had a significant effect on AOD [F(1,272) = 4.96, P = 0.027, ηp2 = 0.02]. Thus, interindividual differences in the TH genotype appear to affect the individual’s tendency to procrastinate, measured by AOD, distinctly in men and women. Post hoc analysis revealed that the differences in the TH genotype were only effective in women. Here, we found a significant AOD difference between the TH genotype groups, with CC genotypes having higher action control in decision-related contexts (CCmean = 8.28, SE = 0.39) than carriers of at least one T-allele (TT/CTmean = 6.38, SE = 0.35, P = 0.000, ηp2 = 0.05; see Figure 2). Thus, women homozygous for the C-allele are less prone to procrastinate than T-allele carriers.
Fig. 2.
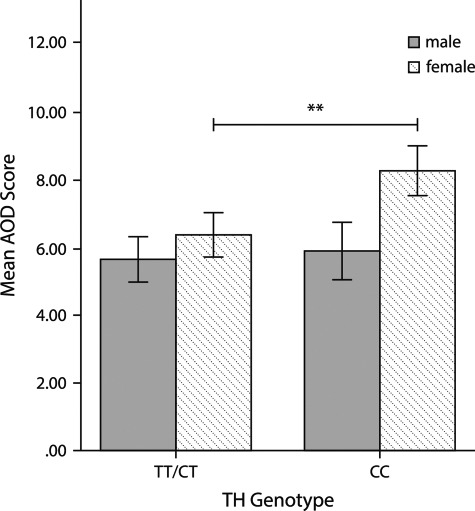
This illustrates the mean AOD scores for the different TH genotype groups indicating that women with the CC genotype having are significantly higher AOD scores than women who carry at least one T-allele. There was no significant AOD difference between the TH genotype groups in men. AOD: prospective and decision-related action orientation. **P < 0.01. Error bars, 95% CI.
Interestingly, there was no significant AOD difference between the TH genotype groups in men (CCmean = 5.66, SE = 0.42; TT/CTmean= 5.90, SE = 0.32; P = 0.126). Finally, our analysis of variances yielded no significant association between the TH genotype and the other two action control scales, AOF and AOP (see Supplementary Figure 2).
To examine, whether the recently detected interindividual differences in the amygdala's structure and function (Schlüter et al., 2018a) are also associated with interindividual differences in the TH genotype, we again undertook an analysis of variances, including sex as a between-subject factor. Interestingly, these analyses yielded a significant effect of TH genotype neither on amygdala volume (see Figure 3a) nor on the functional resting-state connectivity between the amygdala and the dACC (see Figure 3b).
Fig. 3.
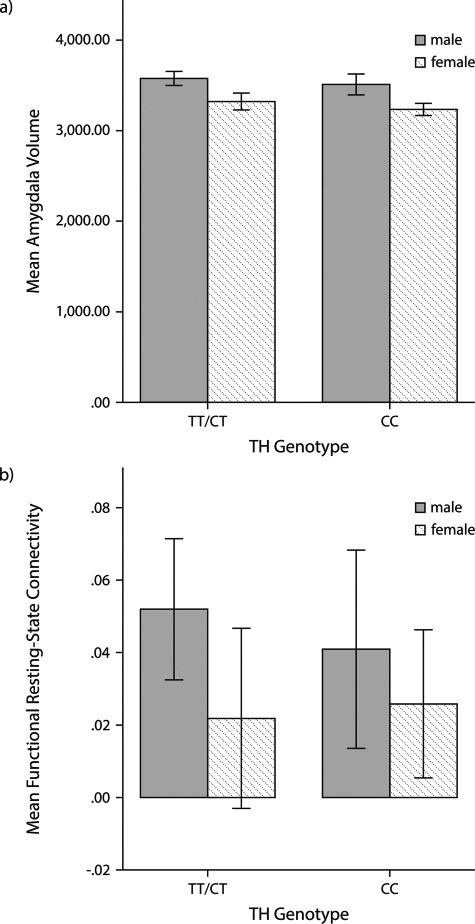
a) This illustrated the mean amygdala volume for the different TH genotype groups indicating that there was no significant differences in amygdala volume between carriers of the CC genotype and carriers of at least one T-allele. b) This illustrates the mean functional connectivity for both TH genotype groups. Again, there was no significant group difference detected. Error bars, 95% CI.
Nonetheless, we were able to confirm our previous findings (Schlüter et al., 2018a) showing that amygdala volume was significantly negatively associated with AOD (r = −0.17, P = 0.004; see Supplementary Figure 3). Thus, individuals with larger amygdala volume are more likely to procrastinate than individuals with a smaller volume of the total amygdala. The same applies to the functional resting-state connectivity of the amygdala. Here, we were able to show that increased functional connectivity between the amygdala and the dACC is positively associated with the individual AOD score (r = 0.16, P = 0.010; see Supplementary Figure 3). Hence, stronger functional connectivity between the amygdala and the dACC appears to be beneficial when it comes to goal-directed behavior. Thus, TH genotype and the amygdala’s structure and function appear to be two significant but independent factors influencing the individual's propensity to procrastination.
Discussion
In the current study, we examined whether different genotypes of the functional TH promoter polymorphism rs10770141 (C-824T) are associated with interindividual differences in the tendency to procrastinate, as measured by Kuhl’s (1994a) AOD scale. Here we could show that there was a significant interaction between TH genotype and sex. While in women homozygous carriers of the C-allele revealed higher AOD scores than carriers of at least one T-allele, there was no significant TH effect on AOD in men. Since T-allele carriers are considered to have higher TH activities and increased levels of dopamine (Zhang et al., 2010; Horiguchi et al., 2014), our study suggests that in women higher dopamine base levels are likely to increase the propensity to procrastinate.
As already stated, dopamine has frequently been related to metacontrol mechanisms (Braver & Cohen, 2000; Dreisbach et al., 2005; Müller et al., 2007; Tai et al., 2012; Howe et al., 2013; Schultz et al., 2017) like goal-shielding (Kuhl, 1994b; Goschke & Bolte, 2014) or the context-sensitive evaluation of how much effort and control should be invested to reach a specific goal (Goschke & Bolte, 2014; Hommel & Wiers, 2017). However, the direction of the dopamine effect appears to be strongly dependent on the respective task used to assess metacontrol (Braver & Cohen, 2000; Goschke & Bolte, 2014). For instance, a review article by Goschke & Bolte (2014) points out that higher dopamine levels, induced by either positive affect (Phillips et al., 2002; Dreisbach & Goschke, 2004; Baumann & Kuhl, 2005) or genetic differences (Dreisbach et al., 2005), elevate cognitive flexibility (Phillips et al., 2002; Baumann & Kuhl, 2005; Müller et al., 2007) and broaden the scope of attention (Friedman & Förster, 2010). Though these processes are considered to facilitate task switching and working memory, updating their apparent benefits occur at the expense of goal shielding (Goschke & Bolte, 2014). It is therefore conceivable that the elevated dopamine level observed in T-allele carriers (Zhang et al., 2010; Horiguchi et al., 2014) increases the amount of context information processed in the working memory of the respective individual. This, in turn, might lead to a higher amount of inferencing information and alternative action plans negatively affecting the individual’s goal shielding ability. The phenomenon of impaired suppression of irrelevant information has also been observed in individuals with low AOD scores (Beckmann & Kuhl, 1984; Kuhl, 1985; Kuhl & Goschke, 1994). For instance, it has been shown that individuals with low action orientation scores toil to reduce the space of information, though they already have a preference for or even committed to a particular goal (Beckmann & Kuhl, 1984; Kuhl & Goschke, 1994).
After all, the exact neurobiological mechanisms behind the dopamine effect on metacontrol and therefore also on the trait-like tendency to procrastinate are challenging to determine. This might be due to the complexity of the dopaminergic system (Goschke & Bolte, 2014). However, some theories suggest that increased dopamine release in the mesocorticolimbic system and especially in the ACC facilitates cognitive flexibility and working memory updating (Ashby & Isen, 1999). As indicated in the Introduction section, unexpected rewards or reward cues elicit a burst of activity in dopaminergic neurons in the VTA, which in turn triggers the encoding of incoming information into working memory (D’Ardenne et al., 2012; Goschke & Bolte, 2014). To understand how this gating function of dopamine might adversely impact procrastination, one needs to consider the updating threshold theory (Braver & Cohen, 2000; Goschke & Bolte, 2014). The theory suggests that if the updating threshold is relatively high, only task-specific reward cues may cause a strong-enough dopamine response in the VTA to open the gates into working memory. Thus, the intended action would be protected, since only goal-relevant stimuli would enter working memory. If, in contrast, the initial dopamine level in the brain is increased, the updating threshold could lower, causing weaker stimuli to trigger the dopaminergic cascade that opens the gate to working memory. This again might facilitate novel or goal-irrelevant information to access working memory, increasing distraction from the initially intended action (Goschke & Bolte, 2014). Regarding the effect of the TH genotype on the tendency to procrastinate, this could imply that carriers of at least one T-allele have lower AOD scores and are therefore more prone to procrastination, due to a lower updating threshold caused by an elevated dopamine baseline. Along these lines, goal-irrelevant stimuli might make their way into the participant’s working memory and thus distract from the actual intention or goal.
However, it is important to mention that TH is rate limiting not only for dopamine but also for norepinephrine (Horiguchi et al., 2014). Thus, also changes in the norepinephrine base level may be responsible for individual differences in metacontrol ability, measured as AOD. Previous research indicated that the norepinephrine system is relevant for the modulation of cognitive control processes, contributing to the optimization of behavioral performance (Aston-Jones & Cohen, 2005). This is especially the case whenever the demands on cognitive control are high (Chmielewski et al., 2017; Mückschel et al., 2017; Wolff et al., 2018). Similar to dopamine, norepinephrine has a neuromodulatory function. Rather than producing direct excitatory or inhibitory signaling, norepinephrine alters effects produced by other neurotransmitters (Aston-Jones & Cohen, 2005). Here a distinction is made between the phasic and tonic release of norepinephrine. While phasic activation of the norepinephrine system is associated with the processing of goal-relevant stimuli and improved performance, high base levels of norepinephrine, as a result of tonic activation, were linked to an impaired signal-to-noise ratio and distractibility (Aston-Jones & Cohen, 2005). Thus, it is conceivable that an increased level of norepinephrine induced by the genetic predisposition of carrying at least one T-allele could affect action control and increase the propensity to procrastination. Future studies may therefore examine the contributions of dopamine and norepinephrine to procrastination separately to untangle their effects.
As already described, dopamine activity in frontal and subcortical brain areas seems to play an essential role in metacontrol (Ashby & Isen, 1999). Interestingly, this is in line with our findings regarding the involvement of the amygdala and dACC in procrastination-related metacontrol processes (Schlüter et al., 2018a). Here we could show that higher amygdala volume and lower functional connectivity between the amygdala and the dACC were associated with lower AOD scores and therefore with a higher propensity to procrastination. The synergy between the dACC and the amygdala is assumed to play a significant role in purposive behavior (Feng et al., 2014) and metacontrol mechanisms. Studies dealing with the neural basis of self-control failure, such as procrastination, support a model of top-down regulation of the amygdala by frontal and anterior cingulate structures (Ochsner et al., 2002). In this context, the ACC is associated with different metacontrol processes, such as action–outcome evaluation and reward-related selection of an action (Shenhav et al., 2013), as well as strategic adjustment of behavior and emotion (Botvinick et al., 2004). For this, the ACC receives information from cortical and subcortical brain regions, such as the amygdala (Shenhav et al., 2013), and in turn regulates them by top-down projections (Ochsner et al., 2002). Based on our previous findings, we assume that a chronic imbalance between both brain regions might lead to behavior that is more strongly motivated by emotions and could therefore lead to an inadequate choice of actions as seen in procrastination and low AOD (Schlüter et al., 2018a). Since dopamine was associated with metacontrol especially in mesocorticolimbic areas (Ashby & Isen, 1999), the question arises whether the previously reported interindividual differences in the morphology and functional connectivity of the amygdala (Schlüter et al., 2018a) are also affected by TH expression. However, there was no significant amygdala volume or connectivity difference between the TH genotype groups, suggesting that genetic, anatomical and functional influences affect AOD independently of each other. Future studies may further investigate if other candidate genes might influence both the individuals’ behavior and their brain's morphology or functional connectivity.
Why TH expression affects AOD in women and men differently remains unresolved. However, our study is not the first to report sex-distinct associations between TH genotype and psychological measures. For instance, Sadahiro et al. (2010) showed that interindividual differences in the TH gene promoter affect the personality trait of novelty seeking in healthy men but not in women. Although the interplay between sex, TH genotype and behavior has not been entirely clarified, it can be assumed that the female sex hormone estradiol alters their relationship. Estradiol is not only considered to increase the number of TH-positive neurons, fostering the development and complexity of dopaminergic neurons, but also assumed to stimulate the expression of TH (Kishi et al., 2005). Therefore, the sex-specific association between TH genotype and AOD observed in our study may be due to the fact that female T-allele carriers show higher responsiveness to the amount of catecholamines in the brain compared to men with the same genetic predisposition. Hence, the neurobiological mechanisms described above could be more pronounced in women causing the observed sex effect.
There are some limitations to the present study that are worth discussing, with the aim of interpreting the results correctly and improving future research. First, our and other studies (Sadahiro et al., 2010) indicate that the relationship between TH genotype and behavior is strongly influenced by estradiol (Kishi et al., 2005). During menstrual cycle, women experience strong fluctuations in sex hormone levels. These fluctuations are considered to alter dopamine-dependent cognitive abilities, like working memory (Jacobs & D'Esposito, 2011). It would therefore be interesting to examine whether the menstrual cycle phase also affects the interaction of TH genotype and AOD. Unfortunately, the menstrual cycle phase was not captured in our study, so further investigation of this aspect is not possible. We therefore recommend future studies inspecting the relationship between TH genotype and behavior to assess the day of menstrual cycle of their female participants.
Second, the trait-like tendency to procrastinate was measured using Kuhl’s (1994a) ACS-90. Since the ASC-90 is a questionnaire, procrastination tendency was assessed based on the participants’ self-report and not based on an objective measuring procedure. Thus, a deviation between the participants’ self-assessment and the actual procrastination behavior cannot be ruled out completely. Still, individual differences in metacontrol abilities assessed by ACS-90 have been frequently associated with real-life outcomes as occupational or academic success (Diefendorff et al., 2000; Hirschauer et al., 2018; Schlüter et al., 2018b).
Finally, our sample comprises mainly students aged 18 to 35 years. Therefore, as in many other studies, transferability to the general population might be limited. Nonetheless, the rather large sample size and the fact that not only students of a certain major took part in our study have to be evaluated positively.
In conclusion, the study at hand is the first to investigate whether genetically induced differences in the dopaminergic system are associated with interindividual differences in the trait-like tendency to procrastinate. We could show that differences in the TH genotype have a sex-dependent effect on AOD. Thus, only women showed significant AOD differences between the TH genotype groups. Here, T-allele carriers of the functional SNP rs10770141 (C-824T) in the TH promoter region had lower AOD values and were therefore more prone to procrastination. This could be due to the higher TH activity and the higher dopamine base levels associated with carrying at least one T-allele. Higher dopamine levels are considered to lower the updating threshold of the working memory leading to impaired gating of information inflow, causing distraction and defective goal shielding, two phenomena that are frequently considered to cause procrastination (Steel, 2007). This work could therefore support Kuhl’s (1994b) assumption that the tendency to procrastinate is not just a behavior but a personality trait, which might be laid out in our genes. Future studies should try to investigate the causal mechanisms behind this detected gene–function relationship experimentally. Finally, we found no significant association between interindividual differences in the TH genotype and differences in the morphology and functional connectivity of the amygdala that were previously associated with AOD (Schlüter et al., 2018a). Thus, this study suggests that genetic, anatomical and functional differences affect trait-like procrastination independently of each other.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (grant numbers GU 227/16-1 and GE 2777/2-1), SFB 940 project B08 and SFB 1280 project A03 (project number 316803389), as well as the Mercur Foundation (grant number An-2015-0044).
Author contributions
E.G. conceived the project and supervised the experiments. C.S., L.A., M.P., O.G., C.B., S.O. and E.G. designed the project. L.A. planned and performed genetic experiments. C.S., C.F. and P.F. collected data. C.S., L.A., C.F., C.B, S.O. and E.G. analyzed the data. C.S., C.B. and E.G. wrote the paper. All authors discussed the results and edited the manuscript.
Supplementary Material
Acknowledgments
The authors thank all research assistants for their support during the behavioral measurements. Furthermore, the authors thank PHILIPS Germany (Burkhard Mädler) for the scientific support with the MRI measurements and Tobias Otto for technical assistance.
References
- Ashby F.G., Isen A.M. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106(3), 529. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. (2005). An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–50. [DOI] [PubMed] [Google Scholar]
- Baumann N., Kuhl J. (2005). Positive affect and flexibility: overcoming the precedence of global over local processing of visual information. Motivation and Emotion, 29(2), 123–34. [Google Scholar]
- Beckmann J., Kuhl J. (1984). Altering information to gain action control: functional aspects of human information processing in decision making. Journal of Research in Personality, 18(2), 224–37. [Google Scholar]
- Beste C., Moll C.K.E., Pötter-Nerger M., Münchau A. (2018). Striatal microstructure and its relevance for cognitive control. Trends in Cognitive Sciences, 22(9), 747–51 doi: 10.1016/j.tics.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Beswick G., Mann L. (1994). State orientation and procrastination. In: Volition and Personality: Action Versus State Orientation, Göttingen, Hogrefe & Huber; Vol. 23 391–396. [Google Scholar]
- Blunt A., Pychyl T.A. (1998). Volitional action and inaction in the lives of undergraduate students: state orientation, procrastination and proneness to boredom. Personality and Individual Differences, 24(6), 837–46. [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–46. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Cohen J.D. (2000). On the control of control: the role of dopamine in regulating prefrontal function and working memory. In: Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA, MIT Press; 713–737. [Google Scholar]
- Chmielewski W.X., Mückschel M., Ziemssen T., Beste C. (2017). The norepinephrine system affects specific neurophysiological subprocesses in the modulation of inhibitory control by working memory demands. Human Brain Mapping, 38(1), 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K., Eshel N., Luka J., Lenartowicz A., Nystrom L.E., Cohen J.D. (2012). Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–80 doi: 10.1016/J.Neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diefendorff J.M., Hall R.J., Lord R.G., Strean M.L. (2000). Action-state orientation: construct validity of a revised measure and its relationship to work-related variables. Journal of Applied Psychology, 85(2), 250. [DOI] [PubMed] [Google Scholar]
- Dreisbach G., Goschke T. (2004). How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. Journal of Experimental Psychology. Learning, Memory, and Cognition, 30(2), 343. [DOI] [PubMed] [Google Scholar]
- Dreisbach G., Müller J., Goschke T., et al. (2005). Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behavioral Neuroscience, 119(2), 483. [DOI] [PubMed] [Google Scholar]
- Durstewitz D., Seamans J.K. (2008). The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biological Psychiatry, 64(9), 739–49. [DOI] [PubMed] [Google Scholar]
- Feng P., Feng T., Chen Z., Lei X. (2014). Memory consolidation of fear conditioning: bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Social Cognitive and Affective Neuroscience, 9(11), 1730–7 doi: 10.1093/scan/nst170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari J.R. (2004). Trait procrastination in academic settings: an overview of students who engage in task delays. In: Counseling the Procrastinator in Academic Settings. Washington, DC, American Psychological Association,19–27. [Google Scholar]
- Fillenz M. (1993). Short-term control of transmitter synthesis in central catecholaminergic neurones. Progress in Biophysics and Molecular Biology, 60(1), 29–46. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. (1999). Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207 doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- Freund A.M., Hennecke M. (2015). On means and ends the role of goal focus in successful goal pursuit. Current Directions in Psychological Science, 24(2), 149–53. [Google Scholar]
- Friedman R.S., Förster J. (2010). Implicit affective cues and attentional tuning: an integrative review. Psychological Bulletin, 136(5), 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T., Bolte A. (2014). Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia, 62, 403–23. [DOI] [PubMed] [Google Scholar]
- Gropel P., Baumeister R.F., Beckmann J. (2014). Action versus state orientation and self-control performance after depletion. Personality and Social Psychology Bulletin, 40(4), 476–87 doi: 10.1177/0146167213516636. [DOI] [PubMed] [Google Scholar]
- Hirschauer A.-K., Aufhammer F., Bode R., Chasiotis A., Künne T. (2018). Parental empathy as a source of child’s scholastic performance. In: Why People Do the Things They Do: Building on Julius Kuhl’s Contributions to the Psychology of Motivation and Volition, 1st edn. Göttingen: Hogrefe. [Google Scholar]
- Hommel B., Colzato L.S. (2017). The social transmission of metacontrol policies: mechanisms underlying the interpersonal transfer of persistence and flexibility. Neuroscience & Biobehavioral Reviews, 81, 43–58. [DOI] [PubMed] [Google Scholar]
- Hommel B., Wiers R.W. (2017). Towards a unitary approach to human action control. Trends in Cognitive Sciences, 21(12), 940–9. [DOI] [PubMed] [Google Scholar]
- Horiguchi M., Ohi K., Hashimoto R., et al. (2014). Functional polymorphism (C-824T) of the tyrosine hydroxylase gene affects IQ in schizophrenia. Psychiatry and Clinical Neurosciences, 68(6), 456–62. [DOI] [PubMed] [Google Scholar]
- Howe M.W., Tierney P.L., Sandberg S.G., Phillips P.E., Graybiel A.M. (2013). Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature, 500(7464), 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., D’Esposito M. (2011). Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. Journal of Neuroscience, 31(14), 5286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Spector P.E. (2004). The effect of action orientation on the academic performance of undergraduate marketing majors. Journal of Marketing Education, 26(3), 250–60. [Google Scholar]
- Jostmann N.B., Koole S. L. (2010). Dealing with high demands: the role of action versus state orientation. In: Handbook of Personality and Self-Regulation, Oxford, UK, Wiley-Blackwell Publishing, Vol. 14 332–352. [Google Scholar]
- Kishi Y., Takahashi J., Koyanagi M., et al. (2005). Estrogen promotes differentiation and survival of dopaminergic neurons derived from human neural stem cells. Journal of Neuroscience Research, 79(3), 279–86. [DOI] [PubMed] [Google Scholar]
- Klingsieck K.B. (2013). Procrastination in different life-domains: is procrastination domain specific? Current Psychology, 32(2), 175–85. [Google Scholar]
- Kobayashi K., Nagatsu T. (2005). Molecular genetics of tyrosine 3-monooxygenase and inherited diseases. Biochemical and Biophysical Research Communications, 338(1), 267–70. [DOI] [PubMed] [Google Scholar]
- Kuhl J. (1984). Volitional aspects of achievement motivation and learned helplessness: toward a comprehensive theory of action control. In: Progress in Experimental Personality Research, Vol. 13 New York, Academic Press, 99–171. [PubMed] [Google Scholar]
- Kuhl J. (1985). Volitional mediators of cognition-behavior consistency: self-regulatory processes and action versus state orientation. In Action Control. New York, Springer, 101–128. [Google Scholar]
- Kuhl J. (1992). A theory of self-regulation: action versus state orientation, self-discrimination, and some applications. Applied Psychology, 41(2), 97–129. [Google Scholar]
- Kuhl J. (1994a). Action versus state orientation: psychometric properties of the Action Control Scale (ACS-90). In: Volition and Personality: Action Versus State Orientation, Göttingen, Hogrefe & Huber, Vol. 47 56. [Google Scholar]
- Kuhl J. (1994b). A theory of action and state orientations. In: Volition and Personality: Action Versus State Orientation. Göttingen, Hogrefe & Huber, 9–46. [Google Scholar]
- Kuhl J., Goschke T. (1994). State orientation and the activation and retrieval of intentions in memory. In: Volition and Personality: Action Versus State Orientation. Göttingen, Hogrefe & Huber, 127–153. [Google Scholar]
- Landman A., Nieuwenhuys A., Oudejans R.R.D. (2016). Decision-related action orientation predicts police officers’ shooting performance under pressure. Anxiety Stress and Coping, 29(5), 570–9 doi: 10.1080/10615806.2015.1070834. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Gohil K., Ziemssen T., Beste C. (2017). The norepinephrine system and its relevance for multi-component behavior. Neuroimage, 146, 1062–70. [DOI] [PubMed] [Google Scholar]
- Müller J., Dreisbach G., Goschke T., Hensch T., Lesch K.P., Brocke B. (2007). Dopamine and cognitive control: the prospect of monetary gains influences the balance between flexibility and stability in a set-shifting paradigm. European Journal of Neuroscience, 26(12), 3661–8. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–29 doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ott T., Nieder A. (2019). Dopamine and cognitive control in prefrontal cortex. Trends in Cognitive Sciences. 23213–234 [DOI] [PubMed] [Google Scholar]
- Palfai T.P. (2002). Action-state orientation and the self-regulation of eating behavior. Eating Behaviors, 3(3), 249–59 doi: 10.1016/s1471-0153(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Palfai T.P., McNally A.M., Roy M. (2002). Volition and alcohol-risk reduction—the role of action orientation in the reduction of alcohol-related harm among college student drinkers. Addictive Behaviors, 27(2), 309–17 doi: 10.1016/s0306-4603(01)00186-1. [DOI] [PubMed] [Google Scholar]
- Phillips L.H., Bull R., Adams E., Fraser L. (2002). Positive mood and executive function: evidence from stroop and fluency tasks. Emotion, 2(1), 12. [DOI] [PubMed] [Google Scholar]
- Sadahiro R., Suzuki A., Shibuya N., et al. (2010). Association study between a functional polymorphism of tyrosine hydroxylase gene promoter and personality traits in healthy subjects. Behavioural Brain Research, 208(1), 209–12. [DOI] [PubMed] [Google Scholar]
- Schlüter C., Fraenz C., Pinnow M., Friedrich P., Güntürkün O., Genç E. (2018a). The structural and functional signature of action control. Psychological Science, 29(10), 1620–30. [DOI] [PubMed] [Google Scholar]
- Schlüter C., Fraenz C., Pinnow M., Voelkle M.C., Güntürkün O., Genç E. (2018b). Volition and academic achievement: interindividual differences in action control mediate the effects of conscientiousness and sex on secondary school grading. Motivation Science, 4(3), 262–73. [Google Scholar]
- Schultz W., Stauffer W.R., Lak A. (2017). The phasic dopamine signal maturing: from reward via behavioural activation to formal economic utility. Current Opinion in Neurobiology, 43, 139–48. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Cohen J.D., Botvinick M.M. (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19(10), 1286. [DOI] [PubMed] [Google Scholar]
- Sirois F.M., Giguère B. (2018). Giving in when feeling less good: procrastination, action control, and social temptations. British Journal of Social Psychology, 57(2), 404–27. [DOI] [PubMed] [Google Scholar]
- Steel P. (2007). The nature of procrastination: a meta-analytic and theoretical review of quintessential self-regulatory failure. Psychology Bulletin, 133(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Tai L.-H., Lee A.M., Benavidez N., Bonci A., Wilbrecht L. (2012). Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nature Neuroscience, 15(9), 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N., Mückschel M., Ziemssen T., Beste C. (2018). The role of phasic norepinephrine modulations during task switching: evidence for specific effects in parietal areas. Brain Structure and Function, 223(2), 925–40. [DOI] [PubMed] [Google Scholar]
- Yee D.M., Braver T.S. (2018). Interactions of motivation and cognitive control. Current Opinion in Behavioral Sciences, 19, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhang L., Rao F., et al. (2010). Human tyrosine hydroxylase natural genetic variation: delineation of functional transcriptional control motifs disrupted in the proximal promoter. Circulation. Cardiovascular Genetics, 3(2), 187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


