Abstract
Irritability is garnering increasing attention in psychiatric research as a transdiagnostic marker of both internalizing and externalizing disorders. These disorders often emerge during adolescence, highlighting the need to examine changes in the brain and in psychological functioning during this developmental period. Adolescents were recruited for a longitudinal study examining the effects of early life stress on the development of psychopathology. The 151 adolescents (73 M/78 F, average age = 11.5 years, standard deviation = 1.1) were scanned with a T1-weighted MRI sequence and parents completed reports of adolescent irritability using the Affective Reactivity Index. Of these 151 adolescents, 94 (46 M/48 F) returned for a second session (average interval = 1.9 years, SD = 0.4). We used tensor-based morphometry to examine cross-sectional and longitudinal associations between irritability and regional brain volume. Irritability was associated with brain volume across a number of regions. More irritable individuals had larger hippocampi, insula, medial orbitofrontal cortex and cingulum/cingulate cortex and smaller putamen and internal capsule. Across the brain, more irritable individuals also had larger volume and less volume contraction in a number of areas that typically decrease in volume over the developmental period studied here, suggesting delayed maturation. These structural changes may increase adolescents’ vulnerability for internalizing and externalizing disorders.
Keywords: irritability, brain structure, tensor-based morphometry, development
Introduction
There is growing interest in the construct of irritability (Vidal-Ribas et al., 2016; Stringaris et al., 2018). Irritability has been conceptualized as ‘proneness to anger,’ although it can also refer to difficulty disengaging from an angry state (Stringaris et al., 2012). Prospective studies indicate that irritability increases individuals’ risk for a number of negative outcomes, including major depressive disorder (Stringaris et al., 2009), suicidality (Pickles et al., 2010) and general functional impairment (Dougherty et al., 2015). Irritability has recently been formulated to be transdiagnostic; indeed, irritability has been associated with both internalizing and externalizing disorders in cross-sectional and longitudinal studies (Leibenluft et al., 2006; Stoddard et al., 2014; Humphreys et al., 2018). Given that these disorders increase in prevalence during adolescence (Lee et al., 2014), examining the neural correlates of irritability during the transition from earlier to later adolescence may help to elucidate the biological mechanisms involved in the development of psychopathology and other negative long-term outcomes.
Several studies have now examined the neural basis of irritability; however, there are specific limitations of these studies that justify additional research in this area. Few studies have examined the association between irritability and alterations in brain structure. Most of these structural studies have used a group-based approach to operationalize irritability. Gold et al. (2016) used voxel-based morphometry (VBM) to examine brain morphometry in children with and without diagnosed psychiatric disorders. In contrast to healthy comparison children, children with disruptive mood dysregulation disorder (DMDD) had lower overall gray matter volume in right dorsolateral prefrontal cortex (dlPFC)/superior frontal gyrus; however, across all groups, irritability was not associated significantly with variation in brain morphometry. Using VBM in a sample of 78, 13- to 14-year-old adolescents with severe mood dysregulation (SMD) and 68 age-matched healthy comparison adolescents (Adleman et al., 2012) found in cross-sectional analyses that youth with SMD had significantly lower gray matter density in bilateral pre-supplementary motor area, right insula and dlPFC than did healthy controls. Longitudinal analyses from these same participants 2 years later yielded no significant group differences or interactions of group and time. In another longitudinal sample, enriched for the presence of early depressive symptoms, in which irritability was operationalized dimensionally, Pagliaccio et al. (2018) found thicker cortex in the left superior frontal gyrus, left superior temporal gyrus and right inferior parietal lobule in a subset of children who exhibited consistently elevated levels of irritability.
There have been a few functional MRI (fMRI) studies focused on irritability, with some focused on individuals with SMD, which is characterized by persistent, elevated and functionally impairing levels of irritability (Leibenluft, 2011) or DMDD (American Psychiatic Association, 2013). fMRI studies of individuals with DMDD or SMD have reported increased amygdala reactivity to both positive and negative emotional faces (Wiggins et al., 2016) and deactivation in the amygdala, striatum, parietal cortex and posterior cingulate in response to frustration (Deveney et al., 2013). The application of findings from these studies to individuals who do not meet criteria for disorder but nonetheless evidence elevated irritability may be limited. In some studies, however, irritability has been operationalized as a dimensional construct, generally assessed using metrics that map onto the Research Domain Criteria construct of ‘frustrative non-reward’ (Insel et al., 2010). In studies using samples recruited based on broader risk metrics, including adolescents with DMDD, anxiety disorders or attention-deficit/hyperactivity disorder (ADHD), higher scores on the Affective Reactivity Index (ARI), reflecting higher levels of irritability, were associated with decreased connectivity between the amygdala and prefrontal cortex (Stoddard et al., 2017), increased fronto-striatal activity in response to frustration (Tseng et al., 2018) and increased activity in the insula, caudate, prefrontal cortex and inferior parietal lobule while orienting away from threat (Kircanski et al., 2018). Examining a sample of young children with clinically significant levels of irritability and at least one psychiatric diagnosis, Perlman et al. (2015) found that dimensionally assessed irritability was associated with decreased activation in the anterior cingulate cortex (ACC) and the striatum during the experience of loss on a reward task. Further, group-based analyses in that study indicated that, relative to healthy comparison children, clinical participants had greater activation in the ACC and middle frontal gyrus (MFG) during a reward task, but reduced activation in these structures during a frustration task. Finally, the clinical participants exhibited less activation of the posterior cingulate than did the healthy control participants during reward, but more activation during frustration.
While it has been useful to use a group-based approach to examine irritability, it is likely that irritability is not a taxon. As employed by some prior studies, it is important that irritability be studied dimensionally, as data indicate that related constructs are best conceptualized dimensionally (Ruscio and Ruscio, 2000). Moreover, including participants across a range of functioning, as opposed to studying irritability in clinical samples of children, increases generalization to the broader population. Finally, most of the studies reviewed above included participants in a large age range (8–20 years old). Given that psychopathology often emerges during adolescence (Lee et al., 2014), it is critical that we assess individuals in this developmental stage—early adolescence prior to the onset of psychopathology. No study to date, however, has investigated associations between brain structure and irritability dimensionally in adolescents who were not selected on the basis of exhibiting symptoms of psychopathology. The present study examined the cross-sectional and longitudinal associations between irritability and brain structure in a cohort of typically developing adolescents. We assessed 151 participants in early adolescence, 94 of whom returned for a longitudinal follow-up session in later adolescence. To measure brain structure at each time point, we used tensor-based morphometry (TBM), which allowed us to conduct a whole-brain search of structural correlates of irritability that was not constrained by a priori hypotheses. Although VBM has been used in previous studies of irritability (Adleman et al., 2012; Gold et al., 2016) and is also a whole-brain approach, TBM has several advantages over VBM (Thompson et al., 2000). For example, TBM does not involve tissue segmentation or spatial smoothing steps, which can introduce error and limit resolution for small effects. Further, VBM depends on tissue density measurements to calculate differences, which require high-quality registration, and inaccuracies can erroneously be identified as differences in volume (Bookstein, 2001). FreeSurfer has a well-validated set of tools for cortical analyses (Fischl, 2012), but these do not allow for whole brain, voxel-wise analysis of volume. Further, in a direct comparison of cortical thickness estimates from FreeSurfer and Advanced Normalization Tools (ANTs—the toolbox used in our TBM workflow), ANTs had stronger neurobiological validity in predicting age and sex (Tustison et al., 2014). We did not limit our analysis to specific regions; indeed, previous studies have not identified structural alterations in a cohesive set of brain regions related to irritability. Based on the fMRI research cited above, however, we hypothesized that irritability would be associated with altered volume in fronto-striatal structures, the amygdala and the insular cortex.
Materials and methods
Participants
Participants in the larger study were 214 children (93 boys, 121 girls) ages 9.1 to 13.9 years (mean age: 11.4 years, SD = 1.0) who were recruited to take part in a longitudinal study of psychopathology across the pubertal transition [see (Humphreys et al., 2016) for more information on the sample]. Participants were selected from the San Francisco Bay community using a combination of flyers and local media; they were recruited on the basis of having experienced a range of early life adversity and were selected for participation if they were in the early stages of puberty (Tanner staging <4). The study was approved by the Stanford University Institutional Review Board; participants and their parents gave assent and informed consent, respectively. Participants were screened for initial inclusion/exclusion criteria through a telephone interview; potentially eligible individuals were then invited to the laboratory for in-person interviews and assessments. Inclusion criteria were that children be between the ages of 9 and 13 years and be proficient in English. Exclusion criteria were factors that would preclude an MRI scan (e.g. metal implants, braces), a history of major neurological or medical illness and severe learning disabilities that would make it difficult for participants to comprehend the study procedures. Females who reported having started menses were excluded, and boys were matched to girls with respect to Tanner stage. Because females enter puberty at an earlier age than males, this meant that the females were significantly younger than the males at both time points (female average age at first study time point = 11.2 years; male average age = 11.9 years, P = 4.4×10−5). Participants were compensated for their time.
A flow chart detailing the final sample size is presented in Figure 1. As detailed in the chart, of the 214 participants who started in the study, 151 (73 boys, 78 girls) had useable MRI and ARI data cross-sectionally at T1. Of these participants, 136 have returned for a Time 2 (T2) scan ~2 years after the T1 session (mean interval: 1.9 years, SD = 0.39) for a second visit at which the same assessments were conducted; 94 of these participants had useable longitudinal MRI and ARI data. Scan quality was visually checked during the scan session so that poor-quality scans could be repeated. Nevertheless, some participants still did not provide usable data on repeated scans due to excessive motion and, therefore, were excluded from analyses. The participants who opted out of the scan did not differ significantly from participants included in this report in levels of irritability. Demographic information for participants included in the present analyses is presented in Table 1. There were no significant differences in T1 irritability between participants who have and who have not yet returned for the T2 assessment (P = 0.48, Figure 2). Participants who were not included in the longitudinal analyses also did not differ from participants who were included in these analyses in age, sex distribution, ethnic distribution, Tanner stage or intracranial volume (all P > 0.10). Correlations among measures are presented in Supplementary Table S1.
Fig. 1.

Flowchart of sample. Of the full study sample of 214 adolescents, 151 had ARI scores and brain scans of adequate quality for cross-sectional analyses and 94 had complete data for longitudinal analyses.
Table 1.
Demographics of sample
| Cross-sectional sample | Longitudinal sample | ||||
|---|---|---|---|---|---|
| N | 151 | N | 94 | ||
| M/F | 73/78 | M/F | 46/48 | ||
| T1 Age | Average | 11.5 | T1 Age | Average | 11.5 |
| SD | 1.1 | SD | 1.0 | ||
| Range | 9.2–14.0 | Range | 9.4–13.9 | ||
| T1 Tanner stage | Average | 2.0 | T1 Tanner stage | Average | 2.0 |
| SD | 0.7 | SD | 0.8 | ||
| Range | 1–4 | Range | 1–4 | ||
| Race/ethnicity | Caucasian | 70 | Race/ethnicity | Caucasian | 49 |
| African American | 12 | African American | 7 | ||
| Hispanic | 9 | Hispanic | 6 | ||
| Asian/Pacific Islander | 19 | Asian/Pacific Islander | 14 | ||
| Multi-racial | 32 | Multi-racial | 13 | ||
| Other or NA | 9 | Other or NA | 4 | ||
| Household income | Less than $5000 | 1 | Household income | Less than $5000 | 1 |
| $5001–$10 000 | 2 | $5001–$10 000 | 2 | ||
| $10 001–$15 000 | 1 | $10 001–$15 000 | 0 | ||
| $15 001–$25 000 | 4 | $15 001–$25 000 | 1 | ||
| $25 001–$35 000 | 3 | $25 001–$35 000 | 2 | ||
| $35 001–$50 000 | 5 | $35 001–$50 000 | 2 | ||
| $50 001–$75 000 | 13 | $50 001–$75 000 | 10 | ||
| $75 001–$100 000 | 16 | $75 001–$100 000 | 10 | ||
| $100 001–$150 000 | 40 | $100 001–$150 000 | 21 | ||
| $150 001 or greater | 58 | $150 001 or greater | 40 | ||
| Not reported | 6 | Not reported | 3 | ||
| T1 ARI score | Average | 2.3 |
T1-T2
Interval |
Average | 1.9 |
| SD | 2.3 | SD | 0.4 | ||
| Range | 1–10 | Range | 1.2–3.8 | ||
|
T2 ARI
score |
Average | 1.8 | |||
| SD | 2.3 | ||||
| Range | 0–9 | ||||
Notes. Sample size, male/female ratio, age at T1 (in years; average, standard deviation and range), Tanner stage at T1 (average, standard deviation and range), race/ethnic category and family income are listed for the cross-sectional and longitudinal samples. T1 ARI score (Affective Reactivity Index; average, standard deviation and range) is listed for the cross-sectional sample and interval between T1 and T2 (in years; average, standard deviation and range) is listed for the longitudinal sample.
Fig. 2.

Histograms showing distribution of ARI scores across sample. Shown are T1 scores across all 151 participants included in the cross-sectional analyses (dark blue, back), T1 scores among only those participants included in the longitudinal analyses (medium blue, middle) and T2 scores among the same longitudinal participants (light blue, front).
Procedure
To assess irritability, the participants’ parents completed the ARI (Stringaris et al., 2012), a seven-item questionnaire that assesses their child’s irritability during the preceding 6 months (six symptom items and one function impairment item). Responses were scored on a three-point scale, from 0 (not true) to 2 (certainly true), and the 6 items that comprise the total score were summed. To limit the impact of extreme values, we excluded participants with ARI scores more than three SD above the sample mean at either T1 or T2. This excluded five participants. Prior work has indicated that the ARI is a reliable and valid measure of irritability in youth (Stringaris et al., 2012), although it does suffer from floor effects (Stoddard et al., 2014). In this sample, the internal consistency of the ARI was high at both T1 (α = 0.86) and T2 (α = 0.89). Informant agreement for child psychopathology is known to be low (De Los Reyes and Kazdin, 2004); because no specific informant is considered the gold standard reporter for irritability, we used parent report rather than child report, given that more parents than children provided ARI data (N with parent ARI = 151; N with child ARI = 143). The correlation between parent and child report for those with both reports was r = 0.37 (P < 0.001).
Scan
MRI scans were acquired at the Center for Cognitive and Neurobiological Imaging at Stanford University using a 3 T Discovery MR750 (GE Medical Systems, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA). Whole-brain T1-weighted images (T1w) were collected using the following spoiled gradient echo pulse sequence: 186 sagittal slices; TR (repetition time)/TE (echo time)/TI (inversion time) = 6.24/2.34/450 ms; flip angle = 12°; voxel size = 0.9 mm × 0.9 mm × 0.9 mm; scan duration = 5:15 min.
Tensor-based morphometry
At both time points, each participant’s T1-weighted anatomical data were N3-corrected using c3d (http://www.itksnap.org) to correct for intensity inhomogeneities. Volumes were automatically skull-stripped using FreeSurfer and brain masks were manually edited to remove extraneous skull or meninges by trained neuroanatomical experts (LS and AC, see Acknowledgements). We used flirt (http://fsl.fmrib.ox.ac.uk) to linearly register each participant to a study-specific template. We used a study-specific registration template to obtain the strongest possible registration results (Hua et al., 2013). We chose a female aged 11 years 5 months (the average for the sample subset included) with a visually normal T1-weighted scan to initialize the linear registration. This exemplar participant was registered to the ICBM template using flirt, using 7 degrees of freedom registration with trilinear interpolation and using mutual information as the similarity function for alignment. Following this, each participant’s masked, N3-corrected T1-weighted image was registered to the participant-template using iterative 6-, 7- and 9-DOF (degrees of freedom) registration. We concatenated transformation files so that only one resampling step was run. This protocol was modified for this data set from the original protocol (Hua et al., 2008; Hua et al., 2009). Thirty participants, selected to be representative of the population, were used to make the minimal deformation template (MDT). This representative group was chosen semi-randomly by dividing the sample into tertiles based on exposure to early life stress and randomly selecting five females and five males from each tertile. The MDT is the template that deviates least from the anatomy of the participants with respect to a mathematically defined metric of difference; in some circumstances, using an MDT can improve statistical power (Lepore et al., 2007). The MDT serves as an unbiased registration target for nonlinear registrations.
For cross-sectional analyses, each participant’s masked, non-uniformity-corrected, template-aligned T1-weighted image was non-linearly aligned to the MDT, using Advanced Normalization Tools’ Symmetric Normalization (SyN; Avants, Epstein, Grossman, and Gee, 2008). SyN registration used a multi-level approach, i.e. the ‘moving’ and fixed T1-weighted images were successively less smoothed at each level, with a full resolution registration occurring at the final level. We used 150, 80, 50 and 10 iterations at each level, with a Gaussian kernel smoothing sigma set to 3, 2, 1 and 0, respectively (7.05, 4.7, 2.35 and 0 voxels full width at half maximum) and shrink factors of 4, 2, 2 and 1, respectively. Image similarity was measured using the ANTs implementation of mutual information (Avants et al., 2011). Image intensities were winsorized, excluding top and bottom 1% of voxels, and histogram matching was used. The output Jacobian determinant image showed the direction and magnitude of volume difference between the participant’s T1 and the MDT. For longitudinal analyses, each participant’s template-aligned T1 from the follow-up scan was non-linearly aligned to the template-aligned T1 from the first scan using the same parameters listed above. The output Jacobian determinant image showed the direction and magnitude of the change between the participant’s Time 1 and Time 2 anatomical images. Output was visually checked for quality.
Statistical analyses
In our voxel-wise linear regression testing for associations with irritability, we included intracranial volume (ICV) as a covariate. The 9-DOF linear registration that is part of our processing protocol accounts for differences in overall brain scale, removing much of the effect of ICV; nevertheless, we included ICV, computed from the linearly registered image, as a covariate. We also ran models without ICV, reported in Supplementary Tables S2–S4. To test associations with irritability, we conducted several models assessing concurrent and predictive associations between irritability and brain volume:
Model 1 (Cross-sectional association at T1):
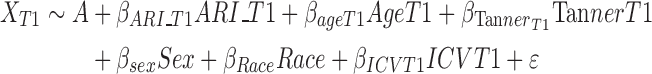 |
Model 2 (T1 brain structure predicting change in irritability):
 |
Model 3 (T1 irritability predicting change in brain structure):
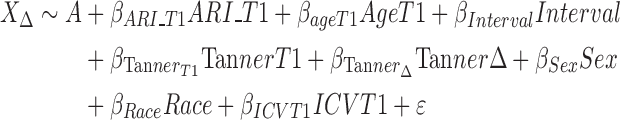 |
Model 4 (Longitudinal changes in irritability and brain structure):
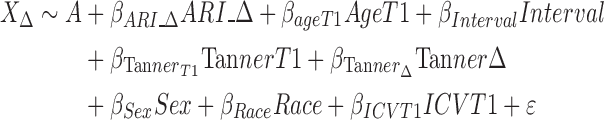 |
where X is the Jacobian determinant value at a given position, A is the constant Jacobian determinant term, the βs are the covariate regression coefficients and ε is an error term. When applicable, variables are noted as being measured at T1 or T2, or as a change from T1 to T2 (Δ). These models can be summarized as follows:
Model 1—the association between ARI and regional brain volume at T1;
Model 2—the association between regional brain volume at T1 and change in ARI score from T1 to T2;
Model 3—the association between T1 ARI and change in brain volume from T1 to T2;
Model 4—the association between change in ARI and change and brain volume from T1 to T2.
We used the ‘lm()’ function from the ‘stats’ package in R (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/lm.html, version 2.9.2) to fit each model using linear regression voxel-wise. For each model, results were corrected for multiple comparisons across all voxels tested using Searchlight FDR [false discovery rate, q < 0.05 (Langers et al., 2007)]. Searchlight FDR uses a sliding window approach to correct for multiple comparisons, yielding improved sensitivity over conventional FDR while maintaining the specificity of conventional FDR and FWE (family-wise error) approaches. We report only those clusters that exceeded 50 voxels. Covariates across the models included age, sex, Tanner stage, race/ethnicity, ICV, interval between T1 and T2 and change in Tanner stage between T1 and T2. We also ran models without pubertal stage, reported in Supplementary Tables S2–S4. For models 2 and 4, we additionally tested models including T1 ARI. A histogram of the change in ARI between T1 and T2 across our sample is presented in Supplementary Figure S1.
Results
Three of the four models tested yielded significant results. We present the results for each model below. Because space precludes a complete discussion of all of the clusters identified, we highlight specific clusters here and present all results in Tables 2–4.
Table 2.
Significant clusters from Model 1 (cross-sectional association at T1)
| Positive | |||||||
|---|---|---|---|---|---|---|---|
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 2993 | 4.7 | −7 | 40 | 13 | L | Cingulate/cingulum | GM/WM |
| 1193 | 3.67 | −46 | −19 | −15 | L | Middle temporal gyrus | WM |
| 904 | 3.81 | 42 | −37 | 19 | R | Angular gyrus | WM |
| 749 | 3.69 | −39 | 23 | 21 | L | Middle frontal gyrus | GM/WM |
| 636 | 3.22 | −48 | −40 | −6 | L | Middle temporal gyrus | WM |
| 564 | 3.51 | −4 | 31 | −24 | L | Medial orbitofrontal cortex | GM |
| 308 | 3.97 | 47 | 29 | 24 | R | Middle frontal gyrus | GM |
| 275 | 3.34 | 46 | −17 | −15 | R | Middle temporal gyrus | WM |
| 254 | 3.08 | 33 | 25 | 46 | R | Middle frontal gyrus | GM/WM |
| 224 | 3.46 | 65 | −55 | 10 | R | Fusiform gyrus | GM |
| 192 | 3.47 | 44 | −28 | −13 | R | Middle temporal gyrus | WM |
| 172 | 3.49 | 52 | 46 | −2 | R | Inferior frontal gyrus | GM |
| 155 | 3.65 | 34 | 8 | 5 | R | Insula | GM |
| 136 | 3.38 | 23 | −6 | −27 | R | Hippocampus | GM |
| 116 | 3.65 | −14 | −6 | 27 | L | Corpus callosum | WM |
| 104 | 3.65 | −59 | −27 | 22 | L | Supramarginal gyrus | GM |
| 85 | 3.62 | 34 | 50 | 33 | R | Superior frontal gyrus | GM |
| Negative | |||||||
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 3789 | −4.25 | −33 | 26 | 10 | L | Putamen/internal capsule | GM/WM |
| 499 | −3.96 | −14 | 19 | 35 | L | Superior frontal gyrus | WM |
| 492 | −4.36 | 42 | −88 | 7 | R | Lateral occipital gyrus | GM |
| 409 | −3.91 | 11 | 43 | 17 | R | Superior frontal gyrus | GM |
| 407 | −3.84 | 43 | 30 | −19 | R | Inferior frontal gyrus | GM |
| 404 | −3.55 | 18 | −69 | 0 | R | Lingual gyrus | GM/WM |
| 390 | −3.27 | 31 | −63 | 13 | R | Posterior thalamic radiation | WM |
| 319 | −3.18 | 20 | −66 | −33 | R | Cerebellum | WM |
| 316 | −3.59 | −26 | −66 | −10 | L | Lingual gyrus | GM |
| 302 | −4.71 | −64 | −7 | 23 | L | Postcentral gyrus | GM |
| 238 | −3.65 | −26 | 64 | 24 | L | Superior frontal gyrus | GM |
| 196 | −3.86 | −10 | −74 | −1 | L | Lingual gyrus | GM |
| 78 | −3.35 | 25 | 13 | −40 | R | Temporal pole | GM |
| 62 | −4.25 | −18 | 57 | 37 | L | Superior frontal gyrus | GM |
| 62 | −3.55 | −60 | −49 | 4 | L | Middle temporal gyrus | GM |
Notes. Clusters showing an association between volume and irritability at T1. Shown are the cluster size, peak regression T-statistic, coordinates (MNI), hemisphere, structure and tissue type.
Table 4.
Significant clusters from Model 3 (T1 irritability predicting change in brain structure)
| Positive | |||||||
|---|---|---|---|---|---|---|---|
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 494 | 3.8 | −17 | 14 | 5 | L | Anterior internal capsule | WM |
| 467 | 4.26 | −16 | −12 | 2 | L | Posterior internal capsule | WM |
| 224 | 4.27 | 19 | 14 | 54 | R | Superior frontal gyrus | WM |
| 104 | 3.35 | −21 | 22 | 22 | L | Anterior corona radiata | WM |
| 86 | 3.86 | 26 | 51 | 34 | R | Superior frontal gyrus | GM |
| 83 | 4.37 | 41 | −7 | 56 | R | Precentral gyrus | GM |
| 69 | 4.43 | −67 | −18 | −6 | L | Middle temporal gyrus | GM |
| 59 | 5.04 | −61 | −11 | 36 | L | Postcentral gyrus | GM |
| Negative | |||||||
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 512 | −4.16 | −33 | 2 | −1 | L | Insula | GM |
| 337 | −3.95 | 20 | −67 | −13 | R | Cerebellum | GM |
| 285 | −4.31 | 17 | 66 | 9 | R | Superior frontal gyrus | GM/WM |
| 252 | −4.16 | −9 | 10 | 13 | L | Caudate | GM |
| 198 | −4.44 | −41 | 23 | 32 | L | Middle frontal gyrus | GM/WM |
| 183 | −4.48 | −10 | 17 | 50 | L | Superior frontal gyrus | WM |
| 119 | −3.94 | −42 | 20 | 22 | L | Middle frontal gyrus | GM |
| 92 | −4.30 | −54 | −18 | −14 | L | Middle temporal gyrus | WM |
| 88 | −3.37 | −12 | 3 | 18 | L | Caudate | GM |
| 83 | −4.69 | −43 | 2 | 28 | L | Precentral gyrus | GM |
| 70 | −4.05 | 36 | −15 | 25 | R | Postcentral gyrus | WM |
| 62 | −4.19 | −44 | −18 | 32 | L | Postcentral gyrus | WM |
| 56 | −4.26 | −41 | 38 | 19 | L | Middle frontal gyrus | WM |
Notes. Clusters showing an association between irritability at T1 and change in volume. Shown are the cluster size, peak regression T-statistic, coordinates (MNI), hemisphere, structure and tissue type.
Model 1: cross-sectional associations between irritability and regional brain volume at T1 (baseline)
The 151 participants provided data for Model 1, which yielded both positive and negative concurrent associations between ARI and brain volume at T1. Positive associations were fairly evenly split between gray matter (GM) and white matter (WM) and between hemispheres. Higher levels of irritability were associated with greater volume in the left dorsal ACC, left medial orbitofrontal cortex, left corpus callosum midbody, right hippocampus and right insula. Negative associations were skewed towards GM over WM. Higher levels of irritability were associated with smaller volume in a large cluster including the putamen and internal capsule and a number of frontal and occipital GM clusters. Clusters are shown in Table 2 and Figure 3.
Fig. 3.

Significant results from cross-sectional imaging analyses (Model 1). Results from Model 1 are shown—those involving cross-sectional brain imaging with cross-sectional irritability scores. Red-yellow are those showing positive associations, blue-purple are clusters showing negative associations. IC = internal capsule, MTG = middle temporal gyrus, SFG = superior frontal gyrus, MFG = middle frontal gyrus, PTR = posterior thalamic radiation, OFC = orbitofrontal cortex. Images are in radiological convention, with the left hemisphere on the right side of the image.
Model 2: longitudinal associations between regional brain volume at T1 and change in irritability from T1 to T2
The 94 participants contributed data to Model 2. There were more positive than negative associations with left hemisphere clusters, and more negative than positive associations with right hemisphere clusters. There were also more negative associations in GM than in WM. There were more associations, both positive and negative, with clusters in the frontal cortex than in other brain lobes. Individuals with greater baseline volume in the right caudate and left corpus callosum midbody had greater increases in irritability over time. Greater baseline volume in the cingulate gyrus, superior frontal gyrus and inferior frontal gyrus was associated with decreases in irritability over time. When we controlled for baseline irritability, the caudate, corpus callosum and cingulate clusters were no longer significant, but many of the frontal cortex clusters remained. Clusters are shown in Table 3 and Figure 4.
Table 3.
Significant clusters from Model 2 (volume at T1 predicting changes in irritability)
| Positive | |||||||
|---|---|---|---|---|---|---|---|
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 683 | 4.41 | −24 | −23 | 58 | L | Precentral gyrus | GM |
| 430 | 3.33 | −55 | −35 | 7 | L | Superior temporal gyrus | GM |
| 416 | 3.23 | 16 | 22 | −1 | R | Caudate | GM |
| 347 | 4.15 | −15 | 39 | 38 | L | Superior frontal gyrus | GM |
| 207 | 3.84 | −10 | 15 | 19 | L | Corpus callosum | WM |
| 181 | 3.84 | −16 | −13 | 49 | L | Superior frontal gyrus | WM |
| 116 | 3.58 | −13 | −38 | 50 | L | Superior parietal lobule | GM/WM |
| 85 | 3.46 | −14 | 19 | 36 | L | Superior frontal gyrus | WM |
| 83 | 4.80 | −10 | −96 | 16 | L | Cuneus | GM |
| 69 | 3.29 | 31 | 27 | −10 | R | Inferior frontal gyrus | GM/WM |
| Negative | |||||||
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 674 | −3.90 | 40 | 8 | 17 | R | Insula/Inferior frontal gyrus | GM/WM |
| 445 | −4.25 | 47 | 24 | 19 | R | Inferior frontal gyrus | GM/WM |
| 414 | −3.72 | 41 | −30 | 13 | R | Transverse temporal gyrus | GM |
| 233 | −3.81 | −28 | −48 | 34 | L | Superior parietal lobule | GM |
| 221 | −4.35 | −6 | −91 | 30 | L | Cuneus | GM |
| 127 | −4.05 | 0 | −13 | 25 | R | Cingulate | GM |
| 121 | −3.75 | 39 | −55 | 35 | R | Angular gyrus | GM/WM |
| 108 | −3.70 | 55 | −4 | 34 | R | Precentral gyrus | GM |
| 105 | −3.86 | 6 | 66 | 5 | R | Superior frontal gyrus | GM |
| 80 | −3.66 | 36 | 54 | 22 | R | Superior frontal gyrus | GM |
| 75 | −4.15 | −4 | 66 | 25 | L | Superior frontal gyrus | GM |
| Including T1 ARI in model | |||||||
| Positive | |||||||
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 409 | 4.04 | −15 | 39 | 38 | L | Superior frontal gyrus | GM/WM |
| 231 | 3.39 | −48 | −35 | 0 | L | Superior temporal gyrus | GM/WM |
| 208 | 5.46 | −10 | −96 | 16 | L | Cuneus | GM |
| 127 | 3.91 | −16 | −14 | 48 | L | Superior frontal gyrus | WM |
| 105 | 4.12 | −13 | 44 | 23 | L | Superior frontal gyrus | WM |
| 51 | 3.62 | −48 | 1 | 16 | L | Precentral gyrus | WM |
| Negative | |||||||
| Voxels | T-stat | X | Y | Z | Side | Structure | Tissue |
| 214 | 4.91 | −6 | −91 | 30 | L | Cuneus | GM |
| 201 | 3.78 | 39 | 6 | 16 | R | Inferior frontal gyrus | WM |
| 98 | 3.98 | 8 | 64 | 6 | R | Superior frontal gyrus | GM |
| 90 | 5.05 | −3 | 66 | 25 | L | Superior frontal gyrus | GM |
| 79 | 3.91 | −38 | −88 | 15 | L | Lateral occipital gyrus | GM |
| 54 | 3.87 | 31 | 49 | 17 | R | Middle frontal gyrus | GM |
Notes. Clusters showing an association between baseline volume and changes in ARI. Shown are the cluster size, peak regression T-statistic, coordinates (MNI), hemisphere, structure and tissue type.
Fig. 4.

Significant results from cross-sectional imaging + longitudinal irritability analyses (Model 2). Results from Model 2 are shown—those involving cross-sectional brain imaging with longitudinal irritability scores. Red-yellow are those showing positive associations, blue-purple are clusters showing negative associations. SFG = superior frontal gyrus, STG = superior temporal gyrus. Images are in radiological convention, with the left hemisphere on the right side of the image.
Model 3: longitudinal associations between irritability at T1 and change in regional brain volume from T1 to T2
The 94 participants contributed data to Model 3. Both positive and negative associations were balanced between GM and WM; negative associations were skewed towards the left hemisphere. Participants who had higher levels of irritability at T1 had greater increases in volume in the left anterior corona radiata, left internal capsule and a number of frontal GM and WM clusters. Higher levels of irritability at T1 were also associated with less expansion, or more contraction, in the left caudate, left insula and a number of frontal GM and WM clusters. Clusters are shown in Table 4 and Figure 5.
Fig. 5.

Significant results from longitudinal imaging analysis (Model 3). Results from Model 3 are shown—those involving longitudinal brain imaging with cross-sectional irritability scores. Red-yellow are those showing positive associations, blue-purple are clusters showing negative associations. SFG = superior frontal gyrus, IC = internal capsule. Images are in radiological convention, with the left hemisphere on the right side of the image.
Model 4: longitudinal associations between change in irritability and change in regional brain volume from T1 to T2
No results survived multiple comparisons correction for Model 4.
Overlap among models
To identify brain regions that were associated consistently with irritability in the three significant models, we overlaid the positive and negative associations across models to create ‘heat maps’ that indicated which voxels were identified by more than one model. Although most of the clusters were significant in only one model, some clusters emerged in two or three of the models. There was overlap between positive associations in Model 1 and negative associations in Models 2 and 3 in the WM of the left MFG. This overlap indicates that higher baseline irritability is associated with greater baseline volume in the left MFG but with smaller increases in volume over time, and that greater baseline volume in the left MFG is associated with smaller increases in irritability over time. There was also overlap between positive associations in Model 3 and negative associations in Model 1 in a large cluster that included the left internal capsule, indicating that higher irritability at T1 is associated with smaller volumes in these regions initially, but with greater expansion in these areas over time. There was overlap between positive associations in Model 1 and negative associations in Model 2 in the WM of the angular gyrus and GM of the right MFG, indicating that higher baseline irritability is associated with greater baseline volume in the right MFG and right angular gyrus, but with smaller increases in volume over time. Finally, there was overlap between positive associations in Model 2 and negative associations in Model 1 in the superior corona radiata and lateral occipital gyrus, indicating that higher baseline irritability is associated with smaller baseline volume in these areas, but with greater volume expansion over time. These clusters are presented in Figure 6 and Supplementary Figure S2.
Fig. 6.

Overlap in results across models. Positive and negative associations found across all seven models are overlaid to show overlap. Several areas of overlap are noted. Blue clusters were reported in one model, purple clusters in two models and pink clusters in three models, as shown in the legend. MFG = middle frontal gyrus, SCR = superior corona radiata, IC = internal capsule, LOG = lateral occipital gyrus. Images are in radiological convention, with the left hemisphere on the right side of the image.
Discussion
This study examined cross-sectional and longitudinal associations between irritability and regional brain volume in a community sample from earlier to later adolescence. Few studies have examined neural correlates of irritability (including SMD and DMDD) in relation to variation in brain structure. Irritability was associated with altered brain volume in a number of striatal, limbic and limbic-associated structures. Most of the brain areas that were positively associated with irritability, either at baseline or longitudinally, typically decrease in volume over the developmental period studied here, suggesting that irritability is associated with delayed brain maturation.
We found larger baseline volumes in a number of fronto-striatal structures in individuals with higher levels of irritability. Cross-sectionally, higher levels of irritability were associated with greater volume of the hippocampus, dorsal ACC/cingulum, medial OFC and insula and with smaller volume in the putamen, internal capsule and a number of GM clusters across the brain. It is noteworthy that the hippocampus, ACC and mOFC are part of, or closely integrated with, the limbic system. The limbic system is central to emotion regulation, and traditionally is conceptualized as including the amygdala, hippocampus, hypothalamus, cingulate cortex, parahippocampal cortex, cingulum bundle and fornix, although this is not a universally accepted set of structures (Nieuwenhuys, 2007; Rajmohan and Mohandas, 2007). Some neuroanatomists include the OFC in the limbic system, as it has structural and functional connectivity with canonical limbic system structures (Rajmohan and Mohandas, 2007). Lesion studies in humans and non-human primates have reported a link between irritability and limbic system functioning (Eichelman, 1983). Prior structural studies of irritability have similarly reported altered volume in frontal regions (Adleman et al., 2012; Gold et al., 2016). In the present study, across both models of cross-sectional brain volume (Models 1 and 2), far more GM than WM clusters were negatively associated with irritability; thus, smaller GM volumes were associated both with higher baseline irritability and with greater increases in irritability over time. Positive associations with irritability were more balanced between GM and WM. Typically, GM volume decreases and WM volume increases throughout childhood and adolescence, although these trajectories vary across the brain (Gogtay et al., 2004). In our sample, the vast majority of the brain areas in which there was a positive association between volume and irritability at baseline decreased in volume over the study period (regional changes in brain volume over time across the whole group shown in Figure 7). Against this background, our finding of larger volumes in individuals with higher levels of irritability could indicate delayed maturation, as the age-expected decreases in volume are lagging (Mills et al., 2016; Tamnes et al., 2017). Clusters in which there was a negative association between baseline volume and irritability were more evenly divided between areas that are increasing and areas that are decreasing in volume over this period. Without additional earlier scans, we cannot determine conclusively whether these effects indicate delayed maturation or irritability-related differences that emerged earlier in childhood. Further, although we cannot determine the degree to which these structural alterations contribute to, rather than result from, higher levels of irritability, our results indicate that structural alterations commonly seen in individuals with clinically significant irritability (Adleman et al., 2012; Gold et al., 2016) are also evident when examining the transdiagnostic construct of irritability as a continuous variable.
Fig. 7.

Longitudinal changes in brain volume across the sample. The images representing change over time for each participant (the Jacobian determinant) were averaged across the group to show age-related changes in the sample as a whole to provide context for our results. The direction and magnitude of the changes are indicated by the color, shown in the color bar, with blue indicating areas of volume contraction and red areas of volume expansion.
Longitudinally, we examined areas in which subsequent neural expansion or contraction was associated with irritability at T1. Higher irritability at T1 was associated with greater volume expansion, or less contraction, in the internal capsule and anterior corona radiata, and with less expansion, or more contraction, in the caudate and a number of frontal clusters. Among clusters in which there was a positive association between baseline irritability and changes in brain volume, all were in areas that typically decrease in volume over this developmental period. This pattern of findings suggests delayed development, given that individuals with higher levels of irritability exhibited less volume contraction across these areas. Clusters showing a negative association between baseline irritability and volume changes were balanced between those increasing and decreasing in volume over time. The volume of the left caudate peaks around age 9 and then decreases (Dima et al., 2015), suggesting that individuals with higher levels of irritability are losing caudate volume more rapidly than are their less irritable peers. The caudate has been found to have both smaller volume and hypoactivation in depressed individuals (Krishnan et al., 1992; Pizzagalli et al., 2009). While these areas have not been implicated in previous studies of irritability and brain structure, studies of brain function in clinical populations have reported decreased activation in the striatum in response to frustration (Deveney et al., 2013; Perlman et al., 2015). We found that more irritable individuals had smaller baseline volume in the putamen and more volume loss in the caudate, suggesting that striatal disruption is present even when examining irritability in a non-clinical sample.
The longitudinal nature of this study yields insights concerning the association between irritability and structural brain development; further follow-up is necessary, however, to determine whether more irritable individuals are simply lagging behind their less irritable counterparts in brain development and will ultimately catch up, or alternatively, will never reach their age-expected targets. Examining the overlap in significant clusters across the models examined in this study provides insight about the temporal nature of the association between regional brain volume and irritability. We found that individuals with higher baseline irritability had larger baseline volume in the bilateral MFG and right angular gyrus, and smaller baseline volume in the internal capsule, superior corona radiata and lateral occipital gyrus. Individuals with higher baseline irritability were more likely to show less volume contraction in the left internal capsule and more volume contraction in the left MFG. Individuals with larger baseline volume in the lateral occipital gyrus and superior corona radiata were more likely to have increased irritability over time, while those with larger baseline volume in the right angular gyrus and bilateral MFG were more likely to have decreased irritability over time. We should note three limitations of this study. First, we measured irritability through parent report; in future studies, data from other informants, such as teachers, will be important in corroborating parents’ reports of their children’s irritability. Second, investigators have found a floor effect for the ARI in community samples (Stoddard et al., 2014); in our sample, too, although we had a reasonable range of scores on the ARI, the majority of participants clustered around a similar value near the floor of the ARI. Although we excluded participants with extreme ARI scores (>3 SD), it is nevertheless possible that the significant associations reported here are driven in part by a smaller subset of participants with higher scores on this measure. In future research, the ARI should be augmented by other measures of irritability.
Finally, as presented in Table 1, we studied a volunteer community sample of adolescents; not surprisingly, the mean irritability score in this sample was lower than has been reported in clinical samples (Stoddard et al., 2017; Kircanski et al., 2018; Tseng et al., 2018).
Despite these limitations, the present findings are important in identifying brain structural correlates of irritability in a sample of young adolescents; these structural differences may be precursors to future internalizing and externalizing disorders. Identifying these risk factors is critical to understanding the emergence of dysfunction and is an important first step in developing more effective interventions. Investigators should continue to conduct longitudinal research to determine whether these alterations predict the development of psychopathology and other adverse outcomes.
Funding
Funding for this study and support for authors was provided by the National Institutes of Health (R37 MH101495, U54 EB020403, R01 AG040060, R01 NS080655, K99 NS096116 and F32 MH107129); the Brain and Behavior Research Foundation (Young Investigator Award 23819); the National Science Foundation; Klingenstein Third Generation Foundation and the Jacobs Foundation Early Career Research Fellowship (2017 1261 05).
Conflict of interest
The authors report no conflicts of interest.
Supplementary Material
Acknowledgements
The authors thank Lucinda Sisk and Anna Cichocki for their assistance in data collection and processing.
References
- Adleman N.E., Fromm S.J., Razdan V., et al. (2012). Cross-sectional and longitudinal abnormalities in brain structure in children with severe mood dysregulation or bipolar disorder. Journal of Child Psychology and Psychiatry, 53(11), 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis, 12(1), 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein F.L. (2001). ‘Voxel-based morphometry’ should not be used with imperfectly registered images. NeuroImage, 14(6), 1454–62. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A., Kazdin A.E. (2004). Measuring informant discrepancies in clinical child research. Psychological Assessment, 16(3), 330. [DOI] [PubMed] [Google Scholar]
- Deveney C.M., Connolly M.E., Haring C.T., et al. (2013). Neural mechanisms of frustration in chronically irritable children. The American Journal of Psychiatry, 170(10), 1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D., Papachristou E., Turner J., et al. (2015). Subcortical brain volumes across the lifespan based on 10, 722 people aged aged 2 to 92. Organization for Human Brian Mapping Annual Meeting (OHBM). Honolulu, Hawaii, USA: Citeseer. [Google Scholar]
- Dougherty L.R., Smith V.C., Bufferd S.J., Kessel E., Carlson G.A., Klein D.N. (2015). Preschool irritability predicts child psychopathology, functional impairment, and service use at age nine. Journal of Child Psychology and Psychiatry, 56(9), 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelman B. (1983). The limbic system and aggression in humans. Neuroscience and Biobehavioral Reviews, 7(3), 391–4. [DOI] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62(2), 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A.L., Brotman M.A., Adleman N.E., et al. (2016). Comparing brain morphometry across multiple childhood psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 55(12), 1027, e1023–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Leow A.D., Parikshak N., et al. (2008). Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage, 43(3), 458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Leow A.D., Levitt J.G., Caplan R., Thompson P.M., Toga A.W. (2009). Detecting brain growth patterns in normal children using tensor-based morphometry. Human Brain Mapping, 30, 209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Hibar D.P., Ching C.R., et al. (2013). Unbiased tensor-based morphometry: improved robustness and sample size estimates for Alzheimer’s disease clinical trials. NeuroImage, 66, 648–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K.L., Kircanski K., Colich N.L., Gotlib I.H. (2016). Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. Journal of Child Psychology and Psychiatry, 57(10), 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K.L., Schoubue N.F., Kirchanski K., Leibenluft E., Stringaris A., Gotlib I.H. (2018). The association between irritability and domains of psychopathology in early adolescence is moderated by sex. J Clinical Child & Adolescent Psychology, 1–9. [DOI] [PMC free article] [PubMed]
- Insel T., Cuthbert B., Garvey M., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–51. [DOI] [PubMed] [Google Scholar]
- Kircanski K., White L.K., Tseng W.L., et al. (2018). A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry, 75(6), 631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K.R., McDonald W.M., Escalona P.R., et al. (1992). Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Archives of General Psychiatry, 49(7), 553–7. [DOI] [PubMed] [Google Scholar]
- Langers D.R., Jansen J.F., Backes W.H. (2007). Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. NeuroImage, 38(1), 43–56. [DOI] [PubMed] [Google Scholar]
- Lee F.S., Heimer H., Giedd J.N., et al. (2014). Adolescent mental health—opportunity and obligation. Science, 346(6209), 547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. (2011). Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. The American Journal of Psychiatry, 168(2), 129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E., Cohen P., Gorrindo T., Brook J.S., Pine D.S. (2006). Chronic versus episodic irritability in youth: a community-based, longitudinal study of clinical and diagnostic associations. Journal of Child and Adolescent Psychopharmacology, 16(4), 456–66. [DOI] [PubMed] [Google Scholar]
- Lepore N., Brun C., Pennec X., et al. (2007). Mean template for tensor-based morphometry using deformation tensors. Medical Image Computing and Computer-assisted Intervention, 10(Pt 2), 826–33. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Herting M.M., et al. (2016). Structural brain development between childhood and adulthood: convergence across four longitudinal samples. NeuroImage, 141, 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R., Voogd J., Van Huijzen C. (2007). The human central nervous system: a synopsis and atlas, Springer Science & Business Media. [Google Scholar]
- Pagliaccio D., Pine D.S., Barch D.M., Luby J.L., Leibenluft E. (2018). Irritability trajectories, cortical thickness, and clinical outcomes in a sample enriched for preschool depression. Journal of the American Academy of Child and Adolescent Psychiatry, 57(5), 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Jones B.M., Wakschlag L.S., Axelson D., Birmaher B., Phillips M.L. (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience, 14, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A., Aglan A., Collishaw S., Messer J., Rutter M., Maughan B. (2010). Predictors of suicidality across the life span: the Isle of Wight study. Psychological Medicine, 40(9), 1453–66. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166(6), 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajmohan V., Mohandas E. (2007). The limbic system. Indian Journal of Psychiatry, 49(2), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio J., Ruscio A.M. (2000). Informing the continuity controversy: a taxometric analysis of depression. Journal of Abnormal Psychology, 109(3), 473–87. [PubMed] [Google Scholar]
- Stoddard J., Stringaris A., Brotman M.A., Montville D., Pine D.S., Leibenluft E. (2014). Irritability in child and adolescent anxiety disorders. Depression and Anxiety, 31(7), 566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J., Tseng W.L., Kim P., et al. (2017). Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry, 74(1), 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A., Cohen P., Pine D.S., Leibenluft E. (2009). Adult outcomes of youth irritability: a 20-year prospective community-based study. The American Journal of Psychiatry, 166(9), 1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A., Goodman R., Ferdinando S., et al. (2012). The affective reactivity index: a concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry, 53(11), 1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A., Vidal-Ribas P., Brotman M.A., Leibenluft E. (2018). Practitioner review: definition, recognition, and treatment challenges of irritability in young people. Journal of Child Psychology and Psychiatry, 59(7), 721–39. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.-L., et al. (2017). Development of the cerebral cortex across adolescence: a multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. Journal of Neuroscience, 37(12), 3302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Giedd J.N., Woods R.P., MacDonald D., Evans A., Toga A. (2000). Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature Letters, 404, 1–4. [DOI] [PubMed] [Google Scholar]
- Tseng W.L., Deveney C.M., Stoddard J., et al. (2018). Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. The American Journal of Psychiatry, 176(1), 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J., Cook P.A., Klein A., et al. (2014). Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage, 99, 166–79. [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P., Brotman M.A., Valdivieso I., Leibenluft E., Stringaris A. (2016). The status of irritability in psychiatry: a conceptual and quantitative review. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Brotman M.A., Adleman N.E., et al. (2016). Neural correlates of irritability in disruptive mood dysregulation and bipolar disorders. The American Journal of Psychiatry, 173(7), 722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


