Abstract
Aims
The aim was to examine associations of insulin resistance and beta cell dysfunction with macrosomia in Chinese women with gestational diabetes mellitus (GDM).
Methods
We performed a secondary analysis of 923 women with GDM enrolled in a randomized controlled trial in 2010–2012 in Tianjin, China. Insulin resistance and beta-cell function were estimated using Homeostasis model assessment. Binary logistic regression was used to obtain adjusted odds ratios (ORs) and 95% confidence intervals (CIs). A two-step adjustment scheme was used to control for effects of potential confounders.
Results
A total of 138 women (16.5%) had excessive weight gain, 127 (7.3%) had macrosomia and 150 (16.3%) had a large for gestational age (LGA) infant. Compared to women in bottom tertile of insulin resistance, women in upper tertile had increased risk of excessive weight gain (OR: 4.32, 95%CI: 1.95–9.62), macrosomia and LGA (OR: 2.61, 95%CI: 1.20–5.69; 2.75, 95%CI: 1.35–5.62, respectively). The observed overall effects were mainly due to their large effect sizes among women with normal pre-pregnancy body weight. However, beta cell function was not found to be associated with either of them.
Conclusions
Increased insulin resistance during pregnancy was associated with excessive weight gain, macrosomia and LGA in Chinese women with GDM.
Keywords: Beta Cell Function, Chinese, Gestational Diabetes Mellitus, Insulin Resistance
Introduction
Gestational diabetes mellitus (GDM) is one of the most common metabolic disorders of pregnancy[1]. More and more pregnant women are being affected, especially in developing countries such as China[1]. In Tianjin, the prevalence of GDM increased by 3.5-fold from 2.3%[2] in 1999 to 8.1%[3] in 2010–2012. It is well established that hyperglycemia in GDM is associated with increased risk of adverse pregnancy outcomes, including maternal excessive weight gain during pregnancy, macrosomia or large for gestational age (LGA), and neonatal morbidity[4], and long-term increased risk of diabetes in the mother and childhood obesity and hypertension in the offspring[5]. Two major randomized controlled trials demonstrated that tight glycaemia control during pregnancy was able to reduce the risk of macrosomia and LGA although its effect on neonatal morbidity was inconsistent [6, 7].
The mechanism underlying fetal overgrowth in mothers complicated by GDM is only partially understood. Maternal hyperglycemia can pass across the placental barrier and stimulate fetal pancreas to secret excessive insulin while fetal hyperinsulinemia leads to accumulation of adipose tissues and protein in the fetus and thus, macrosomia[8]. It is known that pre-pregnancy obesity is associated with increased risk of macrosomia and LGA in normal pregnancy[9], suggesting that maternal insulin resistance might play a role in fetal overgrowth. GDM is characterized by increased insulin resistance and decreased beta cell function[10, 11], and the former may consist of pregnancy-induced insulin resistance and pre-existing insulin resistance before pregnancy that continues in pregnancy. It remains unknown whether insulin resistance or beta cell dysfunction plays a more important role in fetal over-growth in GDM-complicated pregnancy.
Our group conducted a randomized translational trial in a 3-tier antenatal care system in Tianjin, China, which designed to address the effectiveness of lifestyle intervention on pregnancy outcomes. We performed a secondary analysis of the trial data to test whether insulin resistance and beta cell function were associated with excessive weight, macrosomia and LGA in Chinese women with GDM.
Methods
The participants and Settings
Tianjin is a large metropolitan city located in the North of China, with 13 million people, and directly under administration of the central government of China. There are 16 country-level administrative districts or counties and approximately 4.3 million inhabitants live in the six central urban districts.
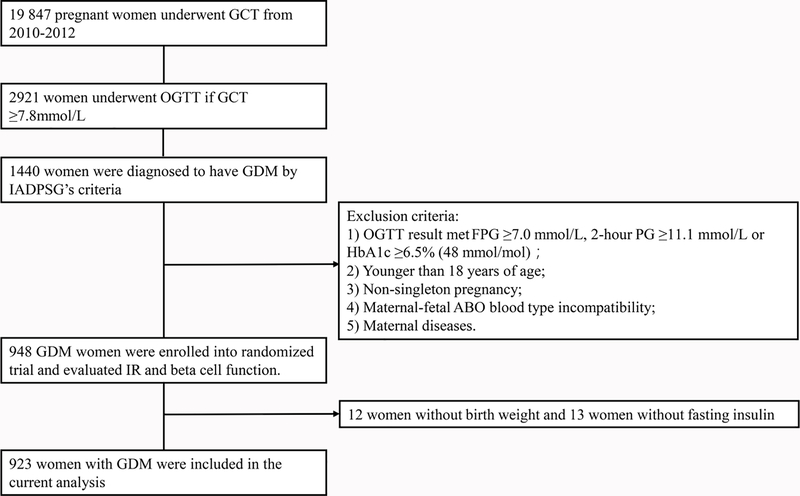
We established a universal screening and management system for GDM in Tianjin 20 years ago[2]. As a quality improvement effort, we conducted a randomized translational trial to test the effectiveness and cost-effectiveness of a lifestyle intervention on pregnancy outcomes of women with GDM. The trial was implemented in the 3-tier’s antenatal care system of the six central urban districts of Tianjin. The study design, participants and methods were described in details previously[12]. Briefly, a total of 19 847 pregnant women underwent a 50-g 1-h glucose challenge test (GCT) at 24–28 gestational weeks from December 2010 to October 2012. Of them, 2921 women with GCT ≥7.8mmol/L were offered a 75-gram 2-h oral glucose tolerance test (OGTT) at central GDM clinic of Tianjin Women and Children’s Health Center (TWCHC). During the trial period, 1440 women were diagnosed to have GDM using the cutoff points by the International Association of Diabetes and Pregnancy Study Groups (IADPSG)[13]. Among these women, 948 women met the criteria and were enrolled in the trial (Figure 1). After exclusion of 12 women with missing birth weight and 13 women with missing fasting insulin, 923 women with GDM were included in the current analysis. The exclusion criteria of the trial were 1). With diabetes defined by fasting plasma glucose (PG) ≥7.0 mmol/L, 2-hour PG ≥11.1 mmol/L or HbA1c ≥6.5% (48 mmol/mol)[14]; 2). Younger than 18 years of age; 3). Non-singleton pregnancy; 4). Maternal-fetal ABO blood type incompatibility; and 5). Maternal diseases such as chronic hypertension, thyrotoxicosis, pre-pregnancy diabetes and use of long-term medications. The approval of the ethics was granted by Tianjin Women and Children’s Health Center Ethics Committee and written informed consent was obtained before data collection.
Fig. 1.
Participant Flow Diagram
Clinical measurement and definitions
Maternal pre-pregnancy weight was recorded as body weight at the first antenatal care visit. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight in kilogram divided by the square of height in meters and categorized according to Chinese adults’ criteria for overweight and obesity[15]. Gestational weight gain was measured as the maternal weight at delivery minus pre-pregnancy. Because the Homeostasis model assessment (HOMA) was validated for estimation of insulin resistance and beta cell function during pregnancy[16], our study estimated insulin resistance and insulin beta-cell functions at OGTT using the HOMA insulin resistance (HOMA-IR) and beta-cell function (HOMA-B) formula:
Clinical outcomes
Excessive gestational weight gain was defined by gestational weight gain more than or equal to the 85th percentile range. Macrosomia was defined as birth weight ≥4000 grams and LGA was defined as birth weight greater than the gestational week and gender specific 90th percentile on the standard growth chart in Tianjin local reference.
Treatment of GDM in the shared care and in the usual care groups
The trial subjects were randomly allocated to receiving either the usual care (UC) or shared care (SC, lifestyle intervention). The details of the SC and UC contents were described previously[12]. Briefly, women who received the SC were offered three individualized counseling sessions and three group diabetes education sessions. Different low total energy intakes by pre-pregnancy BMI were recommended. Meals were evenly spaced during the day. Women in the SC group were asked to walk at least 30 minutes per day, 7 days a week. These women were asked to perform self-monitoring of blood glucose (SMBG). The targets of hyperglycemia control were ≥3.5-≤5.1 mmol/l for fasting capillary blood glucose and ≤7.0 mmol/L for 2-hour postprandial capillary blood glucose up to 36th gestational week and ≤8.0 mmol/L from 36th week onwards. If the targets of glycemia control were exceeded two times or more during a 2-week interval, insulin therapy was recommended. Women who received the UC were offered a group education session at diagnosis of GDM and were also offered a follow-up review of hyperglycemia control one week after the group diabetes education. However, they were not specifically taught to perform SMBG. From Nov 2010 to July 2011, the building of TWCHC was in renovation and separate areas were unavailable, so that the 242 women entering the trial during this period received either the SC or the UC in a same consulting room and contamination was hard to avoid[12]. Although this group of women was excluded in the primary analysis of the trial data, we included them in this secondary analysis of the trial data. To control for the potential contamination, a new variable for women enrolled during this period was created for use in the multivariable analysis in this study.
Statistical Analysis
IBM SPSS Statistics 19.0 (IBM SPSS, Chicago, IL, USA) was used to analyze all data. Data was expressed as mean ± standard deviation (SD) or median (interquartile range, IQR). Student’s t test was used to compare means of continuous variables while Chi-squared test (or Fisher’s exact test where appropriate) was used to compare categorical variables between two groups. Wilcoxon two-sample test was used to compare ordinal variables or continuous variables if they were not normally distributed. Binary logistic regression was used to obtain odds ratios (OR) and 95% confidence intervals of insulin resistance and beta-cell dysfunction for excessive weight gain, macrosomia and LGA. A two-step adjustment scheme was used to control for effects of potential confounders. Model 1 was adjusted for age, pre-pregnancy BMI, gestational age at delivery (only for macrosomia and excessive gestational weight gain), gestational age at OGTT, 2-hour plasma glucose, 2-hour insulin (continuous variables for above all variables), infant gender (only for macrosomia and excessive gestational weight gain), family history of diabetes (first degree relatives), and smoking and drinking status; Model 2 was further adjusted for SC and whether they were enrolled Nov 2010 to 31 Jul 2011 to remove possible confounding effects from potential contamination. P <0.05 was considered to be statistically significant.
Additional subgroup analyses by pre-pregnancy body mass (i.e., by BMI ≥24 kg/m2) and SC status were performed to explore possible subgroups effects of HOMA-IR on outcomes in GDM women.
Results
Clinical and biochemical characteristics
The cohort of 923 women with GDM had a mean age of 29.8 (SD: 3.1) years and a mean gestation age of 10.7 (SD: 2.3) weeks at their first antenatal care visit. Of them, 92 women (10.0%) were obese and 215 (23.3%) were overweight before pregnancy while 66.7% had normal weight (n=616). Primiparas accounted for 95.6% and 97.3% had a Han-ethnicity. Women with excessive weight gain had higher body weight, higher fasting insulin and higher insulin resistance and better beta-cell function but lower pre-pregnancy BMI and relatively low postprandial 2-hour glycemic in OGTT than those women who gained less body weight. Women who delivered a macrosomia or LGA infant were taller and had higher pre-pregnancy BMI. They were also more likely to be smokers and alcohol drinkers, gained more body weight during pregnancy, and had longer gestational age at term. HOMA-B was similar between the two groups, but HOMA-IR was only marginally higher in women who delivery a LGA infant than in those who did not (Table 1).
Table 1:
Clinical and biochemical characteristics of women who delivered macrosomia or large for gestational age infants
| Variables | Excessive weight gain | Macrosomia | Large for gestational age | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | |||
| n | 138 | 699 | 127 | 796 | 150 | 773 | |||||
| Age, year | 29.3(3.0) | 29.8(3.1) | 0.073 | 29.2(2.7) | 29.8(3.2) | 0.035 | 29.7(3.3) | 29.8(3.1) | 0.932 | ||
| Smoker | 10(7.2%) | 29(4.1%) | 0.115 | 15(11.8%) | 28(3.5%) | <0.001 | 17(11.3%) | 26(3.4%) | <0.001 | ||
| Drinker | 40(9.0%) | 204(292) | 0.962 | 46(36.2%) | 229(28.8%) | 0.088 | 57(38.0%) | 218(28.2%) | 0.016 | ||
| Han-ethnicity | 132(95.7%) | 681(97.4%) | 0.389 | 124(97.6%) | 774(97.2%) | 1 | 146(97.3%) | 752(97.3%) | 1 | ||
| Height, cm | 163.9(4.7) | 162.4(4.8) | 0.001 | 164(4.9) | 162(4.8) | <0.001 | 164(5.0) | 162(4.7) | <0.001 | ||
| Weight, kg | 59.7(8.5) | 61.3(10.8) | 0.057 | 65.3(11.6) | 60.5(10.5) | <0.001 | 64.8(11.6) | 60.4(10.6) | <0.001 | ||
| Pre-pregnancy BMI, kg/m2 | 22.2(2.8) | 23.2(3.8) | 0.001 | 24.1(3.8) | 22.9(3.7) | 0.001 | 23.9(3.8) | 22.9(3.7) | 0.004 | ||
| BMI ≥24 to <28 | 27(19.6%) | 170(24.3%) | 0.001 | 39(30.7%) | 176(22.1%) | 0.006 | 42(28.0%) | 173(22.4%) | 0.021 | ||
| BMI ≥28 | 4(2.9%) | 76(10.9%) | 17(13.4%) | 75(9.4%) | 21(14.0%) | 71(9.2%) | |||||
| Gestational age at 1st visit, weeks | 10.8(2.2) | 10.7(2.3) | 0.573 | 10.8(2.4) | 10.7(2.2) | 0.608 | 10.9(2.4) | 10.7(2.2) | 0.305 | ||
| Gestational age at OGTT, weeks | 26.6 | 26.1 | 0.022 | 26 | 26.3 | 0.062 | 26.1 | 26.3 | 0.168 | ||
| (25.9–27.3) | (25.6–27.4) | (25.3–27.1) | (25.6–27.3) | (25.4–27.0) | (25.6–27.3) | ||||||
| GCT, mmol/L | 8.9 | 9.0 | 0.073 | 9.1 | 9.0 | 0.19 | 9.1 | 8.9 | 0.219 | ||
| (8.3–9.5) | (8.4–9.9) | (8.4–10.1) | (8.4–9.8) | (8.4–10.1) | (8.4–9.8) | ||||||
| Fasting PG, mmol/L | 5.2(0.5) | 5.0(0.5) | 0.007 | 5.1(0.6) | 5.1(0.5) | 0.238 | 5.1(0.6) | 5.1(0.5) | 0.063 | ||
| 1-h PG, mmol/L | 9.9(1.5) | 10.1(1.3) | 0.064 | 10.2(1.3) | 10.1(1.4) | 0.429 | 10.2(1.3) | 10.1(1.4) | 0.257 | ||
| 2-h PG, mmol/L | 8.1(1.3) | 8.4(1.3) | 0.011 | 8.4(1.4) | 8.4(1.3) | 0.705 | 8.4(1.3) | 8.4(1.3) | 0.868 | ||
| Fasting insulin, mIU/L | 11.7 (8.0–16.4) | 9.4 (5.9–13.6) | <0.001 | 10.5 (6.7–15.3) | 9.6 (6.1–13.6) | 0.121 | 10.7 (6.8–15.0) | 9.6 (6.0–13.7) | 0.099 | ||
| 2-h insulin, mU/L | 89.7 (57.3–134.8) | 87.9 (55.1–131.0) | 0.588 | 84.3 (55.0–128.3) | 89.3 (56.2–133.1) | 0.342 | 84.3 (56.1–125.7) | 89.5 (55.7–133.7) | 0.207 | ||
| HOMA-IR,m U/L*mmol/l | 2.7 (1.7–3.6) | 2.0 (1.3–3.1) | <0.001 | 2.4 (1.4–3.6) | 2.2 (1.3–3.2) | 0.109 | 2.5 (1.4–3.5) | 2.1 (1.3–3.2) | 0.073 | ||
| HOMA-B,m U/L*(mmol/l) −1 | 148.7 (92.9–211.2) | 127.9 (85.1–185.4) | 0.022 | 137.8 (94.7–207.1) | 129.5 (86.4–188.8) | 0.263 | 135.7 (93.0–196.8) | 129.8 (86.5–190.0) | 0.448 | ||
| Gestational weight gain, kg | 25.8(4.4) | 13.9(5.2) | <0.001 | 19.0(6.9) | 15.3(6.6) | <0.001 | 18.8(7.4) | 15.3(6.5) | <0.001 | ||
| Parity | 0.178 | 0.447 | 0.884 | ||||||||
| 0 | 135(97.8%) | 666(95.3%) | 123(96.9%) | 759(95.4%) | 143(95.3%) | 739(95.6%) | |||||
| ≥1 | 3(2.2%) | 33(4.7%) | 4(3.1%) | 37(4.6%) | 7(4.7%) | 34(4.4%) | |||||
| Gestational week at delivery, week | 39.4(1.4) | 39.3(1.6) | 0.537 | 40.0(1.2) | 39.1(1.8) | <0.001 | 39.3(1.6) | 39.2(1.8) | 0.386 | ||
| Infant male gender | 67(48.6%) | 314(44.9%) | 0.434 | 48(37.8%) | 367(46.1%) | 0.08 | 68(45.3%) | 347(44.9%) | 0.92 | ||
| Birth weight, g | 3595(576.6) | 3408(511.4) | <0.001 | 4252(261) | 3295(467) | <0.001 | 4136(382) | 3289(470) | <0.001 | ||
| Infant stature, cm | 50.5(1.9) | 50.2(1.8) | 0.071 | 52.1(1.3) | 49.9(1.8) | <0.001 | 51.6(1.5) | 49.9(1.8) | <0.001 | ||
Abbreviations: BMI, body mass index; BP, blood pressure; GDM, gestational diabetes mellitus; GCT, glucose challenge test; HbA1c, haemoglobin A1c; OGTT, oral glucose tolerance test; PG, plasma glucose; IR, insulin resistance; B, beta cell function
P values were derived from Chi-square Test, Fisher’s Exact Test, or Student T Test unless specified
Data were reported as median (interquartile range) and P values were derived from Wilcoxon Two-Sample Test.
Associations of insulin resistance with macrosomia and LGA
HOMA-IR at OGTT was associated with excessive weight gain, macrosomia and LGA. HOMA-IR increase per SD was associated with a 1.72-fold increase in the risk of excessive weight gain (95%CI: 1.32–2.23) and 1.32-fold increase in the risk of macrosomia (95%CI: 1.02–1.70) and a 1.33-fold increase in the risk of LGA (95%CI: 1.05–1.68) after adjusting for confounders including pre-pregnancy BMI and weight gain during pregnancy (Table 2). Further adjusting for SC and potential contamination did not change the effect sizes (OR for excessive weight gain: 1.69, 1.29–2.21 and OR for macrosomia: 1.35, 1.04–1.75 and OR for LGA: 1.34, 1.05–1.71, respectively).
Table 2:
Odds ratio of insulin resistance and beta cell function for macrosomia and large for gestational age among Chinese women with gestational diabetes mellitus
| Excessive weight gain† | Macrosomia | LGA | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P§ | OR (95%CI) | P§ | OR(95%CI) | P§ | |||
| HOMA insulin resistance (IR) | ||||||||
| Model 1 | ||||||||
| IR per SD | 1.72(1.32–2.23) | <0.001 | 1.32(1.02–1.70) | 0.037 | 1.33(1.05–1.68) | 0.020 | ||
| IR in tertiles | <0.001 | 0.026 | 0.007 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.75(1.49–5.07) | 1.27(0.70–2.30) | 1.48(0.86–2.54) | |||||
| Upper | 4.57(2.07–10.08) | 2.46(1.15–5.30) | 2.70(1.34–5.46) | |||||
| Model 2 | ||||||||
| IR per SD | 1.69(1.29–2.21) | <0.001 | 1.35(1.04–1.75) | 0.026 | 1.34(1.05–1.71) | 0.018 | ||
| IR in tertiles | 0.001 | 0.019 | 0.006 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.67(1.45–4.95) | 1.32(0.72–2.40) | 1.49(0.87–2.57) | |||||
| Upper | 4.32(1.95–9.62) | 2.61(1.20–5.69) | 2.75(1.35–5.62) | |||||
| HOMA beta cell function (B) | ||||||||
| Model 1 | ||||||||
| B per SD | 0.95(0.73–1.23) | 0.703 | 1.20(0.97–1.48) | 0.091 | 1.14(0.94–1.38) | 0.178 | ||
| B in tertiles | 0.778 | 0.315 | 0.541 | |||||
| Bottom | 1.16(0.59–2.31) | 0.70(0.34–1.44) | 0.83(0.43–1.58) | |||||
| Middle | 0.76(0.46–1.26) | 0.67(0.39–1.15) | 0.79 (0.49–1.27) | |||||
| Upper | Reference | Reference | Reference | |||||
| Model 2 | ||||||||
| B per SD | 0.95(0.73–1.24) | 0.691 | 1.19(0.97–1.47) | 0.102 | 1.13(0.93–1.36) | 0.214 | ||
| B in tertiles | 0.739 | 0.301 | 0.539 | |||||
| Bottom | 1.19(0.60–2.37) | 0.69(0.34–1.43) | 0.82(0.43–1.58) | |||||
| Middle | 0.76(0.45–1.26) | 0.68(0.40–1.16) | 0.80(0.49–1.29) | |||||
| Upper | Reference | Reference | Reference | |||||
Abbreviations: OR, odds ratio; CI: confidence interval; HOMA: homeostasis model assessment; IR: HOMA insulin resistance; B: HOMA beta cell function.
Macrosomia was defined as birth weight >=4000 gram; Large for gestational age (LGA) was defined as birth weight greater than the gestational week and gender specific 90th percentile on the standard growth chart in Tianjin local reference.
Model 1: adjusted for age, pre-pregnancy BMI, 2-h PG, 2-h insulin, gestational age at OGTT, gestational age at delivery (only for macrosomia and excessive gestational weight gain), gender (only for macrosomia and excessive gestational weight gain), family history of diabetes, drinking and smoking
Model 2: adjusted variables in model 1 and for shared care status and unintentional intervention.
Excessive weight gain was >=85th percentile in gestational weight gain (22.0 kg)
P values for trend.
If HOMA-IR was stratified into tertiles, the adjusted ORs of the upper versus bottom tertile in Model 1 were 4.57(2.07–10.08) for excessive weight gain, 2.46 (1.15–5.30) for macrosomia and 2.70 (1.34–5.46) for LGA. Again, further adjustment in Model 2 did not lead to large changes in the effect sizes (OR for excessive weight gain: 4.32, 1.95–9.62, OR for macrosomia: 2.61, 1.20–5.69 and OR for LGA: 2.75, 1.35–5.62), with a significant linear trend (p for trend =0.001 for excessive weight gain, 0.019 for macrosomia and 0.006 for LGA).
Associations of beta-cell function with macrosomia and LGA
Differently from HOMA-IR, HOMA-B was not associated with the risks of excessive weight gain (0.95, 0.73–1.23), macrosomia (1.20, 0.97–1.48) and LGA (1.14, 0.94–1.38) after adjusting for confounders. After further adjustment for treatment, i.e., the SC and potential contamination, the associations with excessive weight gain, macrosomia and LGA remained non-significant (All P values >0.09). In the same way, ORs of the bottom tertiles versus the upper tertiles of HOMA-B for excessive weight gain, macrosomia and LGA were non-significant before and after adjustment for the treatment (Table 2).
3.4. Subgroup analysis
The subgroup analysis by pre-pregnancy overweight or obesity status showed that HOMA-IR was associated with the risk of excessive weight gain before and after further adjusting for the treatment (adjusted OR of HOMA-IR per SD: 1.76, 95%CI: 1.27–2.46) and also associated with increased risks of macrosomia but LGA (macrosomia: 1.45, 95%CI: 1.01–2.08 and LGA: 1.39, 1.00–1.93) among women with normal body weight prior to pregnancy. If tertiles of HOMA-IR used, ORs of the upper versus bottom tertiles for excessive weight gain, macrosomia and LGA were 4.32 (95%CI: 1.75–10.63), 3.33 (1.22–9.08) and 3.36 (1.37–8.26), respectively. In addition, HOMA-IR was not associated with increased risks of macrosomia and LGA and had a weaker association with excessive weight gain (adjusted ORs of HOMA-IR per SD for excessive weight gain: 1.70, 1.02–2.82; macrosomia: 1.15, 0.77–1.72 and LGA: 1.20, 0.83–1.74) among women with pre-pregnancy overweight or obesity (Table 3).
Table 3:
Subgroup analysis of odds ratio of HOMA insulin resistance for macrosomia and large for gestational age by pre-pregnancy overweight or obesity (BMI ≥24kg/m2)
| Excessive weight gain† | Macrosomia | Large for gestational age | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P§ | OR (95%CI) | P§ | OR(95%CI) | P§ | |||
| Among women with pre-pregnancy overweight or obesity* | ||||||||
| Model 1 | ||||||||
| IR per SD | 1.66(1.02–2.72) | 0.043 | 1.11(0.75–1.63) | 0.611 | 1.17(0.82–1.67) | 0.392 | ||
| IR in tertiles | 0.266 | 0.474 | 0.357 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.48(0.54–11.53) | 0.65(0.21–2.04) | 0.76(0.26–2.19) | |||||
| Upper | 3.99(0.63–25.19) | 1.29(0.34–4.85) | 1.23(0.36–4.22) | |||||
| Model 2 | ||||||||
| IR, per SD | 1.70(1.02–2.82) | 0.041 | 1.15(0.77–1.72) | 0.486 | 1.20(0.83–1.74) | 0.335 | ||
| IR in tertiles | 0.215 | 0.406 | 0.319 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.71(0.58–12.57) | 0.66(0.21–2.11) | 0.76(0.26–2.23) | |||||
| Upper | 4.55(0.71–29.09) | 1.36(0.35–5.29) | 1.29(0.37–4.48) | |||||
| Among women with normal pre-pregnancy weight* | ||||||||
| Model 1 | ||||||||
| IR per SD | 1.82(1.31–2.52) | <0.001 | 1.43(1.00–2.05) | 0.050 | 1.39(1.00–1.93) | 0.047 | ||
| IR in tertiles | 0.001 | 0.028 | 0.012 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.61(1.34–5.10) | 1.59(0.78–3.24) | 1.69(0.89–3.20) | |||||
| Upper | 4.65(1.91–11.30) | 3.13(1.17–8.35) | 3.27(1.35–7.92) | |||||
| Model 2 | ||||||||
| IR per SD | 1.76(1.27–2.46) | 0.001 | 1.45(1.01–2.08) | 0.046 | 1.39(1.00–1.93) | 0.053 | ||
| IR in tertiles | 0.002 | 0.021 | 0.011 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.53(1.29–4.97) | 1.68(0.82–3.45) | 1.72(0.90–3.27) | |||||
| Upper | 4.32(1.75–10.63) | 3.33(1.22–9.08) | 3.36(1.37–8.26) | |||||
Abbreviations: OR, odds ratio; CI: confidence interval; HOMA: homeostasis model assessment; IR: HOMA insulin resistance.
Model 1: adjusted for age, pre-pregnancy body mass, 2-h PG, 2-h insulin; gestational age at OGTT, gestational age at delivery and baby gender (for macrosomia and excessive gestational weight gain), family history of diabetes, smoking and drinking
Model 2: adjusted for variables in Model 1 and for shared care status and unintentional intervention
Excessive weight gain was >=85th percentile in gestational weight gain (22.0 kg)
P values for trend.
We also performed subgroup analysis by intervention assignment (Appendix Table 1). The associations of HOMA-IR with excessive weight gain were numerically larger in the UC group than in the SC group but the differences were quite small. However, the associations of HOMA-IR with macrosomia and LGA were significantly larger in SC group.
HOMA-B was not significantly associated with the excessive weight gain, macrosomia and LGA among all GDM women or in subgroup analyses by pre-pregnancy overweight or obesity status and SC status.
Discussion
Our study found that increased HOMA-IR was associated with occurrence of excessive weight gain during pregnancy, macrosomia and LGA. The associations were highly significant in women with normal body weight prior to pregnancy, which highlights the importance of maintaining normal weight gain during pregnancy and the role of undue insulin resistance induced by GDM-implicated pregnancy in fetal overgrowth, and consequently, macrosomia and LGA.
Maternal hyperglycemia can pass through the placenta and stimulate fetal pancreas to secret excessively insulin, which in turn promotes the storage of fetal adipose tissues and growth[8]. Indeed, the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study observed linear associations between fasting and 2-h PG levels during pregnancy and macrosomia[4]. It is further supported by randomized controlled trials showing that intervention (diet plus insulin if necessary) aiming to maintain normal glycaemia was effective to reduce the risk of macrosomia and LGA[6, 7]. Of note, current intervention measures such as diet, physical activity and use of insulin if needed are all targeting hyperglycemia. It is undeniably that most of these measures, if not all, also tend to alleviate insulin resistance. Indeed, levels of hyperglycemia can only account for a small part of variations in birth weight, e.g., 3.4% in Chinese population of pregnant women[17]. Therefore, it remains debatable whether insulin resistance or beta cell dysfunction during pregnancy plays a more important role in overgrowth of the fetus. In this regard, several epidemiological studies observed associations between insulin resistance and birth weight or macrosomia [18–20] although a study failed to find a significant association between insulin resistance in pregnancy and macrosomia in women with GDM [21]. Ong et al [18] analyzed a cohort of 668 pregnant women and reported that maternal insulin resistance was associated with infant’s adiposity at birth. Another study of 804 maternal-neonate pairs observed a strong positive correlation between HOMA-IR and neonatal birth weight, with one unit increase in HOMA-IR increasing birth weight by 25.5 grams and fat mass by 15.4 grams[22].
Insulin resistance before pregnancy may play a causal role in the development of GDM[3]. Of note, normal pregnancy is a state of insulin resistance, commencing from 12th −14th gestational week throughout pregnancy[23]. Besides pre-pregnancy insulin resistance, both insulin resistance induced by pregnancy and beta cell dysfunction due to genetic factors may also contribute to the development of GDM[10, 11]. In this connection, our study found that associations between HOMA-IR and excessive weight gain, macrosomia and LGA were highly significant and stronger in GDM women with normal body weight before pregnancy. The findings are consistent with recent findings that use of metformin, an insulin sensitizer, in obese women with GDM, did not have effects on fetal growth[24]. The findings from our subgroup analysis support the notion that existing insulin resistance before pregnancy and increased insulin resistance during pregnancy have different roles in fetal growth and the latter may play a more important role in macrosomia or LGA.
Our study has clinical and mechanistic implications. The prevalence of GDM has been increasing worldwide[25]. GDM is associated with increased risks of adverse pregnancy outcomes including macrosomia and LGA[4] and increased risk of childhood obesity at the age of 8–10 years[26] and impaired glucose tolerance at the age of 10–16 years in the offspring, irrespective of whether the hyperglycemic status of the mother was gestational or pre-gestational in nature[27]. The offspring born to mothers with GDM also exhibit early features of metabolic syndrome with high blood pressure and low high-density lipoprotein cholesterol[27]. Therefore, pregnancy is a window period and GDM may be a manifestation of short-term and long-term adverse health outcomes for the offspring. Besides, it remains inconclusive whether lifestyle intervention of GDM during pregnancy has long-term benefits on the offspring born to the GDM-implicated pregnancy[28].
Our study had limitations. First, adjustment for pre-pregnancy BMI may not completely remove confounding effects of pre-pregnancy insulin resistance. Second, we only used the HOMA models to estimate insulin resistance and beta cell function because the fasting and 2-h insulin levels were measured in the OGTT. Third, the subjects of our analysis participated in a randomized controlled trial and half of them received intensive lifestyle intervention. The 242 women entering the trial from Nov 2010 to July 2011 received the care in a same consulting room and the UC women were also likely to receive the intervention designed only for the SC group. Although we carefully adjusted for the treatment effect and the possible contamination, the residual confounding effects were unavoidable. Finally, HOMA-IR was measured at the OGTT while maternal weight gain was calculated from the maternal body weight at the first antenatal care visit to the time of being hospitalized for delivery. It is unknown which of pregnancy-induced insulin resistance or excessive maternal weight gain happened first. Therefore, we cannot exclude the possibility of the insulin resistance was a consequence of maternal weight gain.
In conclusion, our study found that insulin resistance during pregnancy was associated with excessive maternal weight gain, macrosomia and LGA. Our findings suggest that more research is warranted to investigate the mechanism of pregnancy induced insulin resistance and its interplays with pre-pregnancy insulin resistance and beta cell function for short-term and long-term effects on the health of mothers and their offspring.
Highlights.
Insulin resistance was related to macrosomia and large for gestational age infant.
It was also related to weight gain in women with gestational diabetes mellitus.
The links were mainly due to the large effect sizes among women with BMI<24 kg/m2.
Acknowledgements
We thank all doctors, nurses and research staffs at 65 primary care hospitals, 6 district-level women and children’s health centers (WCHCs), and Tianjin Women and Children’s Health C enter (TWCHC) for their participation in this study.
Funding
This work was supported by BRIDGES (Grant number: LT09-227). BRIDGES is an International Diabetes Federation program supported by an educational grant from Lilly Diabetes. X. Y. and J.L. will take full responsibility for this manuscript.
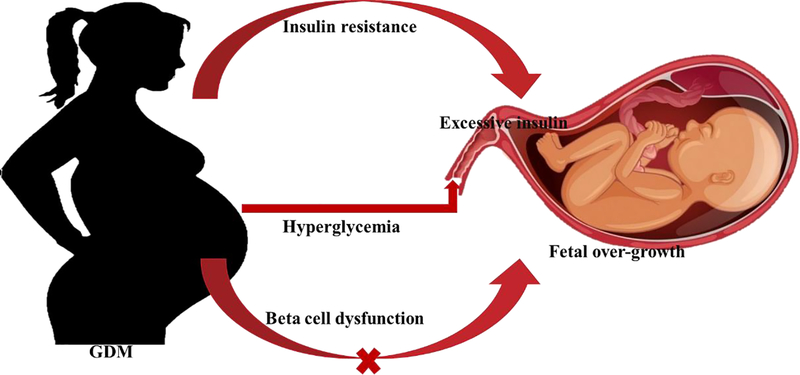
Appendix figure 1.
Conceptual Diagram
Insulin resistance, rather than beta cell dysfunction, was related to macrosomia and large for gestational age infant.
Appendix Table 1:
Subgroup analysis of odds ratio of HOMA insulin resistance for macrosomia and large for gestational age by lifestyle intervention
| Excessive weight gain† | Macrosomia | Large for gestational age | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P§ | OR (95%CI) | P§ | OR(95%CI) | P§ | |||
| Usual care | ||||||||
| Model 1 | ||||||||
| IR per SD | 2.30(1.50–3.54) | <0.001 | 1.19(0.83–1.17) | 0.342 | 1.23(0.89–1.70) | 0.212 | ||
| IR in tertiles | 0.008 | 0.216 | 0.102 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 3.20(1.35–7.58) | 1.17(0.54–2.52) | 1.50(0.75–3.02) | |||||
| Upper | 4.88(1.54–14.45) | 1.95(0.70–5.43) | 2.17(0.86–5.49) | |||||
| Model 2 | ||||||||
| IR, per SD | 2.28(1.47–3.52) | <0.001 | 1.25(0.86–1.81) | 0.235 | 1.25(0.90–174) | 0.183 | ||
| IR in tertiles | 0.011 | 0.142 | 0.084 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 3.15(1.33–7.46) | 1.22(0.56–2.63) | 1.54(0.76–3.10) | |||||
| Upper | 4.62(1.45–14.75) | 2.26(0.79–6.45) | 2.30(0.89–5.89) | |||||
| Shared care | ||||||||
| Model 1 | ||||||||
| IR per SD | 1.37(0.94–1.98) | 0.101 | 1.66(1.13–2.46) | 0.011 | 1.44(0.99–2.10) | 0.056 | ||
| IR in tertiles | 0.007 | 0.008 | 0.030 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.68(1.13–6.41) | 2.09(0.77–5.67) | 1.48(0.63–3.52) | |||||
| Upper | 4.81(1.61–14.40) | 5.53(1.63–18.85) | 3.42(1.17–10.02) | |||||
| Model 2 | ||||||||
| IR per SD | 1.34(0.92–1.96) | 0.127 | 1.64(1.11–2.44) | 0.014 | 1.43(0.98–2.09) | 0.063 | ||
| IR in tertiles | 0.011 | 0.012 | 0.038 | |||||
| Bottom | Reference | Reference | Reference | |||||
| Middle | 2.58(1.07–6.21) | 1.99(0.73–5.45) | 1.44(0.60–3.44) | |||||
| Upper | 4.52(1.49–13.76) | 5.14(1.48–17.77) | 3.27(1.10–9.69) | |||||
Model 1: adjusted for age, smoking and drinking, 2-h PG, 2-h insulin; gestational age at OGTT, gestational age at delivery and baby gender (for macrosomia and excessive gestational weight gain), family history of diabetes; Model 2: adjusted for variables in Model 1 and for unintentional intervention
Excessive weight gain was ≥85th percentile in gestational weight gain (22.0 kg)
P values for trend
Footnotes
Competing interests
The authors report no conflicts of interest to this manuscript.
Availability of data and materials
Data used in this study are not currently publicly available. Requests for access to this dataset should be made to the corresponding author, and it will require all necessary ethical approvals and data sharing agreements to be in place.
Ethics approval and consent to participate
The approval of the ethics was granted by Tianjin Women and Children’s Health Center Ethics Committee and written informed consent was obtained before data collection.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes care, 36 Suppl 1 (2013) S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang X, Hsu-Hage B, Zhang H, Yu L, Dong L, Li J, Shao P, Zhang C, Gestational diabetes mellitus in women of single gravidity in Tianjin City, China, Diabetes care, 25 (2002) 847–851. [DOI] [PubMed] [Google Scholar]
- [3].Leng J, Shao P, Zhang C, Tian H, Zhang F, Zhang S, Dong L, Li L, Yu Z, Chan JC, Hu G, Yang X, Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: a prospective population-based study in Tianjin, China, PloS one, 10 (2015) e0121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].H.S.C.R.G. Group HSCR, Hyperglycemia and adverse pregnancy outcomes, N Engl J Med, 358 (2008) 1991–2002. [DOI] [PubMed] [Google Scholar]
- [5].Bowers K, Liu G, Wang P, Ye T, Tian Z, Liu E, Yu Z, Yang X, Klebanoff M, Yeung E, Hu G, Zhang C, Birth weight, postnatal weight change, and risk for high blood pressure among chinese children, Pediatrics, 127 (2011) e1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr., Sciscione A, Catalano P, Harper M, Saade G, Y Lain K, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB, H. Eunice Kennedy Shriver National Institute of Child, N. Human Development Maternal-Fetal Medicine Units, A multicenter, randomized trial of treatment for mild gestational diabetes, N Engl J Med, 361 (2009) 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian G Carbohydrate Intolerance Study in Pregnant Women Trial, Effect of treatment of gestational diabetes mellitus on pregnancy outcomes, N Engl J Med, 352 (2005) 2477–2486. [DOI] [PubMed] [Google Scholar]
- [8].Kc K, Shakya S, Zhang H, Gestational diabetes mellitus and macrosomia: a literature review, Ann Nutr Metab, 66 Suppl 2 (2015) 14–20. [DOI] [PubMed] [Google Scholar]
- [9].Averett SL, Fletcher EK, Prepregnancy Obesity and Birth Outcomes, Matern Child Health J, 20 (2016) 655–664. [DOI] [PubMed] [Google Scholar]
- [10].Bowes SB, Hennessy TR, Umpleby AM, Benn JJ, Jackson NC, Boroujerdi MA, Sonksen PH, Lowy C, Measurement of glucose metabolism and insulin secretion during normal pregnancy and pregnancy complicated by gestational diabetes, Diabetologia, 39 (1996) 976–983. [DOI] [PubMed] [Google Scholar]
- [11].Kautzky-Willer A, Prager R, Waldhausl W, Pacini G, Thomaseth K, Wagner OF, Ulm M, Streli C, Ludvik B, Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy, Diabetes care, 20 (1997) 1717–1723. [DOI] [PubMed] [Google Scholar]
- [12].Yang X, Tian H, Zhang F, Zhang C, Li Y, Leng J, Wang L, Liu G, Dong L, Yu Z, Hu G, Chan JC, A randomised translational trial of lifestyle intervention using a 3-tier shared care approach on pregnancy outcomes in Chinese women with gestational diabetes mellitus but without diabetes, J Transl Med, 12 (2014) 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].D. International Association of, P. Pregnancy Study Groups Consensus, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy, Diabetes care, 33 (2010) 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes care, 33 Suppl 1 (2010) S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen C, Lu FC, P.R.C. Department of Disease Control Ministry of Health, The guidelines for prevention and control of overweight and obesity in Chinese adults, Biomedical and environmental sciences : BES, 17 Suppl (2004) 1–36. [PubMed] [Google Scholar]
- [16].Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM, Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus, Diabetes care, 24 (2001) 1602–1607. [DOI] [PubMed] [Google Scholar]
- [17].Yang X, Zhang H, Dong L, Yu S, Guo Z, Hsu-Hage BH, The effect of glucose levels on fetal birth weight: a study of Chinese gravidas in Tianjin, China, J Diabetes Complications, 18 (2004) 37–41. [DOI] [PubMed] [Google Scholar]
- [18].Ong KK, Diderholm B, Salzano G, Wingate D, Hughes IA, MacDougall J, Acerini CL, Dunger DB, Pregnancy insulin, glucose, and BMI contribute to birth outcomes in nondiabetic mothers, Diabetes care, 31 (2008) 2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahlsson F, Diderholm B, Jonsson B, Norden-Lindberg S, Olsson R, Ewald U, Forslund A, Stridsberg M, Gustafsson J, Insulin resistance, a link between maternal overweight and fetal macrosomia in nondiabetic pregnancies, Horm Res Paediatr, 74 (2010) 267–274. [DOI] [PubMed] [Google Scholar]
- [20].Yamashita H, Yasuhi I, Fukuda M, Y Kugishima, Yamauchi Y, Kuzume A, Hashimoto T, Sugimi S, Umezaki Y, Suga S, Kusuda N, The association between maternal insulin resistance in mid-pregnancy and neonatal birthweight in uncomplicated pregnancies, Endocr J, 61 (2014) 1019–1024. [DOI] [PubMed] [Google Scholar]
- [21].Voldner N, Qvigstad E, Froslie KF, Godang K, Henriksen T, Bollerslev J, Increased risk of macrosomia among overweight women with high gestational rise in fasting glucose, J Matern Fetal Neonatal Med, 23 (2010) 74–81. [DOI] [PubMed] [Google Scholar]
- [22].Crume TL, Shapiro AL, Brinton JT, Glueck DH, Martinez M, Kohn M, Harrod C, Friedman JE, Dabelea D, Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study, J Clin Endocrinol Metab, 100 (2015) 1672–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Catalano PM, Huston L, Amini SB, Kalhan SC, Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus, Am J Obstet Gynecol, 180 (1999) 903–916. [DOI] [PubMed] [Google Scholar]
- [24].Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, Walker BR, Quenby S, Wray S, Weeks A, Lashen H, Rodriguez A, Murray G, Whyte S, Norman JE, Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial, Lancet Diabetes Endocrinol, 3 (2015) 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH, Global estimates of the prevalence of hyperglycaemia in pregnancy, Diabetes Res Clin Pract, 103 (2014) 176–185. [DOI] [PubMed] [Google Scholar]
- [26].Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH, Gestational diabetes and the risk of offspring obesity, Pediatrics, 101 (1998) E9. [DOI] [PubMed] [Google Scholar]
- [27].Tam WH, Ma RC, Yang X, Ko GT, Tong PC, Cockram CS, Sahota DS, Rogers MS, Chan JC, Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero, Pediatrics, 122 (2008) 1229–1234. [DOI] [PubMed] [Google Scholar]
- [28].Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ, Rouse DJ, Biggio JR Jr., Thorp JM, Chien EK, Saade G, Peaceman AM, Blackwell SC, VanDorsten JP, Mild Gestational Diabetes Mellitus and Long-Term Child Health, Diabetes care, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]