Abstract
Cutaneous wound healing is the process by which skin repairs itself. It is generally accepted that cutaneous wound healing can be divided into 4 phases: haemostasis, inflammation, proliferation, and remodelling. In humans, keratinocytes re-form a functional epidermis (reepithelialization) as rapidly as possible, closing the wound and reestablishing tissue homeostasis. Dermal fibroblasts migrate into the wound bed and proliferate, creating “granulation tissue” rich in extracellular matrix proteins and supporting the growth of new blood vessels. Ultimately, this is remodelled over an extended period, returning the injured tissue to a state similar to that before injury. Dysregulation in any phase of the wound healing cascade delays healing and may result in various skin pathologies, including nonhealing, or chronic ulceration. Indigenous and traditional medicines make extensive use of natural products and derivatives of natural products and provide more than half of all medicines consumed today throughout the world. Recognising the important role traditional medicine continues to play, we have undertaken an extensive survey of literature reporting the use of medical plants and plant-based products for cutaneous wounds. We describe the active ingredients, bioactivities, clinical uses, formulations, methods of preparation, and clinical value of 36 medical plant species. Several species stand out, including Centella asiatica, Curcuma longa, and Paeonia suffruticosa, which are popular wound healing products used by several cultures and ethnic groups. The popularity and evidence of continued use clearly indicates that there are still lessons to be learned from traditional practices. Hidden in the myriad of natural products and derivatives from natural products are undescribed reagents, unexplored combinations, and adjunct compounds that could have a place in the contemporary therapeutic inventory.
1. Introduction
Our skin is the key to our survival, sensing the environment, maintaining physicochemical and thermal homeostasis, acting as a reservoir of essential nutrients, providing passive and active defence, and responding to trauma and injury [1]. Maintaining these critical functions requires robust and effective mechanisms to protect it from trauma and insult and to repair and replace critical skin functions when damaged or lost. Humans have been treating their wounds for millennia [2]. Traditional wound management is limited by what is immediately at hand or can be acquired locally, such as water, soil, and plant and animal products, and is frequently complemented with ceremony and ritual as an added measure. For millions of people across Asia, Africa, the Middle East, and Latin America, traditional medicines derived from local plants, animals, and natural products are the mainstay of wound care; for some, it is the only source of wound care [3]. We discuss herein some of the evidence supporting the use of medicinal plants as effective and affordable treatments for cutaneous wounds.
2. Cutaneous Wound Healing
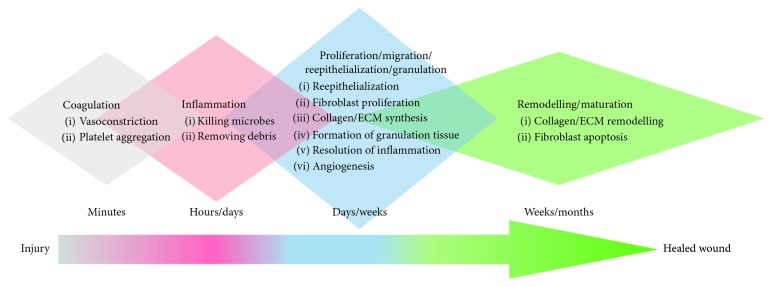
Maintaining homeostasis is critical for the survival of the organism; hence, skin needs and possesses a robust and effective repair mechanism. Cutaneous wound healing is the process by which skin repairs itself following injury caused by surgery, trauma, and burns [4]. The healing process is classically divided into 4 phases (Figure 1): coagulation (a.k.a. haemostasis), inflammation and proliferation (a.k.a. granulation), and remodelling (a.k.a. maturation) [5]. Upon injury, a fibrin clot rapidly forms to restore haemostasis [6, 7]. Platelets present in the blood trigger the clotting cascade and secrete several growth factors, initiating wound healing [8]. In the following inflammation phase, neutrophils migrate into the wound site engulfing foreign debris and killing bacteria by phagocytosis and releasing proteolytic enzymes [8, 9]. Coincidently, blood monocytes infiltrate the injury site and differentiate into macrophages, releasing proteases to debride the wound [8], and secrete a mixture of bioactive molecules, including transforming growth factor-beta 1 (TGF-β1), that stimulates the migration of fibroblasts and epithelial cells [10]. The proliferation phase usually starts about 3 days after wounding; it involves diverse activities including angiogenesis (by endothelial cells), granulation tissue formation (by fibroblasts), and reepithelialization (by keratinocytes) [11, 12]. In this stage, fibroblasts produce a large amount of extracellular matrix (ECM), mainly collagen, to form the granulation tissue which replaces the damaged tissue. Meanwhile, the keratinocytes migrate, proliferate, differentiate, and re-form a functional epidermis (reepithelialization), closing the lesion and protecting underlying tissues from further trauma [13]. As the wound matures, the characteristic disorganized ECM of granulation tissue is actively remodelled by the dermal fibroblast cell population [14], whose numbers are progressively reduced through apoptosis [15]. The outcome of wound healing is scar tissue (aka fibrosis) with sparsely distributed fibroblasts within a collagen-rich ECM. Compared to the original tissue, scar tissue, having distinct texture and reduced biomechanical and functional properties, is characteristically altered [16].
Figure 1.
Wound healing cascade—humans. The wound healing process is an orderly sequence of overlapping, interacting processes commonly categorised into four distinct phases: coagulation, inflammation, proliferation/migration/reepithelialization/granulation, and remodelling/maturation. (1) Coagulation: a clot is formed, providing a temporary barrier to fluid loss and pathogen entry, restores haemostasis; acts as a reservoir of bioactive factors and antimicrobials; provides provisional ECM which supports immune cell infiltration and migration; and initiates tissue repair pathways. (2) Inflammation: damage-associated molecular patterns, free radicals, and reactive molecular species are signals to recruit immune cells; increased blood vessel leakiness; release of antimicrobial species; infiltrating immune cells secretes amplifying alarmin (also known as DAMPs) signals; and activation of keratinocytes and fibroblasts. (3) Proliferation/migration/reepithelialization/granulation: migration and proliferation of keratinocytes, fibroblasts, endothelia; resolution of inflammation; collagen/ECM synthesis; decreased vessel permeability; new capillary and lymphatic vessel angiogenesis; reepithelialization; and de novo formation of granulation tissue. (4) Remodelling/maturation: collagen/ECM turnover (synthesis and degradation); ECM reorganisation and realignment; ECM contraction; endothelia and fibroblast apoptosis; repigmentation.
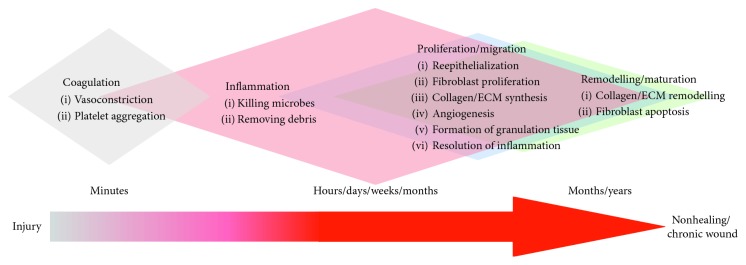
Healing of acute wounds follows an orderly sequence of overlapping, interacting physiological processes (Figure 1). This sequence can take over a few days in juveniles or over a few weeks in adults to occur. Most wounds heal without complication and reestablish homeostasis, skin barrier function, pliability, and physiological functions in less than 4 weeks. Clinical evidence indicates that shorter periods to wound closure are associated with reduced fibrosis and scarring. In contrast, full-thickness wounds and wounds that are slow to heal are associated with increased fibrosis, developing in some individuals into hypertrophic scars and keloids. Deep, full-thickness, and partial-thickness wounds that do not heal within 6 weeks appear to “stall” and fail to progress through the phases of healing described in Figure 1 (Figure 2). These hard-to-heal wounds are considered to be “chronic” wounds [17]. Hard-to-heal wounds become “chronic” for a number of reasons, including underlying conditions such as diabetes, vascular disease, hyperglycaemia, ischemia, and neuropathy. The underlying cause of the wound is often used to describe the wound: diabetic foot ulcers, venous leg ulcers, arterial leg ulcers, and pressure ulcers.
Figure 2.
Nonhealing/chronic wounds—humans. The orderly sequence of overlapping, interacting wound healing processes fails to progress in chronic wounds, frequently due to failure to resolve inflammation. (1) Coagulation: usually unaffected. (2) Inflammation: damage-associated molecular patterns, free radicals, and reactive molecular species; high pH; functional activation of proteases, senescence of keratinocytes, and fibroblasts (vessel permeability sustained-aetiology specific). (3) Proliferation/migration: initiation of de novo granulation tissue formation; failure to sustain proliferation; failure to initiate angiogenesis; failure of keratinocytes to migrate and reepithelialise (failure of wound closure); failure to resolve inflammation; and failure to accumulate ECM. (4) Remodelling/maturation: fails to initiate reorganisation and maturation of ECM.
Nonhealing, chronic wounds clearly pose a risk to the health and well-being of the individual; patients often suffer from pain, impaired mobility, excessive exudates, wound malodour, and restricted social life [18], resulting in substantial disruption, morbidity, and indirect costs to social and healthcare systems [19]. As many as 1-2% of individuals in all populations worldwide will acquire a chronic wound during their life-time [20]. In the USA, chronic wounds are reported to affect 6.5 million people and cost over US $25 billion each year [21]. Alarmingly, the burden of chronic wounds is expected to intensify due to global increases in vascular diseases, diabetes, obesity, metabolic syndrome, and the general aging of the population [21]. Although the mechanisms of wound healing are relatively well known, the pathogenesis of chronic wounds remain poorly defined [22]. It is generally accepted that chronic wounds result from some dysregulation of the normal wound healing process. For example, microbial biofilms, overexpression of inflammatory cytokines, high levels of proteases and reactive oxygen species (ROS), and reduced mitogenic activity stall wound healing in the inflammation phase, inhibiting progression to the proliferation and reepithelialization phases. In addition, overactive matrix metalloproteinases (MMPs) have been shown to contribute to delayed healing [23]. The result is a wound that remains open, does not heal, and becomes chronic [24].
Selecting an appropriate clinical strategy to manage cutaneous wounds is dictated by the aetiology underlying each wound. Consideration is given to (1) removing nonvital (necrotic) tissue, termed debridement; (2) inflammation or infection; (3) controlling moisture (too wet or too dry); and (4) state of the tissue surrounding the wound [25]. This approach has its roots in Greek and Roman medicine [26], where removing these “barriers to healing” was prescribed to allow the healing cascade to progress to completion. Debridement is considered to benefit wounds, restarting the healing process by returning it to an acute presentation. Debridement exposes healthy and well-perfused tissue, facilitating cell proliferation and migration [27]. In addition to removing dead and necrotic tissue, debridement effectively reduces, if not removes, proinflammatory factors, damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs). Debridement also removes “extracellular traps” and microorganisms from the wound. Microorganisms in wounds have long been considered deleterious [28]; however, recent evidence suggests that not all microbes impede healing. Microbial pathogens such as Staphylococcus sp., Streptococcus sp., Propionibacterium sp., and Pseudomonas sp. are commonly equated with infection, while others including Malassezia sp., Candida sp., and Corynebacterium sp. can be isolated from noninfected wounds and may even contribute to wound healing [29]. The optimal frequency and when to perform debridement, however, remains unclear [27]. Dressings provide a physical barrier to reinfection from commensal and adventitious microorganisms, can deliver antimicrobial agents (e.g., honey and iodine), and, in some designs, absorb wound exudates, providing a measure of moisture control.
3. Traditional Medical Practices
Traditional medicine is often described by practitioners of “modern” (western) medicine using sceptical terms such as “alternative,” “nonconventional,” “indigenous,” and “complementary,” when in fact many of the techniques and practices of “modern” medicine are little different from traditional practices when it comes to wounds. Traditional approaches depend almost entirely upon natural resources, such as water, plants, animals, and minerals, and continue to be valued and widely practiced by a majority of the world's population [3]. The practice of traditional Chinese medicine (TCM) is based on the Five-Phases theory and Yin-Yang theory, recorded in the ancient Chinese medical documents such as “Shen Nong Ben Cao Jing” and “Ben Cao Gang Mu.” Many, but not all, TCM makes extensive use of plants, ensuring it is effective, affordable, and accessible [30]. Interestingly, of the new anticancer drugs developed between 1940 and 2002, approximately 54% were derived from natural products [31]. Another study has determined that of all current pharmaceutical products, about 73%, include ingredients derived from natural products [32]. The therapeutic activity of many traditional medicines are conferred by natural ingredients produced within the plant; consequently, the efficiency of TCM preparations can vary widely and are determined by the genotype, environmental, and growing conditions encountered by each source plant [30, 33]. Urbanization and industrialization of pharmaceutical engineering have increased demand for “off the shelf” TCM products with consistent composition, quality, and clinical efficacy. Concomitantly, industrialization has also introduced rigorous product testing for evidence of biological activity and clinical efficacy.
4. Traditional Use of Medical Plants in Wound Healing
4.1. Aloe vera
Applied to wounds for over 5000 years by Egyptians, Romans, indigenous peoples of Africa Asia, and the Americas, Aloe vera continues to be a first-line treatment for burns, ulcers, and surgical wounds [34]. Aloe vera contains many natural bioactive compounds, including pyrocatechol, saponins, acemannan, anthraquinones, glycosides, oleic acid, phytol, as well as simple and complex water-soluble polysaccharides [35]. Acetone extracts from the leaves of Aloe vera exhibit stronger antimicrobial activity than alcohol and aqueous extracts. Gram-positive bacterial species appear to be more sensitive than Gram-negative species to Aloe vera [36]. Compounds with known antimicrobial activity are saponins, acemannan, and anthraquinone derivatives [37].
Acemannan, a major mucopolysaccharide (mesoglycan) from Aloe vera, is a potent stimulator of macrophage and T-cell activity and induces the transcription of proinflammatory mRNAs (including IL-1α, IL-1β, IL-6, TNF-α, PGE2, and nitrous oxide) [38]. Mesoglycan moieties bind and capture endogenous mitogen inhibitors and reactive oxygen species and promote phagocytosis. Coincidently, glycans stabilize secreted cytokines, growth factors, and other bioactives, prolonging their activity. Topically applied acemannan has been reported to significantly reduce the time to wound closure in a rat wound healing model, acting via cyclin D1 and AKT/mTOR signal pathways [39]. Aloe vera glycans are also reported to significantly improve de novo formation of granulation tissue by an unknown mechanism [40].
4.2. Arctium lappa
Arctium lappa, commonly known as burdock, is a widely cultivated perennial herb [41]. Arctium lappa is used in North America, Europe, and Asia to treat sore throat and skin pathologies such as boils, rashes, and acne [42, 43]. Scientific analyses demonstrate Arctium lappa has antioxidant [44], anti-inflammatory [45], antidiabetic [46], antimicrobial [47], antiviral [48], anticancer [49], and hepatoprotective [50] properties. The root extract of Arctium lappa has been shown to significantly improve dermal ECM metabolism, affecting glycosaminoglycan turnover and reducing visible wrinkles in human skin in vivo [51]. Arctium lappa is also reported to regulate cell adhesion and gene expression in canine dermal fibroblasts, affecting the Wnt/β-catenin signalling pathway, known to be a key regulator of wound healing [52]. In a pilot study of one commercial preparation including Arctium lappa, Burns and Wounds™ topical ointment (B&W), pain and healing of first- and second-degree burns in humans was demonstrated to be managed more effectively than the control treatment [53].
4.3. Astragalus propinquus and Rehmannia glutinosa
The root of Astragalus propinquus is a common TCM for the treatment of urinary retention and oedema [54]. The root of Rehmannia glutinosa has been broadly used in hemorheology and diabetes-related diseases [55]. A formulation combining the root of Astragalus propinquus and Rehmannia glutinosa was initially reported to be clinically effective for the treatment of diabetic foot ulcers [56]. This outcome has subsequently been corroborated in diabetic rats [57]. Tam et al. reported that the root of Astragalus propinquus and Rehmannia glutinosa promote diabetic wound healing and postischemic neovascularization by improving angiogenesis and attenuating tissue oxidative stress in diabetic rats [58]. Zhang et al. demonstrated that the root of Astragalus propinquus and Rehmannia glutinosa activate the TGF-β1 signalling pathway and stimulate increased deposition of ECM in human skin fibroblasts [59].
4.4. Ampelopsis japonica
Growing throughout eastern Asia and eastern North America, the roots of Ampelopsis japonica are used as a traditional treatment for burns and ulcers, amongst other indications [60]. Multiple pharmacological activities have been documented for Ampelopsis japonica, including neuroprotective [61], antimicrobial, and anticancer [62] activities. Lee et al. demonstrated that ethanol extracts from dried roots of Ampelopsis japonica accelerated the healing of cutaneous scald injury in rats [63]. Tumour necrosis factor-alpha (TNF-α) and TGF-β1 were observed to be elevated 2 days after injury and declined as healing progressed. In contrast, interleukin-10 (IL-10) was found to be elevated after 14 days, coincident with wound closure [63]. When compared with wounds treated with Vaseline® (petroleum jelly) or silver sulfadiazine, topical treatment with ethanolic Ampelopsis japonica improved reepithelization, granulation tissue formation, vascularization, and collagen deposition [63].
4.5. Andrographis paniculata
Andrographis paniculata, also known as green chiretta, is used in China, India, and south east Asian countries as a traditional treatment for fever, snake bite, dysentery, infections, wounds, and itchiness [64–67]. Extracts from Andrographis paniculata exhibit antioxidant [68], anti-inflammatory [69], antidiabetic [70], anticancer [66], antimicrobial [71], antiviral [72], antimalarial [73], hypotensive [74], immunostimulatory [66], and hepatoprotective [75] activities. In one study, wound closure in rats was observed to be significantly enhanced after treatment with a 10% aqueous leaf extract of Andrographis paniculata [76]. Animals treated with Andrographis paniculata exhibited reduced inflammation, reduced scarring, increased angiogenesis, and an increased number of collagen fibres in healed wounds [76]. Andrographolide, a bicyclic diterpenoid isolated from the leaves of Andrographis paniculata, has been formally evaluated in clinical trials and shown to have positive effects on several autoimmune disorders [77].
4.6. Angelica sinensis
The dried root of Angelica sinensis is widely used in TCM prescriptions for the management of female maladies, inflammation, headaches, mild anemia, fatigue, and hypertension [78]. Angelica sinensis possesses pharmacological activities including anti-inflammatory [79], anticancer [80], antioxidant effects [81], and immune modulator [82]. Extracts from Angelica sinensis have been shown to activate an antiapoptotic pathway and enhance cell proliferation, collagen secretion, and cell mobility in human skin fibroblasts [83]. Extracts have also been shown to stimulate glycolysis and calcium fluxes, increasing cell viability during tissue repair [83]. The role of Angelica sinensis in angiogenesis remains unclear, with several studies reporting contradictory effects of Angelica sinensis on de novo blood vessel growth. An aqueous extract of Angelica sinensis was reported to promote blood vessel growth via activation of JNK1/2 and p38 phosphorylation, resulting in enhanced VEGF expression [84, 85]. In contrast, n-butylidenephthalide, a bioactive isolated from Angelica sinensis, inhibits cell cycle progression, induces apoptosis, and attenuates angiogenesis [86].
4.7. Blumea balsamifera
Endemic throughout the tropics and subtropics of Asia, Blumea balsamifera (also known as ngai camphor) is used widely as a traditional medicine. In the Philippines, Blumea balsamifera is known as sambong and is used as a diuretic. In Ayurveda, Blumea balsamifera is known as kakoranda and is used to treat fevers, coughs, aches, and rheumatism. Leaf extracts are directly applied to treat eczema, dermatitis, skin injury, bruises, beriberi, lumbago, menorrhagia, rheumatism, and skin injury [87]. Extracts from Blumea balsamifera demonstrate a variety of bioactivities; including antimalarial [88], antitumour [89], antifungal [90], and antiobesity [91] properties. Pang et al. reported that oils from Blumea balsamifera improve wound healing in mice by promoting angiogenesis, perfusion, collagen deposition, formation of organised granulation tissue, reepithelialization, and wound closure [92].
4.8. Boswellia sacra
Frankincense, a resinous extract from Boswellia sacra, is valued in Africa, India, and the Middle East for the treatment of trauma and inflammatory diseases such as rheumatoid arthritis [93, 94]. It has also been reported that the boswellic acid acetate extracted from frankincense induces apoptosis and differentiation in melanoma and fibrosarcoma cells [95]. It is a key component of ANBP, a TCM consisting of pulverised Agrimonia eupatoria (A), Nelumbo nucifera (N), Boswellia sacra (B), and pollen from Typha angustifoliae (P). ANBP stimulates Smad-dependent pathways in the TGF-β1 signalling cascade [96]. Using a rabbit ear model of hypertrophic scarring, Hou et al. demonstrated that ANBP moderates inflammation and accelerates the growth of organized granulation tissue and reepithelialization, events that reduce scar formation [96]. Intriguingly, ANBP was also noted to attenuate collagen biosynthesis and accelerate the maturation of the collagen extracellular matrix, contributing to reduced scarring and improved skin tissue repair. Recently, Hou et al. further demonstrated that ANBP reduced the time of wound closure in diabetic mice via direct effects on neovascularization [97].
4.9. Caesalpinia sappan
The heartwood of Caesalpinia sappan is well known for its qualities as a dye and has been used in TCM to improve blood circulation and reduce oedema and pain [98]. Homoisoflavonoids isolated from Caesalpinia sappan have been found to possess antiallergic [99] and anti-inflammatory [100] attributes and to inhibit viral neuraminidase activity [101]. Ethanol extracts of Caesalpinia sappan exhibit effective antibacterial activity against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella pneumoniae [102]. Unexpectedly, the ethanol root extract from Caesalpinia sappan also stimulates dermal fibroblast proliferation, migration, and collagen synthesis [103], in turn improving cutaneous wound healing.
4.10. Calendula officinalis
Calendula officinalis, commonly known as pot marigold, is a very widely distributed plant used for the treatment of a variety of skin conditions, such as wounds, burns, and dermatitis [104, 105]. A range of pharmacological activities are ascribed to Calendula officinalis, including anti-inflammatory, antioxidant, antibacterial, antiviral, antifungal, and anticancer activities [106]. However, the exact mechanisms involved in its activities on the wound healing remain unknown. Studies using cultures of human and murine fibroblasts demonstrated that extracts of Calendula officinalis stimulate fibroblast migration and proliferation in a PI3K-dependent manner [107, 108]. Extracts from the flower of Calendula officinalis stimulate granulation tissue formation by altering the expression of connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA) in excisional wounds of BALB/c mice in vivo [109]. Calendula officinalis is also reported to enhance angiogenesis in vivo, demonstrated using the chicken chorioallantoic membrane (CAM) assay and a cutaneous wound healing model in rats [110].
4.11. Camellia sinensis
Green tea, an aqueous extract made from the leaves of Camellia sinensis, is revered throughout Asia for its reputed health benefits [111]. Centuries of anecdotal evidence has been experimentally validated by demonstrating that Camellia sinensis has antioxidant [112], anti-inflammatory [113], antimicrobial [114], anticarcinogenic [115], antiaging [116], antiobesity [117, 118], cardioprotective [119], and neuroprotective [120] activities. Catechins, the polyphenolic compounds from Camellia sinensis, are primarily responsible for these pharmacological activities [121]. The major catechin, (-)-epigallocatechin-3-gallate (EGCG) [111], stimulates the proliferation and differentiation of keratinocytes [122]. Klass et al. found that EGCG suppresses TGF-β receptors by modifying TGF-β signalling, reducing MMP-1 and MMP-2 expression, and attenuating synthesis of collagen type 1 in human dermal fibroblasts. These properties suggest that EGCG is a potential antiscarring agent [123]. In addition, EGCG was demonstrated to induce keloid shrinkage [124] and inhibit growth and pathological features of keloids by suppressing STAT3 signalling [125]. Methanol extracts from Camellia sinensis reportedly increase fibroblast proliferation and collagen synthesis [115]. Furthermore, in vivo studies have demonstrated that Camellia sinensis significantly improves wound healing by increasing angiogenesis in rats [121, 126]. Extracts from Camellia sinensis are also reported to improve wound healing in a diabetic mouse model [127].
4.12. Carthamus tinctorius
Seeds from Carthamus tinctorius, or safflower, are a popular source for cooking oil in many countries. Less widely known, Carthamus tinctorius also has a long history as an ingredient in TCM formulations for the treatment of blood disorders. Recent experimentation has identified it is associated with a wide range of biological activities, including vasodilation, immune modulation, anticoagulation and thromboprophylaxis, antioxidation, antihypoxic, antiaging, antifatigue, anti-inflammation, antihepatic fibrosis, anticancer, and analgesia [128]. Interestingly, safflower seed oil has also been shown to inhibit melanogenesis in B16 melanoma cells, making it a promising candidate for skin whitening [129]. Hydroxysafflor yellow A (HSYA), the major water-soluble monomer of safflower yellow pigments, has been shown to protect against cerebral and myocardial ischemia [130], conferring antioxidant [131], anti-inflammatory [132], proangiogenic [133], and apoptosis-inhibiting [134] properties. Topical application of HSYA at low dose (4 mg/mL) improves diabetic wound healing, promoting neovascularization, reepithelialization, and granulation tissue formation in streptozotocin-induced diabetic rats [130]. In contrast, at high doses (≥10 mg/mL), wound healing is inhibited [135, 136].
4.13. Celosia argentea
Celosia argentea, also known as silver cock's comb, is used in traditional medicine to treat skin sores, eruptions, ulcers, mouth ulcers, and other skin diseases [137]. Leaf extracts of this plant possess antioxidant [138], hepatoprotective [139], antidiabetic [140], and antimicrobial [141] activities. Priya et al. demonstrated that an alcohol extract of Celosia argentea accelerates burn wound closure in rats by increasing collagen and hexosamine content in granulation tissue wounds. In addition, the extract increased the proliferation and motility of primary rat dermal fibroblasts [137].
4.14. Centella asiatica
Centella asiatica, also known as Asiatic pennywort, has been used to promote wound healing for eons [142]. Extracts from the aerial parts of Centella asiatica are reported to improve the healing of chronic ulcers in Sprague-Dawley rats in terms of width, depth, and length [142]. Wounds associated with acute radiation dermatitis in rats were observed to heal earlier when treated with extracts from Centella asiatica compared to the no-treatment control group [143]. Asiaticoside isolated from Centella asiatica has been found to enhance collagen deposition and epithelialization in a punch wound model in the guinea pig [144]. Triterpenes isolated from Centella asiatica elevate collagen remodelling and glycosaminoglycan synthesis in a rat wound model [145]. Furthermore, oral administration of madecassoside from Centella asiatica was shown to facilitate collagen synthesis and angiogenesis in a mouse wound model [146].
4.15. Cinnamomum cassia
Cinnamomum cassia is a commonly used spice and flavouring agent, and the bark of Cinnamomum cassia is also used to increase blood circulation and as an analgesic [147]. Cinnamomum cassia is frequently formulated with other herbs; it is one of the seven botanical components of Shexiang Baoxin pill (SBP), a well-known TCM prescribed for chest pain and discomfort associated with coronary artery disease [148]. SBP is currently the subject of a randomized double-blinded clinical trial for the treatment of coronary artery disease not amenable to revascularization [149]. Attention is also focussed on SBP anti-inflammatory [150] and anticancer activities [151, 152], as well as its impact on hypertension, insulin resistance, and noninsulin-dependent diabetes mellitus [153]. In vitro and in vivo studies indicate that cinnamaldehyde, a bioactive component from Cinnamomum cassia, is a natural insecticide, is an antimicrobial, antidiabetic, antilipidemic, anti-inflammatory, and neuroprotective agent [154], and activates PI3K/AKT and MAPK signalling pathways, increasing VEGF expression, and stimulating angiogenesis in human umbilical vein endothelial cells [147]. Cinnamaldehyde is also reported to improve wound healing in zebrafish [147].
4.16. Commiphora myrrha
Myrrh, the resinous exudate produced by Commiphora myrrha [155], has well-documented antioxidant [156], anti-inflammatory [157], antibacterial [158], and analgesic [159] activities. Medicinal applications of myrrh include the treatment of gastrointestinal diseases, fractures, arthritis, obesity, parasitic infections, and as an anticoagulant [160–162]. Myrrh has been used topically to clean wounds, reduce oedema, and provide pain relief (analgesia) [163]. Myrrh is commonly used in combination with other ingredients. Galehdari et al. showed that the combination of myrrh, Adiantum capillus-veneris, Aloe vera, and Lawsonia inermis, significantly improved wound healing in diabetic mice [164]. The short-term application of myrrh effectively reduces pain and controls the recurrence of mouth ulcers in humans [165]. In common with several other herbal preparations described here, myrrh is found to modify the expression of TGF-β1 and VEGF in mouse dermal fibroblasts in vitro, suggesting a common mechanism of action [166].
4.17. Curcuma longa
Curcumin, an active substance found in the root of Curcuma longa and a member of the ginger family, has long been used as a medicine and as food seasoning [167]. Practitioners of traditional Ayurveda medicine use curcumin to treat inflammation, respiratory disorders, liver disorders, and diabetes [168]. In traditional Chinese medicine, curcumin is a favoured treatment for abdominal pain. Having widespread use for centuries by diverse ethnic groups, curcumin is one of the most extensively studied nutraceuticals. This highly pleiotropic molecule has been demonstrated to interact with key cellular pathways at transcription, translation, and posttranslational levels. Target pathways include proinflammatory cytokines, apoptosis, NF–κB, cyclooxygenase-2, 5-LOX, STAT3, C-reactive protein, prostaglandin E2, prostate-specific antigen, cell adhesion molecules, phosphorylase kinase, transforming growth factor-β, triglycerides, ET-1, creatinine, heme oxygenase-1, AST, and ALT [169]. The subject of more than 100 clinical trials, in vivo studies, have largely focused on curcumin as a treatment for epithelial cancers. Experimental findings from these in vivo studies and in vitro experiments indicate curcumin elicits most of its beneficial effects via altering the pericellular and extracellular matrix [168]. Perhaps, it is therefore not unexpected that curcumin enhances fibroblast proliferation, granulation tissue formation, and collagen deposition in cutaneous wound healing [170].
4.18. Daphne genkwa
Daphne genkwa, one of the 50 fundamental herbs used in TCM, grows in the Yellow and Yangtze Rivers regions in China. Daphne genkwa is used as an anticonvulsant, analgesic, diuretic, antitussive, expectorant, and mild sedative agent [171–174]. The principal bioactives isolated from Daphne genkwa are biflavonoids, coumarin, diterpenes, and triterpenes. These confer anti-inflammatory [175], antitumour [176, 177], immunoregulatory [178, 179], and antimelanogenesis [172] activities. Flavonoids extracted from the flowers of Daphne genkwa stimulate the ERK/MEK pathway regulating fibroblast proliferation and the expression of collagen (COL1A1 and COL3A1), resulting in improved wound healing [171].
4.19. Entada phaseoloides
Entada phaseoloides, also known as St. Thomas bean, is a liana in the pea family of climbing vines common throughout lowland tropical forests and coastal forests of Africa, Australia, Asia, and Western Pacific. The bark and seeds of Entada phaseoloides are rich in saponins and tannins and are used as analgesic, bacteriocide, haemostatic, and anticancer agents and as a topical treatment for skin lesions [180, 181]. Su et al. reported that extracts enriched with tannins from Entada phaseoloides reduced the time taken to heal infected wounds in rats. Analyses of the data concluded that the improved wound healing was due to the antibacterial, proproliferative, and promigration activity of the Entada phaseoloides extracts [182]. These data are yet to be validated in human patients.
4.20. Hibiscus rosa-sinensis
Hibiscus rosa-sinensis, or shoeblackplant, is an evergreen shrub native to tropical South Eastern Asia [183]. The flowers of Hibiscus rosa-sinensis are edible. Traditional texts describe preparations of the leaves and flowers promote hair growth and prevent greying [184]. Alcoholic extracts of Hibiscus rosa-sinensis flowers are claimed to provide women with control of their fertility [185]. Extracts from Hibiscus rosa-sinensis have also been found to have antibacterial [186] and wound healing properties [187]. They attenuate inflammation, enhance fibroblast proliferation, and collagen deposition, as well as upregulate VEGF and TGF-β1 expression in rat excisional wounds [188].
4.21. Ganoderma lucidum
Ganoderma lucidum, the lingzhi mushroom, is well known to the Chinese, Korean, and Japanese as “the mushroom of immortality” [189, 190]. Used in TCM to boost the patient's immune system [191], Ganoderma lucidum stimulates a variety of pharmacobiological responses including immune modulation, inflammation modulation, anti-infective [192–194], antioxidant [195], cardioprotection [196], and antihyperlipidemia [197] activities. Clinical studies suggest that taking Ganoderma lucidum daily is beneficial and is reported to reduce the number of tumours in patients with colorectal adenomas; circulating viral particles in patients infected with hepatitis B; and symptoms of hypertension [189, 198–202]. Laboratory-based studies reveal that components from Ganoderma lucidum interact with and modulate key enzymes with known roles in lipid metabolism. However, clinical findings remain equivocal and suggest that Ganoderma lucidum is most effective when used as an adjunct with other therapies [203]. Polysaccharide extracts from the fruiting body of Ganoderma lucidum have been shown to improve wound healing in diabetic rats, potentially by stimulating fibroblast proliferation and migration [190], angiogenesis, and quenching oxidative stress [204]. Nevertheless, these responses may also represent indirect responses to Ganoderma lucidum via its established stimulation of humoral immunity.
4.22. Ligusticum striatum
The rhizome of Ligusticum striatum is another one of the 50 fundamental herbs used in TCM. It has a long history of use support cardiovascular and cerebrovascular well-being. It is commonly indicated for the treatment and prevention of ischemic disorders, menstrual disorders, and headache [205–207]. Thus far, about 174 chemical components have been isolated from Ligusticum striatum, among which phthalide lactones and alkaloids are the most numerous, pharmacologically active species [207]. It has been reported that essential oils from Ligusticum striatum inhibit dermal scarring in the rabbit ear scar model [208].
4.23. Lonicera japonica
Lonicera japonica, also known as honeysuckle, has a notable place in traditional medicine throughout its native range of Japan, Korea, and China, where it has been used for thousands of years to treat infectious diseases [209]. In the 1980s, the Chinese State Ministry of Health performed extensive pharmacological and clinical analyses of Lonicera japonica and identified broad-spectrum antimicrobial, anti-inflammatory, antipyretic, antioxidant, anticancer, hepatoprotective, and antihyperlipidemic capabilities [210, 211]. More recently, Chen et al. demonstrated that the ethanol extracts of the flowering aerial parts of Lonicera japonica also support reepithelization, angiogenesis, granulation tissue formation, and contraction during cutaneous wound healing [212]. The plant may be consumed as a “health food,” providing some protection from gastric ulceration although, at high doses, it can cause some neurological pathologies [210].
4.24. Paeonia suffruticosa
Paeonia suffruticosa, also known as moutan peony, has been bred for millennia [213]; over 1000 distinct cultivars are now available. The root bark of Paeonia suffruticosa is the source for bioactive ingredients used for TCM preparations. Pharmacological investigation of Paeonia suffruticosa has demonstrated it has antioxidant [214], neuroprotective [215], antitumour [216], anti-inflammatory [217], and antidiabetic [218] properties. The dried root of Paeonia suffruticosa is commonly applied to cracked skin to assist healing and relieve pain [219]. When tested in vitro at low concentrations (≤10 μg/mL), Paeonia suffruticosa is found to stimulate the viability and proliferation of human primary dermal fibroblasts and HaCaT keratinocytes, suggesting its potential use as a wound healing therapy [220].
4.25. Panax ginseng
Panax ginseng is one of the most popular medicinal plants consumed in China, Japan, Korea, and Eastern Siberia to improve thinking, concentration, and memory. It is also claimed to support immunity and physical stamina and to reduce fatigue [221]. Panax ginseng is thus used to treat depression, anxiety, and chronic fatigue syndrome [222]. Panax ginseng has been demonstrated to induce vasodilation [223], control blood lipids [224], reduce inflammation [225], and confer antioxidant [226], anticancer [227], antibacterial [228], antiallergic [229], antiaging [230], and immunomodulating [231] activities. Panax ginseng contains many bioactive substances, among which a class of saponins (termed ginsenosides by Asian researchers and panaxosides by Russian researchers) represent the most potent active constituents of Panax ginseng [232].
The root extracts of Panax ginseng have been shown to protect skin in C57BL mice from acute UVB irradiation [233] and significantly improve healing after laser burn injury and excisional wounding [221, 234, 235]. Studies demonstrate Panax ginseng extracts enhance keratinocyte migration [221, 236], as well as stimulate proliferation [237] and increase collagen synthesis in human dermal fibroblasts [238] in vitro. In addition, Choi demonstrated that the ginsenoside Rb2, isolated from Panax ginseng, induces the formation of the epidermis in raft culture via increased expression of epidermal growth factor and its receptor, fibronectin and its receptor, and keratin 5/14 and collagenase I [239], all of which have critical roles in wound healing.
4.26. Panax notoginseng
Panax notoginseng, not to be confused with Panax ginseng and other ginsengs, is used to stop bleeding, reduce oedema, reduce bruising, and reduce pain [240, 241]. Terpene saponins isolated from the leaves of Panax notoginseng possess substantial pharmacological activities, including antioxidative effects [242], anti-inflammatory effects [243], immunostimulation [244], neuroprotective effects [245], anticancer [246], and antidiabetic activities [247]. Terpene saponins stimulate VEGF expression and angiogenesis, key factors in wound healing [240, 241]. Mechanism of action studies has found Panax notoginseng flower extracts block NF-κB signalling [248, 249], thus affecting the expression of inflammatory cytokines, including IL-6, known to contribute to keloid pathogenesis [250, 251].
Interestingly, saponins isolated from Panax notoginseng exhibit antihaemostatic (antiplatelet and anticoagulant) activity when assayed in vitro and in vivo in a rat model [252]. It was proposed that when administered orally, key bioactive constituents responsible for the haemostatic activity could be modified, which does not occur when administered topically. Of particular note, it is now evident that ginsenosides exhibit significant stereospecific differences in pharmacokinetic properties, including absorption, distribution, and metabolism [253]. These findings may account for some of the confusing and contradictory experimental observations. For example, 20(R)-ginsenoside Rh2 inhibits osteoclastgenesis without cytotoxicity. In contrast, 20(S)-ginsenoside Rh2 is strongly cytotoxic for osteoclasts [254]. Such observation highlights the crucial importance of reagent preparation and the need for rigorous quality control.
4.27. Polygonum cuspidatum
The root of Polygonum cuspidatum is usually formulated with several other ingredients and is most commonly prescribed for treating coughs, hepatitis, jaundice, amenorrhea, leucorrhea, arthralgia, burns, and snake bite [255]. A diversity of compounds have been isolated from Polygonum cuspidatum, dominated by resveratrol, polydatin, and anthraquinones and are presumed to be responsible for Polygonum cuspidatum's anti-inflammatory, estrogenic, antitumour, antiaging, neuroprotective, and cardioprotective activities [256–258]. In one recent in vivo study examining wound healing in rats, extracts of Polygonum cuspidatum were found to increase TGF-β1 expression and to significantly improve wound healing in terms of reepithelization, granulation tissue formation, collagen synthesis, and angiogenesis [259]. Novel anthraquinones isolated from Polygonum cuspidatum have been verified to inhibit tyrosinase, the rate-limiting enzyme controlling the synthesis of melanin that gives colour to skin [260].
4.28. Lithospermum erythrorhizon
The dried root of Lithospermum erythrorhizon is indigenous to northeast China and has potent biological activities, including anti-inflammatory, antibacterial, antiangiogenic, and antitumour qualities [261]. Shikonin, a naphthoquinone, is extracted from the root of Lithospermum erythrorhizon and stimulates the activity of caspases, poly-(ADP-ribosyl) polymerase (PARP) and reactive oxygen species (ROS), triggering programmed cell death in cancer cell lines [262]. These characteristics prompted investigation of shikonin as a novel scar remediation therapy. These studies found that shikonin inhibits cell proliferation and collagen production in hypertrophic scar-derived human skin fibroblasts [263]. Arnebin-1, a related naphthoquinone extracted from Lithospermum erythrorhizon, has been reported to synergise with VEGF, resulting in significantly improved wound healing in a rat diabetic model [264].
4.29. Rheum officinale
Rheum officinale, also known as Chinese rhubarb, is one of the best known traditional herbal medicines with pharmacological activities. Extracts from the roots of Rheum officinale have strong antibacterial [265], antioxidative [266], anti-inflammatory [267], and haemostatic [268] effects, validating its widespread use for constipation, chronic liver and kidney diseases [265, 269], and skin lesions [270]. Using a rat excisional wound model, Tang et al. found healing was stimulated via TGF-β1-related pathways [270]. The nature of the active component responsible for this activity is not clear. Emodin [1,3,8-trihydroxy-6-methyl-anthraquinone], an anthraquinone derived from the roots of Rheum officinale, has been shown to act as a ligand for PPAR-γ and interact with HSP90 and androgen receptors, in part explaining its therapeutic benefit for chronic diseases [271]. Experimental evidence also indicates a direct association of emodin with NF-κB, AP-1, and STAT3, known regulators of proinflammatory cytokine and mitogenic kinase pathways [272, 273].
4.30. Rhodiola imbricata
Rhodiola imbricata, a perennial herb native to high altitudes (4000–5000 m) of the western Himalayas, is known to contain bioactive flavonoids, coumarins, and phenyl glycosides. These compounds are commonly found in botanical herbal medicines. Ethanolic extracts of rhizomes from Rhodiola imbricata stimulate a robust wound healing response when applied to excisional wounds in rats [274]. Others have reported related functions that may contribute to tissue repair, namely, immunomodulation [275], antioxidation [276], hepatoprotection [277], radioprotection [278], and anticancer [279] properties.
4.31. Salvia miltiorrhiza
The root of the perennial plant Salvia miltiorrhiza (also known as red sage) is highly valued in TCM and used to treat cerebrovascular and cardiovascular diseases, such as stroke, coronary heart disease, and hyperlipidemia [280–283]. To date, Salvia miltiorrhiza has been demonstrated to reduce ischemia and necrosis and to improve the survival of skin flaps after mastectomy [284, 285]. Salvianolic acids isolated from Salvia miltiorrhiza have potent antioxidative capabilities due to their polyphenolic structure [286]. Although hepatoprotective [287], neuroprotective [288], antimicrobial [289], anti-inflammatory [290], and anticancer [291] activities have been reported, the greatest clinical benefit of salvianolic acids appears to be cardiovascular protection, via the promotion of cardiac angiogenesis and inhibition of ischemia and hypoxia during myocardial injury [292]. Water-soluble extracts from Salvia miltiorrhiza, containing danshensu (DSU) and salvianolic acid B (SAB), have been shown to enhance the proliferation of fibroblasts and increase collagen synthesis [293]. Salvianolic acid B is also a potent antagonist of epithelial-to-mesenchymal transition, necessary for wound closure [294]. In contrast, cryptotanshinone, a lipid-soluble terpenoid isolated from Salvia miltiorrhiza, has been demonstrated to downregulate the expression of COL1A1, COL3A1, and α-SMA in hypertrophic scar-derived fibroblasts (HSF), as well as reduce HSF migration and HSF contraction, thus ameliorating fibrosis and scarring [295].
4.32. Sanguisorba officinalis
Sanguisorba officinalis, a member of the family Rosaceae and commonly known as great burnet, is widely distributed in the cooler northern districts of Asia, Europe, and North America [296]. Roots of this plant are a potent haemostatic [297], with antioxidant [298], immunomodulatory [298], anti-inflammatory [299], and antiallergy [300] properties. The traditional use of Sanguisorba officinalis is to control bleeding disorders. It is also applied to heal scalds, burns, allergic skin diseases, urticaria, eczema, and allergic dermatitis [299]. Aqueous extracts made from the root of Sanguisorba officinalis suppress mast cell degranulation, as well as inhibit activation of STAT-1, Jak-2, p38, and JNK pathways and release of inflammatory cytokines [301]. In mouse studies, the oral administration of polysaccharides isolated from Sanguisorba officinalis is claimed to stimulate wound contraction, reduce the time required for reepithelization (wound closure), increase collagen synthesis, and improve angiogenesis [296]. Administration of the polysaccharide extract also resulted in elevated IL-1β and VEGF in mice [296].
4.33. Sophora flavescens
Sophora flavescens is a species from a genus of over 50 plants distributed throughout Asia, Oceania, and the islands of the Pacific. The root of Sophora flavescens is used for conditions involving the heart, liver, intestinal tract, and skin. Experimental investigations indicate extracts from Sophora flavescens stimulate anticancer, antibacterial, antiviral, anti-inflammatory, and antipruritic responses and benefits wound healing [302]. One recent report claims it is a potent inhibitor of tyrosinase, the enzyme responsible for synthesizing melanin, thus has potential cosmetic applications as a skin whitener [303]. Other reports claim specific compounds present in Sophora flavescens benefit individuals with androgenetic alopecia [304]. Recently, Xu et al. demonstrated that a mixture of Sophora flavescens and other herbs significantly reduced perianal ulceration in a rat model, finding that the expression of prostaglandin E2 and IL-8 was concomitantly reduced in treated animals [302].
4.34. Stemona tuberosa
Stemona tuberosa is another of the 50 fundamental herbs used in TCM. It has strong insecticidal activity, the foundation property for its traditional use in treating impetigo, scabies, louse, lice, and ticks. It is also used as a mosquito repellent and preservative to protect stored cereals from insects [305]. In traditional medicine, it is used to treat coughs and lung infections. Alkaloid and stilbenoid isolated from the root of Stemona tuberosa are reported to have anti-inflammatory [306] and antibacterial [307] effects, while the dehydrotocopherol derivatives have been found to scavenge oxygen and free radicals [308]. Tocopherols isolated from the root of Stemona tuberosa increase cell proliferation in the mouse fibroblast NIH3T3 cells, suggesting the potential use of these compounds as wound healing agents [309].
4.35. Wedelia trilobata
The plant Wedelia trilobata, which is also known as Sphagneticola trilobata, was originally native to the tropical Americas; however, as one of the world's most invasive species, it is now ubiquitous throughout the tropics. Alcohol extracts made from the leaves of Wedelia trilobata have been used to treat rheumatism, stubborn wounds, and arthritic painful joints [310]. Luteolin, a flavonoid present in the leaves, has been demonstrated to contribute to the medicinal value of Wedelia trilobata, conferring neuroprotective, anticancer, antioxidant, and immunomodulatory activities [311]. Traditional healers use the leaves of Wedelia trilobata to treat skin wounds. Luteolin inhibits the expression of NF-κB-regulated proinflammatory cytokines, a characteristic feature of skin infection and psoriasis [312]. In a study designed to validate this traditional use, Balekar et al. fractionated ethanolic extracts from the leaves of Wedelia trilobata and assayed them in vitro [310]. Specific subfractions were found to support fibroblast viability, proliferation, and migration. Different subfractions were also found to be active against Staphylococcus aureus and Staphylococcus epidermidis [310].
4.36. Zanthoxylum bungeanum
Zanthoxylum bungeanum is a flowering plant belonging to the Rutaceae family, native to eastern provinces of China. It yields important food ingredients such as sichuan pepper [313]. Over 140 compounds have been isolated from Zanthoxylum bungeanum, including alkaloids, terpenoids, flavonoids, and free fatty acids, eliciting a wide variety of biological responses, including analgesic [314], anticancer [315], antioxidant [316], anti-inflammatory [317], antibacterial, antifungal, and antiasthma properties [318]. Zanthoxylum bungeanum are known in traditional Western folk medicine as “toothache trees,” useful for treating pruritus (itch) and chronic pain. The pericarp from the fruit berry is commonly used to formulate TCM oils, powders, tinctures, elixirs, and pills [319]. Extracts from Zanthoxylum bungeanum are also prescribed for skin infections, including acne, eczema, scalds, and wound healing [320]. One unique property of fruit husk extracts from Zanthoxylum bungeanum is as a lifting agent for skin wrinkles. When applied topically to skin, subcutaneous muscles are relaxed, reducing skin wrinkles, thus has attracted the attention of cosmetic manufacturers [321]. Another interesting property reportedly associated with essential oils of Zanthoxylum bungeanum is the capacity to enhance percutaneous drug delivery [322].
5. Conclusion
We have surveyed and presented an overview of evidence that explains why many medicinal plants are used as traditional treatments for cutaneous wounds and clinical skin disorders. Medicinal plants have been the first line of treatment for trauma, infection, disease, and injury from prehistory. Over millennia, humans have learned to identify and transform the botanical resources from the immediate environment, and with the development of trade, as food and medicine. A great many of these “ancient” and traditional medical plants have been validated to confer therapeutic benefits, albeit not always in controlled clinical trials. One unexpected outcome from validation studies is just how many medical plants synthesize equivalent or closely related compounds. Consequently, it is not surprising that many biological properties are also shared by unrelated species. Also shared are many of the same biological targets and pathways; many of these are also key events in the mammalian wound healing cascade. Many of the identified compounds target mitogenic pathways (e.g., AKT, PI3K, SMAD, and cyclins), the proinflammatory NF-κB pathway (e.g., caspases, interleukins, TNF-α, and TGF-β1), angiogenesis pathway (e.g., VEGF), extracellular matrix synthesis (e.g., MMPs), and differentiation pathways (e.g., α-SMA).
The active ingredients, part of use, type of extract, assessment methods, bioactivities, clinical use, formulation, and commercial product of the medicinal plants are summarized in Table 1. While experimental evidence has been acquired for each documented plant from in vitro or in vivo analyses, not every mechanism of action has been verified. On the contrary, several compounds, including acemannan (from Aloe vera), hydroxysafflor yellow A (from Carthamus tinctorius), polysaccharide (from Ganoderma lucidum), phthalide lactones, and alkaloids (from Ligusticum striatum), saponins (from Panax ginseng), shikonin and arnebin-1 (from Lithospermum erythrorhizon), salvianolic acids (from Salvia miltiorrhiza), polysaccharides (from Sanguisorba officinalis), and alkaloid and stilbenoid (from Stemona tuberosa) are well characterised and have been demonstrated to have properties that benefit wound healing. In particular, Centella asiatica, Curcuma longa, and Paeonia suffruticosa are popular medicinal products in several global markets.
Table 1.
Summary of the medicinal plants used in wound healing.
| Botanical name | Traditional name | Plant family | Active ingredients | Part used | Type of extract | Assessment methods | Bioactivities | Clinical use | Formulation | Commercial product |
|---|---|---|---|---|---|---|---|---|---|---|
| Aloe vera | Lu Hui | Asphodelaceae | Acemannan [40] | Leaves | Ethanol | Punch biopsy wounds in Sprague-Dawley rats [40] | Immunomodulatory [40] Antiviral [40] |
Wound healing [34] | Gel [323] | Aloe vera gel |
| Arctium lappa | Niu Bang Zi | Asteraceae | Arctigenin [324] Arctiin [42] Caffeic acid [42] Chlorogenic acid [42] Diarctigenin [42] Inulin [42] Lappaol F [49] Tannin [42] Trachelogenin 4 [42] |
Leaves Whole root |
No extraction, whole leaves Aqueous |
Human burn wounds [53] Canine dermal fibroblast adhesion assay [52] |
Anticancer [49] Antidiabetic [46] Anti-inflammatory [45] Antimicrobial [47] Antiviral [48] Hepatoprotective [50] Radical scavenging [44] |
Acne vulgaris [43] Boils [42] Burns [53] Rashes [42] Sore throat [42] Wrinkles [51] |
Ointment [53] | Not available |
|
Astragalus propinquus
Rehmannia glutinosa |
Huang Qi Di Huang |
Fabaceae Orobanchaceae |
Astragaloside IV [57] Calycosin [57] Catalpol [57] |
Roots | Aqueous | In vitro scratch wound healing and quantitative cell migration assays [59] | Anti-inflammatory [57] Proangiogenic [57] |
Diabetic foot ulcer [56] | Herbal drink [56] | Not available |
| Ampelopsis japonica | Bai Lian | Vitaceae | Catechins [325] Epicatechin gallate [325] Resveratrol [325] |
Root tuber | Ethanol | Cutaneous scald injury in rats [63] | Anticancer [62] Neuroprotective [61] |
Antipyretic detoxicate [60] Burns [60] Ulcers [60] |
Wound plaster (Patent: CN105748741A) | Hydrating Moisturizer |
| Andrographis paniculata | Chuan Xin Lian | Acanthaceae | Andrographolide [64] Kalmeghin [64] |
Leaves | Aqueous | Excision model in albinos Wistar rats [76] | Anticancer [66] Antidiabetic [70] Anti-inflammatory [69] Antimalarial [73] Antimicrobial [71] Antioxidant [68] Antiviral [72] Hepatoprotective [74] Hypotensive [75] Immunostimulatory [66] |
Dysentery [65] Fever [64] Snake bites [66] Sores [67] |
Paste (applied externally), juice (internally) [326] | Chuan Xin Lian Nei Zhi Di Wan (穿心莲内酯滴丸) |
| Angelica sinensis | Dang Gui | Apiaceae | Ferulic acid [83] n-Butylidenephthalide [86] | Whole plant | Ethanol | Cell line antioxidant activity assay [83] | Anticancer [80] Anti-inflammatory [79] Antioxidant [81] Immunomodulatory [82] |
Amenorrhea [78] Dysmenorrhea [78] Menstrual disorders [78] |
Ointment [327] Nanosilver hydrocolloid dressing [328] |
Dang Gui Shao Yao San (当归芍药散) |
| Blumea balsamifera | Ai Na Xiang | Asteraceae | L-Borneol [92] | Leaves | Violate oil | Excision wound model in mice [92] | Antifungal [90] Antiobesity [91] Antiplasmodial [88] Antitumour [89] |
Beriberi [87] Dermatitis [87] Eczema [87] Skin bruises [87] Skin injury [87] |
Oil | Blumea leaf oil |
| Boswellia sacra | Ru Xiang | Burseraceae | Boswellic acids [329] | Resin | Dry extract Dry extract |
Rabbit ear hypertrophic scar model of full-thickness scar defect [96] Excision wound model in diabetic C57BL/6 mice [97] |
Anticancer [329] Antifibrotic [97] |
Improvement of blood circulation [95] Pain treatment [94] Rheumatoid arthritis [93] |
Spray [93] | Frankincense oil |
| Caesalpinia sappan | Su Mu | Fabaceae | Brazilin [103] Sappanchalcone [103] |
Roots Roots |
Ethanolic Ethanol |
In vitro antibacterial assay [102] In vitro anti-inflammatory and wound healing assays [103] |
Antiallergic [99] Antibacterial [102] Anti-inflammatory [100] Viral neuraminidase inhibitory [101] |
Improvement of blood circulation [98] Pain treatment [98] Oedema [98] |
Tablets | Lukol™, Vicco Vajradanti™ |
| Calendula officinalis | Jin Zhan Ju | Asteraceae | Esculetin [108] Quercetin-3-O-glucoside [108] |
Flower Flower Flower |
Hexane and ethanol Hydroethanol Hexane and ethanol |
Scratch assay [107] Excision wound model in BALB/c mice [109] Punch wound model in rats [110] |
Antibacterial [106] Anticancer [106] Antifungal [106] Anti-inflammatory [106] Antioxidant [106] Antiviral [106] |
Burns [105] Dermatitis [105] Wounds [104] |
Topical spray [330] Oil [331] |
Calendula Herbal-Extract Toner Plenusdermax® |
| Camellia sinensis | Cha Shu | Theaceae | Epcatechin-3-gallate [111] Epicatechin [111] Epigallocatechin [111] Epigallocatechin-3-gallate [111] |
Leaves Leaves Leaves |
Methanol Methanol Ethanol |
In vitro and in vivo keloid fibroblasts models [125] NIH3T3 fibroblast proliferation assay [115] Excision wound model in Sprague-Dawley rats [121] |
Antiaging [116] Anticarcinogenic [115] Anti-inflammatory [113] Antimicrobial [114] Antiobesity [117, 118] Antioxidant [112] Cardioprotective [119] Neuroprotective [120] |
Angina pectoris [332] Asthma [332] Bacterial infections [332] Cancer [332] |
Oil | Tea tree oil |
| Carthamus tinctorius | Hong Hua | Asteraceae | Hydroxysafflor yellow A [130] | Seeds | Reflux | Antioxidant enzyme assay in zebrafish [333] | Anti-inflammatory [132] Antioxidant [131] Apoptosis-inhibiting [134] Melanogenesis-inhibitory [129] Proangiogenic [133] |
Blood stasis [334, 335] Osteoporosis [334, 335] Promotion of bone formation [334, 335] |
Oil | Safflower oil (红花油) |
| Celosia argentea | Qing Xiang | Amaranthaceae | Celosin I [139] Celosin II [139] |
Leaves | Ethanol | Rat burn wound model [137] | Antidiabetic [140] Antimicrobial [141] Antioxidant [138] Hepatoprotective [139] |
Skin sores [137] Ulcers [137] |
Poultice of stems and leaves (topically) [336] | Not available |
| Centella asiatica | Ji Xue Cao | Apiaceae | Asiaticoside [144] Madecassoside [146] |
Aerial parts | Hexane, ethyl acetate, methanol, and water | Incision and partial-thickness burn wound models in rats [142] | Anti-inflammatory [142] Antioxidant [142] Proangiogenic [142] |
Wounds [142] | Oral form (tablets, drops) Topical medication (ointments and powder) Injections (subcutaneous and intramuscular) [337, 338] |
Madecassol® [143] Centellase® Blastoestimulina® [337] Collaven® [338] |
| Cinnamomum cassia | Rou Gui | Lauraceae | Cinnamaldehyde [147] | Bark Whole plant |
Volatile oil Ethanol |
In vitro and in vivo angiogenic activity assay [147] Excision wound model in rats [339] |
Anticancer [151, 152] Antidiabetic [340] Anti-inflammatory [150] Antimicrobial [341] Antioxidant [342] |
Analgesia [147] Improvement of blood circulation [147] |
Oil | Cinnamon cassia oil |
| Commiphora myrrha | Mo Yao | Burseraceae | Furanoeudesma-1,3-diene [165] Terpene [165] |
Leaves and resin Resin Leaves and resin |
Dry extract Dry extract Dry extract |
Excision wound model in diabetic rats [164] Human recurrent aphthous stomatitis [165] In vitro cell migration assay [166] |
Analgesic [159] Antibacterial [158] Anti-inflammatory [157] Antioxidant [156] |
Gastrointestinal diseases [161, 162] Wounds and pain [163, 165] |
Oil | Myrrh essential oil |
| Curcuma longa | Jiang Huang | Zingiberaceae | Curcuminoids [170] | Rhizomes | Nanosuspension | Antioxidant analysis [343] | Antibacterial [344] Anti-inflammatory [345] Antioxidant [346] |
Digestive diseases [347] Liver disorders [347] Menstrual difficulties [347] Pain disorders [347] Sprains [347] Wounds [347] |
Capsules [348] | Kordel's Theracurmin™ BCM-95®, Theracurmin™, CurcuVIVA™, CurcuMIND, Long-vida RD CAVACURMIN®, Biocurcumax™ [349] |
| Daphne genkwa | Yuan Hua | Thymelaeaceae | Daphnodorin B [177] Daphnodorin G [177] Daphnodorin G 3ʺ-methylether [177] Daphnodorin H [177] Daphnodorin H 3-methyl [177] Daphnodorin H 3ʺ-methylether [177] Genkwanin [177] Genkwanol A [177] Yuanhuacine [350] Yuenkanin [177] |
Flower Roots |
Aqueous | Human wounds from anal fistula therapy [171] | Anti-inflammatory [175] Antitumour [177] Immunoregulatory [178, 179] Melanogenesis inhibitory [172] |
Coughs [174] Wounds [171] |
Not available | Not available |
| Entada phaseoloides | Ke Teng | Fabaceae | Tannin [182] | Stem skin and seeds | Ethanol | Acetic acid-induced mouse writhing experiment [351] | Antibacterial [180] Antioxidant [181] |
Aging [352] Atherosclerosis [352] Cancer [352] Diabetes [352] Neurodegenerative disorders [352] |
Not available | Not available |
| Hibiscus rosa-sinensis | Zhu Jin | Malvaceae | Anthocyanins [353] Flavonoids [353] Polyphenolic acids [353] Protocatechuic acid [353] |
Flower | Ethanol | Excision, incision and dead space wound models in rats [187, 188] | Antibacterial [186] | Antitumour [353] Hair growth [184] |
Powder | Lustrous Henna® Shampoo powder |
| Ganoderma lucidum | Ling Zhi | Ganodermataceae | Ganoderma lucidum polysaccharide [204] | Fruiting body Fruiting body |
Aqueous Aqueous |
Excision wound model in diabetic rats [190] Full-thickness excision wound model in diabetic mice [204] |
Antihyperlipidemic [197] Anti-infective [192–194] Anti-inflammatory [354] Antioxidant [195] Cardioprotective [196] Immunomodulating [191] |
Cancer [198] Diabetes [200] Hepatitis [189] Leukaemia [189] Ulcer [201] |
Not available | Ganoderma lucidum spores powder capsules |
| Ligusticum striatum | Chuan Xiong | Apiaceae | Ferulic acid [206] Ligustilide [206] Senkyunolide A [206] Tetramethylpyrazine [206] |
Rhizome | Extracted by hydrodistillation | Hypertrophic scar rabbit model [208] | Antiatherosclerotic [355] Antioxidant [356] Neuroprotective [357] Vasorelaxant [358] |
Headache [206] Ischemic disorders [205] Menstrual disorders [207] |
Not available | Chuanxiong chatiao Wan (川芎茶调丸) |
| Lonicera japonica | Jin Yin Hua | Caprifoliaceae | Biflavonoids [209] Dicaffeoylquinic acid [209] Phenolic acids [209] Quercetin [209] |
Flowering aerial parts | Ethanol | Rat excision wound model [212] | Anticancer [210] Antihyperlipidemic [210] Anti-inflammatory [211] Antimicrobial [211] Antioxidant [211] Hepatoprotective [210] |
Infectious diseases [209] | Essential oils [210] | Not available |
| Paeonia suffruticosa | Mu Dan | Paeoniaceae | Suffruticosides A, B, C, and D [359] Galloyl-oxypaeoniflorin [359] Galloyl-paeoniflorin [359] |
Root bark | Water, ethanol-water | In vitro cell viability and proliferation assays [220] | Antidiabetic [218] Anti-inflammatory [217] Antioxidant [214] Antitumour [216] Neuroprotective [215] |
Genital diseases [360] Improvement of blood circulation [361] |
Ointment | Winvivo (Puji) ointment |
| Panax ginseng | Ren Shen | Araliaceae | Ginsenosides Rb1, Rb2, Rc, and Rd [232] | Leaves Leaves, root, and whole plant |
Ethanol Dichloromethane, ethanol, butanol, and methanol |
Laser burn and excision wounds models in mice [236] Cell migration and wound healing assays [221,237–239] |
Antiaging [230] Antiallergic [229] Anticancer [227] Anti-inflammatory [225] Antimicrobial [228] Antioxidant [226] Immunomodulating [231] |
Wound healing [221] | Not available | Ginseng Strong 200 mg Ginsemax® Ginseng Vita-Complex |
| Panax notoginseng | San Qi | Araliaceae | Notoginsenoside Ft1 [240] | Leaves, flower, roots, and rhizome | Steamed extraction | In vitro anticoagulation and antioxidation test; in vivo hemostasis and anti-inflammation test [362] | Angiogenesis-stimulatory [240, 241] Anticancer [246] Antidiabetes [247] Anti-inflammatory [243] Antioxidative [242] Immunostimulatory [244] Neuroprotective [245] |
Trauma [240, 241] | Powder on wound Spray on wound |
San Qi Fen (三七粉) Yunnan Baiyao (云南白药) |
| Polygonum cuspidatum | Hu Zhang | Polygonaceae | Emodin [260] Polydatin [258] Resveratrol [256] |
Roots | Ethanol | Full-thickness excision wounds in rats [259] | Antiaging [256, 257] Antibacterial [260] Anticancer [256, 257] Anti-inflammatory [256, 257] Antioxidant [256, 257] Antiviral [260] Cardioprotective [256, 257] |
Hepatitis [255] Hyperlipemia [255] Jaundice [255] Scald [255] Skin burns [255] Suppurative dermatitis [255] |
Capsules | Resveratrol supplement |
| Lithospermum erythrorhizon | Zi Cao | Boraginaceae | Arnebin-1 [264] Shikonin [363] |
Roots | Frozen and ground | Apoptotic effects against murine primary peritoneal macrophages [364] | Antiangiogenic [261] Antibacterial [261] Anti-inflammatory [261] Antiscarring [263] Antitumorigenic [261] |
Wounds [264] | Oil formulations, gel [365] | Burt's bee Res-Q ointment (神奇紫草膏) |
| Rheum officinale | Da Huang | Polygonaceae | Emodin [270] | Roots | Ethanol | Rat excisional wound model [270] | Antibacterial [265] Anti-inflammatory [267] Antioxidative [266] Haemostatic [268] |
Chronic kidney disease [269] Hepatitis [265] Wounds [270] |
Pills | Dahuang Zhechong Wan (大黄蜇虫丸) |
| Rhodiola imbricate | Hong Jing Tian | Crassulaceae | Gallic acid [277] p-Tyrosol [277] Rosavin [277] Rosin [277] |
Rhizome | Ethanol | Rat excision wound model [274] | Anticancer [279] Antioxidative [276] Hepatoprotective [277] Immunomodulatory [275] Radioprotective [278] |
Asthma [277] Fatigue [277] Hemorrhage [277] Impotence [277] Gastrointestinal diseases [277] |
Not available | Not available |
| Salvia miltiorrhiza | Dan Shen | Lamiaceae | Cryptotanshinone [366] Danshensu [293] Salvianolic acid B [293] |
Leaves Leaves |
Aqueous Aqueous |
In vitro proliferative and angiogenic assays; second-degree burn wound model in rats [367] Cell proliferation assay; in vitro assays for collagen and melanin synthesis [293] |
Anticancer [291] Anti-inflammatory [290] Antimicrobial [289] Antioxidant [286] Antiplatelet aggregation [368] Proangiogenic [367, 369, 370] |
Blood stasis [371, 372] Cardiovascular diseases [282] |
Pills | Compound danshen dripping pills (复方丹参滴丸) |
| Sanguisorba officinalis | Di Yu | Rosaceae | Tannins [296] Triterpenoid glycosides [296] Triterpenoids [296] |
Roots | Ethanol | Burn wound model in mice [296] | Antiallergy [300] Anti-inflammatory [299] Antioxidant [298] Haemostatic [297] Immunomodulatory [298] |
Burns [299] Chronic intestinal infections [373] Haemorrhoids [373] Menorrhagia [373] Scalds [299] |
Oral administration | Sanguisorba officinalis Mother Tincture |
| Sophora flavescens | Ku Shen | Fabaceae | Kushenol [374] Sophoraflavanone B [374] |
Roots | Cellulose column | Human liver LO2 proliferation and viability assay [375] | Analgesic [376] Anthelmintic [376] Antipyretic [376] Skin whitening [376] Stomachic [376] |
Asthma [374] Burns [374] Dysentery [374] Eczema [374] Fever [374] Hematochezia [374] Inflammatory [374] Jaundice [374] Oliguria [374] Vulvar swelling [374] |
Gel | Kushen gel (苦参凝胶) Gatuline® Spot-Light |
| Stemona tuberosa | Bai Bu | Stemonaceae | Tuberostemonine N [306] | Roots | Methanol | Mouse fibroblast NIH3T3 proliferation assay [309] | Antibacterial [307] Anti-inflammatory [306] Antioxidant [308] Antitussive [305] Neuroprotective [377] |
Insect pests [307] | Not available | Not available |
| Wedelia trilobata | Asteraceae | Kaurenoic acid [310] Luteolin [311] |
Leaves | Ethanol | In vitro antimicrobial assay, cell proliferation, and viability assays [310] | Antimicrobial [310] Antioxidant [311] Antitumour [311] |
Arthritic painful joints [310] Rheumatism [310] Stubborn wounds [310] |
Not available | Not available | |
| Zanthoxylum bungeanum | Hua Jiao | Rutaceae | Afzelin [378] Hyperoside [378] Quercitrin [378] Rutin [378] |
Seed oil | Press extraction | Rat scald wound model [378] | Anaesthetic [314] Antiasthma [318] Anti-inflammatory [317] Antoxidant [316] Antitumour [315] |
Skin wrinkles [321] | Cream | ZANTHALENE® |
We provide these data in the belief that we still have much to learn from traditional practices, some of which undoubtedly could deliver novel reagents and therapies for today's therapeutic challenges. Notwithstanding, we recognise that modern medicine and drugs remain effectively inaccessible (and unaffordable) to the majority of the world's population. For this reason alone, traditional medicine continues to be the first line of treatment, indeed, frequently the only line of treatment for many. With greater understanding of traditional practices comes appreciation and benefit to more of the world's peoples. We would like to see that this knowledge is not discarded by “modern medicine” but leveraged through investigation to benefit all.
Acknowledgments
This work was supported by funding from Singapore's Agency for Science, Technology and Research, “SPF 2013/004: Skin Biology Basic Research” and the “Wound Care Innovation for the Tropics” IAF-PP/2017 (HBMS) (H17/01/a0/009).
Abbreviations
- a.k.a.:
Also known as
- AKT:
Protein kinase B
- ALT:
Alanine aminotransferase
- ANBP:
Agrimonia eupatoria (A), Nelumbo nucifera (N), Boswellia sacra (B), and pollen from Typha angustifoliae (P)
- AP-1:
Activator protein 1 (transcription factor)
- α-SMA:
Alpha smooth muscle actin
- AST:
Aspartate aminotransferase
- COX-2:
Cyclooxygenase-2
- CTGF:
Connective tissue growth factor
- DAMPs:
Damage-associated molecular patterns
- DSU:
Danshensu
- ECM:
Extracellular matrix
- EGCG:
(-)-Epigallocatechin-3-gallate
- ERK:
Extracellular signal-regulated kinase
- ET-1:
Endothelin 1
- HSF:
Hypertrophic scar-derived fibroblasts
- HSYA:
Hydroxysafflor yellow A
- IL-1α:
Interleukin-1 alpha
- IL-1β:
Interleukin-1 beta
- IL-6:
Interleukin-6
- IL-8:
Interleukin-8 (CXCL8)
- IL-10:
Interleukin-10
- iNOS:
Inducible nitic oxide synthase
- Jak-2:
Janus kinase 2
- JNK:
c-Jun N-terminal kinase
- MAPK:
Mitogen-activated protein kinase
- MEK:
Mitogen-activated protein kinase, MAPK/ERK kinase
- MMP-1:
Matrix metalloproteinase-1 (interstitial collagenase)
- MMP-2:
Matrix metalloproteinase-2 (gelatinase A)
- MMP-3:
Matrix metalloproteinase-3 (stromelysin-1)
- MMPs:
Matrix metalloproteinases
- mRNA:
Messenger ribonucleic acid
- mTO:
Mammalian target of rapamycin
- NF–κB:
Nuclear factor-kappa B
- PAMPs:
Pathogen-associated molecular patterns
- PARP:
Poly-ADP-ribosyl polymerase
- PGE2:
Prostaglandin E2
- PI3K:
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- ROS:
Reactive oxygen species
- SAB:
Salvianolic acid B
- SBP:
Shexiang Baoxin Pill
- SMAD:
Sapien homologue of mothers against decapentaplegic
- STAT-1:
Signal transducer and activator of transcription 1
- STAT3:
Signal transducer and activator of transcription 3
- TCM:
Traditional Chinese medicine
- TGF-β1:
Transforming growth factor-beta 1
- TNF-α:
Tumour necrosis factor-alpha
- TXA2:
Thromboxane A2
- VEGF:
Vascular endothelial growth factor
- WHO:
World Health Organisation
- 5-LOX:
Arachidonate 5-lipoxygenase.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Xu R., Luo G., Xia H., et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials. 2015;40:1–11. doi: 10.1016/j.biomaterials.2014.10.077. [DOI] [PubMed] [Google Scholar]
- 2.Talbott J. H. A short history of medicine. JAMA. 1962;180(9):p. 794. doi: 10.1001/jama.1962.03050220086023. [DOI] [Google Scholar]
- 3. WHO Traditional Medicine Strategy: 2014–2023, ISBN: 9789241506090, http://www.who.int/traditional-complementary-integrative-medicine/en/
- 4.Ghahary A., Ghaffari A. Role of keratinocyte–fibroblast cross-talk in development of hypertrophic scar. Wound Repair and Regeneration. 2007;15(1):46–53. doi: 10.1111/j.1524-475x.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 5.Eming S. A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine. 2014;6(265) doi: 10.1126/scitranslmed.3009337.265sr6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arya A. K., Tripathi K., Das P. Promising role of ANGPTL4 gene in diabetic wound healing. The International Journal of Lower Extremity Wounds. 2014;13(1):58–63. doi: 10.1177/1534734614520704. [DOI] [PubMed] [Google Scholar]
- 7.Golebiewska E. M., Poole A. W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Reviews. 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enoch S., Leaper D. J. Basic science of wound healing. Surgery (Oxford) 2008;26(2):31–37. doi: 10.1016/j.mpsur.2007.11.005. [DOI] [Google Scholar]
- 9.Martin P., Leibovich S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends in Cell Biology. 2005;15(11):599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Delavary B. M., van der Veer W. M., van Egmond M., Niessen F. B., Beelen R. H. J. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Kasuya A., Tokura Y. Attempts to accelerate wound healing. Journal of Dermatological Science. 2014;76(3):169–172. doi: 10.1016/j.jdermsci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Fan D., Takawale A., Lee J., Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & Tissue Repair. 2012;5(1):p. 15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kioka N., Ito T., Yamashita H., et al. Crucial role of vinexin for keratinocyte migration in vitro and epidermal wound healing in vivo. Experimental Cell Research. 2010;316(10):1728–1738. doi: 10.1016/j.yexcr.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar S. K., Marmer B., Goldberg G., Neuman K. C. Single-molecule tracking of collagenase on native type I collagen fibrils reveals degradation mechanism. Current Biology. 2012;22(12):1047–1056. doi: 10.1016/j.cub.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akasaka Y., Ono I., Kamiya T., et al. The mechanisms underlying fibroblast apoptosis regulated by growth factors during wound healing. The Journal of Pathology. 2010;221(3):285–299. doi: 10.1002/path.2710. [DOI] [PubMed] [Google Scholar]
- 16.McDougall S., Dallon J., Sherratt J., Maini P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2006;364(1843):1385–1405. doi: 10.1098/rsta.2006.1773. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R., John J. Role of stem cells in the management of chronic wounds. Indian Journal of Plastic Surgery. 2012;45(2):237–243. doi: 10.4103/0970-0358.101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deufert D., Graml R. Disease-specific, health-related quality of life (HRQoL) of people with chronic wounds-a descriptive cross-sectional study using the wound-QoL. Wound Medicine. 2017;16:29–33. doi: 10.1016/j.wndm.2017.01.006. [DOI] [Google Scholar]
- 19.Frykberg R. G., Banks J. Challenges in the treatment of chronic wounds. Advances in Wound Care. 2015;4(9):560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Orue I., Pedraz J. L., Hernandez R. M., Igartua M. Nanotechnology-based delivery systems to release growth factors and other endogenous molecules for chronic wound healing. Journal of Drug Delivery Science and Technology. 2017;42:2–17. doi: 10.1016/j.jddst.2017.03.002. [DOI] [Google Scholar]
- 21.Budovsky A., Yarmolinsky L., Ben-Shabat S. Effect of medicinal plants on wound healing. Wound Repair and Regeneration. 2015;23(2):171–183. doi: 10.1111/wrr.12274. [DOI] [PubMed] [Google Scholar]
- 22.Schreml S., Szeimies R.-M., Prantl L., Landthaler M., Babilas P. Wound healing in the 21st century. Journal of the American Academy of Dermatology. 2010;63(5):866–881. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Caley M. P., Martins V. L. C., O’Toole E. A. Metalloproteinases and wound healing. Advances in Wound Care. 2015;4(4):225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Advances in Therapy. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robson M. C., Barbul A. Guidelines for the best care of chronic wounds. Wound Repair and Regeneration. 2006;14(6):647–648. doi: 10.1111/j.1524-475x.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya S. Wound healing through the ages. Indian Journal of Plastic Surgery. 2012;45(2):177–179. doi: 10.4103/0970-0358.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Advances in Therapy. 2017;34(3):599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards R., Harding K. G. Bacteria and wound healing. Current Opinion in Infectious Diseases. 2004;17(2):91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kalan L., Zhou M., Labbie M., Willing B. Measuring the microbiome of chronic wounds with use of a topical antimicrobial dressing-a feasibility study. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187728.e0187728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han F., Li Y., Zhang X., Song A., Zhu H., Yin R. A pilot study of direct infusion analysis by FT-ICR MS for rapid differentiation and authentication of traditional Chinese herbal medicines. International Journal of Mass Spectrometry. 2016;403:62–67. doi: 10.1016/j.ijms.2016.01.012. [DOI] [Google Scholar]
- 31.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5) doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wangchuk P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. Journal of Biologically Active Products from Nature. 2018;8(1):1–20. doi: 10.1080/22311866.2018.1426495. [DOI] [Google Scholar]
- 33.Qi Y., Li S., Pi Z., et al. Chemical profiling of Wu-tou decoction by UPLC-Q-TOF-MS. Talanta. 2014;118:21–29. doi: 10.1016/j.talanta.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Orue I., Gainza G., Gutierrez F. B., et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. International Journal of Pharmaceutics. 2017;523(2):556–566. doi: 10.1016/j.ijpharm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Salehi B., Albayrak S., Antolak H., et al. Aloe genus plants: from farm to food applications and phytopharmacotherapy. International Journal of Molecular Sciences. 2018;19(9):p. 2843. doi: 10.3390/ijms19092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence R., Tripathi P., Jeyakumar E. Isolation, purification and evaluation of antibacterial agents from Aloe vera. Brazilian Journal of Microbiology. 2009;40(4):906–915. doi: 10.1590/s1517-83822009000400023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-Romero D., Alburquerque N., Valverde J. M., et al. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: a new edible coating. Postharvest Biology and Technology. 2006;39(1):93–100. doi: 10.1016/j.postharvbio.2005.09.006. [DOI] [Google Scholar]
- 38.Ali P., Chen Y.-F., Sargsyan E. Chapter 12-bioactive molecules of herbal extracts with anti-infective and wound healing properties. In: Kon K., Rai M., editors. Microbiology for Surgical Infections. Amsterdam, Netherlands: Academic Press; 2014. pp. 205–220. [Google Scholar]
- 39.Xing W., Guo W., Zou C.-H., et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. Journal of Dermatological Science. 2015;79(2):101–109. doi: 10.1016/j.jdermsci.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Jettanacheawchankit S., Sasithanasate S., Sangvanich P., Banlunara W., Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. Journal of Pharmacological Sciences. 2009;109(4):525–531. doi: 10.1254/jphs.08204fp. [DOI] [PubMed] [Google Scholar]
- 41.Lin S.-C., Lin C.-H., Lin C.-C., et al. Hepatoprotective effects of Arctium lappa linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. Journal of Biomedical Science. 2002;9(5):401–409. doi: 10.1159/000064549. [DOI] [PubMed] [Google Scholar]
- 42.Chan Y.-S., Cheng L.-N., Wu J.-H., et al. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2011;19(5):245–254. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 43.Miglani A., Manchanda R. K. Observational study of Arctium lappa in the treatment of acne vulgaris. Homeopathy. 2014;103(3):203–207. doi: 10.1016/j.homp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Fierascu R. C., Georgiev M. I., Fierascu I., et al. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Plantaginaceae) Food and Chemical Toxicology. 2018;111:44–52. doi: 10.1016/j.fct.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 45.de Almeida A. B. A., Sánchez-Hidalgo M., Martín A. R., et al. Anti-inflammatory intestinal activity of Arctium lappa L. (Asteraceae) in TNBS colitis model. Journal of Ethnopharmacology. 2013;146(1):300–310. doi: 10.1016/j.jep.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 46.Ahangarpour A., Heidari H., Oroojan A. A., Mirzavandi F., Nasr Esfehani K., Dehghan Mohammadi Z. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna Journal of Phytomedicine. 2017;7(2):169–179. [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira J. V., Bergamo D. C. B., Pereira J. O., França S. d. C., Pietro R. C. L. R., Silva-Sousa Y. T. C. Antimicrobial activity of Arctium lappa constituents against microorganisms commonly found in endodontic infections. Brazilian Dental Journal. 2005;16(3):192–196. doi: 10.1590/s0103-64402005000300004. [DOI] [PubMed] [Google Scholar]
- 48.Dias M. M., Zuza O., Riani L. R., et al. In vitro schistosomicidal and antiviral activities of Arctium lappa L. (Asteraceae) against Schistosoma mansoni and Herpes simplex virus-1. Biomedicine & Pharmacotherapy. 2017;94:489–498. doi: 10.1016/j.biopha.2017.07.116. [DOI] [PubMed] [Google Scholar]
- 49.Sun Q., Liu K., Shen X., et al. Lappaol F, a novel anticancer agent isolated from plant Arctium Lappa L. Molecular Cancer Therapeutics. 2014;13(1):49–59. doi: 10.1158/1535-7163.mct-13-0552. [DOI] [PubMed] [Google Scholar]
- 50.de Souza Predes F., da Silva Diamante M. A., Foglio M. A., et al. Hepatoprotective effect of Arctium lappa root extract on cadmium toxicity in adult Wistar rats. Biological Trace Element Research. 2014;160(2):250–257. doi: 10.1007/s12011-014-0040-6. [DOI] [PubMed] [Google Scholar]
- 51.Knott A., Reuschlein K., Mielke H., et al. Natural Arctium lappa fruit extract improves the clinical signs of aging skin. Journal of Cosmetic Dermatology. 2008;7(4):281–289. doi: 10.1111/j.1473-2165.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 52.Pomari E., Stefanon B., Colitti M. Effect of Arctium lappa (burdock) extract on canine dermal fibroblasts. Veterinary Immunology and Immunopathology. 2013;156(3-4):159–166. doi: 10.1016/j.vetimm.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Amish Burn Study G., Kolacz N. M., Jaroch M. T., et al. The effect of Burns & Wounds (B&W)/burdock leaf therapy on burn-injured Amish patients: a pilot study measuring pain levels, infection rates, and healing times. Journal of Holistic Nursing. 2014;32(4):327–340. doi: 10.1177/0898010114525683. [DOI] [PubMed] [Google Scholar]
- 54.Liu P.-P., Shan G.-S., Zhang F., Chen J.-N., Jia T.-Z. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chinese Journal of Natural Medicines. 2018;16(9):714–720. doi: 10.1016/s1875-5364(18)30111-0. [DOI] [PubMed] [Google Scholar]
- 55.Chiu C.-Y., Hsu W.-H., Liu H.-K., Liu S.-H., Lin Y.-L. Prepared Rehmanniae Radix oligosaccharide regulates postprandial and diabetic blood glucose in mice. Journal of Functional Foods. 2018;41:210–215. doi: 10.1016/j.jff.2017.12.031. [DOI] [Google Scholar]
- 56.Wong M. W., Leung P. C., Wong W. C. Limb salvage in extensive diabetic foot ulceration-a preliminary clinical study using simple debridement and herbal drinks. Hong Kong Medical Journal. 2001;7(4):403–407. [PubMed] [Google Scholar]
- 57.Tam J. C. W., Lau K. M., Liu C. L., et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. Journal of Ethnopharmacology. 2011;134(3):831–838. doi: 10.1016/j.jep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Tam J. C.-W., Ko C.-H., Lau K.-M., et al. A Chinese 2-herb formula (NF3) promotes hindlimb ischemia-induced neovascularization and wound healing of diabetic rats. Journal of Diabetes and its Complications. 2014;28(4):436–447. doi: 10.1016/j.jdiacomp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q., Fong C. C., Yu W. K., et al. Herbal formula Astragali Radix and Rehmanniae Radix exerted wound healing effect on human skin fibroblast cell line Hs27 via the activation of transformation growth factor (TGF-β) pathway and promoting extracellular matrix (ECM) deposition. Phytomedicine. 2012;20(1):9–16. doi: 10.1016/j.phymed.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Mi J., Wu C., Li C., Xi F., Wu Z., Chen W. Two new triterpenoids from Ampelopsis japonica (Thunb.) Makino. Natural Product Research. 2014;28(1):52–56. doi: 10.1080/14786419.2013.838237. [DOI] [PubMed] [Google Scholar]
- 61.Park H., Shim J. S., Kim H. G., Lee H., Oh M. S. Ampelopsis radix protects dopaminergic neurons against 1-methyl-4-phenylpyridinium/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced toxicity in parkinson’s disease models in vitro and in vivo. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/346438.346438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nho K. J., Chun J. M., Kim D.-S., Kim H. K. Ampelopsis japonica ethanol extract suppresses migration and invasion in human MDA-MB-231 breast cancer cells. Molecular Medicine Reports. 2015;11(5):3722–3728. doi: 10.3892/mmr.2015.3179. [DOI] [PubMed] [Google Scholar]
- 63.Lee K., Lee B., Lee M.-H., et al. Effect of Ampelopsis Radix on wound healing in scalded rats. BMC Complementary and Alternative Medicine. 2015;15(1):p. 213. doi: 10.1186/s12906-015-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akbar S. Andrographis paniculata: a review of pharmacological activities and clinical effects. Alternative Medicine Review. 2011;16(1):66–77. [PubMed] [Google Scholar]
- 65.Kabir M., Hasan N., Rahman M., et al. A survey of medicinal plants used by the Deb barma clan of the Tripura tribe of Moulvibazar district, Bangladesh. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 19. doi: 10.1186/1746-4269-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar R. A., Sridevi K., Kumar N. V., Nanduri S., Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. Journal of Ethnopharmacology. 2004;92(2-3):291–295. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Chen L.-X., He H., Xia G.-Y., Zhou K.-L., Qiu F. A new flavonoid from the aerial parts of Andrographis paniculata. Natural Product Research. 2014;28(3):138–143. doi: 10.1080/14786419.2013.856907. [DOI] [PubMed] [Google Scholar]
- 68.Adedapo A. A., Adeoye B. O., Sofidiya M. O., Oyagbemi A. A. Antioxidant, antinociceptive and anti-inflammatory properties of the aqueous and ethanolic leaf extracts of Andrographis paniculata in some laboratory animals. Journal of Basic and Clinical Physiology and Pharmacology. 2015;26(4):327–334. doi: 10.1515/jbcpp-2014-0051. [DOI] [PubMed] [Google Scholar]
- 69.Shen T., Yang W. S., Yi Y.-S., et al. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from Andrographis paniculata. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/210736.210736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhtar M., Mohd Sarib M. B., Ismail I., et al. Anti-diabetic activity and metabolic changes induced by andrographis paniculata plant extract in obese diabetic rats. Molecules. 2016;21(8) doi: 10.3390/molecules21081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman M. M., Ahmad S. H., Mohamed M. T. M., Ab Rahman M. Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Scientific World Journal. 2014;2014 doi: 10.1155/2014/635240.635240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiart C., Kumar K., Yusof M. Y., Hamimah H., Fauzi Z. M., Sulaiman M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytotherapy Research. 2005;19(12):1069–1070. doi: 10.1002/ptr.1765. [DOI] [PubMed] [Google Scholar]
- 73.Mishra K., Dash A. P., Dey N. Andrographolide: a novel antimalarial diterpene lactone compound from Andrographis paniculata and its interaction with curcumin and artesunate. Journal of Tropical Medicine. 2011;2011 doi: 10.1155/2011/579518.579518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C., Tan B. Hypotensive activity of aqueous extract of Andrographis paniculata in rats. Clinical and Experimental Pharmacology and Physiology. 1996;23(8):675–678. doi: 10.1111/j.1440-1681.1996.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 75.Nagalekshmi R., Menon A., Chandrasekharan D. K., Nair C. K. K. Hepatoprotective activity of Andrographis paniculata and Swertia chirayita. Food and Chemical Toxicology. 2011;49(12):3367–3373. doi: 10.1016/j.fct.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 76.Al-Bayaty F. H., Abdulla M. A., Hassan M. I. A., Ali H. M. Effect of Andrographis paniculata leaf extract on wound healing in rats. Natural Product Research. 2012;26(5):423–429. doi: 10.1080/14786419.2010.496114. [DOI] [PubMed] [Google Scholar]
- 77.Shamsizadeh A., Roohbakhsh A., Ayoobi F., Moghaddamahmadi A. Chapter 25-the role of natural products in the prevention and treatment of multiple sclerosis. In: Watson R. R., Killgore W. D. S., editors. Nutrition and Lifestyle in Neurological Autoimmune Diseases. Cambridge, MA, USA: Academic Press; 2017. pp. 249–260. [Google Scholar]
- 78.Jin M., Zhao K., Huang Q., Xu C., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: a review. Carbohydrate Polymers. 2012;89(3):713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W.-q., Hua Y.-l., Zhang M., et al. Metabonomic analysis of the anti-inflammatory effects of volatile oils of Angelica sinensis on rat model of acute inflammation. Biomedical Chromatography. 2015;29(6):902–910. doi: 10.1002/bmc.3372. [DOI] [PubMed] [Google Scholar]
- 80.Cheng Y.-L., Chang W.-L., Lee S.-C., et al. Acetone extract of Angelica sinensis inhibits proliferation of human cancer cells via inducing cell cycle arrest and apoptosis. Life Sciences. 2004;75(13):1579–1594. doi: 10.1016/j.lfs.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Lei T., Li H., Fang Z., et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regeneration Research. 2014;9(3):260–267. doi: 10.4103/1673-5374.128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang T., Jia M., Meng J., Wu H., Mei Q. Immunomodulatory activity of polysaccharide isolated from Angelica sinensis. International Journal of Biological Macromolecules. 2006;39(4-5):179–184. doi: 10.1016/j.ijbiomac.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Hsiao C. Y., Hung C.-Y., Tsai T.-H., Chak K.-F. A Study of the wound healing mechanism of a traditional Chinese medicine, Angelica sinensis, using a proteomic approach. Evidence-Based Complementary and Alternative Medicine. 2012;2012:14. doi: 10.1155/2012/467531.467531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J., Yuan Z., Zhao H., et al. Ferulic acid promotes endothelial cells proliferation through up-regulating cyclin D1 and VEGF. Journal of Ethnopharmacology. 2011;137(2):992–997. doi: 10.1016/j.jep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Lam H.-W., Lin H.-C., Lao S.-C., et al. The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo. Journal of Cellular Biochemistry. 2008;103(1):195–211. doi: 10.1002/jcb.21403. [DOI] [PubMed] [Google Scholar]
- 86.Yeh J.-C., Cindrova-Davies T., Belleri M., et al. The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis. 2011;14(2):187–197. doi: 10.1007/s10456-011-9202-8. [DOI] [PubMed] [Google Scholar]
- 87.Chen M., Qin J.-J., Fu J.-J., et al. Blumeaenes A-J, sesquiterpenoid esters from Blumea balsamifera with NO inhibitory activity. Planta Medica. 2010;76(9):897–902. doi: 10.1055/s-0029-1240800. [DOI] [PubMed] [Google Scholar]
- 88.Noor Rain A., Khozirah S., Mohd Ridzuan M. A., et al. Antiplasmodial properties of some Malaysian medicinal plants. Tropical Biomedicine. 2007;24(1):29–35. [PubMed] [Google Scholar]
- 89.Li J., Zhao G.-Z., Chen H.-H., et al. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Letters in Applied Microbiology. 2008;47(6):574–580. doi: 10.1111/j.1472-765x.2008.02470.x. [DOI] [PubMed] [Google Scholar]
- 90.Ragasa C. Y., Kristin A. L., Rideout J. A. Antifungal metabolites from Blumea balsamifera. Natural Product Research. 2005;19(3):231–237. doi: 10.1080/14786410410001709773. [DOI] [PubMed] [Google Scholar]
- 91.Kubota H., Kojima-Yuasa A., Morii R., et al. Anti-obesity effect of Blumea balsamifera extract in 3T3-L1 preadipocytes and adipocytes. The American Journal of Chinese Medicine. 2009;37(05):843–854. doi: 10.1142/s0192415x09007326. [DOI] [PubMed] [Google Scholar]
- 92.Pang Y., Wang D., Hu X., et al. Effect of volatile oil from Blumea balsamifera (L.) DC. leaves on wound healing in mice. Journal of Traditional Chinese Medicine. 2014;34(6):716–724. doi: 10.1016/s0254-6272(15)30087-x. [DOI] [PubMed] [Google Scholar]
- 93.Jahandideh M., Hajimehdipoor H., Mortazavi S. A., Dehpour A., Hassanzadeh G. A wound healing formulation based on iranian traditional medicine and its HPTLC fingerprint. Iranian Journal of Pharmaceutical Research. 2016;15:149–157. [PMC free article] [PubMed] [Google Scholar]
- 94.Banno N., Akihisa T., Yasukawa K., et al. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. Journal of Ethnopharmacology. 2006;107(2):249–253. doi: 10.1016/j.jep.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 95.Zhao W., Entschladen F., Liu H., et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cells. Cancer Detection and Prevention. 2003;27(1):67–75. doi: 10.1016/s0361-090x(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 96.Hou Q., He W.-J., Hao H.-J., et al. The four-herb Chinese medicine ANBP enhances wound healing and inhibits scar formation via bidirectional regulation of transformation growth factor pathway. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0112274.e112274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou Q., He W.-J., Chen L., et al. Effects of the four-herb compound ANBP on wound healing promotion in diabetic mice. The International Journal of Lower Extremity Wounds. 2015;14(4):335–342. doi: 10.1177/1534734615575244. [DOI] [PubMed] [Google Scholar]
- 98.Zhao H., Wang X., Li W., Koike K., Bai H. A new minor homoisoflavonoid from Caesalpinia sappan. Natural Product Research. 2014;28(2):102–105. doi: 10.1080/14786419.2013.847439. [DOI] [PubMed] [Google Scholar]
- 99.Yodsaoue O., Cheenpracha S., Karalai C., Ponglimanont C., Tewtrakul S. Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen-induced β-hexosaminidase release. Phytotherapy Research. 2009;23(7):1028–1031. doi: 10.1002/ptr.2670. [DOI] [PubMed] [Google Scholar]
- 100.Min B. S., Cuong T. D., Hung T. M., Min B. K., Shin B. S., Woo M. H. Compounds from the heartwood of Caesalpinia sappan and their anti-inflammatory activity. Bioorganic & Medicinal Chemistry Letters. 2012;22(24):7436–7439. doi: 10.1016/j.bmcl.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 101.Jeong H. J., Kim Y. M., Kim J. H., et al. Homoisoflavonoids from Caesalpinia sappan displaying viral neuraminidases inhibition. Biological and Pharmaceutical Bulletin. 2012;35(5):786–790. doi: 10.1248/bpb.35.786. [DOI] [PubMed] [Google Scholar]
- 102.Temrangsee P., Kondo S., Itharat A. Antibacterial activity of extracts from five medicinal plants and their formula against bacteria that cause chronic wound infection. Journal of the Medical Association of Thailand. 2011;94(7):S166–S171. [PubMed] [Google Scholar]
- 103.Tewtrakul S., Tungcharoen P., Sudsai T., Karalai C., Ponglimanont C., Yodsaoue O. Antiinflammatory and wound healing effects of Caesalpinia sappan L. Phytotherapy Research. 2015;29(6):850–856. doi: 10.1002/ptr.5321. [DOI] [PubMed] [Google Scholar]
- 104.Nicolaus C., Junghanns S., Hartmann A., Murillo R., Ganzera M., Merfort I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. Journal of Ethnopharmacology. 2017;196:94–103. doi: 10.1016/j.jep.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Leach M. J. Calendula officinalis and wound healing: a systematic review. Wounds. 2008;20(8):236–243. [PubMed] [Google Scholar]
- 106.Chandran P. K., Kuttan R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. Journal of Clinical Biochemistry and Nutrition. 2008;43(2):58–64. doi: 10.3164/jcbn.2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fronza M., Heinzmann B., Hamburger M., Laufer S., Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. Journal of Ethnopharmacology. 2009;126(3):463–467. doi: 10.1016/j.jep.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 108.Dinda M., Dasgupta U., Singh N., Bhattacharyya D., Karmakar P. PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: implication in wound healing. Phytotherapy Research. 2015;29(4):607–616. doi: 10.1002/ptr.5293. [DOI] [PubMed] [Google Scholar]
- 109.Dinda M., Mazumdar S., Das S., et al. The water fraction of Calendula officinalis hydroethanol extract stimulates in vitroand in vivo proliferation of dermal fibroblasts in wound healing. Phytotherapy Research. 2016;30(10):1696–1707. doi: 10.1002/ptr.5678. [DOI] [PubMed] [Google Scholar]
- 110.Parente L. M., Lino Júnior R. d. S., Faustino Tresvenzol L. M., Vinaud M. C., de Paula J. R., Paulo N. M. Wound healing and anti-inflammatory effect in animal models of Calendula officinalis L. growing in Brazil. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/375671.375671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang C. S., Chen G., Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. Journal of Traditional and Complementary Medicine. 2014;4(1):17–23. doi: 10.4103/2225-4110.124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Espinosa C., López-Jiménez J. A., Pérez-Llamas F., et al. Long-term intake of white tea prevents oxidative damage caused by adriamycin in kidney of rats. Journal of the Science of Food and Agriculture. 2016;96(9):3079–3087. doi: 10.1002/jsfa.7483. [DOI] [PubMed] [Google Scholar]
- 113.Chen B. T., Li W.-X., He R.-R., et al. Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxidative Medicine and Cellular Longevity. 2012;2012:7. doi: 10.1155/2012/537923.537923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anwar Ibrahim D., Noman Albadani R. Evaluation of the potential nephroprotective and antimicrobial effect of Camellia sinensis leaves versus Hibiscus sabdariffa (in vivo and in vitro studies) Advances in Pharmacological Sciences. 2014;2014:5. doi: 10.1155/2014/389834.389834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Er S., Dikmen M. Camellia sinensis increased apoptosis on U2OS osteosarcoma cells and wound healing potential on NIH3T3 fibroblast cells. Cytotechnology. 2017;69(6):901–914. doi: 10.1007/s10616-017-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jadoon S., Karim S., Bin Asad M. H. H., et al. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxidative Medicine and Cellular Longevity. 2015;2015:17. doi: 10.1155/2015/709628.709628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He R.-r., Chen L., Lin B.-h., Matsui Y., Yao X.-s., Kurihara H. Beneficial effects of oolong tea consumption on diet-induced overweight and obese subjects. Chinese Journal of Integrative Medicine. 2009;15(1):34–41. doi: 10.1007/s11655-009-0034-8. [DOI] [PubMed] [Google Scholar]
- 118.Hasani-Ranjbar S., Jouyandeh Z., Abdollahi M. A systematic review of anti-obesity medicinal plants-an update. Journal of Diabetes & Metabolic Disorders. 2013;12(1):p. 28. doi: 10.1186/2251-6581-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khan G., Haque S. E., Anwer T., Ahsan M. N., Safhi M. M., Alam M. F. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Poloniae Pharmaceutica. 2014;71(5):861–868. [PubMed] [Google Scholar]
- 120.Levites Y., Weinreb O., Maor G., Youdim M. B. H., Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. Journal of Neurochemistry. 2001;78(5):1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 121.Hajiaghaalipour F., Kanthimathi M. S., Abdulla M. A., Sanusi J. The effect of Camellia sinensis on wound healing potential in an animal model. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/386734.386734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsu S., Bollag W. B., Lewis J., et al. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. Journal of Pharmacology and Experimental Therapeutics. 2003;306(1):29–34. doi: 10.1124/jpet.103.049734. [DOI] [PubMed] [Google Scholar]
- 123.Klass B. R., Branford O. A., Grobbelaar A. O., Rolfe K. J. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-β1-stimulated wound contraction. Wound Repair and Regeneration. 2010;18(1):80–88. doi: 10.1111/j.1524-475x.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 124.Syed F., Bagabir R. A., Paus R., Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Laboratory Investigation. 2013;93(8):946–960. doi: 10.1038/labinvest.2013.82. [DOI] [PubMed] [Google Scholar]
- 125.Park G., Yoon B. S., Moon J.-H., et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. Journal of Investigative Dermatology. 2008;128(10):2429–2441. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 126.Asadi S. Y., Parsaei P., Karimi M., et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. International Journal of Surgery. 2013;11(4):332–337. doi: 10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 127.Kim H., Kawazoe T., Han D.-W., et al. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair and Regeneration. 2008;16(5):714–720. doi: 10.1111/j.1524-475x.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 128.Yao D., Wang Z., Miao L., Wang L. Effects of extracts and isolated compounds from safflower on some index of promoting blood circulation and regulating menstruation. Journal of Ethnopharmacology. 2016;191:264–272. doi: 10.1016/j.jep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 129.Roh J. S., Han J. Y., Kim J. H., Hwang J. K. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biological & Pharmaceutical Bulletin. 2004;27(12):1976–1978. doi: 10.1248/bpb.27.1976. [DOI] [PubMed] [Google Scholar]
- 130.Gao S.-Q., Chang C., Niu X.-Q., Li L.-J., Zhang Y., Gao J.-Q. Topical application of Hydroxysafflor yellow A accelerates the wound healing in streptozotocin induced T1DM rats. European Journal of Pharmacology. 2018;823:72–78. doi: 10.1016/j.ejphar.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 131.Wei X., Liu H., Sun X., et al. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neuroscience Letters. 2005;386(1):58–62. doi: 10.1016/j.neulet.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 132.Song L., Zhu Y., Jin M., Zang B. Hydroxysafflor yellow a inhibits lipopolysaccharide-induced inflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia. 2013;84:107–114. doi: 10.1016/j.fitote.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Zhang N., Xing M., Wang Y., et al. Hydroxysafflor yellow A improves learning and memory in a rat model of vascular dementia by increasing VEGF and NR1 in the hippocampus. Neuroscience Bulletin. 2014;30(3):417–424. doi: 10.1007/s12264-013-1375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ji D. B., Zhang L. Y., Li C. L., Ye J., Zhu H. B. Effect of hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vascular Pharmacology. 2009;50(3-4):137–145. doi: 10.1016/j.vph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 135.Yuan W., Yang D., Sun X., et al. Effects of hydroxysafflor yellow A on proliferation and collagen synthesis of rat vascular adventitial fibroblasts induced by angiotensin II. Int J Clin Exp Pathol. 2014;7(9):5772–5781. [PMC free article] [PubMed] [Google Scholar]
- 136.Yang F., Li J., Zhu J., Wang D., Chen S., Bai X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling pathway in H22 tumor-bearing mice. European Journal of Pharmacology. 2015;754:105–114. doi: 10.1016/j.ejphar.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 137.Priya K. S., Arumugam G., Rathinam B., Wells A., Babu M. Celosia argentea Linn. leaf extract improves wound healing in a rat burn wound model. Wound Repair and Regeneration. 2004;12(6):618–625. doi: 10.1111/j.1067-1927.2004.12603.x. [DOI] [PubMed] [Google Scholar]
- 138.Malomo S. O., Ore A., Yakubu M. T. In vitro and in vivo antioxidant activities of the aqueous extract of Celosia argentea leaves. Indian Journal of Pharmacology. 2011;43(3):278–285. doi: 10.4103/0253-7613.81519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu Q. B., Wang Y., Liang L., Jiang Q., Guo M. L., Zhang J. J. Novel triterpenoid saponins from the seeds of Celosia argentea L. Natural Product Research. 2013;27(15):1353–1360. doi: 10.1080/14786419.2012.740034. [DOI] [PubMed] [Google Scholar]
- 140.Hamzah R. U., Lawal A. R., Madaki F. M., Erukainure O. L. Methanolic extract of Celosia argentea var. crista leaves modulates glucose homeostasis and abates oxidative hepatic injury in diabetic rats. Comparative Clinical Pathology. 2018;27(4):1065–1071. doi: 10.1007/s00580-018-2702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wiart C., Mogana S., Khalifah S., et al. Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia. 2004;75(1):68–73. doi: 10.1016/j.fitote.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 142.Somboonwong J., Kankaisre M., Tantisira B., Tantisira M. H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complementary and Alternative Medicine. 2012;12:p. 103. doi: 10.1186/1472-6882-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Y.-J., Dai Y.-S., Chen B.-F., et al. The effect of tetrandrine and extracts of Centella asiatica on acute radiation dermatitis in rats. Biological & Pharmaceutical Bulletin. 1999;22(7):703–706. doi: 10.1248/bpb.22.703. [DOI] [PubMed] [Google Scholar]
- 144.Shukla A., Rasik A. M., Jain G. K., Shankar R., Kulshrestha D. K., Dhawan B. N. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. Journal of Ethnopharmacology. 1999;65(1):1–11. doi: 10.1016/s0378-8741(98)00141-x. [DOI] [PubMed] [Google Scholar]
- 145.Maquart F. X., Chastang F., Simeon A., Birembaut P., Gillery P., Wegrowski Y. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. European Journal of Dermatology. 1999;9(4):289–296. [PubMed] [Google Scholar]
- 146.Liu M., Dai Y., Li Y., et al. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Medica. 2008;74(08):809–815. doi: 10.1055/s-2008-1074533. [DOI] [PubMed] [Google Scholar]
- 147.Yuan X., Han L., Fu P., et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Laboratory Investigation. 2018;98(6):783–798. doi: 10.1038/s41374-018-0025-8. [DOI] [PubMed] [Google Scholar]
- 148.Zhang K. J., Zhu J.-Z., Bao X.-Y., Zheng Q., Zheng G.-q., Wang Y. Shexiang baoxin pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Frontiers in Pharmacology. 2017;8:p. 404. doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tian P.-p., Li J., Gao J., Li Y. Efficacy and safety of the Shexiang Baoxin Pill for the treatment of coronary artery disease not amenable to revascularisation: study protocol for a randomised, placebo-controlled, double-blinded trial. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-018052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee S. H., Lee S. Y., Son D. J., et al. Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochemical Pharmacology. 2005;69(5):791–799. doi: 10.1016/j.bcp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 151.Koppikar S. J., Choudhari A. S., Suryavanshi S. A., et al. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:p. 210. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwon H.-K., Jeon W. K., Hwang J.-S., et al. Cinnamon extract suppresses tumor progression by modulating angiogenesis and the effector function of CD8 + T cells. Cancer Letters. 2009;278(2):174–182. doi: 10.1016/j.canlet.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 153.Ye H., Du J., Shen D., et al. [Effect of shexiang baoxin pill on the function of vascular endothelium in patients with diabetes mellitus type 2 complicated with angina pectoris] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(12):1077–1079. [PubMed] [Google Scholar]
- 154.Rao P. V., Gan S. H. Cinnamon: a multifaceted medicinal plant. Evidence-Based Complementary and Alternative Medicine. 2014;2014:p. 12. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walsh M. E., Reis D., Jones T. Integrating complementary and alternative medicine: use of myrrh in wound management. Journal of Vascular Nursing. 2010;28(3):p. 102. doi: 10.1016/j.jvn.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Fatani A. J., Alrojayee F. S., Parmar M. Y., Abuohashish H. M., Ahmed M. M., Al-Rejaie S. S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Experimental and Therapeutic Medicine. 2016;12(2):730–738. doi: 10.3892/etm.2016.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manjula N., Gayathri B., Vinaykumar K. S., Shankernarayanan N. P., Vishwakarma R. A., Balakrishnan A. Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-α, IL-1β and IL-2. International Immunopharmacology. 2006;6(2):122–132. doi: 10.1016/j.intimp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 158.Shuaib M., Ali A., Ali M., Panda B., Ahmad M. Antibacterial activity of resin rich plant extracts. Journal of Pharmacy and Bioallied Sciences. 2013;5(4):265–269. doi: 10.4103/0975-7406.120073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shalaby M., Hammouda A. Analgesic, anti-inflammatory and antihyperlipidemic activities of Commiphora molmol extract (myrrh) Journal of Intercultural Ethnopharmacology. 2014;3(2):56–62. doi: 10.5455/jice.20140130015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.al-Harbi M. M., Qureshi S., Raza M., Ahmed M. M., Afzal M., Shah A. H. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. Journal of Ethnopharmacology. 1997;55(2):141–150. doi: 10.1016/s0378-8741(96)01488-2. [DOI] [PubMed] [Google Scholar]
- 161.Abdul-Ghani R. A., Loutfy N., Hassan A. Myrrh and trematodoses in Egypt: an overview of safety, efficacy and effectiveness profiles. Parasitology International. 2009;58(3):210–214. doi: 10.1016/j.parint.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 162.Shen T., Li G.-H., Wang X.-N., Lou H.-X. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2012;142(2):319–330. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 163.Nomicos E. Y. H. Myrrh. Holistic Nursing Practice. 2007;21(6):308–323. doi: 10.1097/01.hnp.0000298616.32846.34. [DOI] [PubMed] [Google Scholar]
- 164.Galehdari H., Negahdari S., Kesmati M., Rezaie A., Shariati G. Effect of the herbal mixture composed of Aloe Vera, Henna, Adiantum capillus-veneris, and Myrrha on wound healing in streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine. 2016;16(1):p. 386. doi: 10.1186/s12906-016-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mansour G., Ouda S., Shaker A., Abdallah H. M. Clinical efficacy of new aloe vera- and myrrh-based oral mucoadhesive gels in the management of minor recurrent aphthous stomatitis: a randomized, double-blind, vehicle-controlled study. Journal of Oral Pathology & Medicine. 2014;43(6):405–409. doi: 10.1111/jop.12130. [DOI] [PubMed] [Google Scholar]
- 166.Negahdari S., Galehdari H., Kesmati M., Rezaie A., Shariati G. Wound healing activity of extracts and formulations of Aloe vera, Henna, Adiantum capillus-veneris, and Myrrh on mouse dermal fibroblast cells. International Journal of Preventive Medicine. 2017;8:p. 18. doi: 10.4103/ijpvm.ijpvm_338_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sciences. 2014;116(1):1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 168.Fadus M. C., Lau C., Bikhchandani J., Lynch H. T. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine. 2017;7(3):339–346. doi: 10.1016/j.jtcme.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gupta S. C., Patchva S., Aggarwal B. B. Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS Journal. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Joe B., Vijaykumar M., Lokesh B. R. Biological properties of curcumin-cellular and molecular mechanisms of action. Critical Reviews in Food Science and Nutrition. 2004;44(2):97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 171.Yang D., Xu J. H., Shi R. J. Root extractive from Daphne genkwa benefits in wound healing of anal fistula through up-regulation of collagen genes in human skin fibroblasts. Bioscience Reports. 2017;37(2) doi: 10.1042/bsr20170182.BSR20170182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bang K. K., Yun C.-Y., Lee C., et al. Melanogenesis inhibitory daphnane diterpenoids from the flower buds of Daphne genkwa. Bioorganic & Medicinal Chemistry Letters. 2013;23(11):3334–3337. doi: 10.1016/j.bmcl.2013.03.096. [DOI] [PubMed] [Google Scholar]
- 173.Zhan Z.-J., Fan C.-Q., Ding J., Yue J.-M. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorganic & Medicinal Chemistry. 2005;13(3):645–655. doi: 10.1016/j.bmc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 174.Du W. J., Ji J., Wang L., et al. Relationship between the UPLC-Q-TOF-MS fingerprinted constituents from Daphne genkwa and their anti-inflammatory, anti-oxidant activities. Biomedical Chromatography. 2017;31(12) doi: 10.1002/bmc.4012.e4012 [DOI] [PubMed] [Google Scholar]
- 175.Lee M. Y., Park B.-Y., Kwon O.-K., et al. Anti-inflammatory activity of (-)-aptosimon isolated from Daphne genkwa in RAW264.7 cells. International Immunopharmacology. 2009;9(7-8):878–885. doi: 10.1016/j.intimp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 176.Hong J.-Y., Chung H.-J., Lee H.-J., Park H. J., Lee S. K. Growth inhibition of human lung cancer cells via down-regulation of epidermal growth factor receptor signaling by yuanhuadine, a daphnane diterpene from Daphne genkwa. Journal of Natural Products. 2011;74(10):2102–2108. doi: 10.1021/np2003512. [DOI] [PubMed] [Google Scholar]
- 177.Zheng W., Gao X., Chen C., Tan R. Total flavonoids of Daphne genkwa root significantly inhibit the growth and metastasis of Lewis lung carcinoma in C57BL6 mice. International Immunopharmacology. 2007;7(2):117–127. doi: 10.1016/j.intimp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 178.Uyangaa E., Choi J. Y., Ryu H. W., Oh S.-R., Eo S. K. Anti-herpes activity of vinegar-processed Daphne genkwa flos via enhancement of natural killer cell activity. Immune Network. 2015;15(2):91–99. doi: 10.4110/in.2015.15.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang S. Z., Zhang X. J., Li X. Y., et al. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry. 2012;75:99–107. doi: 10.1016/j.phytochem.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 180.Widsten P., Cruz C. D., Fletcher G. C., Pajak M. A., McGhie T. K. Tannins and extracts of fruit byproducts: antibacterial activity against foodborne bacteria and antioxidant capacity. Journal of Agricultural and Food Chemistry. 2014;62(46):11146–11156. doi: 10.1021/jf503819t. [DOI] [PubMed] [Google Scholar]
- 181.Figueroa-Espinoza M. C., Zafimahova A., Alvarado P. G. M., Dubreucq E., Poncet-Legrand C. Grape seed and apple tannins: emulsifying and antioxidant properties. Food Chemistry. 2015;178:38–44. doi: 10.1016/j.foodchem.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 182.Su X., Liu X., Wang S., et al. Wound-healing promoting effect of total tannins from Entada phaseoloides (L.) Merr. in rats. Burns. 2017;43(4):830–838. doi: 10.1016/j.burns.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 183.Bhaskar A., Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian Journal of Pharmacology. 2012;44(6):694–698. doi: 10.4103/0253-7613.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Adhirajan N., Ravi Kumar T., Shanmugasundaram N., Babu M. In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn. Journal of Ethnopharmacology. 2003;88(2-3):235–239. doi: 10.1016/s0378-8741(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 185.Jadhav V. M., Kamble S. S., Kadam V. J. Herbal medicine: Syzygium cumini: a review. Journal of Pharmacy Research. 2009;2(8):1212–1219. [Google Scholar]
- 186.Khan Z. A., Naqvi S. A., Mukhtar A., et al. Antioxidant and antibacterial activities of Hibiscus Rosa-sinensis Linn flower extracts. Pakistan Journal of Pharmaceutical Sciences. 2014;27(3):469–474. [PubMed] [Google Scholar]
- 187.Shivananda Nayak B., Sivachandra Raju S., Orette F. A., Chalapathi Rao A. V. Effects of Hibiscus rosa sinensis L (Malvaceae) on wound healing activity: a preclinical study in a sprague dawley rat. The International Journal of Lower Extremity Wounds. 2007;6(2):76–81. doi: 10.1177/1534734607302840. [DOI] [PubMed] [Google Scholar]
- 188.Shen H.-M., Chen C., Jiang J.-Y., et al. The N-butyl alcohol extract from Hibiscus rosa-sinensis L. flowers enhances healing potential on rat excisional wounds. Journal of Ethnopharmacology. 2017;198:291–301. doi: 10.1016/j.jep.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 189.Sanodiya B., Thakur G., Baghel R., Prasad G., Bisen P. Ganoderma lucidum: a potent pharmacological macrofungus. Current Pharmaceutical Biotechnology. 2009;10(8):717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 190.Cheng P. G., Phan C.-W., Sabaratnam V., Abdullah N., Abdulla M. A., Kuppusamy U. R. Polysaccharides-rich extract of Ganoderma lucidum (M.A. Curtis:Fr.) P. Karst accelerates wound healing in streptozotocin-induced diabetic rats. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/671252.671252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boh B. Ganoderma lucidum: a potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Patents on Anti-Cancer Drug Discovery. 2013;8(3):255–287. doi: 10.2174/1574891x113089990036. [DOI] [PubMed] [Google Scholar]
- 192.Baig M. N., Shahid A. A., Ali M. In vitro assessment of extracts of the lingzhi or reishi medicinal mushroom, Ganoderma lucidum (higher basidiomycetes) against different plant pathogenic fungi. International Journal of Medicinal Mushrooms. 2015;17(4):407–411. doi: 10.1615/intjmedmushrooms.v17.i4.90. [DOI] [PubMed] [Google Scholar]
- 193.Oluba O. M., Olusola A. O., Fagbohunka B. S., Onyeneke E. C. Antimalarial and hepatoprotective effects of crude ethanolic extract of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.) P.Karst. (higher Basidiomycetes), in Plasmodium berghei-infected mice. International Journal of Medicinal Mushrooms. 2012;14(5):459–466. doi: 10.1615/intjmedmushr.v14.i5.30. [DOI] [PubMed] [Google Scholar]
- 194.Zhang W., Tao J., Yang X., et al. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochemical and Biophysical Research Communications. 2014;449(3):307–312. doi: 10.1016/j.bbrc.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 195.Rani P., Lal M. R., Maheshwari U., Krishnan S. Antioxidant potential of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Higher Basidiomycetes) cultivated on Artocarpus heterophyllus sawdust substrate in India. International Journal of Medicinal Mushrooms. 2015;17(12):1171–1177. doi: 10.1615/intjmedmushrooms.v17.i12.70. [DOI] [PubMed] [Google Scholar]
- 196.Xie Y. Z., Yang F., Tan W., et al. The anti-cancer components of Ganoderma lucidum possesses cardiovascular protective effect by regulating circular RNA expression. Oncoscience. 2016;3(7-8):203–207. doi: 10.18632/oncoscience.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pan D., Zhang D., Wu J., et al. Antidiabetic, antihyperlipidemic and antioxidant activities of a novel proteoglycan from Ganoderma lucidum fruiting bodies on db/db mice and the possible mechanism. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068332.e68332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hsieh T.-C., Wu J. M. Regulation of cell cycle transition and induction of apoptosis in HL-60 leukemia cells by the combination of Coriolus versicolor and Ganoderma lucidum. International Journal of Molecular Medicine. 2013;32(1):251–257. doi: 10.3892/ijmm.2013.1378. [DOI] [PubMed] [Google Scholar]
- 199.Suarez-Arroyo I. J., Rosario-Acevedo R., Aguilar-Perez A., et al. Anti-tumor effects of Ganoderma lucidum (reishi) in inflammatory breast cancer in In Vivo and In Vitro models. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057431.e57431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li F., Zhang Y., Zhong Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. International Journal of Molecular Sciences. 2011;12(9):6135–6145. doi: 10.3390/ijms12096135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gao Y., Tang W., Gao H., Chan E., Lan J., Zhou S. Ganoderma lucidum polysaccharide fractions accelerate healing of acetic acid-induced ulcers in rats. Journal of Medicinal Food. 2004;7(4):417–421. doi: 10.1089/jmf.2004.7.417. [DOI] [PubMed] [Google Scholar]
- 202.Gao Y., Zhou S., Wen J., Huang M., Xu A. Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sciences. 2002;72(6):731–745. doi: 10.1016/s0024-3205(02)02301-9. [DOI] [PubMed] [Google Scholar]
- 203.Jin X., Ruiz Beguerie J., Sze D. M., Chan G. C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database of Systematic Reviews. 2012;(6) doi: 10.1002/14651858.CD007731.pub2.CD007731 [DOI] [PubMed] [Google Scholar]
- 204.Tie L., Yang H.-Q., An Y., et al. Ganoderma lucidum polysaccharide accelerates refractory wound healing by inhibition of mitochondrial oxidative stress in type 1 diabetes. Cellular Physiology and Biochemistry. 2012;29(3-4):583–594. doi: 10.1159/000338512. [DOI] [PubMed] [Google Scholar]
- 205.Gu J., Chen J., Yang N., et al. Combination of Ligusticum chuanxiong and Radix Paeoniae ameliorate focal cerebral ischemic in MCAO rats via endoplasmic reticulum stress-dependent apoptotic signaling pathway. Journal of Ethnopharmacology. 2016;187:313–324. doi: 10.1016/j.jep.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 206.Ran X., Ma L., Peng C., Zhang H., Qin L.-P. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharmaceutical Biology. 2011;49(11):1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 207.Chen Z., Zhang C., Gao F., et al. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong) Food and Chemical Toxicology. 2018;119:309–325. doi: 10.1016/j.fct.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 208.Wu J.-G., Wei Y.-J., Ran X., Zhang H., Nian H., Qin L.-P. Inhibitory effects of essential oil from rhizomes of Ligusticum chuanxiong on hypertrophic scarring in the rabbit ear model. Pharmaceutical Biology. 2011;49(7):764–769. doi: 10.3109/13880209.2010.542761. [DOI] [PubMed] [Google Scholar]
- 209.Fu J., Yang L., Khan M. A., Mei Z. Genetic characterization and authentication of Lonicera japonica Thunb. by using improved RAPD analysis. Molecular Biology Reports. 2013;40(10):5993–5999. doi: 10.1007/s11033-013-2703-3. [DOI] [PubMed] [Google Scholar]
- 210.Shang X., Pan H., Li M., Miao X., Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology. 2011;138(1):1–21. doi: 10.1016/j.jep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li Y., Cai W., Weng X., et al. Lonicerae Japonicae Flos and Lonicerae Flos: a systematic pharmacology review. Evidence-Based Complementary and Alternative Medicine. 2015;2015:16. doi: 10.1155/2015/905063.905063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen W. C., Liou S.-S., Tzeng T.-F., Lee S.-L., Liu I.-M. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complementary and Alternative Medicine. 2012;12:p. 226. doi: 10.1186/1472-6882-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou S. L., Zou X.-H., Zhou Z.-Q., et al. Multiple species of wild tree peonies gave rise to the ‘king of flowers’, Paeonia suffruticosa Andrews. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1797) doi: 10.1098/rspb.2014.1687.20141687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rho S., Chung H.-S., Kang M., et al. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of Moutan Cortex Radicis in oxidative stressed PC12 cells. Biological & Pharmaceutical Bulletin. 2005;28(4):661–666. doi: 10.1248/bpb.28.661. [DOI] [PubMed] [Google Scholar]
- 215.Kim H. G., Park G., Piao Y., et al. Effects of the root bark of Paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson’s disease. Food and Chemical Toxicology. 2014;65:293–300. doi: 10.1016/j.fct.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 216.Xing G., Zhang Z., Liu J., Hu H., Sugiura N. Antitumor effect of extracts from moutan cortex on DLD-1 human colon cancer cells in vitro. Molecular Medicine Reports. 2010;3(1):57–61. doi: 10.3892/mmr_00000218. [DOI] [PubMed] [Google Scholar]
- 217.Wu M., Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evidence-Based Complementary and Alternative Medicine. 2009;6(1):63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hong H., Wang Q. M., Zhao Z. P., et al. Studies on antidiabetic effects of cortex Moutan polysaccharide-2b in type 2 diabetes mellitus rats. Yao Xue Xue Bao. 2003;38(4):255–259. [PubMed] [Google Scholar]
- 219.He D. Y., Dai S. M. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional Chinese herbal medicine. Frontiers in Pharmacology. 2011;2:p. 10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang R., Lechtenberg M., Sendker J., Petereit F., Deters A., Hensel A. Wound-healing plants from TCM: in vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia. 2013;84:308–317. doi: 10.1016/j.fitote.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 221.Kim W. K., Song S.-Y., Oh W. K., et al. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. European Journal of Pharmacology. 2013;702(1–3):285–293. doi: 10.1016/j.ejphar.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 222.Arring N. M., Millstine D., Marks L. A., Nail L. M. Ginseng as a treatment for fatigue: a systematic review. The Journal of Alternative and Complementary Medicine. 2018;24(7):624–633. doi: 10.1089/acm.2017.0361. [DOI] [PubMed] [Google Scholar]
- 223.Jovanovski E., Peeva V., Sievenpiper J. L., et al. Modulation of endothelial function by Korean red ginseng (Panax ginseng C.A. Meyer) and its components in healthy individuals: a randomized controlled trial. Cardiovascular Therapeutics. 2014;32(4):163–169. doi: 10.1111/1755-5922.12077. [DOI] [PubMed] [Google Scholar]
- 224.Lee L.-S., Cho C.-W., Hong H.-D., Lee Y.-C., Choi U.-K., Kim Y.-C. Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits. Molecules. 2013;18(10):12548–12560. doi: 10.3390/molecules181012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang Y., Yang W. S., Yu T., et al. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. Journal of Ethnopharmacology. 2014;154(1):218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 226.Jiao L., Li B., Wang M., Liu Z., Zhang X., Liu S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C.A. Meyer. Carbohydrate Polymers. 2014;106:293–298. doi: 10.1016/j.carbpol.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 227.Li C., Tian Z.-N., Cai J.-P., et al. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer. Carbohydrate Polymers. 2014;102:103–109. doi: 10.1016/j.carbpol.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 228.Fukuyama N., Shibuya M., Orihara Y. Antimicrobial polyacetylenes from Panax ginseng hairy root culture. Chemical and Pharmaceutical Bulletin. 2012;60(3):377–380. doi: 10.1248/cpb.60.377. [DOI] [PubMed] [Google Scholar]
- 229.Sumiyoshi M., Sakanaka M., Kimura Y. Effects of red ginseng extract on allergic reactions to food in Balb/c mice. Journal of Ethnopharmacology. 2010;132(1):206–212. doi: 10.1016/j.jep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 230.Hwang E., Park S.-Y., Yin C. S., Kim H.-T., Kim Y. M., Yi T. H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. Journal of Ginseng Research. 2017;41(1):69–77. doi: 10.1016/j.jgr.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee J., Hwang H., Ko E.-J., et al. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 2014;6(2):517–529. doi: 10.3390/nu6020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cho J.-G., Lee M.-K., Lee J.-W., et al. Physicochemical Characterization and NMR Assignments of Ginsenosides Rb1, Rb2, Rc, and Rd Isolated from Panax ginseng. Journal of Ginseng Research. 2010;34(2):113–121. doi: 10.5142/jgr.2010.34.2.113. [DOI] [Google Scholar]
- 233.Kim Y. G., Sumiyoshi M., Kawahira K., Sakanaka M., Kimura Y. Effects of Red Ginseng extract on ultraviolet B-irradiated skin change in C57BL mice. Phytotherapy Research. 2008;22(11):1423–1427. doi: 10.1002/ptr.2339. [DOI] [PubMed] [Google Scholar]
- 234.Kimura Y., Sumiyoshi M., Kawahira K., Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. British Journal of Pharmacology. 2006;148(6):860–870. doi: 10.1038/sj.bjp.0706794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Morisaki N., Watanabe S., Tezuka M., et al. Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. British Journal of Pharmacology. 1995;115(7):1188–1193. doi: 10.1111/j.1476-5381.1995.tb15023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kim Y.-S., Cho I.-H., Jeong M.-J., et al. Therapeutic effect of total ginseng saponin on skin wound healing. Journal of Ginseng Research. 2011;35(3):360–367. doi: 10.5142/jgr.2011.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen X., Wang M., Xu X., et al. Panax ginseng total protein promotes proliferation and secretion of collagen in NIH/3T3 cells by activating extracellular signal-related kinase pathway. Journal of Ginseng Research. 2017;41(3):411–418. doi: 10.1016/j.jgr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee J., Jung E., Lee J., et al. Panax ginseng induces human type I collagen synthesis through activation of Smad signaling. Journal of Ethnopharmacology. 2007;109(1):29–34. doi: 10.1016/j.jep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 239.Choi S. Epidermis proliferative effect of the Panax ginseng Ginsenoside Rb2. Archives of Pharmacal Research. 2002;25(1):71–76. doi: 10.1007/bf02975265. [DOI] [PubMed] [Google Scholar]
- 240.Shen K., Ji L., Gong C., et al. Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochemical Pharmacology. 2012;84(6):784–792. doi: 10.1016/j.bcp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 241.Hong S.-J., Wan J.-B., Zhang Y., et al. Angiogenic effect of saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytotherapy Research. 2009;23(5):677–686. doi: 10.1002/ptr.2705. [DOI] [PubMed] [Google Scholar]
- 242.Zhou N., Tang Y., Keep R. F., Ma X., Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21(10):1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Huang X.-P., Ding H., Lu J.-D., Tang Y.-H., Deng B.-X., Deng C.-Q. Effects of the combination of the main active components of Astragalus and Panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. The American Journal of Chinese Medicine. 2015;43(7):1419–1438. doi: 10.1142/s0192415x15500809. [DOI] [PubMed] [Google Scholar]
- 244.Sun H. X., Ye Y.-P., Pan H.-J., Pan Y.-J. Adjuvant effect of Panax notoginseng saponins on the immune responses to ovalbumin in mice. Vaccine. 2004;22(29-30):3882–3889. doi: 10.1016/j.vaccine.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 245.Zeng X.-S., Zhou X.-S., Luo F.-C., et al. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Canadian Journal of Physiology and Pharmacology. 2014;92(2):102–108. doi: 10.1139/cjpp-2013-0274. [DOI] [PubMed] [Google Scholar]
- 246.Yang Q., Wang P., Cui J., Wang W., Chen Y., Zhang T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. Journal of Ethnopharmacology. 2016;193:255–265. doi: 10.1016/j.jep.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 247.Uzayisenga R., Ayeka P. A., Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytotherapy Research. 2014;28(4):510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 248.Peng X.-d., Dai L.-l., Huang C.-q., He C.-m., Yang B., Chen L.-j. Relationship between anti-fibrotic effect of Panax notoginseng saponins and serum cytokines in rat hepatic fibrosis. Biochemical and Biophysical Research Communications. 2009;388(1):31–34. doi: 10.1016/j.bbrc.2009.07.099. [DOI] [PubMed] [Google Scholar]
- 249.Jung H.-W., Seo U.-K., Kim J.-H., Leem K.-H., Park Y.-K. Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-κB signaling pathway in murine macrophages. Journal of Ethnopharmacology. 2009;122(2):313–319. doi: 10.1016/j.jep.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 250.Makino S., Mitsutake N., Nakashima M., et al. DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I collagen accumulation in keloid fibroblasts. Journal of Dermatological Science. 2008;51(3):171–180. doi: 10.1016/j.jdermsci.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 251.Ghazizadeh M. Essential role of IL-6 signaling pathway in keloid pathogenesis. Journal of Nippon Medical School. 2007;74(1):11–22. doi: 10.1272/jnms.74.11. [DOI] [PubMed] [Google Scholar]
- 252.Lau A.-J., Toh D.-F., Chua T.-K., Pang Y.-K., Woo S.-O., Koh H.-L. Antiplatelet and anticoagulant effects of Panax notoginseng: comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium. Journal of Ethnopharmacology. 2009;125(3):380–386. doi: 10.1016/j.jep.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 253.Peng M., Yi Y. X., Zhang T., Ding Y., Le J. Stereoisomers of saponins in Panax notoginseng (Sanqi): a review. Frontiers in Pharmacology. 2018;9:p. 188. doi: 10.3389/fphar.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu J., Shiono J., Shimizu K., et al. 20(R)-ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorganic & Medicinal Chemistry Letters. 2009;19(12):3320–3323. doi: 10.1016/j.bmcl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 255.Peng W., Qin R., Li X., Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. Journal of Ethnopharmacology. 2013;148(3):729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 256.Lee M.-H., Kao L., Lin C.-C. Comparison of the antioxidant and transmembrane permeative activities of the different Polygonum cuspidatum extracts in phospholipid-based microemulsions. Journal of Agricultural and Food Chemistry. 2011;59(17):9135–9141. doi: 10.1021/jf201577f. [DOI] [PubMed] [Google Scholar]
- 257.Su P.-W., Yang C.-H., Yang J.-F., Su P.-Y., Chuang L.-Y. Antibacterial activities and antibacterial mechanism of Polygonum cuspidatum extracts against nosocomial drug-resistant pathogens. Molecules. 2015;20(6):11119–11130. doi: 10.3390/molecules200611119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jiao Y., Wu Y., Du D. Polydatin inhibits cell proliferation, invasion and migration, and induces cell apoptosis in hepatocellular carcinoma. Brazilian Journal of Medical and Biological Research. 2018;51(4) doi: 10.1590/1414-431x20176867.e6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wu X.-b., Luo X.-q., Gu S.-y., Xu J.-h. The effects of Polygonum cuspidatum extract on wound healing in rats. Journal of Ethnopharmacology. 2012;141(3):934–937. doi: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 260.Liu Z., Wei F., Chen L.-J., et al. In vitro and in vivo studies of the inhibitory effects of emodin isolated from Polygonum cuspidatum on coxsakievirus B4. Molecules. 2013;18(10):11842–11858. doi: 10.3390/molecules181011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chen X., Yang L., Oppenheim J. J., Howard O. M. Z. Cellular pharmacology studies of shikonin derivatives. Phytotherapy Research. 2002;16(3):199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 262.Li X., Fan X.-X., Jiang Z.-B., et al. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacological Research. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 263.Fan C., Xie Y., Dong Y., Su Y., Upton Z. Investigating the potential of Shikonin as a novel hypertrophic scar treatment. Journal of Biomedical Science. 2015;22:p. 70. doi: 10.1186/s12929-015-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zeng Z., Zhu B.-H. Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. Journal of Ethnopharmacology. 2014;154(3):653–662. doi: 10.1016/j.jep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 265.Yanwen W., Wenyuan G., Xiaohe X., Yi L. Calorimetric investigation of the effect of hydroxyanthraquinones in Rheum officinale Baill on Staphylococcus aureus growth. Thermochimica Acta. 2005;429(2):167–170. doi: 10.1016/j.tca.2005.03.008. [DOI] [Google Scholar]
- 266.Clementi E. M., Misiti F. Bioactive Foods in Promoting Health Fruits and Vegetables. Cambridge, MA, USA: Academic Press; 2010. Potential health benefits of rhubarb; pp. 407–423. [Google Scholar]
- 267.Chu X., Wei M., Yang X., et al. Effects of an anthraquinone derivative from Rheum officinale Baill, emodin, on airway responses in a murine model of asthma. Food and Chemical Toxicology. 2012;50(7):2368–2375. doi: 10.1016/j.fct.2012.03.076. [DOI] [PubMed] [Google Scholar]
- 268.Li W.-Y., Chan S.-W., Guo D.-J., et al. Water extract of Rheum officinale Baill. induces apoptosis in human lung adenocarcinoma A549 and human breast cancer MCF-7 cell lines. Journal of Ethnopharmacology. 2009;124(2):251–256. doi: 10.1016/j.jep.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 269.Wang H., Song H., Yue J., Li J., Hou Y. B., Deng J. L. Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane Database of Systematic Reviews. 2012;(7) doi: 10.1002/14651858.cd008000.pub2.CD008000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tang T., Yin L., Yang J., Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. European Journal of Pharmacology. 2007;567(3):177–185. doi: 10.1016/j.ejphar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 271.Sung B. Chapter 3-regulation of inflammation-mediated chronic diseases by botanicals. In: Shyur L.-F., Lau A. S. Y., editors. Advances in Botanical Research. Cambridge, MA, USA: Academic Press; 2012. pp. 57–132. [Google Scholar]
- 272.Kumar A., Dhawan S., Aggarwal B. B. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-κB activation, IκB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17(7):913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- 273.Subramaniam A., Shanmugam M. K., Ong T. H., et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. British Journal of Pharmacology. 2013;170(4):807–821. doi: 10.1111/bph.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gupta A., Kumar R., Upadhyay N., Pal K., Kumar R., Sawhney R. Effects of Rhodiola imbricata on dermal wound healing. Planta Medica. 2007;73(8):774–777. doi: 10.1055/s-2007-981546. [DOI] [PubMed] [Google Scholar]
- 275.Mishra K. P., Ganju L., Singh S. B. Anti-cellular and immunomodulatory potential of aqueous extract of Rhodiola imbricatarhizome. Immunopharmacology and Immunotoxicology. 2012;34(3):513–518. doi: 10.3109/08923973.2011.638307. [DOI] [PubMed] [Google Scholar]
- 276.Gupta V., Lahiri S. S., Sultana S., Tulsawani R. K., Kumar R. Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C-H-R) exposure and post-stress recovery. Food and Chemical Toxicology. 2010;48(4):1019–1025. doi: 10.1016/j.fct.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 277.Senthilkumar R., Chandran R., Parimelazhagan T. Hepatoprotective effect of Rhodiola imbricata rhizome against paracetamol-induced liver toxicity in rats. Saudi Journal of Biological Sciences. 2014;21(5):409–416. doi: 10.1016/j.sjbs.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Goel H. C., Bala M., Prasad J., Singh S., Agrawala P. K., Swahney R. C. Radioprotection by Rhodiola imbricata in mice against whole-body lethal irradiation. Journal of Medicinal Food. 2006;9(2):154–160. doi: 10.1089/jmf.2006.9.154. [DOI] [PubMed] [Google Scholar]
- 279.Senthilkumar R., Parimelazhagan T., Chaurasia O. P., Srivastava R. B. Free radical scavenging property and antiproliferative activity of Rhodiola imbricata Edgew extracts in HT-29 human colon cancer cells. Asian Pacific Journal of Tropical Medicine. 2013;6(1):11–19. doi: 10.1016/s1995-7645(12)60194-1. [DOI] [PubMed] [Google Scholar]
- 280.Su C.-Y., Ming Q.-L., Rahman K., Han T., Qin L.-P. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chinese Journal of Natural Medicines. 2015;13(3):163–182. doi: 10.1016/s1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 281.Zhang J., Li Y., Chen X., Pan Y., Zhang S., Wang Y. Systems pharmacology dissection of multi-scale mechanisms of action for herbal medicines in stroke treatment and prevention. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0102506.e102506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Luo J., Song W., Yang G., Xu H., Chen K. Compound Danshen (Salvia miltiorrhiza) dripping pill for coronary heart disease: an overview of systematic reviews. The American Journal of Chinese Medicine. 2015;43(1):25–43. doi: 10.1142/s0192415x15500020. [DOI] [PubMed] [Google Scholar]
- 283.Zhou L., Zuo Z., Chow M. S. S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. The Journal of Clinical Pharmacology. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 284.Chen J., Lv Q., Yu M., Zhang X., Gou J. Randomized clinical trial of Chinese herbal medications to reduce wound complications after mastectomy for breast carcinoma. British Journal of Surgery. 2010;97(12):1798–1804. doi: 10.1002/bjs.7227. [DOI] [PubMed] [Google Scholar]
- 285.Lay I.-S., Hsieh C.-C., Chiu J.-H., Shiao M.-S., Lui W.-Y., Wu C.-W. Salvianolic acid b enhances in vitro angiogenesis and improves skin flap survival in sprague-dawley rats1. Journal of Surgical Research. 2003;115(2):279–285. doi: 10.1016/s0022-4804(03)00226-9. [DOI] [PubMed] [Google Scholar]
- 286.Tang Y., Wang M., Le X., et al. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011;18(12):1024–1030. doi: 10.1016/j.phymed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 287.Tsai M.-K., Lin Y.-L., Huang Y.-T. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicology and Applied Pharmacology. 2010;242(2):155–164. doi: 10.1016/j.taap.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 288.Chen T., Liu W., Chao X., et al. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Research Bulletin. 2011;84(2):163–168. doi: 10.1016/j.brainresbull.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 289.Zhao J., Lou J., Mou Y., Li P., Wu J., Zhou L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules. 2011;16(3):2259–2267. doi: 10.3390/molecules16032259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gao H., Sun W., Zhao J., et al. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen) Scientific Reports. 2016;6 doi: 10.1038/srep33720.33720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sung B., Chung H. S., Kim M., et al. Cytotoxic effects of solvent-extracted active components of Salvia miltiorrhiza Bunge on human cancer cell lines. Experimental and Therapeutic Medicine. 2015;9(4):1421–1428. doi: 10.3892/etm.2015.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wang J., Xiong X., Feng B. Cardiovascular effects of salvianolic acid B. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/247948.247948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chen Y.-S., Lee S.-M., Lin Y.-J., Chiang S.-H., Lin C.-C. Effects of danshensu and salvianolic acid B from Salvia miltiorrhiza bunge (Lamiaceae) on cell proliferation and collagen and melanin production. Molecules. 2014;19(2):2029–2041. doi: 10.3390/molecules19022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang Q.-L., Tao Y.-Y., Yuan J.-L., Shen L., Liu C.-H. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biology. 2010;11(1):p. 31. doi: 10.1186/1471-2121-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Li Y., Shi S., Gao J., et al. Cryptotanshinone downregulates the profibrotic activities of hypertrophic scar fibroblasts and accelerates wound healing: a potential therapy for the reduction of skin scarring. Biomedicine & Pharmacotherapy. 2016;80:80–86. doi: 10.1016/j.biopha.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 296.Zhang H., Chen J., Cen Y. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. International Journal of Biological Macromolecules. 2018;112:862–867. doi: 10.1016/j.ijbiomac.2018.01.214. [DOI] [PubMed] [Google Scholar]
- 297.Sun W., Zhang Z.-L., Liu X., et al. Terpene glycosides from the roots of Sanguisorba officinalis L. and their hemostatic activities. Molecules. 2012;17(7):7629–7636. doi: 10.3390/molecules17077629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang L., Koyyalamudi S. R., Jeong S. C., et al. Antioxidant and immunomodulatory activities of polysaccharides from the roots of Sanguisorba officinalis. International Journal of Biological Macromolecules. 2012;51(5):1057–1062. doi: 10.1016/j.ijbiomac.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 299.Yang J.-H., Hwang Y.-H., Gu M.-J., Cho W.-K., Ma J. Y. Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine. 2015;22(14):1262–1268. doi: 10.1016/j.phymed.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 300.Su X. D., Guo R. H., Li H. X., et al. Anti-allergic inflammatory components from Sanguisorba officinalis L. Bioorganic & Medicinal Chemistry Letters. 2018;28(12):2210–2216. doi: 10.1016/j.bmcl.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 301.Yang J. H., Yoo J.-M., Cho W.-K., Ma J. Y. Anti-inflammatory effects of sanguisorbae radix water extract on the suppression of mast cell degranulation and STAT-1/Jak-2 activation in BMMCs and HaCaT keratinocytes. BMC Complementary and Alternative Medicine. 2016;16:p. 347. doi: 10.1186/s12906-016-1317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Xu X., Li X., Zhang L., et al. Enhancement of wound healing by the traditional Chinese medicine herbal mixture Sophora flavescens in a rat model of perianal ulceration. In Vivo. 2017;31(4):543–549. doi: 10.21873/invivo.11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shin D. H., Cha Y. J., Joe G. J., et al. Whitening effect of Sophora flavescens extract. Pharmaceutical Biology. 2013;51(11):1467–1476. doi: 10.3109/13880209.2013.799708. [DOI] [PubMed] [Google Scholar]
- 304.Takahashi T., Ishino A., Arai T., et al. Improvement of androgenetic alopecia with topical Sophora flavescens aiton extract, and identification of the two active compounds in the extract that stimulate proliferation of human hair keratinocytes. Clinical and Experimental Dermatology. 2016;41(3):302–307. doi: 10.1111/ced.12753. [DOI] [PubMed] [Google Scholar]
- 305.Fan L.-L., Zhu S., Chen H.-B., Yang D.-H., Cai S.-Q., Komatsu K. Molecular analysis of stemona plants in china based on sequences of four chloroplast DNA regions. Biological & Pharmaceutical Bulletin. 2009;32(8):1439–1446. doi: 10.1248/bpb.32.1439. [DOI] [PubMed] [Google Scholar]
- 306.Jung K.-H., Kil Y.-S., Jung J., et al. Tuberostemonine N, an active compound isolated from Stemona tuberosa, suppresses cigarette smoke-induced sub-acute lung inflammation in mice. Phytomedicine. 2016;23(1):79–86. doi: 10.1016/j.phymed.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 307.Lin L.-G., Yang X.-Z., Tang C.-P., Ke C.-Q., Zhang J.-B., Ye Y. Antibacterial stilbenoids from the roots of Stemona tuberosa. Phytochemistry. 2008;69(2):457–463. doi: 10.1016/j.phytochem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 308.Brem B., Seger C., Pacher T., et al. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry. 2004;65(19):2719–2729. doi: 10.1016/j.phytochem.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 309.Kil Y.-S., Park J., Han A.-R., Woo H., Seo E.-K. A new 9,10-dihydrophenanthrene and cell proliferative 3,4-δ-dehydrotocopherols from Stemona tuberosa. Molecules. 2015;20(4):5965–5974. doi: 10.3390/molecules20045965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Balekar N., Nakpheng T., Katkam N. G., Srichana T. Wound healing activity of ent-kaura-9(11),16-dien-19-oic acid isolated from Wedelia trilobata (L.) leaves. Phytomedicine. 2012;19(13):1178–1184. doi: 10.1016/j.phymed.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 311.Govindappa M. Antimicrobial, antioxidant and in vivo anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. Journal of Medicinal Plants Research. 2011;5(24):5718–5729. [Google Scholar]
- 312.Weng Z., Patel A. B., Vasiadi M., Therianou A., Theoharides T. C. Luteolin inhibits human keratinocyte activation and decreases NF-kappaB induction that is increased in psoriatic skin. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090739.e90739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lan Y., Wu Q., Mao Y.-q., et al. Cytotoxicity and enhancement activity of essential oil from Zanthoxylum bungeanum Maxim. as a natural transdermal penetration enhancer. Journal of Zhejiang University Science B. 2014;15(2):153–164. doi: 10.1631/jzus.b1300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rong R., Cui M.-Y., Zhang Q.-L., et al. Anesthetic constituents of Zanthoxylum bungeanum Maxim.: a pharmacokinetic study. Journal of Separation Science. 2016;39(14):2728–2735. doi: 10.1002/jssc.201600295. [DOI] [PubMed] [Google Scholar]
- 315.Zhang Y., Dong H., Zhang J., Zhang L. Inhibitory effect of hyperoside isolated from Zanthoxylum bungeanum leaves on SW620 human colorectal cancer cells via induction of the p53 signaling pathway and apoptosis. Molecular Medicine Reports. 2017;16(2):1125–1132. doi: 10.3892/mmr.2017.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang Y., Wang D., Yang L., Zhou D., Zhang J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105725.e105725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhang Z., Liu J., Shen P., et al. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. International Immunopharmacology. 2016;41:127–135. doi: 10.1016/j.intimp.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 318.Tang W., Xie Q., Guan J., Jin S., Zhao Y. Phytochemical profiles and biological activity evaluation of Zanthoxylum bungeanum Maxim seed against asthma in murine models. Journal of Ethnopharmacology. 2014;152(3):444–450. doi: 10.1016/j.jep.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 319.Bautista D. M., Sigal Y. M., Milstein A. D., et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nature Neuroscience. 2008;11(7):772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhang M., Wang J., Zhu L., et al. Zanthoxylum bungeanum Maxim. (Rutaceae): a systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. International Journal of Molecular Sciences. 2017;18(10) doi: 10.3390/ijms18102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Artaria C., Maramaldi G., Bonfigli A., Rigano L., Appendino G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. International Journal of Cosmetic Science. 2011;33(4):328–333. doi: 10.1111/j.1468-2494.2010.00629.x. [DOI] [PubMed] [Google Scholar]
- 322.Lan Y., Li H., Chen Y.-y., et al. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: a comparative study. Journal of Zhejiang University Science B. 2014;15(11):940–952. doi: 10.1631/jzus.b1400158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Molazem Z., Mohseni F., Younesi M., Keshavarzi S. Aloe vera gel and cesarean wound healing; a randomized controlled clinical trial. Global Journal of Health Science. 2014;7(1):203–209. doi: 10.5539/gjhs.v7n1p203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Gao Q., Yang M., Zuo Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacologica Sinica. 2018;39(5):787–801. doi: 10.1038/aps.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Choi M.-R., Choi D.-K., Kim K.-D., et al. Ampelopsis japonica makino extract inhibits the inflammatory reaction induced by pathogen-associated molecular patterns in epidermal keratinocytes. Annals of Dermatology. 2016;28(3):352–359. doi: 10.5021/ad.2016.28.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 326.Okhuarobo A., Ehizogie Falodun J., Erharuyi O., Imieje V., Falodun A., Langer P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pacific Journal of Tropical Disease. 2014;4(3):213–222. doi: 10.1016/s2222-1808(14)60509-0. [DOI] [Google Scholar]
- 327.Huang K.-F., Hsu Y.-C., Lin C.-N., Tzeng J.-I., Chen Y.-W., Wang J.-J. Shiunko promotes epithelization of wounded skin. The American Journal of Chinese Medicine. 2004;32(03):389–396. doi: 10.1142/s0192415x04002041. [DOI] [PubMed] [Google Scholar]
- 328.Zhao H., Deneau J., Che G. O. L., et al. Angelica sinensis isolate SBD.4: composition, gene expression profiling, mechanism of action and effect on wounds, in rats and humans. European Journal of Dermatology. 2012;22(1):58–67. doi: 10.1684/ejd.2011.1599. [DOI] [PubMed] [Google Scholar]
- 329.Dozmorov M. G., Yang Q., Wu W., et al. Differential effects of selective frankincense (Ru Xiang) essential oil versus non-selective sandalwood (Tan Xiang) essential oil on cultured bladder cancer cells: a microarray and bioinformatics study. Chinese Medicine. 2014;9(1):p. 18. doi: 10.1186/1749-8546-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Buzzi M., Freitas F. d., Winter M. d. B. Cicatrização de úlceras por pressão com extrato Plenusdermax de Calendula officinalis L. Revista Brasileira de Enfermagem. 2016;69(2):250–257. doi: 10.1590/0034-7167.2016690207i. [DOI] [PubMed] [Google Scholar]
- 331.Carvalho A. F. M. d., Feitosa M. C. P., Coelho N. P. M. d. F., et al. Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Revista da Escola de Enfermagem da USP. 2016;50(4):628–634. doi: 10.1590/s0080-623420160000500013. [DOI] [PubMed] [Google Scholar]
- 332.Akram M., Hamid A., Khalil A., et al. Review on medicinal uses, pharmacological, phytochemistry and immunomodulatory activity of plants. International Journal of Immunopathology and Pharmacology. 2014;27(3):313–319. doi: 10.1177/039463201402700301. [DOI] [PubMed] [Google Scholar]
- 333.Xia Q., Ma Z., Mei X., et al. Assay for the developmental toxicity of safflower (Carthamus tinctorius L.) to zebrafish embryos/larvae. Journal of Traditional Chinese Medical Sciences. 2017;4(1):71–81. doi: 10.1016/j.jtcms.2017.05.004. [DOI] [Google Scholar]
- 334.Zhao G., Gai Y., Chu W.-J., Qin G.-W., Guo L.-H. A novel compound N1,N5-(Z)-N10-(E)-tri-p-coumaroylspermidine isolated from Carthamus tinctorius L. and acting by serotonin transporter inhibition. European Neuropsychopharmacology. 2009;19(10):749–758. doi: 10.1016/j.euroneuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 335.Zhou X., Tang L., Xu Y., Zhou G., Wang Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. Journal of Ethnopharmacology. 2014;151(1):27–43. doi: 10.1016/j.jep.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 336.Kanu C. L., Imosemi I. O., Malomo A. O. A review of the multifaceted usefulness of Celosia argentea Linn. European Journal of Pharmaceutical and Medical Research. 2017;4(10):72–79. [Google Scholar]
- 337.Bylka W., Znajdek-Awiżeń P., Studzińska-Sroka E., Brzezińska M. Centella asiatica in cosmetology. Advances in Dermatology and Allergology. 2013;1(1):46–49. doi: 10.5114/pdia.2013.33378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Brinkhaus B., Lindner M., Schuppan D., Hahn E. G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine. 2000;7(5):427–448. doi: 10.1016/s0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 339.Farahpour M., Habibi M. Evaluation of the wound healing activity of an ethanolic extract of Ceylon cinnamon in mice. Veterinární Medicína. 2012;57(1):53–57. doi: 10.17221/4972-vetmed. [DOI] [Google Scholar]
- 340.Khan A., Safdar M., Ali Khan M. M., Khattak K. N., Anderson R. A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 341.Ooi L. S. M., Li Y., Kam S.-L., Wang H., Wong E. Y. L., Ooi V. E. C. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. The American Journal of Chinese Medicine. 2006;34(3):511–522. doi: 10.1142/s0192415x06004041. [DOI] [PubMed] [Google Scholar]
- 342.Yang C.-H., Li R.-X., Chuang L.-Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules. 2012;17(6):7294–7304. doi: 10.3390/molecules17067294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Carvalho D. d. M., Takeuchi K. P., Geraldine R. M., Moura C. J. d., Torres M. C. L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Science and Technology. 2015;35(1):115–119. doi: 10.1590/1678-457x.6515. [DOI] [Google Scholar]
- 344.Mun S. H., Joung D.-K., Kim Y.-S., et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20(8-9):714–718. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 345.Liang G., Yang S., Zhou H., et al. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. European Journal of Medicinal Chemistry. 2009;44(2):915–919. doi: 10.1016/j.ejmech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 346.Meng B., Li J., Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Current Pharmaceutical Design. 2013;19(11):2101–2113. doi: 10.2174/1381612811319110011. [DOI] [PubMed] [Google Scholar]
- 347.Thangapazham R. L., Sharad S., Maheshwari R. K. Skin regenerative potentials of curcumin. Biofactors. 2013;39(1):141–149. doi: 10.1002/biof.1078. [DOI] [PubMed] [Google Scholar]
- 348.Ryan J. L., Heckler C. E., Ling M., et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiation Research. 2013;180(1):34–43. doi: 10.1667/rr3255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mohanty C., Sahoo S. K. Curcumin and its topical formulations for wound healing applications. Drug Discovery Today. 2017;22(10):1582–1592. doi: 10.1016/j.drudis.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 350.Zhang R., Wang Y., Li J., Jin H., Song S., Huang C. The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M arrest in human cancer cells by up-regulating p21 protein expression through an p53 protein-independent cascade. Journal of Biological Chemistry. 2014;289(10):6394–6403. doi: 10.1074/jbc.m113.513960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zhao Y. H., Lin Y. F., Zhao Y. The analgesic activity study on Entada phaseoloides total saponin. Journal of Medicine & Pharmacy of Chinese Minorities. 2011;2:53–55. [Google Scholar]
- 352.Dong Y., Shi H., Yang H., Peng Y., Wang M., Li X. Antioxidant phenolic compounds from the stems of Entada phaseoloides. Chemistry & Biodiversity. 2012;9(1):68–79. doi: 10.1002/cbdv.201100002. [DOI] [PubMed] [Google Scholar]
- 353.Chang Y.-C., Huang K.-X., Huang A.-C., Ho Y.-C., Wang C.-J. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food and Chemical Toxicology. 2006;44(7):1015–1023. doi: 10.1016/j.fct.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 354.Son H.-U., Lee S., Heo J.-C., Lee S.-H. The solid-state fermentation of Artemisia capillaris leaves with Ganoderma lucidum enhances the anti-inflammatory effects in a model of atopic dermatitis. International Journal of Molecular Medicine. 2017;39(5):1233–1241. doi: 10.3892/ijmm.2017.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Xiao Y., Wang Y.-C., Li L.-L., et al. Lactones from Ligusticum chuanxiong Hort. reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-kB -dependent adhesion molecules. Fitoterapia. 2014;95:240–246. doi: 10.1016/j.fitote.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 356.Liu J.-L., Zheng S.-L., Fan Q.-J., et al. Optimisation of high-pressure ultrasonic-assisted extraction and antioxidant capacity of polysaccharides from the rhizome of Ligusticum chuanxiong. International Journal of Biological Macromolecules. 2015;76:80–85. doi: 10.1016/j.ijbiomac.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 357.Lin Y.-L., Wang G.-J., Huang C.-L., et al. Ligusticum chuanxiong as a potential neuroprotectant for preventing serum deprivation-induced apoptosis in rat pheochromocytoma cells: functional roles of mitogen-activated protein kinases. Journal of Ethnopharmacology. 2009;122(3):417–423. doi: 10.1016/j.jep.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 358.Liang M.-J., He L.-C., Yang G.-D. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong. Life Sciences. 2005;78(2):128–133. doi: 10.1016/j.lfs.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 359.Matsuda H., Ohta T., Kawaguchi A., Yoshikawa M. Bioactive constituents of Chinese natural medicines. VI. Moutan cortex. (2): structures and radical scavenging effects of suffruticosides A, B, C, D, and E and galloyl-oxypaeoniflorin. Chemical and Pharmaceutical Bulletin. 2001;49(1):69–72. doi: 10.1248/cpb.49.69. [DOI] [PubMed] [Google Scholar]
- 360.Sun L., Liu L., Zong S., et al. Traditional Chinese medicine Guizhi Fuling capsule used for therapy of dysmenorrhea via attenuating uterus contraction. Journal of Ethnopharmacology. 2016;191:273–279. doi: 10.1016/j.jep.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 361.Dan H., Zhang L., Qin X., et al. Moutan cortex extract exerts protective effects in a rat model of cardiac ischemia/reperfusion. Canadian Journal of Physiology and Pharmacology. 2016;94(3):245–250. doi: 10.1139/cjpp-2015-0168. [DOI] [PubMed] [Google Scholar]
- 362.Xiong Y., Chen L., Man J., Hu Y., Cui X. Chemical and bioactive comparison of Panax notoginseng root and rhizome in raw and steamed forms. Journal of Ginseng Research. 2019;43(3):385–393. doi: 10.1016/j.jgr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Jang S. Y., Lee J. K., Jang E. H., Jeong S. Y., Kim J.-H. Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncology Reports. 2014;31(6):2827–2833. doi: 10.3892/or.2014.3159. [DOI] [PubMed] [Google Scholar]
- 364.Azuma H., Li J., Youda R., et al. Improved isolation procedure for shikonin from the root of the Chinese medicinal plant Lithospermum erythrorhizon and its solubilization with cyclodextrins. Journal of Applied Research on Medicinal and Aromatic Plants. 2016;3(2):58–63. doi: 10.1016/j.jarmap.2016.01.002. [DOI] [Google Scholar]
- 365.Chen J., He Y., Gao T., Zhang L., Zhao Y. Preparation and properties of compound Arnebiae Radix microemulsion gel. African Journal of Traditional, Complementary and Alternative medicines. 2017;14(3):274–279. doi: 10.21010/ajtcam.v14i3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhang Y., Jiang P., Ye M., Kim S.-H., Jiang C., Lü J. Tanshinones: sources, pharmacokinetics and anti-cancer activities. International Journal of Molecular Sciences. 2012;13(10):13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Tan Y., Wang K., Wang N., Li G., Liu D. Ectopic expression of human acidic fibroblast growth factor 1 in the medicinal plant, Salvia miltiorrhiza, accelerates the healing of burn wounds. BMC Biotechnology. 2014;14(1):p. 74. doi: 10.1186/1472-6750-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Liu J.-Q., Lee T.-F., Miedzyblocki M., Chan G. C. F., Bigam D. L., Cheung P.-Y. Effects of tanshinone IIA, a major component of Salvia miltiorrhiza, on platelet aggregation in healthy newborn piglets. Journal of Ethnopharmacology. 2011;137(1):44–49. doi: 10.1016/j.jep.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 369.Lay I.-S., Chiu J.-H., Shiao M.-S., Lui W.-Y., Wu C.-W. Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Medica. 2003;69(1):26–32. doi: 10.1055/s-2003-37034. [DOI] [PubMed] [Google Scholar]
- 370.Zhang Z. R., Li J.-H., Li S., et al. In vivo angiogenesis screening and mechanism of action of novel tanshinone derivatives produced by one-pot combinatorial modification of natural tanshinone mixture from Salvia miltiorrhiza. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100416.e100416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ma P., Liu J., Zhang C., Liang Z. Regulation of water-soluble phenolic acid biosynthesis in Salvia miltiorrhiza Bunge. Applied Biochemistry and Biotechnology. 2013;170(6):1253–1262. doi: 10.1007/s12010-013-0265-4. [DOI] [PubMed] [Google Scholar]
- 372.Dang X., Miao J.-j., Chen A.-q., et al. The antithrombotic effect of RSNK in blood-stasis model rats. Journal of Ethnopharmacology. 2015;173:266–272. doi: 10.1016/j.jep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 373.Yu T., Lee Y. J., Yang H. M., et al. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE2 production is mediated by suppression of NF-κB and AP-1 activation signaling cascade. Journal of Ethnopharmacology. 2011;134(1):11–17. doi: 10.1016/j.jep.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 374.He X., Fang J., Huang L., Wang J., Huang X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology. 2015;172:10–29. doi: 10.1016/j.jep.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 375.Yang H., Zhou Z., He L., et al. Hepatoprotective and inhibiting HBV effects of polysaccharides from roots of Sophora flavescens. International Journal of Biological Macromolecules. 2018;108:744–752. doi: 10.1016/j.ijbiomac.2017.10.171. [DOI] [PubMed] [Google Scholar]
- 376.Ryu Y. B., Westwood I. M., Kang N. S., et al. Kurarinol, tyrosinase inhibitor isolated from the root of Sophora flavescens. Phytomedicine. 2008;15(8):612–618. doi: 10.1016/j.phymed.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 377.Lee K. Y., Sung S. H., Kim Y. C. Neuroprotective bibenzyl glycosides of Stemona tuberosa roots. Journal of Natural Products. 2006;69(4):679–681. doi: 10.1021/np0504154. [DOI] [PubMed] [Google Scholar]
- 378.Li X. Q., Li X.-Q., Kang R., Huo J.-C., Xie Y.-H., Wang S.-W. Wound-healing activity of Zanthoxylum bungeanum maxim seed oil on experimentally burned rats. Pharmacognosy Magazine. 2017;13(51):363–371. doi: 10.4103/pm.pm_211_16. [DOI] [PMC free article] [PubMed] [Google Scholar]