Abstract
Background/Objectives:
Anti-inflammatory agents play a crucial role in controlling inflammatory diseases such as Inflammatory Bowel Disease (IBD) but their use is restricted due to their vast side effects. M2000 (β-D-mannuronic acid) is a new immunomodulatory drug. According to the capacity of M2000 in suppressing some molecules involved in Toll Like Receptors (TLRs) signaling and reducing oxidative stress we hypothesize that, this molecule may have a potential role in decreasing inflammatory responses in IBD. The aim of this study was to evaluate the cytotoxicity of M2000 and its effect on the gene expression of TLR2 and TLR4.
Methods:
HEK293 cell line was grown and divided into 96-well cell plate and MTT assay was performed. HT29 cells were cultured and treated with low and high doses of M2000. Total RNA was extracted and cDNA synthesized and quantitative real-time PCR was done to quantify the TLR2 and TLR4 mRNA expression.
Results:
We found that M2000 at the concentration of ≤ 1000µg/ml had no obvious cytotoxicity effect on the HEK293 cells. Also, low and high doses of M2000 could significantly down-regulate both TLR2 and TLR4 mRNA expression. Moreover, a significant reduction in gene expression of TLR2 and TLR4 in an inflammatory condition resulted in high doses of M2000 in the presence of LPS.
Conclusion:
Our study which was conducted in colonic epithelial cell model, shows that M2000 can be considered as a new anti-inflammatory agent in IBD. However, more comprehensive experimental and clinical studies are required to recognize the molecular mechanism of M2000 and also its safety and efficacy.
Keywords: HT29, IBD, M2000, β-D-Mannuronic acid, TLR2, TLR4
1. INTRODUCTION
Inflammatory Bowel Disease (IBD) is defined as a chronic and incurable inflammatory disease of intestines. Ulcerative Colitis (UC) and Crohn's Disease (CD) are the main types of IBD [1]. IBD highly decreases the quality of life of the patients (1) and has remained a worldwide problem in public health [2]. In the US, IBD is the second most prevalent inflammatory disease which mostly affects people between 15-30 year of age [3]. Recently, we are encountering a rapid increase in the prevalence and incidence of IBD worldwide, especially in Asia [3-5].
Several factors are proposed as etiology of IBD including genetic susceptibility, alterations in Intestinal Epithelial Cells (IECs), immune dysregulation, environmental factors, microbiota intolerance, and signaling of oxidative stress. The results of both experimental models and clinical studies showed that oxidative stress damages the mucosal layer of intestines and leads to bacterial invasion which in turn activates the immune responses and thus IBD development [6]. The IECs express Pattern Recognition Receptors (PRRs), such as Toll-Like Receptors (TLRs), to differentiate commensal and attacking bacteria [7, 8].
TLRs have been found in IECs and other cell types of the digestive system. Animal and human studies revealed that TLR1, 2, 3, 4, 5, and 9 are present in the small and large intestine while TLR6, 7, and 8 are express just in the mice small intestine and large intestine of human [9]. The expression of TLRs varies throughout the intestine and small levels of various TLRs are expressed by IECs of small intestine, whereas they are overexpressed by colonic IECs. The expression of TLR5 is limited to Paneth cells in the epithelium of small intestine [10].
TLRs encompass two different pathways for signal transduction: the canonical pathway using MyD88 adaptor protein and the non-canonical pathway using TRIF adaptor protein [11, 12]. Excluding TLR3, canonical pathway activates Mitogen-Activated Protein Kinase (MAPK) and NF-kB leading to the secretion of inflammatory cytokines such as IL-6 and TNF-α [13]. But the non-canonical pathway stimulates Interferon Regulatory Factor 3 (IRF3) resulting in the production of interferon [13, 14].
During inflammatory conditions, bacteria can invade the gut epithelial barrier, initiate TLRs signaling and lead to TLRs expression [15]. It is established that TLRs especially TLR2 and TLR4 are involved in a variety of autoimmune and inflammatory disorders such as Multiple Sclerosis (MS), Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Ankylosing Spondylitis (AS) and Type 1 Diabetes (TID) as well as IBD [16, 17].
Hence, it is suggested that the antagonists of TLR2 and TLR4 and their signaling pathway could be useful in designing the novel anti-inflammatory drugs for treating IBD [18].
The principal aim of IBD traditional therapeutic strategy is to reduce inflammation; therefore, anti-inflammatory drugs including corticosteroids, sulfasalazine, mesalazine, infliximab, are used to reduce the inflammation and IBD symptoms. But, substantial side effects of classic IBD drugs leads them unfavorable [19].
M2000 (β-D-Mannuronic Acid) is a novel patented small molecule (with Patent No. of DE/102016113018.4 - PCT/EP2017/067919) Anti-Inflammatory Drug (NSAID) [20]. M2000 is extracted from alginate by chemical hydrolysis method in Immunology Department of Tehran University of Medical Sciences. Tolerability and anti-inflammatory effects of M2000 have been established in various animal models of Experimental Autoimmune Encephalomyelitis (EAE), Adjuvant Induced Arthritis (AIA), nephrotic syndrome, and acute glomerulonephritis [21-24]. Recently Mirshafiey et al. indicated the satisfactory effect of M2000 on several antioxidant enzymes and their gene expression, including SOD2, CAT, GPX1, and GST [25]. Recently, the efficacy and safety of M2000 were approved in clinical trial Phase I/II on RA (IRCT2014011213739N2) and clinical trial Phase I/II on AS (IRCT2013062213739N1) [26, 27].
Due to the capacity of M2000 in suppressing some molecules involved in TLRs signaling [28-31] and reducing oxidative stress [25, 32], we hypothesize that this molecule may have a potential role in decreasing inflammatory responses in IBD. The aim of this study was to evaluate the cytotoxicity of M2000 and to determine whether M2000 is able to inhibit the gene expression of TLR2 and TLR4 in HT29 cells.
2. MATERIAL AND METHODS
2.1. Cell Culture of HEK293
We purchased Cell lines of HEK 293 from National Cell Bank of Iran at Pasteur Institute (NCBI). At first, HEK 293 cells were cultured in RPMI1640 improved with 10% heat-inactivated Fetal Bovine Serum (FBS), 1% L-glutamine, 100U/ml penicillin, 100µg/ml streptomycin (Gibco, Life Technologies USA). The cells were incubated at 37ºC and 5% CO2. The medium was changed 3 times a week. Then, 1 × 104 cells were divided into 96-well cell culture plate.
2.2. Cell Viability Test
After 24hr of cell seeding into a 96-well cell culture plate, we performed MTT assay for determining the cell cytotoxicity of M2000. Serial dilutions of β-D-Mannuronic acid (62.5-1000µg/ml) were added to wells and cells were incubated 20-24 hours. Then, 100µl of MTT dye solution (0.5mg/ml) was added into each well and incubated for 4hr. After incubation time dimethyl sulfoxide was added to dissolve the formazan crystals. Finally, absorbance was measured by microplate reader at 570nm.
2.3. Cell Culture of HT29
HT29 cells are human colon adenocarcinoma cell line and repeatedly is used as a model of luminal surface colonic epithelial cells. We purchased HT29 from the Cell Bank at the International genetic and biological center. Cells were cultured in RPMI enriched with 10% FBS, 20mM HEPES and 1% L-glutamine and 100U/ml penicillin, 100µg/ml streptomycin. Cell culture medium was renewed every 1-2 days for 4-5 days until when the full polarization of the HT-29 cell monolayer was achieved. One million HT29 cells were divided into each well of 12-well cell culture plates.
2.4. Treatment of HT29 Cells
Twenty-four hours after seeding cells, the cells were treated by 1µg/ml LPS, 5µg/ml (low dose) and 25µg/ml (high dose) of M2000. The cells incubated at 37ºC and 5% CO2 for 20-24hr. Additionally, after 2hr of incubation, we added 1µg/ml LPS (Sigma, USA) to cells which had been treated with low and high dose of M2000 and returned to the cell culture incubator at the same condition.
2.5. RNA Extraction and Reverse Transcription
We used manual method consuming Qiazole (Qiagen, USA) to extract total RNA. The concentration and quality of isolated RNA were assessed by UV spectrophotometry (NanoDrop ND1000) using the 260/280 and 280/230 ratio. The RNA was reversely transcribed to cDNA by reverse transcription kit (Takara, Japan) according to the instruction of the company.
2.6. Quantitative Real-time PCR (RT-PCR)
We utilized the SYBR Green PCR Master Mix (Takara, Japan) to perform Quantitative Real-time PCR. The expression of TLR2, TLR4, and β-actin gene were assessed using primer pairs as follows: β-actin: F; CGTTGACATCCGTAAAGACC -3', R; 3'- TAGAGCCACCAATCCACACA -5', TLR2: F; 5'- TGTCTCTCACCGAACCG -3', R; 3'- CAACTCCATTAAGGGTGC -5', and TLR4: F; 5'- GAGCACCTGGACCTTTCAAATAAC -3', R; 3'-GTAGGACATGACGAGGTTGATC-5'. The transcripts were quantified by ABI Step One Plus Real-time PCR system (ABI System, USA). Amplification conditions were: 95ºC for 15s followed by 40 cycles of 95ºC for 30s, 60ºC for 30s, and 72ºC for 30s. The β-actin housekeeping gene was used for normalization of amplification. The relative frequency of TLR2 and TLR4 gene expression was calculated by 2-ΔΔCt method.
2.7. Statistical Analysis
The results were analyzed by SPSS software (version 16). The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine whether our data were normally distributed. The Paired samples t-test was used for detecting statistical differences in parametric analysis and Wilcoxon tests were used for non-parametric analysis. P values ≤ 0.05 were considered statistically significant. Prism software (version 5) was used for drawing graphs.
3. RESULTS
3.1. Cytotoxicity Findings of M2000
We tested cytotoxicity of some concentrations of M2000 including, 1000, 250, 62.5µg/ml using MTT assay. The outcomes of MTT assay showed that M2000 at concentrations less than 1000μg/ml had no cytotoxicity on the HEK 293 TLR4 cells (Fig 1).
Fig. (1).
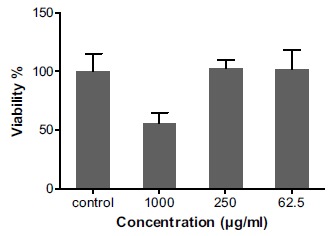
The percent of the viability of HEK293-TLR4 cells after treatment with different concentrations of M2000 by MTT assay. Treatment of HEK293 cells with concentrations ≤ 1000µg/ml of M2000 is associated with more than 50% of cells viability in MTT assay.
3.2. Effect of M2000 on the Gene Expression of TLR2 and TLR4
To determine whether the immunomodulatory effect of M2000 is able to change the TLR2 and TLR4 gene expression, quantitative real-time PCR was performed to reveal the mRNA expression of these molecules in HT29 cells.
The results of quantitative real-time PCR showed that the expression of TLR2 mRNA in HT29 cells in low-dose M2000 treatment group was decreased significantly compared with untreated control group, 0.32 + 0.03 vs 1.00 + 0.16 (p = 0.02). Also, we showed that the high dose of M2000 can significantly decrease the gene expression of TLR2 compared to control group 0.05 + 0.03 vs 1.00 + 0.16 (p = 0.01).
Treating the cells with low-dose M2000 led to significant reduction in the gene expression of TLR4 compared to control cells, 0.52 + 0.08 vs 1.00 + 0.09 (p = 0.01); similarly, high dose of M2000 decreased the gene expression of TLR4, 0.16 + 0.03 vs 1.00 + 0.09 (p = 0.008).
3.3. Effect of M2000 on the Gene Expression of TLR2 and TLR4 in the Presence of LPS
The gene expression of TLR2 and TLR4 were assessed in the presence of LPS to simulate the effect of M2000 in an inflammatory condition. As expected, treating the HT29 cells with LPS significantly increased TLR2 and TLR4 gene expression compared with their baseline levels 1.52 + 0.19 vs 1.00 + 0.16 (p = 0.001) and 3.17 + 0.25 vs 1.00 + 0.09 (p = 0.0003) respectively (Figs. 2 and 3). Although low-dose of M2000 in the presence of LPS had no effect on the TLR2 gene expression compared to the untreated control group, the high-dose M2000 in the presence of LPS significantly reduced the expression of TLR2 mRNA compared to the control group, 0.07 + 0.02 vs 1.00 + 0.16 (p = 0.01). Comparing the results with the gene expression of TLR2 in exclusively LPS treating group showed that co-treatment of LPS with low and high doses of M2000 significantly reduce the gene expression of TLR2, 0.91 + 0.09 vs 1.52 + 0.19 (p = 0.009) and 0.07 + 0.07 vs 1.52 + 0.19 (p = 0.007) respectively (Fig. 2).
Fig. (2).
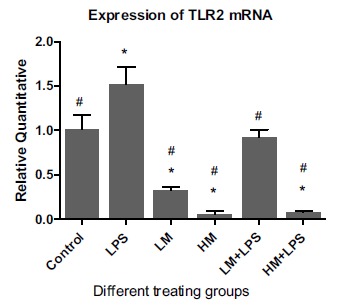
Effect of M2000 on the expression of TLR2 mRNA in HT29 cells. Abbreviations: LM: low dose of M2000 (5µg/ml); HM: high dose of M2000 (25µg/ml); LPS: lipopolysaccharide. Data are shown as mean + SD. The sign of * displays significant statistical difference with the control group. The sign of # displays significant difference with LPS.
Fig. (3).
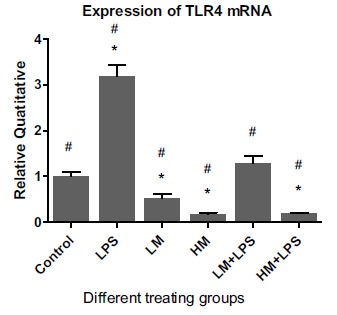
Effect of M2000 on the expression of TLR4 mRNA in HT29 cells. Abbreviations: LM: low dose of M2000 (5µg/well); HM: high dose of M2000 (25µg/well); LPS: lipopolysaccharide. Data are shown as mean + SD. The sign of * displays significant statistical difference with the control group. The sign of # displays significant difference with LPS.
Moreover, Low-dose M2000 in the presence of LPS was unable to significantly alter the gene expression of TLR4 whereas high-dose M2000 accompanied by LPS significantly reduced the gene expression of TLR4 on the surface of HT29 cells, 1.00 + 0.09 vs 0.18 + 0.01 (p = 0.005).
The culture of HT29 cells with the combination of LPS and low-dose M2000 did not significantly alter the gene expression of TLR4 compared to the gene expression of TLR4 in exclusively LPS treating group 1.28 + 0.16 vs 3.17 + 0.25 (p = 0.001). Whereas co-treatment of LPS with low- and high-dose of M2000 could significantly reduce the gene expression of TLR4 compared to its gene expression in merely LPS treating group, 1.28 + 0.16 vs 3.17 + 0.25 (p = 0.001) and 0.18 + 0.01 vs 3.17 + 0.25 (p = 0.002) respectively (Fig. 3).
4. DISCUSSION
Toll-Like Receptors (TLR) are pattern recognition receptors which recognize conserved molecular patterns of microorganisms. Exposure of IECs to PAMPs potentially initiates the inflammatory reaction, the most important mechanism of UC and its pathogenesis, by chemokines secretion and recruitment of inflammatory cells pathogenesis [33]. Therefore, IECs could be a potential target for anti-inflammatory treatment strategies [34]. Corticosteroids and immunomodulators such as NSAIDs are traditional favorable therapeutic choices for patients with IBD [35]. The NSAIDs play a significant role in the management of inflammatory disorders [36]. Corticosteroids and immunomodulators have profound adverse effects, possibly including a higher risk of latent infection recurrence, such as tuberculosis [37]. Recent findings show that manipulation of the non-canonical NF-κB pathway may provide a new way for designing novel therapeutic targets for treating IBD [38]. Non-canonical NF-κB signaling can be a leading cause of chronic and non-severe inflammation predisposing IBD. The first phase of the non-canonical NF-κB pathway is the recognition of a TNF family signaling molecules, such as TNF, lymphotoxin β, CD40L, or BAFFR [39]. Therefore, utilizing anti-TNF monoclonal antibodies (mAbs) has proved us noteworthy therapeutic effects. Current anti-TNF treatments such as infliximab, adalimumab, and certolizumab are reported to have a great efficacy in mucosal healing of UC patients [35, 40, 41]. However, the effects of these drugs are often variable and temporary. Even though most of the patients, in the beginning, respond well to such anti-TNF drugs, but there is a high risk of becoming irresponsive in long term.
Due to their various side effects, so much effort has been put into finding an immunosuppressive agent with acceptable amount of efficacy and safety.
The crucial role of TLRs in the pathogenesis of inflammatory disorders suggests that inhibiting TLRs signal transmission can be beneficial in the treatment of inflammatory diseases in general [42]. Inappropriately, clinical use of TLR inhibitors is very limited due to lack of their available ingredients. Thereby, exploring new molecules with the ability to interfere TLRs, their co-receptors, and their signal transduction is of great importance.
Epidemiological studies showed that reducing intestinal helminthic infections is associated with a high incidence of autoimmune diseases; for instance, according to a study, soluble products from the helminth parasite Trichuris suis (TsSP) suppress TLR4 responses in human macrophages and dendritic cells [43, 44].
Interestingly, it has been suggested that probiotic helminth administration of the porcine T. suis (TsSP) species can suppress the signaling of TLRs and could be considered for the treatment of inflammatory and autoimmune diseases such as MS, UC, and CD [45-49]. Detection of molecular components of TsSp is suggested for future researches to extract agents which has potential TLR inhibitory role.
Totally, two major methods are described for TLRs inhibition: 1. Prohibiting TLR ligands from binding to their receptors and 2. Interrupting TLRs signaling pathways by stopping the signal transmission to the nucleus [50]. For this purpose, a number of therapeutic agents are developed to inhibit TLRs and consequently control unwanted inflamma- tion; that can be categorized as follows:
4.1. Small Molecule Inhibitors
Small Molecule Inhibitors (SMIs) are popular pharmaceutical agents. They are chemical components with natural or synthetic origin with the ability to stop TLR signal transduction. Their chemical properties such as their small size, and amphipathic nature enable these molecules to easily pass the cell membranes and modify the intercellular targets such as TLRs adaptor proteins as well as other molecules in downstream signaling cascade. Although SMIs show appropriate bioavailability, their specificity to recognize their targets is low.
TAK-242 (Resatorvid) is a SMIs with anti-sepsis effect that inhibits the communication between TLR4 and two adaptor proteins (TIRAP and TRAM) and therefore diminishes the inflammation [51]. After preclinical achievements, TAK-242 advanced into clinical trials. clinical trial NCT00143611 investigated its effect on severe sepsis and other clinical trial NCT00633477 for sepsis-induced cardiovascular and respiratory; but both clinical trials have been ended in Phase III due to their weak effects and business decision respectively.
Other SMIs are previously developed drugs while their suppressing effect on TLR2 and TLR4 has been recently discovered such as statins, and Angiotensin II Receptor Blockers (ARBs).
For Instance, valsartan (a member of ARB family) can reduce the secretion of proinflammatory cytokines release and thereby, myocardial myocardial infarction size by inhibiting TLR4 signaling [52], while vandesartan another member of this family can suppress both TLR2 and TLR4 activation [53].
Statins are other small molecules with the lipid-lowering effect such as fluvastatin, simvastatin, and atorvastatin. These drugs have also been shown to effectively suppress TLR4 and its induced inflammation [54-56].
4.2. Antibodies
Despite SMIs, monoclonal antibodies have high specificity in targeting certain molecules. Accordingly, they have been extensively used to inhibit receptor-ligand interaction, neutralize soluble molecules, and promote targeted cytotoxicity [57].
1A6 is another monoclonal antibody interfering MD2, a co-receptor for TLR4 activation [58]. It has been found to be protective against septic shock [58, 59]. Moreover, an animal study showed that 1A6 has a protective role in colitis. Unfortunately, the TLR4 inhibiting property of 1A6 was accompanying blemished mucosal healing in recovery period [60]. This finding reveals the different functions of TLRs in disease courses including relapses and remissions. Consideration of diverse abilities of TLRs is important to design new TLR-based therapies.
NI-0101 is a promising anti-TLR4 antibody that acts by inhibiting of TLR4 dimerization but its function is not associated with the kind or concentratioof ligand [61]. NI-0101 can prevent cytokine release in monocytes of rheumatoid arthritis patients [62].
The results of Phase I clinical trial (NCT01808469) of NI-0101 were hopeful as it showed high safety, banning cytokine production, and inhibiting LPS induced flu-like syndrome [63].
4.3. Oligonucleotides
Certain nucleotide sequences can act as the antagonist of endosomal TLRs like TLR3, TLRs7, TLR8, and TLR9. These antagonistic nucleotides are able to interfere with binding dsRNA, ssRNA, and CpG-DNA ligands to the endosomes-expressing TLRs and inhibit their signal transduction. Consequently, a set of oligonucleotide-based antagonist is designed for treatment of endosomal TLRs associated with autoimmune and inflammatory diseases. For instance, IRS-954 [64], IMO-3100 [65, 66], DV-1179 [67], and INH-ODN-24888 [68] are nucleotide-based inhibitors of TLR7 and TLR9 advanced to clinical examinations for SLE therapy. Interestingly, IMO 8400 with the ability to inhibit more TLRs (TLR7, TLR8, and TLR9) was potent in the treatment of mouse models of both lupus and psoriasis [65, 66, 69].
4.4. Lipid A Analogs
Lipid A is a lipid part of LPS with a highly conserved structure associated with the toxicity of an endotoxin molecule. Hence, lipid A is a good therapeutic target for modulating TLR4 signaling pathway [70]. Analogs of lipid A are TLR antagonists, for example, eritoran (E5564) is a synthetic lipid A analog of Rhodobacter sphaeroides. E5564 competitively bind to the MD2 and inhibit the TLR4 signaling [71]. Phase II clinical trial of eritoran in severe sepsis patients (NCT00046072) resulted in a lower mortality rate [72]. But Phase III was unsuccessful because of its poor survival outcome [73].
4.5. MicroRNAs
MicroRNAs (miRNAs) are a category of small non-coding RNA molecule act as post-transcription regulator factors [74]. They target messenger RNAs to reduce or inhibit their translation. At least 20 microRNAs are recognized regulating TLRs signaling cascades [75]. There are well-known TLRs-regulating microRNAs like miR-146a, miR-155, and miR-21a which attracted the attention of many studies in the area of autoimmune disorders. MiR-146a was found to inhibit the translation of the TRAF6 and IRAK1 in the downstream signaling cascade of TLR4 [76]. Additional studies have demonstrated that miR-146a plays a central role as an intrinsic brake on inflammation [77].
miR-21 has been shown to decrease the expression of programmed cell death 4 gene (PDCD4) resulting in IL-10 release and promoting anti-inflammatory responses [78].
miR-155 has been reported to show controversial function in direction of inflammation; for instance, miR-155 damp down the inflammation by interfering TAK1 and blocking the activation of NF-κB [79]. On the other hand, there are data demonstrating the role of miR-155 in progress of SLE [80] and septic shock [81], and neuroinflammation [82].
4.6. Nano-Inhibitors
Nano-inhibitors serve as favorable and potent TLR-inhibitors. They have improved bio-distribution and adequate accumulation because of their ultrafine dimensions [83, 84].
Widely held TLR nano-inhibitors act on TLR4 signaling pathway. Lipid-modified Non-Anticoagulant Heparin Nanoparticle (NAHNP) is an intriguing self-assembling nanodevice with an inhibitory effect on chronic inflammation in an animal model of RA. NAHNP interacts with TLR4/MD2 and preventing MyD88-dependent NF-kB pathway [85].
Other TLR4 nano-inhibitors use emerging Gold Nanoparticles (GNPs) because of their interesting medical and physical properties [86], including HDL-like NP [87], bare GNP [88], and glycolipid-coated GNP Peptide [89].
According to the previous studies, the immunosuppressive effects of M2000 were found in animal models and cell lines [21-24, 90]; Also, anti-oxidant property of M2000 and its monomer, G2013 has been proven in animal models [25, 32]. Recently, the results of a clinical trial showed that M2000 has an inhibitory effect on Anti-Cyclic Citrullinated Peptide Antibodies (anti-CCP), Rheumatoid Factor (RF), anti-double strand DNA (anti-dsDNA) and acute phase reactants in RA patients [91]. Also, a reduction in IL17 and RORγt gene expression was observed in RA patients after oral administration of M2000 [92].
The previous researches have demonstrated that M2000 lowers the expression of the molecules involved in the downstream signaling pathway of TLR2 and TLR4 [28-31]; therefore, we hypothesized that these receptors can be considered as specific targets of M2000.
Interestingly, we found that M2000-treated HT29 cells downregulated the expression of TLR2 mRNA and TLR4 mRNA. This finding was congruent with the previous studies. In 2017, Aletaha et al. showed that M2000 efficiently can inhibit the mRNA expression of MyD88 and NF-κB as well as reduce the production of TNF-α and IL-6 in HEK-293 cells stimulated by LTA and LPS [28]. Mortazavi Jahromi et al. in 2017 showed that M2000 significantly reduces the gene expression of miR-146a, IRAK1, TRAF6, and NF-κB [29]. Sharifi et al. in 2017 showed that G2013 (analog of M2000) significantly reduces NF-κB, IkB and MyD88 mRNA expression and also decreases the secretion of IL-1β in human mononuclear cells [30]. According to the other studies, this drug can significantly reduce the Mean Fluorescence Intensity (MFI) of TLR2 and TLR4 as well as gene expression of NF-κB in Common Variable Immunodeficiency (CVID) [31], whose patients are more prone to inflammatory and autoimmune diseases [93, 94].
We know that oxidative stress damages the mucosal layer of intestines, leading to bacterial invasion, and IBD development [6]. Therefore, due to the capacity of M2000 in suppressing TLR2 and TLR4 and its role in oxidative stress reduction M2000 [25, 32] we anticipate a potent capacity of M2000 for preventing IBD. More comprehensive in vitro and in vivo studies are needed to recognize the molecular mechanism of M2000 and also its probable side effects. Although ongoing clinical trials on ankylosing spondylitis (IRCT2013062213739N1), rheumatoid arthritis (IRCT20140 11213739N2), osteoarthritis (IRCT2014113013739N3), multiple sclerosis (IRCT2016111313739N6), and breast cancer (IRCT2017012213739N7) can be beneficial to evaluate the clinical effects of M2000 and its probable side effects, we suggest that M2000 should advance to clinical investigation phase because of its promising TLR inhibiting effect on colonic epithelial cells.
CONCLUSION
Here, we showed that M2000 exerts its suppressive effect by down-regulating the surface expression of TLR2 and TLR4. The established role of this drug in the reduction of some signaling molecules of TLRs' can be probably due to the suppression of TLR2 and TLR4.
According to the results of our research on TLR2 and TLR4 mRNA expression in colonic epithelial cell model, we conclude that M2000 could be considered as a new treatment of inflammation in IBD.
CURRENT & FUTURE DEVELOPMENTS
Recently, using inhibitors or antagonists of TLRs are considered as emerging immunosuppressive treatments for inflammatory and autoimmune diseases. To explore proper inflammation-modulating TLR inhibitors, their safety and efficacy should be controlled in the clinical studies. Although TLR-based therapeutic approaches are well studied experimentally, there are few TLR inhibitors that have been experienced in clinical trials and unfortunately, most of them did not pass the phases of these clinical trials. In addition to safety and efficacy, it should be answered accurately how these molecules can suppress the immune responses.
Launching a clinical trial in the future to evaluate the efficacy and safety of M2000 in the clinical manifestations of IBD according to the properties of M2000 as a novel TLR inhibitor may be promising.
Furthermore, TLR nano-inhibitors is an emerging field in immunosuppressive therapies which are at preclinical investigations. Before undergoing any clinical trials pharmacokinetic and pharmacodynamics properties of these nano-drugs should be done in animal models. The effectiveness of nanoparticles in treating cancer makes TLR nano-inhibitors a great hope for clinical use in the future.
ACKNOWLEDGEMENTS
Declared none.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research project was supported by a grant from Tehran University of Medical Sciences (TUMS).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Tian T.Z. Wang, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky N.A., Soon S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Loftus E.V., Sandborn W.J. Epidemiology of inflammatory bowel disease. Gastroenterol. Clin. North Am. 2002;31(1):1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 4.Ng S.C., Tang W., Ching J.Y., Wong M., Chow C.M., Hui A.J., et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145(1):158–165. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Z., Zhu Z., Yang Y., Ruan W., Peng X., Su Y., et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province China: A prospective population-based study. J. Gastroenterol. Hepatol. 2013;28(7):1148–1153. doi: 10.1111/jgh.12164. [DOI] [PubMed] [Google Scholar]
- 6.Goyette P., Labbé C., Trinh T.T., Xavier R.J., Rioux J.D. Molecular pathogenesis of inflammatory bowel disease: Genotypes, phenotypes and personalized medicine. Ann. Med. 2007;39(3):177–199. doi: 10.1080/07853890701197615. [DOI] [PubMed] [Google Scholar]
- 7.Fukata M., Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6(3):451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastorelli L., De Salvo C., Mercado J.R., Vecchi M., Pizarro T.T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front. Immunol. 2013;4:280–287. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moossavi S., Rezaei N. Toll-like receptor signalling and their therapeutic targeting in colorectal cancer. Int. Immunopharmacol. 2013;16(2):199–209. doi: 10.1016/j.intimp.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Price A.E., Shamardani K., Lugo K.A., Deguine J., Roberts A.W., Lee B.L., et al. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49(3):560–575. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Akira S., Takeda K. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Akira S., Takeda K. TLR signaling pathways. Semin. Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Akira S., Takeda K. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461–468. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidemann J., Domschke W., Kucharzik T., Maaser C. Intestinal microvascular endothelium and innate immunity in inflammatory bowel disease: A second line of defense? Infect. Immun. 2006;74(10):5425–5432. doi: 10.1128/IAI.00248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jager P.L., Franchimont D., Waliszewska A., Bitton A., Cohen A., Langelier D., et al. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007;8(5):387–397. doi: 10.1038/sj.gene.6364398. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Yin H., Zhao M., Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 2014;47(2):136–147. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K., Sugi Y., Hosono A., Kaminogawa S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J. Immunol. 2009;183(10):6522–6529. doi: 10.4049/jimmunol.0901271. [DOI] [PubMed] [Google Scholar]
- 19.Dahan A., Amidon G.L., Zimmermann E.M. Drug targeting strategies for the treatment of inflammatory bowel disease: A mechanistic update. Expert Rev. Clin. Immunol. 2010;6(4):543–550. doi: 10.1586/eci.10.30. [DOI] [PubMed] [Google Scholar]
- 20.Mirshafiey A. Pharmaceutical use of beta-D-mannuronic acid. EP067919. 2017
- 21.Mirshafiey A., Cuzzocrea S., Rehm B., Mazzon E., Saadat F., Sotoude M. Treatment of experimental arthritis with M2000, a novel designed non-steroidal anti-inflammatory drug. Scand. J. Immunol. 2005;61(5):435–441. doi: 10.1111/j.1365-3083.2005.01594.x. [DOI] [PubMed] [Google Scholar]
- 22.Mirshafiey A., Cuzzocrea S., Rehm B., Matsuo H. M2000: A revolution in pharmacology. Med. Sci. Monit. 2005;11(8):I53–I63. [PubMed] [Google Scholar]
- 23.Mirshafiey A., Matsuo H., Nakane S., Rehm B.H., Koh C.S., Miyoshi S. Novel immunosuppressive therapy by M2000 in experimental multiple sclerosis. Immunopharmacol. Immunotoxicol. 2005;27(2):255–265. doi: 10.1081/iph-200067751. [DOI] [PubMed] [Google Scholar]
- 24.Fattahi M.J., Abdollahi M., Agha Mohammadi A., Rastkari N., Khorasani R., Ahmadi H. Preclinical assessment of beta-D-mannuronic acid (M2000) as a non-steroidal anti-inflammatory drug. Immunopharmacol. Immunotoxicol. 2015;37(6):535–540. doi: 10.3109/08923973.2015.1113296. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini S., Abdollahi M., Azizi G., Fattahi M.J., Rastkari N., Zavareh F.T., et al. Anti-aging effects of M2000 (beta-D-mannuronic acid) as a novel immunosuppressive drug on the enzymatic and non-enzymatic oxidative stress parameters in an experimental model. J. Basic Clin. Physiol. Pharmacol. 2017;28(3):249–255. doi: 10.1515/jbcpp-2016-0092. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi H. A Phase I/II randomized, controlled, clinical trial for assessment of the efficacy and safety of beta-D-mannuronic acid in rheumatoid arthritis patients. Inflammopharm. 2018;26(3):737–745. doi: 10.1007/s10787-018-0475-z. [DOI] [PubMed] [Google Scholar]
- 27.Fattahi M.J., Jamshidi A.R., Mahmoudi M., Vojdanian M., Yekaninejad M.S., Jafarnezhad-Ansariha F., et al. Evaluation of the efficacy and safety of beta-D-mannuronic acid in patients with ankylosing spondylitis: A 12-week randomized, placebo-controlled, Phase I/II clinical trial. Int. Immunopharmacol. 2018;54:112–117. doi: 10.1016/j.intimp.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Aletaha S., Haddad L., Roozbehkia M., Bigdeli R., Asgary V., Mahmoudi M., et al. M2000 (beta-D-mannuronic acid) as a novel antagonist for blocking the TLR2 and TLR4 downstream signalling pathway. Scand. J. Immunol. 2017;85(2):122–129. doi: 10.1111/sji.12519. [DOI] [PubMed] [Google Scholar]
- 29.Mortazavi-Jahromi S., Jamshidi M.M., Farazmand A., Aghazadeh Z., Yousefi M., Mirshafiey A. Pharmacological effects of beta-d-mannuronic acid (M2000) on miR-146a, IRAK1, TRAF6 and NF-kappaB gene expression, as target molecules in inflammatory reactions. Pharmacol. Rep. 2017;69(3):479–484. doi: 10.1016/j.pharep.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Sharifi L., Mirshafiey A. Immunomodulatory effect of G2013 (a-L-guluronic acid) on theTLR2 and TLR4 in human mononuclear cells. Curr. Drug Discov. Technol. 2017:143–149. doi: 10.2174/1570163814666170605111331. [DOI] [PubMed] [Google Scholar]
- 31.Sharifi L., Aghamohammadi A., Mohsenzadegan M., Rezaei N., Tofighi Zavareh F., Moshiri M., et al. Immunomodulation of TLR2 and TLR4 by G2013 (alpha-L-guluronic acid) in CVID Patients. Int. J. Pediatr. 2017;5(7):5327–5337. [Google Scholar]
- 32.Mirshafiey A., Hosseini S., Afraei S., Rastkari N., Zavareh F., Azizi G. Anti-aging property of G2013 molecule as a novel immunosuppressive agent on enzymatic and non-enzymatic oxidative stress determinants in Rat model. Curr. Drug Discov. Technol. 2016;13(1):25–33. doi: 10.2174/1570163813666160224123851. [DOI] [PubMed] [Google Scholar]
- 33.Böcker U., Yezerskyy O., Feick P., Manigold T., Panja A., Kalina U., et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int. J. Colorectal Dis. 2003;18(1):25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 34.Böcker U. Influence of Therapeutic Intervention with Interleukins on Epithelial Cell Function. In: Andus T., editor. Cytokines and Cell Homeostasis in the Gastrointestinal Tract. Lancaster: Kluwer; pp. 61–71. [Google Scholar]
- 35.Danese S., Colombel J.F., Peyrin‐Biroulet L., Rutgeerts P., Reinisch W. The role of anti-TNF in the management of ulcerative colitis: Past, present and future. Aliment. Pharmacol. Ther. 2013;37(9):855–866. doi: 10.1111/apt.12284. [DOI] [PubMed] [Google Scholar]
- 36.Sidiropoulos P.I., Hatemi G., Song I.H., Avouac J., Collantes E., Hamuryudan V., et al. Evidence-based recommendations for the management of ankylosing spondylitis: systematic literature search of the 3E Initiative in Rheumatology involving a broad panel of experts and practising rheumatologists. Rheumatology. 2008;47(3):355–361. doi: 10.1093/rheumatology/kem348. [DOI] [PubMed] [Google Scholar]
- 37.Wheat C.L., Ko C.W., Clark-Snustad K., Grembowski D., Thornton T.A., Devine B. Inflammatory Bowel Disease (IBD) pharmacotherapy and the risk of serious infection: a systematic review and network meta-analysis. BMC Gastroenterol. 2017;17(1):52–67. doi: 10.1186/s12876-017-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDaniel D.K., Eden K., Ringel V.M., Allen I.C. Emerging Roles for Noncanonical NF-kappaB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel Dis. 2016;22(9):2265–2279. doi: 10.1097/MIB.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.Y., Morgan M., Kim D.G., Lee J.Y., Bai L., Lin Y., et al. TNFalpha induced noncanonical NF-kappaB activation is attenuated by RIP1 through stabilization of TRAF2. J. Cell Sci. 2011;124(Pt 4):647–656. doi: 10.1242/jcs.075770. [DOI] [PubMed] [Google Scholar]
- 40.Rutgeerts P., Sandborn W.J., Feagan B.G., Reinisch W., Olson A., Johanns J., et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 41.van Heel D.A., Udalova I.A., De Silva A.P., McGovern D.P., Kinouchi Y., Hull J., et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-Kappa)B transcription factors. Hum. Mol. Genet. 2002;11(11):1281–1289. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- 42.Hennessy EJ, Parker AE, O’neill LA. Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun. 2015;16(6):378–387. doi: 10.1038/gene.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klaver E.J., van der Pouw Kraan T.C., Laan L.C., Kringel H., Cummings R.D., Bouma G., et al. Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun. 2015;16(6):378–387. doi: 10.1038/gene.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottow M.K., Klaver E.J., van der Pouw Kraan T.C., Heijnen P.D., Laan L.C., Kringel H., et al. The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun. 2014;15(7):477–486. doi: 10.1038/gene.2014.38. [DOI] [PubMed] [Google Scholar]
- 45.Garg S.K., Croft A.M., Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 2014;(1):CD009400. doi: 10.1002/14651858.CD009400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming J.O., Isaak A., Lee J.E., Luzzio C.C., Carrithers M.D., Cook T.D., et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: A Phase 1 study. Mult. Scler. 2011;17(6):743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summers R.W. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology. 2005;128(4):825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Summers R.W., Elliott D.E., Urban J.F., Thompson R., Weinstock J.V. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54(1):87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun S., Wang X., Wu X., Zhao Y., Wang F., Liu X., et al. Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit. Vectors. 2011;4:186–192. doi: 10.1186/1756-3305-4-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao W., Xiong Y., Li Q., Yang H. Inhibition of Toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Front. Physiol. 2017;8:508–517. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danese S., Colombel J.F., Peyrin‐Biroulet L., Rutgeerts P., Reinisch W. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011;79(1):34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 52.Yang J., Jiang H., Yang J., Ding J.W., Chen L.H., Li S., et al. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol. Cell. Biochem. 2009;330(1-2):39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- 53.Dasu M.R., Riosvelasco A.C., Jialal I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis. 2009;202(1):76–83. doi: 10.1016/j.atherosclerosis.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Földes G., von Haehling S., Okonko D.O., Jankowska E.A., Poole-Wilson P.A., Anker S.D. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int. J. Cardiol. 2008;124(1):80–85. doi: 10.1016/j.ijcard.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 55.Fang D., Yang S., Quan W., Jia H., Quan Z., Qu Z. Atorvastatin suppresses Toll-like receptor 4 expression and NF-kappaB activation in rabbit atherosclerotic plaques. Eur. Rev. Med. Pharmacol. Sci. 2014;18(2):242–246. [PubMed] [Google Scholar]
- 56.Methe H., Kim J.O., Kofler S., Nabauer M., Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler. Thromb. Vasc. Biol. 2005;25(7):1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki M., Kato C., Kato A. Therapeutic antibodies: Their mechanisms of action and the pathological findings they induce in toxicity studies. J. Toxicol. Pathol. 2015;28(3):133–139. doi: 10.1293/tox.2015-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spiller S., Elson G., Ferstl R., Dreher S., Mueller T., Freudenberg M., et al. TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives gram-negative sepsis in mice. J. Exp. Med. 2008;205(8):1747–1754. doi: 10.1084/jem.20071990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lima C.X., Souza D.G., Amaral F.A., Fagundes C.T., Rodrigues I.P., Alves-Filho J.C., et al. Therapeutic effects of treatment with anti-tlr2 and anti-tlr4 monoclonal antibodies in polymicrobial sepsis. PLoS One. 2015;10(7):e0132336. doi: 10.1371/journal.pone.0132336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ungaro R., Fukata M., Hsu D., Hernandez Y., Breglio K., Chen A., et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(6):G1167–G1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monnet E., Shang L., Lapeyre G., Hatterer E., Buatois V., Elson G., et al. AB0451 NI-0101, a monoclonal antibody targeting toll like receptor 4 (TLR4) being developed for rheumatoid arthritis (RA) treatment with a potential for personalized medicine. Ann. Rheum. Dis. 2015;74(Suppl. 2):1046–1053. [Google Scholar]
- 62.Hatterer E., Shang L., Simonet P., Herren S., Daubeuf B., Teixeira S., et al. A specific anti-citrullinated protein antibody profile identifies a group of rheumatoid arthritis patients with a toll-like receptor 4-mediated disease. Arthritis Res. Ther. 2016;18(1):224–229. doi: 10.1186/s13075-016-1128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monnet E., Lapeyre G., van Poelgeest E., Jacqmin P., de Graaf K., Reijers J., Moerland M., et al. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized Phase I dose escalation study in healthy volunteers receiving LPS. Clin. Pharmacol. Ther. 2017;101(2):200–208. doi: 10.1002/cpt.522. [DOI] [PubMed] [Google Scholar]
- 64.Barrat F.J., Meeker T., Gregorio J., Chan J.H., Uematsu S., Akira S., et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202(8):1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suárez-Fariñas M., Arbeit R., Jiang W., Ortenzio F.S., Sullivan T., Krueger J.G. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8(12):e84634. doi: 10.1371/journal.pone.0084634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu F.G., Jiang W., Bhagat L., Wang D., Yu D., Tang J.X., Kandimalla E.R., et al. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 2013;46(7):419–428. doi: 10.3109/08916934.2013.798651. [DOI] [PubMed] [Google Scholar]
- 67.Gao W., Xiong Y., Li Q., Yang H. Inhibition of Toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Front. Physiol. 2017;8:508–513. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Römmler F., Jurk M., Uhlmann E., Hammel M., Waldhuber A., Pfeiffer L., et al. Guanine modification of inhibitory oligonucleotides potentiates their suppressive function. J. Immunol. 2013;191(6):3240–3253. doi: 10.4049/jimmunol.1300706. [DOI] [PubMed] [Google Scholar]
- 69.Jiang W., Zhu F.G., Bhagat L., Yu D., Tang J.X., Kandimalla E.R., et al. A Toll-like receptor 7, 8, and 9 antagonist inhibits Th1 and Th17 responses and inflammasome activation in a model of IL-23-induced psoriasis. J. Invest. Dermatol. 2013;133(7):1777–1784. doi: 10.1038/jid.2013.57. [DOI] [PubMed] [Google Scholar]
- 70.Rietschel E.T., Kirikae T., Schade F.U., Mamat U., Schmidt G., Loppnow H., et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8(2):217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 71.Park B.S., Song D.H., Kim H.M., Choi B.S., Lee H., Lee J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 72.Tidswell M., Tillis W., LaRosa S.P., Lynn M., Wittek A.E., Kao R. Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis. Crit. Care Med. 2010;38(1):72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 73.Opal S.M., Laterre P.F., Francois B., LaRosa S.P., Angus D.C., Mira J.P., et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 74.Liu B., Li J., Cairns M.J. Identifying miRNAs, targets and functions. Brief. Bioinform. 2014;15(1):1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He X., Jing Z., Cheng G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. BioMed Res. Int. 2014;2014:945169. doi: 10.1155/2014/945169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boldin M.P., Taganov K.D., Rao D.S., Yang L., Zhao J.L., Kalwani M., et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheedy F.J., Palsson-McDermott E., Hennessy E.J., Martin C., O’leary J.J., Ruan Q., et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010;11(2):141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 79.Ceppi M., Pereira P.M., Dunand-Sauthier I., Barras E., Reith W., Santos M.A., et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. USA. 2009;106(8):2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’connell R.M., Kahn D., Gibson W.S., Round J.L., Scholz R.L., Chaudhuri A.A., et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33(4):607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tili E., Michaille J.J., Cimino A., Costinean S., Dumitru C.D., Adair B., et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 82.Barker K.R., Lu Z., Kim H., Zheng Y., Chen J., Conroy A.L., Hawkes M., et al. miR-155 modifies inflammation, endothelial activation and blood-brain barrier dysfunction in cerebral malaria. Mol. Med. 2017;23:24–33. doi: 10.2119/molmed.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He C., Hu Y., Yin L., Tang C., Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 84.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Babazada H., Yamashita F., Hashida M. Suppression of experimental arthritis with self-assembling glycol-split heparin nanoparticles via inhibition of TLR4-NF-kappaB signaling. J. Control. Release. 2014;194:295–300. doi: 10.1016/j.jconrel.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 86.Pedrosa P. Gold Nanotheranostics: Proof-of-concept or clinical tool? Nanomat (Basel) 2015;5(4):1853–1879. doi: 10.3390/nano5041853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foit L., Thaxton C.S. Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials. 2016;100:67–75. doi: 10.1016/j.biomaterials.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 88.Pereira D.V. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2012;53(13):8036–8041. doi: 10.1167/iovs.12-10743. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez Lavado J., Sestito S.E., Cighetti R., Aguilar Moncayo E.M., Oblak A., et al. Trehalose- and glucose-derived glycoamphiphiles: small-molecule and nanoparticle Toll-like receptor 4 (TLR4) modulators. J. Med. Chem. 2014;57(21):9105–9123. doi: 10.1021/jm501182w. [DOI] [PubMed] [Google Scholar]
- 90.Mirshafiey A., Rehm B.H., Sahmani A.A., Naji A., Razavi A. M-2000, as a new anti-inflammatory molecule in treatment of experimental nephrosis. Immunopharmacol. Immunotoxicol. 2004;26(4):611–619. doi: 10.1081/iph-200042362. [DOI] [PubMed] [Google Scholar]
- 91.Ahmadi H., Jamshidi A.R., Mahmoudi M., Cuzzocrea S., Fattahi M.J., Barati A. The potent inhibitory effect of beta-D-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property on anti-cyclic citrullinated peptide antibodies, rheumatoid factor and anti-dsDNA antibodies in patients with rheumatoid arthritis. Curr. Drug Discov. Technol. 2017;14(3):206–214. doi: 10.2174/1570163814666170321113059. [DOI] [PubMed] [Google Scholar]
- 92.Barati A., Jamshidi A.R., Ahmadi H., Aghazadeh Z., Mirshafiey A. Effects of beta-D-mannuronic acid, as a novel non-steroidal anti-inflammatory medication within immunosuppressive properties, on IL17, ROR gamma T, IL4 and GATA3 gene expressions in rheumatoid arthritis patients. Drug Des. Devel. Ther. 2017;11:1027–1033. doi: 10.2147/DDDT.S129419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abolhassani H., Amirkashani D., Parvaneh N., Mohammadinejad P., Gharib B., Shahinpour S. Autoimmune phenotype in patients with common variable immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2013;23(5):323–329. [PubMed] [Google Scholar]
- 94.Sharifi L., Mirshafiey A., Rezaei N., Azizi G., Magaji Hamid K., Amirzargar A.A. The role of Toll-like receptors in B-cell development and immunopathogenesis of common variable immunodeficiency. Expert Rev. Clin. Immunol. 2016;12(2):195–207. doi: 10.1586/1744666X.2016.1114885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


