Abstract
Background:
Garcinia indica also known as kokum is used in traditional system of medicine for relieving inflammation and rheumatic pain. Garcinol, a benzophenone obtained from its fruit rind is reported to have anti-inflammatory effect via modulating arachidonic acid metabolism, suppressing iNOS expression, NF-κB activation and COX-2 expression. It has also been studied for antioxidant and anti-cancer activity. Apart from these, few patents claim that garcinol also has anti-obesity and hepatoprotec-tive effect and has a potential to be used for the treatment of renal disorders, endometriosis and cardiac dysfunction.
Objective:
Garcinol Enriched Fraction (GEF) from the fruit rind of Garcinia indica should be effective in the treatment of arthritis, one of the chronic inflammatory disorder owing to its anti-inflammatory property as indicated by earlier experiments.
Methods:
GEF was prepared from the fruit rind of Garcinia indica and quantified using LC-MS/MS. It was found to contain 89.4% w/w of garcinol. GEF was evaluated at the dose of 10mg/kg for its efficacy against Complete Freund’s Adjuvant (CFA) induced arthritis in Wistar albino rats. Paw volumes of both sides were measured by Plethysmometer and body weight was recorded on 0, 1, 5, 12 and 21st day. The hyperalgesic response was also measured by motility test and stair climbing test.
Results:
GEF showed a significant reduction in paw swelling (p < 0.0001) and arthritis index (p < 0.0001) exhibiting anti-inflammatory potential. It also improves the motility and stair climbing ability of experimental animals (p < 0.05), thus reducing hyperalgesia.
Conclusion:
Garcinol enriched fraction shows anti-arthritic activity in experimental animals.
Keywords: Benzophenone, Complete Freund’s Adjuvant, Garcinia indica, inflammation, kokum, rheumatoid arthritis
1. INTRODUCTION
Garcinia indica of the Clusiaceae family (Mangosteen family) is a slender evergreen tree and is endemic to India in the tropical evergreen rain forests of Western Ghats of Maharashtra. The fruits, commonly known as kokum, are used for culinary purposes in India. A syrup formulated from the fruits is a popular drink in the states of Maharashtra and Goa and is believed to avoid skin damage or allergies due to sun. The fruit has been used as a coloring agent and for imparting sour taste in traditional dishes. Kokum is used in the preparation of kokum beverages, dehydrated kokum, kokum butter, which is used in chocolates and confectionary preparations and in the production of soaps and candles [1]. Apart from its uses in cooking, it has been used in traditional system of medicine to relieve inflammation, rheumatic pain and bowel complaints [2]. It is also prescribed for treating constipation, delayed menstruation, oedema, intestinal parasites and for weight loss [3]. Garcinia indica is reported to have cardioprotective effect [4], antioxidant and hepatoprotective effect [5], useful in Parkinson’s disease [6], antibacterial effect [7] and anti- ulcer effect [8]. There are a number of patents described below which claim that garcinol, the active component of Garcinia indica, is responsible for the aforementioned effects. A herbal formulation containing hydromethanolic extract of Garcinia indica at the dose of 175-300mg/day is effective for the prevention and management of type-2 diabetes mellitus and diabetic complications [9]. Nutraceutical grade tablets containing 75mg of Garcinia indica are reported to be useful for treatment of elevated blood cholesterol [10].
Kokum fruits contain 23-26% of fat, which is used for commercial and medical purposes. The fruit rind contains proteins, tannins, pectins, sugars, organic acids like (-) hydroxycitric acid, hydroxycitric acid lactone and citric acid; anthocyanins like cyanidin-3-glucoside and cyanidin-3-sambubioside; and polyisoprenylated phenolics-garcinol and isogarcinol [2]. Hydroxycitric acid, a component from the fruit rind is a potential anti-obesity agent [11]. Kokum pigments have the potential to absorb UV light. It is used in the cosmetic industry in the manufacturing of sun-screen lotions and pastes. The same anthocyanin pigments change their color from red to blue/violet as pH increases beyond 5. This property can be utilized in making pH sensitive indicators [12]. The fruit rind is a potential source of natural food colorant in many food and food formulations [1].
A very interesting application of the fruit rind is that its extract has been used as a mordant for dying silk fabric which may also protect the skin from the damaging effects of sun [13]. In fact, one of the patent claims that a composition containing Garcinia indica, by the virtue of having anti-oxidant, anti-inflammatory, UV-A and UV-B protective activity and hyper proliferation inhibitory activity has anti-skin damaging effect [14]. A patent filed in 1998, has claimed that garcinol, a polyisoprenylated benzophenone derivative, present in Garcinia indica has hyaluronidase inhibitory effect and hence can be expected to have skin ageing prevention, anti-inflammatory and anti-tumor effect [15]. Accordingly, garcinol has been studied for anticancer effect and has also shown in vitro anti-inflammatory activity [16, 17]. It has been reported to modulate arachidonic acid metabolism, when added prior to Lipopolysaccharide (LPS), it also suppresses NF-κB activation and COX-2 expression through the interruption of LPS binding to toll-like receptors [18]. Besides, one of the reports states that garcinol may exhibit effect on lipoxygenase and prostaglandin E2 synthase [19]. It is found to be useful to treat skin inflammation and tumorigenesis [20]. A Japanese patent also, claims it to be an anti-tumor agent in the dose range of 10-2,000mg [21]. Further to this, it has been claimed that since garcinol has a histone deacetylase inhibitory activity, it can be considered as a novel chemical entity for the development of anti-cancer drugs [22].
Fig. (1) depicts the chemical structure of garcinol. It is clear that the presence of ketonic group, phenyl ring with hydroxyl groups and the double bond of isoprenyl group provide the oxidation sites. Moreover, the isoprenyl chain being hydrophobic in nature provides the site of attachment to biological targets [23, 24]. Garcinol can be considered as a prenylated chalcone, containing two aromatic rings separated by carbonyl group having a structural similarity to curcumin when opened. Synthetic chalcones are reported to show anti-inflammatory activity [25].
Fig. (1).
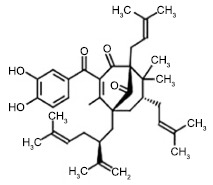
Structure of garcinol.
Rheumatoid Arthritis (RA) is a chronic, inflammatory, systemic auto-immune disorder that mainly affects small diarthrodial joints of hands and feet [26]. Genes and environmental factors are important causes for RA. Smoking/ consumption of tobacco remains one of the major environmental risk factor for development of RA [27]. Smoking along with other risk factors like pollutants, xenobiotics, radiations and chemotherapeutic agents lead to generation of Reactive Oxygen Species (ROS). ROS in turn enhance the levels of synovial lipid peroxides and cause degradation of ligament and cartilage. It can further lead to the formation of advanced glycation end products and advanced oxidative protein products, both of which might be responsible for autoimmune responses observed in RA. ROS also activates NF-κB pathway which contributes to the inflammatory response in RA. Thus, countering the actions of ROS by administration of anti-oxidants can be one of the strategies of treating RA [28]. Gut microbiome is responsible to maintain the homeostasis and integrity of the gut. Mucosal inflammation and altered gut microflora due to infection or smoking is also an established cause for autoimmunity and plays an important role in the progression of RA [29]. Shared Epitope (SE) alleles, an MHC region encoding HLA protein is one amongst the several genetic factors believed to cause autoimmunity, increase inflammation and thus contribute for RA risk [30]. Due to the several causative factors, personalized therapy needs to be tailored for individual patient. Precision medicine is not yet clinically used [31] and hence, currently the ultimate goals in RA management are to decrease pain and inflammation, prevent and control joint damage as well as loss of function. Medications currently used for RA treatment can be divided into three classes, namely, general analgesics and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), glucocorticoids and Disease Modifying Anti-Rheumatic Drugs (DMARDs) (synthetic or biologic) [26, 32]. However, these drugs have limited effect to halt joint destruction. NSAIDs cause gastric damage by causing ulceration. Glucocorticoid causes acute adrenal insufficiency and withdrawal syndrome. DMARDs cause stomach upset, which may affect the immune system and increase the risk of infection. Thus, each has its own limitations and one needs to find safer and effective therapy for RA. The new molecule should also have a favourable pharmacokinetic data and should be non-toxic. Although, currently there is no data which robustly confirm the bioavailability of garcinol, preliminary animal studies indicate that garcinol is well absorbed after oral/intraperitoneal administration and can readily cross the cell membrane to reach the interiors of mammalian cells [33]. Moreover, earlier studies have indicated that garcinol does not show apparent toxicity in animals [34].
A plant like Garcinia indica having the presence of garcinol, an anti-inflammatory and anti-oxidant moiety with favourable bioavailability, should have applications in the treatment of rheumatoid arthritis. Moreover, the traditional system prescribes it for the treatment of inflammation and symptoms associated with inflammatory disorders like rheumatic pain. Therefore, this study was undertaken to evaluate the anti-inflammatory effect of garcinol enriched fraction in rheumatoid arthritis.
2. MATERIALS AND METHODS
2.1. Plant Name and Parts Used
Fresh fruits of Garcinia indica were procured from Dr. Badhe Waadi, Phoolpada Road, Virar (East) and were authenticated from Agharkar Research Institute, Pune, Maharashtra, India.
2.2. Chemical Compounds
Standard garcinol was purchased from Cayman Chemical Company-USA. Methanol (HPLC grade) was purchased from SD Fine chemical Limited (Mumbai, India). N-hexane, toluene, ethyl acetate, formic acid, and other chemical were purchased from Loba Chemie (Mumbai, India).
2.3. Extraction
700g of air dried fruit rind powder was extracted with hexane using soxhlet apparatus for 72hrs. The extract was concentrated in rotary vacuum evaporator to get a pasty mass (10g). This extract was subjected to phytochemical screening to detect the presence of different phytoconstituents.
2.4. Fractionation & LC-MS/MS Analysis of Enriched Fraction
The extract (9gm) was adsorbed on silica and loaded onto silica column (100 - 120 mesh size). Stepwise gradient elution with n-hexane- ethyl acetate (1:0 - 0:1) gave four major fractions, fraction A, B, C, D. All the four fractions were subjected to thin layer chromatography using silica gel GF254 as stationary phase, Toluene: Ethyl acetate: Formic acid (4:1:0.5) as mobile phase, vanillin sulphuric acid as spraying reagent and standard garcinol as reference standard. Fraction C (800mg) obtained from n-hexane: Ethyl acetate (0.95:0.05) showed maximum concentration of garcinol. The enriched fraction was evaluated for the content of garcinol by LC-MS / MS (Shimadzu LC-MS 8040) using methanol as the solvent. Table 1 gives the details of LC-MS/MS analysis. This garcinol enriched fraction was used for animal studies and would henceforth be referred to as GEF.
Table 1. Parameters for LC-MS/MS Analysis of Garcinol Enriched Fraction (GEF).
| Column | C18 |
|---|---|
| Flow rate | 10µl |
| Mobile Phase | Methanol: water (0.1% formic acid), 80:20 |
| Nebulizing gas (N2) flow | 3.0L/min |
| Drying gas(N2) flow | 15.0L/min |
| Heat block temperature | 400°C |
| DL (Desolvation) temperature | 250°C |
| Mode of Ionization | Positive |
| Ionization probe | ESI |
| Scanning modes | Q1 Scan |
2.5. Animals
Male Wistar rats weighing between 180-200g respectively, were purchased from Bharat serum, Thane, Maharashtra, India. All animals were placed in cages and kept in standard environmental conditions [23°C + 5, 60% + 5 RH, and 12:12h dark and light cycle], fed with standard diet and allowed free access to drinking water during the period of acclimatization (one week).
2.6. Establishment of Rat Adjuvant Arthritis and Treatment Regimen
Adjuvant Arthritis (AA) rat model was developed by single injection of 0.1ml of Complete Freund’s Adjuvant (CFA) procured from Sigma Aldrich, Mumbai, India containing 0.1mg of heat killed Mycobacterium tuberculosis in 0.85ml liquid paraffin and 0.15ml mannide monooleate into the sub plantar region of left hind paw as described [35].
Rats were divided into groups of four having six animals each
Group I- Normal Control, Group II- Disease Control, Group III- Positive control (diclofenac sodium, 10mg/kg) and Group IV- GEF (10mg/kg).
From day 0-day 12, AA rats in group IV were treated with GEF (10mg/kg, p.o.) and in group III with diclofenac sodium (10mg/kg, p.o.) once daily. Normal and disease control groups were given vehicle simultaneously. From day 13-21 the animals were not dosed, but monitoring of the parameters was continued.
2.7. Evaluation of Arthritis Swelling
The volume of left hind paw was measured using electronic water plethysomometer on day 0, 1, 5 12, 16 and 21 indicating the primary lesion and the influence of therapeutic agents in this Phase. Right hind paw volume was also measured to understand the severity of secondary lesions. Visual arthritis index was measured to evaluate the severity of AA. For this, the rats were examined visually for the presence of inflammatory lesions in ears, nose, tail, forepaws and hind paws and were scored for arthritis severity. The paw, ears, nose, eyes and tail were graded separately and cumulative scoring depended on redness, swelling and presence/absence of nodules [35]. Observations were recorded by the person who was blind to the study.
2.8. Measurement of Body Weight
The body weights were measured for all the animals on day 0, 1, 5 12, 16 and 21.
2.9. Stair Climbing Test
Animals were trained for one week to climb wooden stairs, placed in the observation cage, with 3 steps respectively 5, 10cm and 15cm high having water on the second step and food on the third step. The climbing ability was scored as 0 if the rat does not climb; 1if the rat climbs onto step 1; 2 if the rat climbs onto steps 1 and 2; 3 if the rat climbs onto steps 1, 2 and 3. This test was performed on day 0, 1, 5, 12, 16, and 21 after the CFA injection.
2.10. Evaluation of Motility Test
Each animal was trained for a period of one week in a wooden box for motility test. The motility of each rat was performed on the day 0, 1, 5, 12, 16, and 21 after the CFA injection. The motility of animals was observed for 5min in an observation cage. The motility pattern was scored 0 if the rat lies down; 1 if the rat crawls; 2 if the rat walks; 3 if the rat runs and climbs with some difficulty and 4 if the rat runs and climbs well [36, 37].
2.11. Statistical Analysis
All the data were expressed as Mean + SEM. Statistical analysis was carried out by one-way ANOVA followed by either Sidak’s/ Dunnett’s multiple comparison test. The criteria for significance was p < 0.05.
3. RESULTS
3.1. Extraction
The fresh fruits of kokum were confirmed to be that of Garcinia indica, Choisy (Family: Clusiaceae). A sample specimen is deposited at the institute with specimen number 15-147. The air dried fruit rind was extracted with hexane. Based on the results of phytochemical analysis, the extract was found to contain phenols and flavonoids. The percentage yield of hexane extract was found to 1.43% w/w.
3.2. Fractionation and LC-MS/MS of Enriched Fraction
Percentage yield of GEF was found to be 8.88% w/w and the fraction contained 89.4% w/w of garcinol by LC-MS/MS (Fig. 2A, B). Remaining 10.6% w/w of the fraction comprised phenols and flavonoids as evident by qualitative phytochemical tests performed on the fraction.
Fig. (2).
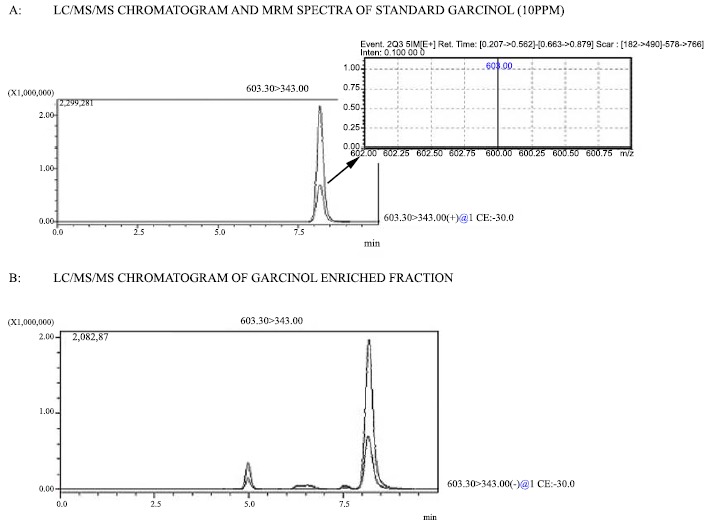
LC-MS/MS chromatogram of garcinol and GEF.
3.3. Effect of GEF on Paw Swelling Arthritis Index
As shown in Table 2, treatment with CFA, induced primary arthritis in the left hind paw of animals as observed by significant swelling (p < 0.0001) in the paw and is maintained for 21 days as compared to control. Table 2 also depicts the swelling measured in the right hind paw indicative of secondary lesions of arthritis. Although there was an increase in the paw volume of CFA treated animals it was not significant to term it as secondary arthritis. Treatment with GEF significantly inhibited paw swelling from 5th to 21st day (p < 0.0001) as compared to disease control animals. Diclofenac also showed a significant reduction in the paw volume (p < 0.0001). The treated groups also showed reduction in right hind paw swelling although it was not significant.
Table 2. Effect of GEF on Various Parameters in CFA Induced Arthritis in Rats.
| Number of Days | Paw Volume (ml) | Arthritis Index | Body Weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Disease Control | Diclofenac Treated | GEF Treated | Control | Disease Control | Diclofenac Treated | GEF Treated | Control | Disease Control | Diclofenac Treated | GEF Treated | |
| 0 | 0.021 + 0.001 | 0.025 + 0.002 |
0.026 + 0.001 | 0.031 + 0.002 | 0 | 0 | 0 | 0 | 244.52 + 17.13 | 227.98 + 11.23 | 258.3 + 12.8 | 249.36 + 9.01 |
| 1 | 0.0220 + 0.001 |
0.750 + 0.065a |
0.790 + 0.052 | 0.960 + 0.085c | 0 | 2.6 + 0.44a | 1.4 + 0.21b | 1.6 + 0.245 b | 245.34 + 16.9 |
228.96 + 11.17 | 259.44 + 12.58d | 250.14 + 8.85 |
| 5 | 0.0351 + 0.002 |
2.080 + 0.084 a |
0.618 + 0.041b | 0.746 + 0.070 b | 0 | 2.8 + 0.32 a | 1.2 + 0.28 b | 1. 4+ 0.38 b | 250.66 + 17.27 |
226.98 + 11* | 261.06 + 12.37 d | 253.52 + 8.74 e |
| 12 | 0.0480 + 0.004 |
1.926 + 0.063 a |
0.490 + 0.036 b | 0.690 + 0.063 b | 0 | 2.4 + 0.48 a | 1.2 + 0.26 b | 1.0 + 0.20 b | 257.86 + 20.43 |
240.02 + 12.09 | 269.66 + 9.45 d | 262.16 + 6.90 |
| 16 | 0.0740 + 0.006 |
1.890 + 0.097 a |
0.490 + 0.032 b | 0.670 + 0.059 b | 0 | 2.0 + 0.36 a | 1.0 + 0.31 b | 1.0 + 0.34c | 259.38 + 20.31 |
241.58 + 11.42 | 272.2 + 8.97 d | 265.26 + 6.86 e |
| 21 | 0.1140 + 0.015 |
1.510 + 0.054 a |
0.250 + 0.022 b | 0.240 + 0.021 b | 0 | 1.6 + 0.51 a | 1.0+ 0.33d | 1.0 + 0.26d | 262.5 + 19.9 |
246.46 + 9.8 | 275.34 + 9.57 d | 268.32 + 7.71 e |
ap < 0.0001, *p < 0.05 as compared to control, bp < 0.0001, cp < 0.001, dp < 0.01, ep < 0.05 as compared to disease control animals, using one way ANOVA followed by Sidak’s multiple comparison test. Each value represents Mean + S.E.M. (n = 6, the number of animals utilized during experiment).
As shown in Table 2, rats treated with CFA showed a significant increase in arthritis index as compared to control. The index steadily increased and reached a maximum value on 5th day followed by a gradual reduction. Treatment with GEF and diclofenac significantly reduced the arthritis index as compared to the disease control animals.
3.4. Effect of GEF on Body Weight
Treatment with CFA reduced the body weight as compared to control although it was not significant except on day 5 after which there was a steady increase in body weight of CFA treated animals. However, treatment with GEF and diclofenac significantly inhibited this weight loss from the 5th day to 21st day as compared to disease control animals as shown in Table 2.
3.5. Effect of GEF on Stair Climbing and Motility Test
CFA treatment significantly reduced the stair climbing activity from day 5 to day 21 in all animals when compared to normal animals indicating the induction of hyperalgesia, as shown in Fig. (3). Diclofenac and GEF treatment improved the score from day 12 and day 16 respectively and showed a significant increase till day 21.
Fig. (3).
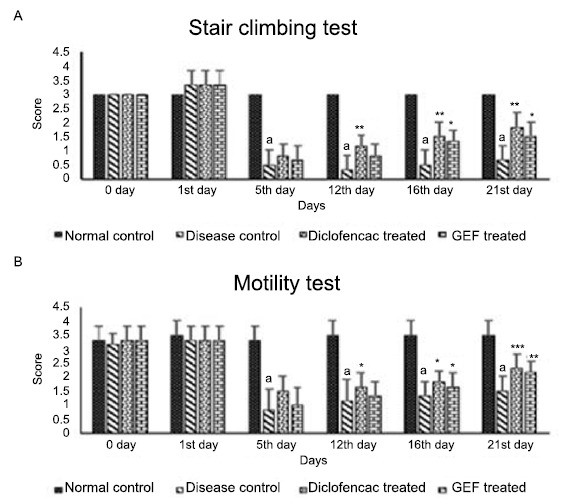
Effect of GEF on hyperalgesic response induced by CFA.
a p < 0.0001 as compared to control.
***p < 0.001, **p < 0.01, *p < 0.05 as compared to disease control animals using one-way ANOVA followed by Dunnett’s multiple comparison. Each value represents Mean + S.E.M. (n = 6, the number of animals utilized during experiment).
As shown in Fig. (3), all rats showed a significant decrease in motility from day 5 to day 21 as compared to normal rats. The motility score of animals treated with Diclofenac showed a steady and significant increase from day 12 to day 21 whereas the animals treated with GEF showed a steady and significant increase from day 16 to day 21.
4. DISCUSSION
Acute and chronic pain can be relieved effectively by non-steroidal anti-inflammatory drugs that inhibit cyclooxygenases (COX-1and COX-2) and consequently, are widely used [38]. Opioid analgesic drugs remain the most effective therapy available for the treatment of moderate to severe pain. However, the problems arising from unwanted side effects persist. A range of new therapies has been developed in recent decades to treat inflammation and chronic inflammatory diseases. Nonetheless, the number of such drugs remains small and in addition to the fact that they all display side effects, limit their use. Thus, the development of new pain management strategies can allow clinicians to have additional options for management of pain. Here we have demonstrated anti-arthritic activity of garcinol enriched fraction obtained from hexane extract of Garcinia indica in CFA induced arthritis in rats which is a chronic model of inflammation for the first time.
CFA induced paw edema is a chronic 21-day inflammatory model with paw swelling as a factor of assessing inflammation and hence the anti-arthritic activity of various drugs. The inflammation peaks at 5th day and continues till 12th day as shown by clinic-behavioural parameters that include increased paw swelling, arthritis index and reduction in motility and stair climbing ability depicting the acute phase of inflammation. Thereafter the parameters show an improvement till 21st day depicting the post-acute phase of inflammation. The aforementioned parameters do not return to normalcy till 21 days depicting the transition to late phase of inflammation.
This model induces synovial inflammation, cartilage and bone destruction [39]. In the articular chondrocytes activation of NF-κB mediates response to pro-inflammatory cytokines like TNF-α, IL-1β, increases the expression of cytokines like IL-6, IL-1, matrix metalloproteinases (MMP1, MMP-3, MMP-13), which in turn stimulate the proliferation of fibroblasts, stimulate the production of PGE2, and increase the expression of other cytokines and synthesis of collagen by synovial cells, contributing to cartilage and bone destruction [40]. This results into inflammatory and histological changes in arthritis [41].
In the present study, GEF showed a significant reduction in the paw swelling and arthritis index from the 5th day to 21st day of treatment as compared to disease control, suggesting that GEF which is reported to inhibit NF-κB in vitro, also inhibits it in the articular chondrocytes, and thus suppressing the inflammatory cascade and reducing paw swelling.
GEF treatment improves the motility and stair climbing ability of rats from day 16 till day 21 implying that it also works in the post-acute phase of inflammation. Thus, continuous treatment for a chronic period of 2 months and evaluation of all the aforementioned parameters along with serum and tissue levels of cytokines like IL-6, IL-10 and TNF-α involved in chronic inflammation would give a clear picture of the effects of GEF in the post-acute and late phase of inflammation.
Previous reports have established a correlation between arthritis induction and weight loss [42]. Accordingly, in the present study, there was weight loss in animals treated with CFA as compared to control. Treatment with GEF showed a significant attenuation of this weight loss.
CONCLUSION
The study indicates that GEF has anti-arthritic activity in CFA induced arthritis model. Garcinol, the major component of GEF may be the key player in exhibiting the anti-arthritic potential probably due to its ability to inhibit NF-κB [39] in the articular chondrocytes and thus suppressing the inflammatory cascade, reduce the paw swelling, arthritis index and hyperaglesic response. However, the exact mechanism needs to be elucidated.
CURRENT & FUTURE DEVELOPMENTS
Garcinol is currently proposed as an anti-cancer and anti-inflammatory molecule. The authors have positively investigated the use of garcinol enriched fraction in one of the inflammatory condition-rheumatoid arthritis. Table 3 enlists the current therapeutic applications of garcinol [43-51]. Garcinol may further be explored for its efficacy in other acute and chronic inflammatory disorders of the immune, endocrine, nervous or reproductive system. Kokum fruit which is the source of garcinol, is a part of diet, thus a nutraceutical enriched with garcinol may be formulated and used as a dietary approach for the treatment of rheumatoid arthritis.
Table 3. Therapeutic Applications of Garcinol.
| Patent No. | Applications | References |
|---|---|---|
| JP2000044468 | Anti-obesity agent due to lipase inhibitory effect | [43] |
| EP1254209 | Garcinol along with hydoxycitric acid and anthocyanins- cyanidine-3-glucoside & cyanidine-3-sambubioside, is effective in reducing total body weight, body fat and increasing the lean body mass | [44] |
| US20090062231 | A composition containing garcinol along with alginate and hydroxycitric acid used as appetite suppressant which not only claim to reduce total cholesterol, triglycerides, glucose but also inhibits gastric reflux | [45] |
| US8329743 | Composition along with other phytoconstituents reduces adipogenesis and hence is useful in treating obesity | [46] |
| US20130281544 | Hepatoprotective agent showing in vitro and in vivo activity | [47] |
| WO2016123044 | Treatment of endometriosis | [48] |
| US9872840 | Treatment of renal disorders specifically for diabetic nephropathy | [49] |
| WO2016063296 | Derivatives of garcinol, acting as p-glycoprotein inducers, as therapeutic agents for treatment of Alzheimer’s | [50] |
| US9956301 | Garcinol complexed with cyclodextrin used in prevention and management of cardiac dysfunction induced by chemotherapy or lifestyle/disease conditions | [51] |
ACKNOWLEDGEMENTS
The authors would like to acknowledge the institute SPP-School of Pharmacy and Technology Management, SVKM’s NMIMS, Mumbai, Maharashtra, India for providing the facility required to conduct the experiments.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN & ANIMAL RIGHTS
No humans were used in this study. All animal experiments were carried out in accordance with the guidelines of Committee for the Purpose and Supervision of Experiments on Animals (CPCSEA), Government of India and the study was approved by the Institutional Animal Ethics committee (IAEC) with approval number- CPCSEA/IAEC/SPTM/P-03/2016.
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
REFERENCES
- 1.Nayak C.A., Rastogi N.K., Raghavarao K.S. Bioactive constituents present in Garcinia indica choisy and its potential food applications: A review. Int. J. Food Prop. 2010;13:441–453. [Google Scholar]
- 2.Baliga M.S., Bhat H.P., Pai R.J., Boloor R., Palatty P.L. The chemistry and medicinal uses of the underutilized Indian fruit tree Garcinia indica Choisy (kokum): A review. Food Res. Int. 2011;44:1790–1799. [Google Scholar]
- 3.Kunnumakkara A.B., Koca C., Dey S., Gehlot P., Yodkeeree S., Danda D., et al. Molecular targets and therapeutic uses of spices. New Jersey: World Scientific; 2009. pp. 1–24. [Google Scholar]
- 4.Patel K.J., Panchasara A.K., Barvaliya M.J., Purohit B.M., Baxi S.N., Vadgama V.K., et al. Evaluation of cardio protective effect of aqueous extract of Garcinia indica Linn. Fruit rinds on isoprenaline-induced myocardial injury in Wistar albino Rats. RPS. 2015;10:388. [PMC free article] [PubMed] [Google Scholar]
- 5.Panda V., Ashar H., Srinath S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol induced hepatic damage in rodents. Interdiscip. Toxicol. 2012;5:207–213. doi: 10.2478/v10102-012-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antala B.V., Patel M.S., Bhuva S.V., Gupta S., Rabadiya S., Lahkar M. Protective effect of methanolic extract of Garcinia indica fruits in 6-OHDA rat model of Parkinson’s disease. Indian J. Pharmacol. 2012;44:83. doi: 10.4103/0253-7613.103242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshmi C., Kumar K.A., Dennis T.J., Kumar T.S. Antibacterial activity of polyphenols of Garcinia indica. Indian J. Pharm. Sci. 2011;73:470. doi: 10.4103/0250-474X.95655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi F., Saito M., Ariga T., Yoshimura Y., Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000;48:2320–2325. doi: 10.1021/jf990908c. [DOI] [PubMed] [Google Scholar]
- 9.Govind P.D., Aruna A., Nirupama D., Shipra D., Rajesh D., Mercy D.S. A novel herbal formulation for the prevention and management of type-2 diabetes mellitus and vascular complications associated with diabetes. WO2011158247. 2011
- 10.Amit P. Nutraceutical formulation for treatment of elevated cholesterol and cardiovascular disease. US20140314729. 2014
- 11.Heymsfield S.B., Allison D.B., Vasselli J.R., Pietrobelli A., Greenfield D., Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: A randomized controlled trial. JAMA. 1998;280:1596–1600. doi: 10.1001/jama.280.18.1596. [DOI] [PubMed] [Google Scholar]
- 12.Nayak C.A., Srinivas P., Rastogi N.K. Characterisation of anthocyanins from Garcinia indica Choisy. Food Chem. 2010;118:719–724. [Google Scholar]
- 13.Hegde M.G., Goutham N. Application of medicinal dye (turmeric and annatto) on silk fabrics using eco-friendly mordants. MSRUAS-SASTech J. 2015;14:33–36. [Google Scholar]
- 14.Muhammed M. Anti-skin damage compositions with complimentary dual mode of action. US2009169651. 2009
- 15.Toshiaki A., Norio Y. Hyaluronidase inhibitor. JP2000072665. 2000
- 16.Wang Y., Tsai M.L., Chiou L.Y., Ho C.T., Pan M.H. Antitumor activity of garcinol in human prostate cancer cells and xenograft mice. J. Agric. Food Chem. 2015;63:9047–9052. doi: 10.1021/acs.jafc.5b03851. [DOI] [PubMed] [Google Scholar]
- 17.Yu S.Y., Liao C.H., Chien M.H., Tsai T.Y., Lin J.K., Weng M.S. Induction of p21Waf1/Cip1 by garcinol via down regulation of p38-MAPK signalling in p53-independent H1299 lung cancer. J. Agric. Food Chem. 2014;62:2085–2095. doi: 10.1021/jf4037722. [DOI] [PubMed] [Google Scholar]
- 18.Hong J., Sang S., Park H.J., Kwon S.J., Suh N., Huang M.T., et al. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis. 2006;27:278–286. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 19.Koeberle A., Northoff H., Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem. Pharmacol. 2009;77:1513–1521. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Hung W.L., Liu C.M., Lai C.S., Ho C.T., Pan M.H. Inhibitory effect of garcinol against 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumorigenesis in mice. J. Funct. Foods. 2015;18:432–444. [Google Scholar]
- 21.Toshiaki A., Shigehiro K., Koichi K., Akira M., Norio Y. Antitumor agent. JPH11139965. 1999
- 22.Kundu T.K., Karnam B., Kempegowda M., Altaf M., Venkatesh S., Radhika A.V. Polyisoprenylated benzophenones and their isomers as inhibitors of histone acetyltransferases and uses thereof. US7402706. 2008
- 23.Padhye S., Ahmad A., Oswal N., Sarkar F.H. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J. Hematol. Oncol. 2009;2:1. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang W., Pan M.H., Sang S., Li S., Ho C.T. Garcinol from Garcinia indica: Chemistry and Health Beneficial Effects. In: Patil B.S., Jayaprakasha G.K., Roa C.O., Mahattanatawee K., editors. Tropical and subtropical fruits: Flavors, color, and health benefits. Washington, DC: American Chemical Society; 2013. pp. 33–145. [Google Scholar]
- 25.Won S.J., Liu C.T., Tsao L.T., Weng J.R., Ko H.H., Wang J.P., et al. Synthetic chalcones as potential anti-inflammatory and cancer chemo preventive agents. Eur. J. Med. Chem. 2005;40:103–112. doi: 10.1016/j.ejmech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.O’Dell J.R. Therapeutic strategies for rheumatoid arthritis. N. Engl. J. Med. 2004;350:2591–2602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Esquide V., Sanmartí R. Tobacco and other environmental risk factors in rheumatoid arthritis. Reumatol. Clin. 2012;8(6):342–350. doi: 10.1016/j.reuma.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Phull A.R., Nasir B., Haq I.U., Kim S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018;281:121–136. doi: 10.1016/j.cbi.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014;588(22):4244–4249. doi: 10.1016/j.febslet.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deane K.D., Demoruelle M.K., Kelmenson L.B., Kuhn K.A., Norris J.M., Holers V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017;31(1):3–18. doi: 10.1016/j.berh.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulielmos G.N., Zervou M.I., Myrthianou E., Burska A., Niewold T.B., Ponchel F. Genetic data: The new challenge of personalized medicine, insights for rheumatoid arthritis patients. Gene. 2016;583(2):90–101. doi: 10.1016/j.gene.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Bansback N.J., Regier D.A., Ara R., Brennan A., Shojania K., Esdaile J.M., et al. An overview of economic evaluations for drugs used in rheumatoid arthritis: Focus on tumour necrosis factor-alpha antagonists. Drugs. 2005;65:473–496. doi: 10.2165/00003495-200565040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Banarjee S., Parsramka M.A., Paruthy S.B. Bharti AC, Aggarwal BB, Eds. Role of Nutraceuticals in Cancer Chemosensitization Volume 2 in Cancer Sensitizing Agents for Chemotherapy. United States, Academic Press; 2018. Garcinol: Preclinical Perspective Underpinning Chemo- and Radiosensitization of Cancer. p. 297-324. [Google Scholar]
- 34.Liu C., Ho P.C., Wong F.C., Sethi G., Wang L.Z., Goh B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015;362(1):8–14. doi: 10.1016/j.canlet.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Vogel H.G. Drug discovery and Evaluation Pharmacological Assay. 2nd ed. Germany: Springer; 2002. [Google Scholar]
- 36.Costa M.D., De Sutter P., Gybels J., Van Hees J. Adjuvant-induced arthritis in rats: A possible animal model of chronic pain. Pain. 1981;10(2):173–185. doi: 10.1016/0304-3959(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V.L., Roy S., Sehgal R., Padhy B.M. A comparative study on the efficacy of rofecoxib in monoarticular arthritis induced by latex of Calotropis procera and Freund’s complete adjuvant. Inflammopharmacology. 2006;14:17–21. doi: 10.1007/s10787-006-1512-x. [DOI] [PubMed] [Google Scholar]
- 38.Hoogstraate J., Andersson L.I., Berge O.G., Jonzon B., Öjteg G. COX-inhibiting nitric oxide donators (CINODs): A new paradigm in the treatment of pain and inflammation. Inflammopharmacology. 2003;11:423–428. doi: 10.1163/156856003322699591. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y.L., Lin H.M., Zou R., Wu J.C., Han R., Raymond L.N., et al. Suppression of complete Freund’s adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol. Sin. 2009;30:219–227. doi: 10.1038/aps.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tak P.P., Firestein G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roman-Blas J.A., Jimenez S.A. NF-κB as a potential therapeutic target in steoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Wang S., Wang Y., Liu X., Guan L., Yu L., Zhang X. Anti-inflammatory and anti-arthritic effects of taraxasterol on adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2016;187:42–48. doi: 10.1016/j.jep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Toshiaki A., Norio Y. Lipase inhibitor and antiobestic medicine or hyperlipidemia inhibitor. JP2000044468. 2000
- 44.Muhammed M., Vladimir B. Composition comprising hydroxycitric acid and garcinol for weight-loss. EP1254209. 2006
- 45.Ann M.O., Brendan J.O. Appetite suppressant composition and method of appetite suppression. US20090062231. 2009
- 46.Muhammed M. Compositions and its use in treating obesity or inducing weight loss. US8329743. 2012
- 47.Muhammed M., Sarang B. Hepatoprotectant activity of garcinol. US20130281544. 2013
- 48.Gaurang S.D. Methods and materials for treating endometriosis. WO2016123044. 2016
- 49.Sonia G., Srinivasan B.P. Effect of garcinol in delaying the progression of diabetic nephropathy. US9872840. 2016
- 50.Sandip B., Ajay K., Jaideep B., Prashant J., Abubukar W., Ramesh M., Rohit S., Ram V. Polyprenylated phloroglucinol compounds as potent p-glycoprotein inducers. WO2016063296. 2016
- 51.Sunil B., Moharn V. Complex of garcinol, cyclodextrin and method thereof. US9956301. 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


