Abstract
Background and Objective
The distal cholangiocarcinoma (dCCA) is associated with many factors: genes, environment, infection, etc. The current changes in biliary flora are thought to be involved in the formation of many gastrointestinal tract (GIT) diseases, like colon adenocarcinoma. Therefore we want to investigate whether the dCCA has a certain correlation with biliary microecology, and to detect specific strains.
Methods
A total of 68 adults were enrolled, of whom 8 with dCCA, 16 with recurrent choledocholithiasis, and 44 with the onset of common bile duct stones. Endoscopic Retrograde Cholangiopancretography (ERCP) was utilized to collect bile samples for DNA extraction and 16S rRNA gene sequencing, followed by analysis of bile microbiota composition.
Results
First, Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria are the most dominant phyla in the bile of patients with dCCA and the onset of common bile duct stoes. Secondly, compared with the onset of common bile duct stones patients, we got a significant increase in the phylum Gemmatimonadetes, Nitrospirae, Chloroflexi, Latescibacteria, and Planctomycetes in dCCA patients. Finally, at the genus level, we obtained sequencing results of 252 bacterial genera from patients with dCCA, recurrent choledocholithiasis, and the new onset of common bile duct stones, revealing heterogeneity among individuals.
Conclusion
To the best of our knowledge, this is the first study of the dysbiosis of bile flora in patients with dCCA. This micro-ecological disorder may be a decisive factor in the formation of dCCA. At the same time, for the first time, this study provides a test chart of biliary microbial populations that may be associated with recurrent choledocholithiasis. The compositional changes of the core microbial group of the biliary tract have potentially important biological and medical significance for the microbiological biliary disorders of dCCA.
1. Introduction
Cholangiocarcinoma (CCA) includes intrahepatic, perihilar, and distal CCAs according to the anatomic origin of the tumor. Three subtypes have different clinical behaviors and molecular patterns. As the most common biliary tract malignancy, CCA has a dismal prognosis. Only 10–15% of all patients with CCA are amenable to curative surgery at the time of diagnosis. Majority of patients present at an advanced stage owe to the lack of effective screening strategies [1]. During the past 40 years, the incidence rate of CCA increased significantly in American, with the subtype of intrahepatic CCA dramatically increasing from 0.44 to 1.18 cases per 100,000 person-year [2]. In China, there is no publicly available data about the incidence rate so far, and the median overall survival period is reported to be around 20 months, and the 5-year cancer cause-specific survival varies from 0% to 20% among different subgroups [3]. Given this high fatality rate, and the silent progression of early disease, identifying risk factors for the prevention and early detection of biliary tract cancer is critical to reducing its mortality. Genomic factors, chronic inflammation, biliary cysts, virus are all causes of CCA, but none of them can be assumed as explicit motivation of a certain subtype [4]. Exploration into the mechanism of carcinogenic effects of mutation promotes the newly development of anticancer drugs. However, the total outcome is not as promising as expected due to the highly heterogeneity of CCA. Hepatolithiasis is regarded as a risk factor of CCA, and after hepatic resection for treatments of both stones and strictures in bile duct, tumors still arise in some patients within some years after surgery [5]. No causal association of common bile duct (CBD) stones and distal CCA is described so far, but phenomenal relation of choledocholithiasis and proximal malignant obstruction was reported before [6].
Microbiota now is considered as an irreplaceable composition of the human body. They are indispensable for specific physiological function of the human body. At the same time, a large variety of diseases are thought to have a correlation with gut microbiome. Microbiota modulate carcinogenesis through altered composition of its components (dysbiosis), harmful properties of some bacteria, shift in local distribution of communities, and change in bacterial metabolic activity. Dysbiosis and a defect in host defense cause bacterial translocation leading to inflammation and the activation of TLRs in different cells by microorganism-associated molecular patterns (MAMPs) [7]. Bacteria may cause genotoxic effects (ie., colibactin) which will damage host DNA and activate signaling cascades. The resulting chromosomal aberrations and translocation of microbial process will lead to the activation of IL-23-producing myeloid cells which will promote tumor growth [8]. Another analysis of the whole microbiota revealed differences between extrahepatic cholangiocarcinoma (ECCA) and benign biliary pathology (BBP): the relative proportions of Fusobacteria, Acidobacteria, and Planctomycetes were significantly higher in ECCA [9]. Microbiota differences have been observed in other gastrointestinal tumors like colon adenocarcinoma, where an increasement of Fusobacterium, Prevotella, and Campylobacter, and reduction of short chain fatty acids (SCFA) producers may represent a pattern that distinguish colon adenocarcinoma patients [10]. Of note, Fusobacterium, Prevotella, and Campylobacter were three genera that Francisco Avilés-Jiménez also found significantly more frequently in ECCA, further suggesting they might have a role in gastrointestinal cancers, probably by inducing an unregulated inflammatory response [9]. An interesting fact is that microorganism is detected in more and more organs where they are expected to be sterile before. Female reproductive tract and human urinary are all proved to be a place of residence for microorganism under normal conditions of the body [11, 12]. Traditional opinion is challenged so frequently as to no surprise will be anticipated if new proof of residence is found in other clean region in human. Bile duct has already been proved to carry bacteria under disease condition. The relationship of chronic inflammation of the bile duct and gut microbiota has been investigated in PSC patients [13]. Recurrence of bile duct stones is also ascribed to migration of bacteria from the small intestine. However, whether there is microbiome in the bile duct of healthy individuals and patients without infectious disease is not known yet. In the light of the ethnic dilemma to obtain bile from healthy people, we enrolled dCCA patients and choledocholithiasis patients to do a preliminary research of microbiota in the biliary system.
Recent advances in the technology (e.g., a cost-effective method based on culture-independent 454 pyrosequencing of the bacterial 16S rRNA gene) used to identify and to analyze components of the microbiome have substantially improved our understanding of the microbial communities associated with this human disease [14]. In this study, we undertook a large-scale molecular analysis of 16S rRNA sequences in order to gain a clearer picture of three crucial issues: (1) the structure and component of biliary tract microbial communities in patients with dCCA; (2) the characteristics of biliary tract core microbiome and its potential connection in the formation of dCCA; and (3) the disorder of biliary microbiota with recurrence of common bile duct stones.
2. Material and Methods
2.1. Patients Enrollment
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Shanghai General Hospital.
From February 2016 to January 2017, patients who were in the process of being performed ERCP with a diagnosis of distal CCA or CBD stones were recruited to the study in Shanghai General Hospital. If they met the inclusion criteria, informed consent will be obtained for enrollment. For patients with a diagnosis of dCCA, the inclusion criteria are as follows, diagnosis of distal CCA with evidence of abdominal imaging including abdominal ultrasound, computed tomography or magnetic resonance imaging. Exclusion criteria for dCCA patients are as follows: sign of CBD stones, caroli disease, PSC, and previous cancer diagnosis. Patients with diagnosis of dCBD stones were further categorized into two groups, one group is patients who were diagnosed as common bile duct stones for the first without a history of ERCP, and the other group is patients who had recurrent common bile duct stones and had been performed sphincterotomy during previous ERCP procedure.
All of the patients were enrolled into the dCCA group(Tumor group, T), new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P). Important past history and main laboratory results were collected and compared among three groups.
2.2. Collection of Samples
ERCP were conducted in a dedicated ERCP room. Ultraviolet disinfection was applied 30 min before each ERCP operation. Side-viewing endoscopes (TJF240/JF-260V; Olympus Optical, Tokyo, Japan) were used which were strictly sterile before operation to keep its working channel sterile. New plugs for the working channel were applied to every patient. During standard ERCP procedure, the bile samples were collected through a sterilied catheter which went through the working channel into the bile duct. After successful intubation of the catheter into the bile duct, the tip of the catheter should be in the lower third of CBD, which was confirmed under fluoroscopy. Then the bile was aspirated out through the catheter into a 10 ml syringe, and immediately put into −80°C refrigerator for storage. 5–10 ml of bile was necessary for each patient.
2.3. DNA Extraction and MiSeq Pyrosequencing of 16S Ribosomal RNA Gene Amplicons
2.3.1. DNA Extraction and Quality Control
DNA was purified and stored by standard methods [15]. Total DNA was extracted from each noncentrifuged sample with the OMEGA DNA Kit (Omega Bio-tek, Norcross, Georgia, USA) following manufacturer's instruction. A Qubit picogreen Fluorescence quantification system was used to quantify all the DNA, and 1% agarose gel electrophoresis was used to examine the DNA quality.
2.3.2. 16S rRNA Amplicon Sequencing
Specific primers with barcode were made according to the region to be sequenced. To ensure the accuracy and consistency of data analysis, low cycle number amplification is used to ensure that the cycle number of sample amplification is consistent. TaKaRa EX Taq (Takara Bio, Mountain View, CA, USA) DNA polymerase is used for PCR (Applied Biosystems 2720 Thermal Cycler). The amplified products were identified by 2% agarose gel electrophoresis. The AxyPrep Mag PCR Clean-Up Kit (Thermofisher scientific, CA, USA) magnetic beads were recovered and purified, and then utilized for quantitative construction. Detailed protocols for the two-step 16S rRNA gene amplification and library construction procedures are reported before [16, 17].
2.4. Data Analysis
Ages were presented as mean ± SD. Gender differences were assessed using Chi-squared-tests. Comparison of past history and laboratory results among three groups were done by using Chi-squared-test. Comparison of the relative abundance of microbiota among three groups were done by nonparametric tests. Alpha diversity is used to analyze the species diversity of individual samples, measured by species richness (Richness, Chao, ACE) and diversity index (Shannon, Simpson). Linear discriminant analysis effect size (LEfSe) was introduced to identify bacterial biomarkers for the three groups. Differences with P < 0.05 were considered to be statistically significant.
3. Results
3.1. Characteristics of Patients
Totally 68 patients were enrolled in the study including 8 with dCCA (5 males, 3 females), 44 with the new onset of common bile duct stones (18 males, 26 females), and 16 with recurrent choledocholithiasis (9 males, 7 females). There was no significant difference among these three groups in regard of average age, proportion of gender, co-existing chronic disease, presence of gallbladder stone, and abnormal ratio of laboratory tests (Table 1).
Table 1.
Characteristics of patients.
| Characteristic | Group T n = 8 | Group C n = 44 | Group P n = 16 | p value |
|---|---|---|---|---|
| Gender | ||||
| Male (n, %) | 3 (37.5%) | 18 (40.9%) | 9 (56.2%) | 0.527 |
| Female (n, %) | 5 (62.5%) | 26 (59.1%) | 7 (43.8%) | — |
| Age (range) | 72.13 (60–95) | 66.98 (44–90) | 73.94 (49–92) | 0.132 |
| Diabetes ( n , %) | 0 (0.0%) | 10 (22.7%) | 4 (25%) | 0.303 |
| Dyslipidemia ( n , %) | 1 (12.5%) | 15 (34.1%) | 4 (25%) | 0.424 |
| Hypertension (n, %) | 5 (62.5%) | 17 (38.6%) | 6 (37.5%) | 0.426 |
| Elevated ALT or AST (IU/L) ( n, %) | 7 (87.5%) | 25 (56.8%) | 7 (43.75%) | 0.123 |
| Elevated Tbil and/or Dbil (µmol/L) ( n , %) | 6 (75.0%) | 19 (43.2%) | 7 (43.75%) | 0.241 |
| Elevated Scr (µmoi/l) ( n , %) | 2 (25.0%) | 6 (13.6%) | 2 (12.5%) | 0.678 |
| Elevated WBC or NE (10^9/L) ( n , %) | 0 (0.0%) | 7 (15.9%) | 0 (0.0%) | 0.119 |
| Cholecystolithiasis (n, %) | 1 (12.5%) | 23 (52.3%) | 7 (43.7%) | 0.114 |
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; Tbil, total bilirubin; Dbil, Direct Bilirubin; Cre, creatinine; WBC, White blood cell; NE, neutrophilicgranulocyte. The dCCA group (Tumor group, T), the new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P) denoted as “Group T”, “Group C”, and “Group P”, respectively, in the table.
3.2. Increase in Biliary Microbial Diversity Is Closely Related to the Presence of dCCA
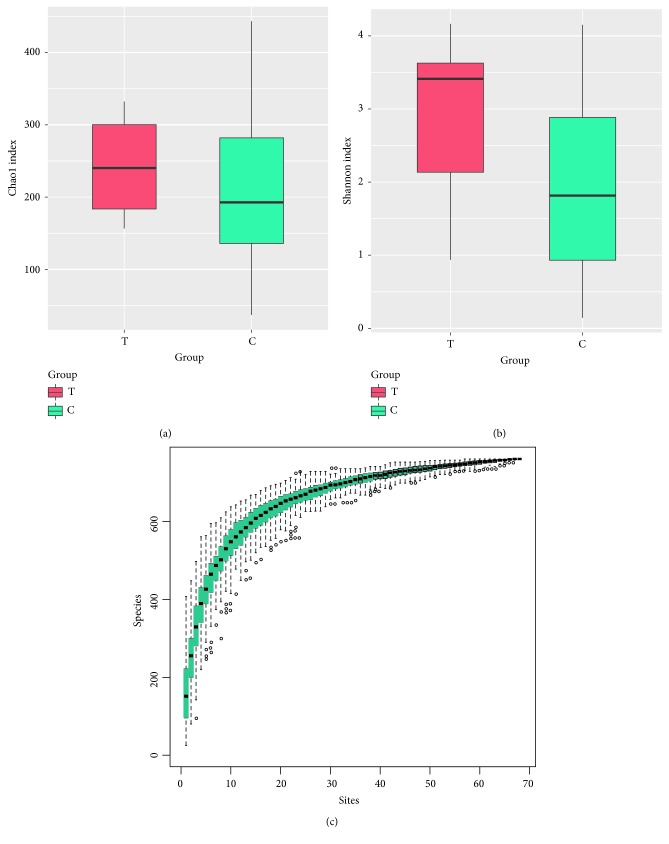
We compared the richness of the bacterial community (Chao index) and diversity (Shannon index) in the bile of patients with dCCA and patients with common bile duct stones (Table 2). We observed no difference in the Chao index among the three groups. The Chao index of Group T, Group C, and Group P is 243.42 ± 73.27, 202.26 ± 94.64, and 181.2 ± 71.31 separately. Comparing to the other two groups, the Shannon index for dCCA patients (2.88 ± 1.19) was significantly higher than that of Group C (1.95 ± 1.11) (P = 0.36) ().
Table 2.
Alpha diversity analysis of biliary microbiota in patients with dCCA and the onset of common bile duct stones.
| Sample | mean (group_T) | sd (group_T) | mean (group_C) | sd (group_C) | mean (group_P) | sd (group_P) | p value |
|---|---|---|---|---|---|---|---|
| Observed_OTU | 209.6250 | 74.5576 | 165.0682 | 90.5750 | 134.2500 | 66.9423 | 0.0976 |
| Chao | 243.4245 | 73.2746 | 202.2659 | 94.6487 | 181.1733 | 71.3074 | 0.2413 |
| Shannon | 2.8807 | 1.1989 | 1.9514 | 1.1148 | 1.8863 | 0.8409 | 0.0989 |
| Simpson | 0.7738 | 0.2312 | 0.6270 | 0.2620 | 0.6687 | 0.2271 | 0.2010 |
| Goodscoverage | 0.9986 | 0.0006 | 0.9983 | 0.0008 | 0.9983 | 0.0008 | 0.8360 |
| Shannoneven | 0.5355 | 0.2064 | 0.3794 | 0.1877 | 0.3874 | 0.1515 | 0.0887 |
OTU, operational taxonomic unit; sd, Standard Deviation. The dCCA group (Tumor group, T), the new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P) denoted as “Group T”, “Group C”, and “Group P”, respectively, in the table.
The results of the Specaccum species (Figure 1(c)) accumulation curve show that as the sample size increases, the curve shows a sharp rise, indicating that a large number of species are found in the community, as the number of test samples continues to increase, the curve tends to be flat, indicating that species in this environment do not increase significantly as the sample size increases. This curves shows that the experiment is adequately sampled and data analysis can be carried out.
Figure 1.
The richness and diversity of biliary microbiota in patients with dCCA and the onset of common bile duct stones(a&b). Richness rerefaction plot from all samples,the abscissa indicates the number of sequences randomly extracted from the sample, and the ordinate indicates the number of OTUs obtained by clustering the corresponding sequence numbers (c). The dCCA group (Tumor group, T) and the new onset of CBD stones group (CBD stones group, C) denoted as “Group T” and “Group C”, respectively, in the figure.
Similar richness (P = 0.24, Figure 1(a)) and higher diversity (P < 0.05Figure 1(b)) measures were observed in patients with dCCA and the new onset of common bile duct stones.
3.3. Relative Taxon Abundance in the Microbiota of Patients with dCCA and the New Onset of Common Bile Duct Stones
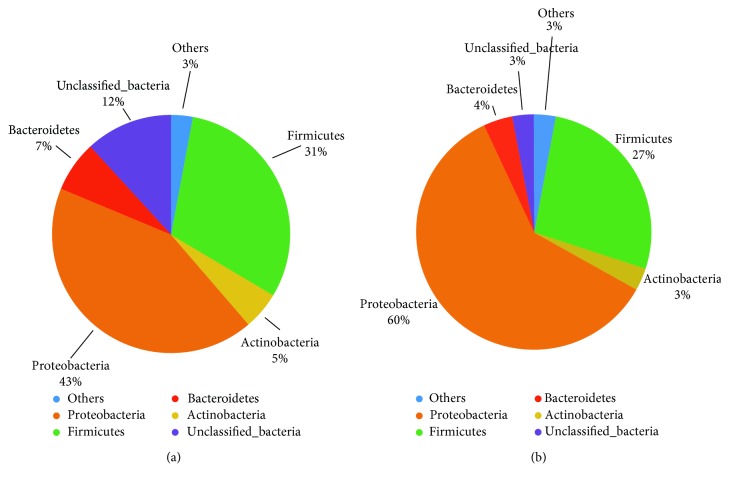
We explored the biliary microbial community characteristics of patients with dCCA, and compared the relative abundance of microbiota between patients with dCCA and the new onset of common bile duct stones. The overall microbial composition at the phylum and genus level illustrated that the taxonomic composition varies widely among individuals, but Proteobacteria and Firmicutes were the dominant phylum among all individuals (Figure 2(a)), and Escherichia/Shigella and Halomonas were the major genus (Figure 2(b)).
Figure 2.
Relative taxa abundance and OTUs comparison in patients with dCCA and the onset of common bile duct stones. The overall microbial composition at the phylum (a) and genus (b) level illustrated that the taxonomic composition varies widely among individuals, but Proteobacteria and Firmicutes were the dominant phylum among all individual, and Escherichia/Shigella and Halomonas were the major genus. It indicated that the core OTUs from patients with dCCA and the new onset of common bile duct stones were composed of 536 and 703 OTUs, respectively, of which 490 were shared between the two groups (c). The dCCA group (Tumor group, T) and the new onset of CBD stones group (CBD stones group, C) denoted as “Group T” and “Group C”, respectively, in the figure.
The core bacterial community at the OTU level was shared by at least 50% of the samples in each group and was shown in the Venn diagrams constructed, to evaluate the shared OTUs in patients with dCCA and the new onset of common bile duct stones (Figure 2(c)). It indicated that the core OTUs from patients with dCCA and the new onset of common bile duct stones were composed of 536 and 703 OTUs, respectively, of which 490 were shared between the two groups.
We compared the biliary microbial community of patients with dCCA and the new onset of common bile duct stones. Patients from both groups contained five dominant phyla: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Unclassified_Bacteria, which accounted for 97% of the biliary microbes. Relative abundance of Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria is similar between patients with dCCA and the new onset of common bile duct stones (Figure 3).
Figure 3.
Relative abundances of dominant phyla in patients with dCCA (a), and the onset of common bile duct stones (b), patients from both groups contained five dominant phyla: Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Unclassified_Bacteria, which accounted for 97% of the biliary microbes. Relative abundance of Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria is similar between patients with dCCA and the new onset of common bile duct stones. The dCCA group (Tumor group, T) and the new onset of CBD stones group (CBD stones group, C) denoted as “Group T” and “Group C”, respectively, in the figure.
Besides abundance of Gemmatimonadetes, Nitrospirae, Chloroflexi, Latescibacteria, Unclassified_Bacteria, and Planctomycetes (P < 0.05) increased in patients with dCCA, and notably Gemmatimonadetes, Latescibacteria, and Nitrospirae were not detected in the samples from patients with the new onset of common bile duct stones (Table 3).
Table 3.
Relative abundance at the phylum level in patients with dCCA and the onset of common bile duct stones.
| Phylum | mean (group_T) | sd (group_T) | mean (group_C) | sd (group_C) | p value |
|---|---|---|---|---|---|
| Chloroflexi | 0.001255 | 0.001756 | 0.000036 | 0.000142 | 0.000650 |
| Gemmatimonadetes | 0.000295 | 0.000547 | 0 | 0 | 0.000930 |
| Nitrospirae | 0.000704 | 0.001973 | 0 | 0 | 0.000930 |
| Unclassified_Bacteria | 0.118056 | 0.095475 | 0.029619 | 0.074029 | 0.002429 |
| Latescibacteria | 0.000600 | 0.001697 | 0 | 0 | 0.021911 |
| Planctomycetes | 0.000545 | 0.001069 | 0.000002 | 0.000009 | 0.039550 |
The dCCA group (Tumor group, T), the new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P) denoted as “Group T”, “Group C”, and “Group P”, respectively, in the table.
At the genus level, 252 genera were identified; the five genera with the highest relative abundances in patients with dCCA were Escherichia/Shigella, Staphylococcus, Klebsiella, Unclassified_Enterobacteriaceae, and Faecalibacterium, whereas those inpatients with the new onset of common bile duct stones were Escherichia/Shigella, Halomonas, Klebsiella, Streptococcus, and Enterococcus (). And the abundance of Staphylococcus, Okibacterium, and Corynebacterium (P < 0.001) is more abundant in patients with dCCA (Table 4).
Table 4.
Relative abundance at the genus level in patients with dCCA and the onset of common bile duct stones.
| Genus | mean (group_T) | sd (group_T) | mean (group_C) | sd (group_C) | p value |
|---|---|---|---|---|---|
| Staphylococcus | 0.1065 | 0.2852 | 0.0320 | 0.1476 | 0.0002 |
| Okibacterium | 0.0055 | 0.0051 | 0.0011 | 0.0048 | 0.0004 |
| Corynebacterium | 0.0043 | 0.0071 | 0.0002 | 0.0004 | 0.0009 |
| Sphingomonas | 0.0047 | 0.0035 | 0.0006 | 0.0014 | 0.0018 |
| Stenotrophomonas | 0.0119 | 0.0110 | 0.0016 | 0.0042 | 0.0021 |
| Acidovorax | 0.0008 | 0.0009 | 0.0002 | 0.0006 | 0.0026 |
| Gemmiger | 0.0049 | 0.0071 | 0.0004 | 0.0010 | 0.0027 |
| Providencia | 0.0030 | 0.0067 | 0.0009 | 0.0041 | 0.0027 |
| Comamonas | 0.0071 | 0.0079 | 0.0006 | 0.0022 | 0.0028 |
| Methylobacterium | 0.0011 | 0.0009 | 0.0003 | 0.0005 | 0.0029 |
The dCCA group (Tumor group, T), the new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P) denoted as “Group T”, “Group C”, and “Group P”, respectively, in the table.
3.4. Biliary Microbiota Variability and Its Association with Biliary Disease
We tested bile in patients with biliary tract tumors and compared all bile microbial organisms in patients with dCCA, the new onset of common bile duct stones, and the recurrent choledocholithiasis. A total of 252 bacterial species were identied, clustering was not consistent with patient grouping (). Analysis of bile microbial colonies with an abundance ≥0.2% reveals heterogeneity between individuals (Figure 4). Rough literature mining, Cluster II contained most of the intestinal microbes, whereas Cluster I contained most of the oral cavity and environmental inhabitants. High within-sample abundance was observed for some species, such as Pseudomonas (77.39%) in C44 and Citrobacter (66.52%) in P9. Escherichia/Shigella, Klebsiella, Enterococcus, Haemophilus, and Streptococcus were the species identied in almost all individuals (). The current changes in biliary flora are thought to be involved in the formation of common bile duct stones and the recurrence of calculi after ERCP. Therefore, we want to prove that the recurrence of calculi after ERCP has a certain correlation with biliary microecology. We applied LEfSe analysis to further identify the significantly different abundance between patients with recurrent choledocholithiasis and the new onset of common bile duct stones.
Figure 4.
Heatmap of bile microbial from all samples. We tested bile in patients with biliary tract tumors and compared all bile microbial organisms in patients with biliary tract tumors, recurrent choledocholithiasis, and the new onset of common bile duct stones. Analysis of bile microbial colonies reveals heterogeneity between individuals. The dCCA group (Tumor group, T), the new onset of CBD stones group (CBD stones group, C), and recurrent CBD stones group (Post-ERCP CBD stones group, P) denoted as “Group T”, “Group C”, and “Group P”, respectively, in the figure.
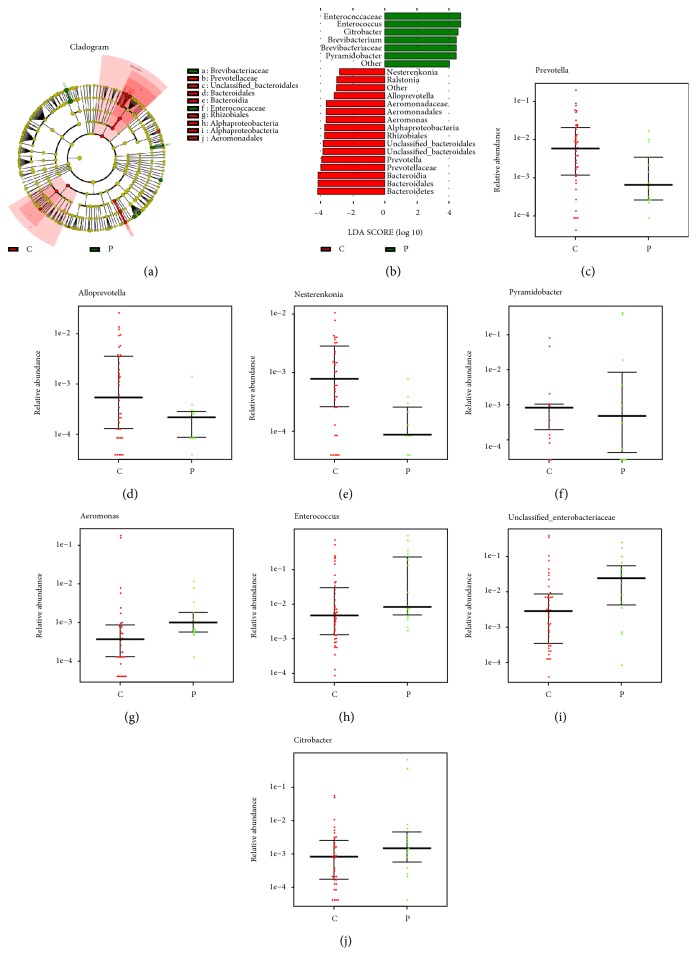
A cladogram (Figure 5(a)) was used to represent the predominant bacteria and the structure of the microbiota in both groups. LEfSe analysis revealed 23 taxa, distinguishing biliary microbial communities of patients with recurrent choledocholithiasis and the new onset of common bile duct stones by a LDA score above 2.8. In these taxa, 7 and 16 were identified as enriched within the patients with recurrent choledocholithiasis and the new onset of common bile duct stones, respectively (Figure 5(b)). At the genus level, abundance of Prevotella, Alloprevotella, Nesterenkonia, and Pyramidobacter (P < 0.05) increased in patients with the new onset of common bile duct stones (Figure 5(c)–5(f)), whereas Aeromonas, Enterococcus, Unclassified_Enterobacteriaceae, and Citrobacter (P < 0.05) were more abundant in patients with recurrent choledocholithiasis (Figures 5(g)–5(j)).
Figure 5.
Characteristics of microbial community composition in patients with recurrent choledocholithiasis and the onset of common bile duct stones. (a) Cladogram representing taxa enriched in biliary microbiota community of the two groups detected by the LEfSe tool. Differences were represented by the color of the most abundant class (red, patients with the onset of common bile duct stones; green, patients with recurrent choledocholithiasis). The diameter of each circle is proportional to the taxon's abundance. The central point represents the root of the tree (Bacteria), and each ring represents the next lower taxonomic level. (b) Histogram of the LDA scores computed for different abundance levels between patients with recurrent choledocholithiasis and the onset of common bile duct stones, as detected by the LEfSe tool. (c–j) Relative abundance of Prevotella, Alloprevotella, Nesterenkonia, Pyramidobacter, Aeromonas, Enterococcus, Unclassified_Enterobacteriaceae, and Citrobacter in biliary microbiota community of patients with recurrent choledocholithiasis and the onset of common bile duct stones.
4. Discussion
Existence of microbiota in healthy bile duct is not acceptable widely as a matter of fact till now. Inner part of the body should be free of microorganisms under no infectious condition. Due to the ethical dilemma to collect bile on normal recipients, we selected dCCA patients as research subjects, who are supposed to be without clinical inflammation. Since microbiota can be found in human bile duct, another question we always want to figure out is what's the relation of them with the microform in the gut. The potential relationship of bile duct microbiota and the disease itself is also of our interest. The role of gut bacteremia in the formation of the CBD stones has been discussed before [18–20]. Infection of the gallbladder and migration of germ is also a proven origin of CBD habitants. Oddi's sphincter is an inborn barrier between intestine and bile duct. However, during the process of ERCP for CBD stone patients, sphinterectomy is probably performed to achieve total removal of calculi. Therefore the reflux of bacteria from the gut to the bile duct is inevitable in addition to the imaging evidence of reflux of content of duodenum [21]. High recurrence rate of CBD stones after ERCP was partially ascribed to the anatomic change of protection [22].
By using a targeted amplicon sequencing approach for 16s rRNA, our study demonstrates an underlying biliary microbiota dysbiosis present with dCCA. This is the first study to use 16S rRNAs to clarify the composition of biliary tract microbiota in patients with dCCA and compare them with patients with the new onset of common bile duct stones. Methods such as Alpha diversity analysis can identify differences of microbial components and diversity both between and within the biliary tract microbiota from patients with cancer background and without cancer background.
Our results discovered, in terms of species diversity, biliary tract, like the intestine, has highly abundant microbial colonies. We also discovered that biliary tract microbiota has the heterogeneity between individuals. The reason may be explained by the complex source of bacteria including other parts of gut and mouth. Under pathological condition, the connection of bile germ and intestine was also confirmed. A high microbial diversity in the bile duct was recently reported in the only other study in humans, although that was done in patients with cholesterol gallstones [18]. In addition, whereas in a previous research Proteobacteria comprised around 30% of phyla in the stomach [23], in the bile duct Proteobacteria represented 60%, which is more similar to values described in the small intestine [24]. Surprisingly though, in our research Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria, which account for 97% of the biliary flora, are all intestinal flora. This raises a possibility that biliary tract microbiota could be originated from the duodenum. Further evidence is obviously needed to explore this possibility. If is there any possibilities those bacteria are from the small intestine is unknown. For tumors located near to the orifice of the bile duct, it will probably affect the function of the sphincter of Oddi. However, obstruction will definitely lead to the increasing of inner pressure of the proximal duct.
Our research also confirmed significant changes in biliary microbial components between patients with dCCA and patients with first episode of common bile duct stones. Within the biliary tree of patients with dCCA, there exists an over growth of the bacterial phylum Gemmatimonadetes, Nitrospirae, Chloroflexi, Latescibacteria, and Planctomycetes. Planctomycetes are a set of fastidious Gram-negative bacteria related to Verrucomicrobia and Chlamydia in a so-called “PVC (Planctomycetes-Verrucomicrobia-Chlamydiae) superphylum” [25]. These organisms are mainly environmental bacteria found in water sources, but recent searches detected DNA sequences specific for Planctomycetes in the digestive tract of healthy individuals [26]. A study found Planctomycetes DNA in 2 out of 100 blood samples from patients suffering from leukemia with neutropenia induced by chemotherapy, as well as fever, rash, pneumonia, and diarrhea. Therefore they suggest antibiotic-resisting Planctomycetes may be pathogenic in these patients [27]. The phylum Gemmatimonadetes was present in both the sediment and microbialite contigs, and comprised 7-8% of the protein coding open reading frames (ORFs) [28]. The Gemmatimonadetes contigs annotated mainly as hypothetical proteins; however, positive Gemmatimonadetes annotations included ATPases, Zn-dependent peptidases, and glucose/sorbosone dehydrogenase-like genes. Glucose/sorbosone dehydrogenases transform various sugar moieties into vitamins, including L-ascorbic acid (vitamin C), or can make D-glucono1,5-lactone from D-glucose, which can acidify the extracellular environment, which may lead to the dissolution of carbonate by heterotrophic process [29–31]. However, whether the bacteria have the same physiological function in the intestine is worthy further study. Nitrospira, one of microorganisms derived from biofilm in oligotrophic environments, was detected from dental unit water system (DUW) [32]. Chloroflexi was significantly different between patients with adenomas and healthy volunteers, supporting that colorectal pre-neoplastic lesion maybe the most important factor leading to alterations in bacterial community composition [33]. Changes in microbiota have also been reported in mouth and esophagus cancers [34–36].
Previous studies demonstrated an association between biliary microbiota dysbiosis and human diseases, e.g., cholesterol gallstones [18–20], validating the biliary microbiota dysbiosis may be a key contributor to the presence of biliary diseases. In a compromised and obstructed bile duct, bacteria may find a more favorable environment to colonize. Our results found Bacteroidetes accounted for 7% of bile bacterial colonies from patients with dCCA, partially supporting the above inference. Second, Tumors may alter the biliary microenvironment, implying that the dCCA themselves may actually aggravate the over growth of bacteria in the biliary tract.
The biliary microbiota showed significant inter-individual variation in our test results. For example, the highest abundance of Proteobacteria phylum in one dCCA patient was 74.27% and the lowest was 12.83% in another (). High person-to-person variation in biliary tract microbiota could be related to a number of factors: host diets, lifestyles, genotypes, disease status, etc. Aside from interpersonal variation, there are several potential factors that may affect the colonization or survival of bacteria in biliary tract of dCCA patients. First, MDR efflux pump proteins expressed by bacteria can produce bile resistance, allowing bacteria to survive in their ecological niche [37–41], as well as BSH activity [42] produced by bacteria that can protect the bacterial cells which produce it from the toxicity of conjugated bile salts.
In conclusion, we reported for the first time the microbiota in the biliary tract in cancer and non-cancer conditions, and found a significant increment in the phylum Gemmatimonadetes, Nitrospirae and Planctomycetes, and a reduction in Chloroflexi in dCCA. We recognized the need for larger studies to confirm our observations. It remains to be determined if these OTUs have a role in carcinogenesis or if they result from changes in the microenvironment of dCCA. We reported the presence of bacteria commonly described in the environment but rarely in humans, such as Latescibacteria isolated from groundwater and temperate freshwater [43]. We then compared biliary microbiology from patients with recurrent choledocholithiasis and the new onset of common bile duct stones. Notably, all the bacteria detected in the bile of patients with the new onset of common bile duct stones were identified in the bile of patients with the recurrent choledocholithiasis, and there is no significant difference between the Shannon index and the Chao index (). We suspect that the mechanism by which the flora causes recurrence of stones may be the change of dominant bacteria, not changes in the entire biliary biological population.
To the best of our knowledge, this is the first study to discover a potential association of the biliary microbiota dysbiosis that is present among dCCA patients. Likewise, our characterization of the biliary tract core microbiome provides potentially significant biological implications about both the unexpected diversity of the microbiome among patients with dCCA, as well as the probable roles of bacteria in the formation of dCCA. Our further research on the bile tract microbiome from recurrent choledocholithiasis patients will likely complement our findings on biliary tract microbiome and clarify some of the implications that arose from our conclusions. Ultimately, these findings have numerous medical implications for both prevention and therapeutics for dCCA or common bile duct stones, warranting further follow-up studies that are needed to verify these findings and move forward.
5. Conclusions
We applied 16S sequencing methods to samples collected from the bile of Chinese dCCA patients. Microbial communities of these individuals were significantly different from patients with the new onset of common bile duct stones. We also paid attention to the biliary flora factors associated with stone recurrence. On the other hand, many patient-specific bacteria were found, implying the strong individuality of the biliary microbial community. Taken together, our results provide novel insights into the biliary microbiota with respect to microbial composition, which could be valuable for clinical applications, such as the diagnosis and treatment of bile duct diseases.
Acknowledgments
The authors are grateful to patients who amidst their busy schedule took out time to participate in this study and Shanghai General Hospital for their support of this research.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Shanghai General Hospital.
Consent
informed consent was obtained from all participants.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Hang Zhao was responsible for guiding study design, conception and design of the study data analysis. Bingrong Chen and Seng Wang Fu were responsible for data collection and reviewing of the manuscript. Lungen Lu was in charge of critical review of the manuscript and provided intellectual guidance. Both the authors approved the submission of the final manuscript.
Supplementary Materials
Figure S1: heatmap of bile microbial from all samples. We tested bile in patients with biliary tract tumors and compared all bile microbial organisms in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis. A total of 252 bacterial species were identied ,clustering was not consistent with patient grouping.
Figure S2: The highest and lowest abundance of Proteobacteria phylum in dCCA patients. The biliary microbiota showed significant inter-individual variation in our test results. For example, the highest abundance of Proteobacteria phylum in one dCCA patient was 74.27% and the lowest was 12.83% in another.
Table S1: Alpha diversity analysisof biliary microbiota in patients with dCCA and the onset of common bile duct stones.
Table S2: Relative abundance at the genus level in patients with dCCA and the onset of common bile duct stones.
Table S3: Relative abundance of 252 bacterial species in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis.
Table S4: Relative abundance of 252 bacterial species in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis.
References
- 1.Nathan H., Pawlik T. M., Wolfgang C. L., Choti M. A., Cameron J. L., Schulick R. D. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. Journal of Gastrointestinal Surgery. 2007;11(11):1488–1496. doi: 10.1007/s11605-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 2.Saha S. K., Zhu A. X., Fuchs C. S. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. The Oncologist. 2016;21(5):594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H. J., Mao H., Shrestha A., et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: a single-institution experience in China. World Journal of Gastroenterology. 2016;22(8):2601–2610. doi: 10.3748/wjg.v22.i8.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S. A., Tavolari S., Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver International. 2019;39(1):19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 5.Meng Z.-W., Han S.-H., Zhu J.-H., Zhou L.-Y., Chen Y.-L. Risk factors for cholangiocarcinoma after initial hepatectomy for intrahepatic stones. World Journal of Gastroenterology. 2017;41(3):835–843. doi: 10.1007/s00268-016-3752-2. [DOI] [PubMed] [Google Scholar]
- 6.Nichols D. M., Macleod A. J. M. Choledocholithiasis associated with malignant biliary obstruction–significance and management. Clinical Radiology. 1998;53(1):49–52. doi: 10.1016/s0009-9260(98)80034-2. [DOI] [PubMed] [Google Scholar]
- 7.Tözün N., Vardareli E. Gut microbiome and gastrointestinal cancer: les liaisons dangereuses. Journal of Clinical Gastroenterology. 2016;50(2):S191–S196. doi: 10.1097/MCG.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 8.Parekh P. J., Balart L. A., Johnson D. A. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease Clinical and Translational. Gastroenterology. 2015;6(6):p. e91. doi: 10.1038/ctg.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avilés-Jiménez F., Guitron A., Segura-López F., et al. Microbiota studies in the bile duct strongly suggest a role for Helicobacter pylori in extrahepatic cholangiocarcinoma. Clinical Microbiology and Infection. 2016;22(2):178.e11–178.e22. doi: 10.1016/j.cmi.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Tan J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., Macia L. The role of short-chain fatty acids in health and disease. Advances in Immunology. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen C., Song X., Wei W., et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases Nature. Communications. 2017;8(1):875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu P., Zhang G., Zhao J., et al. Profiling the urinary microbiota in male patients with bladder cancer in China Frontiers in Cellular and Infection. Microbiology. 2018;8:167. doi: 10.3389/fcimb.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabino J., Vieira-Silva S., Machiels K., et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65(10):1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczynski J., Lauber C. L., Walters W. A., et al. Experimental and analytical tools for studying the human microbiome. Nature Reviews Genetics. 2012;13(1):47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manichanh C., Rigottier-Gois L., Bonnaud E., et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelzer E. S., Willner D., Buttini M., Huygens F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek. 2018;111(6):933–943 . doi: 10.1007/s10482-017-0992-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen G. L., Zhang Y., Wang W. Y., et al. Partners of patients with ulcerative colitis exhibit a biologically relevant dysbiosis in fecal microbial metacommunities. World Journal of Gastroenterology. 2017;23(25):4624–4631. doi: 10.3748/wjg.v23.i25.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T., Zhang Z., Liu B., et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14(1):p. 669. doi: 10.1186/1471-2164-14-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen H., Ye F., Xie L., et al. Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Scientific Reports. 2015;5(1):p. 17450. doi: 10.1038/srep17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye F., Shen H., Li Z., et al. Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. PLOS One. 2016;11(3):p. e0150519. doi: 10.1371/journal.pone.0150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochar R., Banerjee S. Infections of the biliary tract. Gastrointestinal Endoscopy Clinics of North America. 2013;23(2):199–218. doi: 10.1016/j.giec.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Cai J.-S., Qiang S., Bao-Bing Y. Advances of recurrent risk factors and management of choledocholithiasis. Scandinavian Journal of Gastroenterology. 2017;52(1):34–43. doi: 10.1080/00365521.2016.1224382. [DOI] [PubMed] [Google Scholar]
- 23.Aviles-Jimenez F., Vazquez-Jimenez F., Medrano-Guzman R., Mantilla A., Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Scientific Reports. 2014;4(1):p. 4202. doi: 10.1038/srep04202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamada N., Chen G. Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nature Immunology. 2013;14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuerst J. A., Sagulenko E. Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function. Nature Reviews Microbiology. 2011;9(6):403–413. doi: 10.1038/nrmicro2578. [DOI] [PubMed] [Google Scholar]
- 26.Cayrou C., Sambe B., Armougom F., Raoult D., Drancourt M. Molecular diversity of the planctomycetes in the human gut microbiota in France and Senegal. APMIS. 2013;121(11):1082–1090. doi: 10.1111/apm.12087. [DOI] [PubMed] [Google Scholar]
- 27.Drancourt M., Prebet T., Aghnatios R., et al. Planctomycetes DNA in febrile aplastic patients with leukemia, rash, diarrhea, and micronodular pneumonia. Journal of Clinical Microbiology. 2014;52(9):3453–3455. doi: 10.1128/JCM.01207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White R. A., Power I. M., Dipple G. M., Southam G., Suttle C. A. Metagenomic analysis reveals that modern microbialites and polar microbial mats have similar taxonomic and functional potential. Frontiers in Microbiology. 2015;6:966. doi: 10.3389/fmicb.2015.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupraz C., Visscher P. T. Microbial lithification in marine stromatolites and hypersaline mats. Trends in Microbiology. 2005;13(9):429–438. doi: 10.1016/j.tim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki T., Sugisawa T., Hoshino T. Pyrroloquinoline quinone-dependent dehydrogenases from Ketogulonicigenium vulgare catalyze the direct conversion of L-sorbosone to L-ascorbic acid. Applied and Environmental Microbiology. 2006;72(2):1487–1495. doi: 10.1128/AEM.72.2.1487-1495.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fender J. E., Bender C. M., Stella N. A., Lahr R. M., Kalivoda E. J., Shanks R. M. Q. Serratia marcescens quinoprotein glucose dehydrogenase activity mediates medium acidification and inhibition of prodigiosin production by glucose. Applied and Environmental Microbiology. 2012;78(17):6225–6235. doi: 10.1128/AEM.01778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeon E. H., Han J. H., Ahn T. Y. Comparison of bacterial composition between human saliva and dental unit water system. Journal of Microbiology. 2007;45(1):1–5. [PubMed] [Google Scholar]
- 33.Lu Y., Chen J., Zheng J., et al. Mucosal adherent bacterial dysbiosis in patients with colorectal adenomas. Scientific Reports. 2016;6(1):p. 26337. doi: 10.1038/srep26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt B. L., Kuczynski J., Bhattacharya A., et al. Changes in abundance of oral microbiota associated with oral cancer. PLOS One. 2014;9(6):p. e98741. doi: 10.1371/journal.pone.0098741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L., Francois F., Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clinical Cancer Research. 2012;18(8):2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu G., Gail M. H., Shi J., et al. Association between upper digestive tract microbiota and cancer-predisposing states in the esophagus and stomach. Cancer Epidemiology Biomarkers and Prevention. 2014;23(5):735–741. doi: 10.1158/1055-9965.EPI-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piddock L. J. V. Multidrug-resistance efflux pumps ? Not just for resistance. Nature Reviews Microbiology. 2006;4(8):629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 38.Lin J., Sahin O., Michel L. O., Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infection and Immunity. 2003;71(8):4250–4259. doi: 10.1128/iai.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kullak-Ublick G. A., Stieger B., Meier P. J. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126(1):322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Begley M., Gahan C. G., Hill C. The interaction between bacteria and bile. FEMS Microbiology Reviews. 2005;29(4):625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Glavinas H., Krajcsi P., Cserepes J., Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity Current. Drug Delivery. 2004;1(1):27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 42.Jones B. V., Begley M., Hill C., Gahan C. G. M., Marchesi J. R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proceedings of the National Academy of Sciences. 2008;105(36):13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farag I. F., Youssef N. H., Elshahed M. S. Global distribution patterns and pangenomic diversity of the candidate phylum "latescibacteria" (WS3) Applied and Environmental Microbiology. 2017;83(10) doi: 10.1128/AEM.00521-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: heatmap of bile microbial from all samples. We tested bile in patients with biliary tract tumors and compared all bile microbial organisms in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis. A total of 252 bacterial species were identied ,clustering was not consistent with patient grouping.
Figure S2: The highest and lowest abundance of Proteobacteria phylum in dCCA patients. The biliary microbiota showed significant inter-individual variation in our test results. For example, the highest abundance of Proteobacteria phylum in one dCCA patient was 74.27% and the lowest was 12.83% in another.
Table S1: Alpha diversity analysisof biliary microbiota in patients with dCCA and the onset of common bile duct stones.
Table S2: Relative abundance at the genus level in patients with dCCA and the onset of common bile duct stones.
Table S3: Relative abundance of 252 bacterial species in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis.
Table S4: Relative abundance of 252 bacterial species in patients with dCCA, the new onset of common bile duct stones and the recurrent choledocholithiasis.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.