Abstract
Background:
Visfatin is an adipokine that plays a role in the inflammatory process of atherosclerosis. This study aimed to investigate whether adipokine is associated with the extent of stable coronary artery disease (CAD).
Methods:
The study population included 110 patients who underwent elective coronary angiography (CAG) due to stable angina pectoris. The severity of CAD was assessed by the ‘Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX)’ score. We evaluated patients in two groups: group 1 with a SYNTAX score <22 (low) and group 2 with a SYNTAX score ⩾22 (intermediate to high).
Results:
Serum visfatin (8.6 ± 4.2 ng/ml versus 13.4 ± 5.2 ng/ml, p < 0.001) and serum C-reactive protein (CRP) levels [0.46 (0.25–0.77) mg/dl versus 0.71 (0.32–1.10) mg/dl, p < 0.001] were lower in group 1. A positive significant correlation was found between serum visfatin level and SYNTAX score (r = 0.559, p < 0.001). In a multivariate logistic regression analysis, visfatin [odds ratio (OR) 1.22, 95% confidence interval (CI) 1.10–1.36; p < 0.001], CRP (OR 6.22, 95% CI 1.70–22.7; p = 0.006), and diabetes mellitus (OR 3.83, 95% CI 1.10–13.2; p = 0.034) were found to be independent predictors of SYNTAX score.
Conclusions:
Serum visfatin level was positively correlated with CAD severity in patients with high SYNTAX score. Serum visfatin level can be a useful biomarker for predicting high SYNTAX scores in patients with angina pectoris undergoing CAG.
Keywords: Visfatin, SYNTAX score, inflammation, stable coronary artery disease
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality and leads to an increased financial burden in healthcare systems in developing countries.1 Despite advances in therapeutic strategy, including optimal pharmacotherapy and myocardial revascularization, the prognosis of CVD is still unsatisfactory. The pathophysiology of myocardial ischemia should be better understood to overcome this issue. The severity of coronary artery disease (CAD) is associated with mortality.2 The ‘Synergy Between Percutaneous Coronary Intervention (PCI) with TAXUS and Cardiac Surgery (SYNTAX)’ score (www.syntaxscore.com), is an anatomically based scoring system used to determine the complexity of CAD, and is a guide for decision-making between coronary artery bypass grafting (CABG) surgery and PCI. The SYNTAX score is related to mortality and morbidity in stable CAD.3,4 Stable angina pectoris is characterized by reversible ischemia caused by a demand–supply imbalance due to ischemia or hypoxia.5 In patients with stable CAD, epicardial atherosclerotic lesions and, less often, endothelial erosions are observed. The lesion is fibrotic, has a small necrotic core and thick fibrous cap, with little or no thrombus.6 As inflammation plays a crucial role in coronary plaque rupture, new determinants such as visfatin, an adipokine, appears to be relevant in this process. Adipocytokines are locally effective in the adipose tissue and affect distal organs via the systemic circulation. Visfatin has become a prominent research topic in recent years owing to its potential effects. Visfatin may be related to endothelial function, which is indispensable for coronary circulation, and may be an indicator of an inflammatory event in the coronary arteries.7 Many studies have investigated the relationship between inflammation markers and the extent of CAD.8–10 In this study, we emphasized that inflammation involved with the severity and extent of coronary atherosclerosis might be related to serum visfatin level in patients with stable CAD.
Methods
Study population
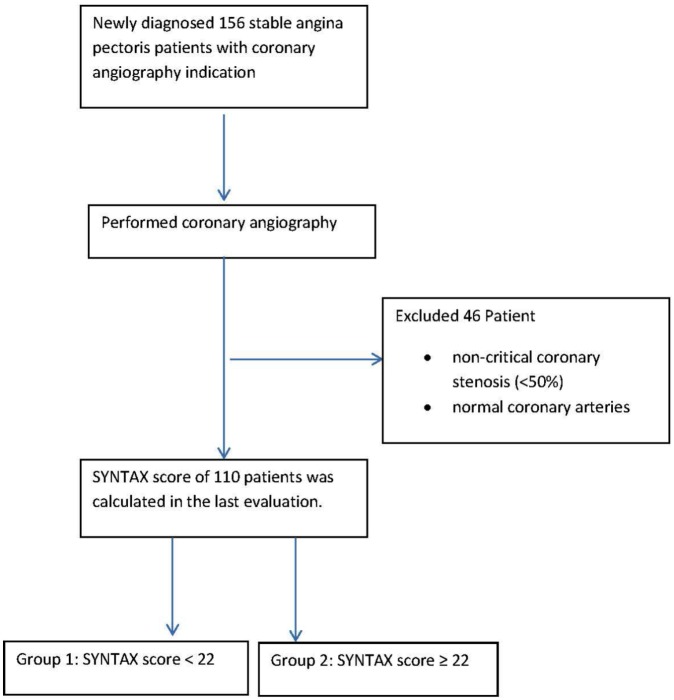
The research was carried out under the principles set out in Helsinki Declaration, after approval by a local ethics committee (Recep Tayyip Erdoğan University Non-invasive Clinical Research Ethics Committee decision/protocol number: 40465587-153/164; decision date: 27 August 2018). This single-center cross-sectional study included 156 consecutive patients who underwent elective coronary angiography (CAG). CAG was performed to investigate ischemic heart disease in patients with a positive treadmill test, myocardial perfusion scintigraphy, or typical chest pain. After the patients gave written consent, clinical data were recorded, and blood samples were taken. According to the CAG results, we divided patients into three groups: patients with nonstenotic coronary arteries; patients with a SYNTAX score <22 (group 1); and patients with SYNTAX score ⩾22 (group 2). Since the aim of the study was to investigate the relationship between the visfatin and the extent of CAD, patients with nonstenotic coronary arteries were not included (Figure 1). We excluded patients with acute coronary syndrome, liver, kidney or systemic inflammatory diseases, malignancies, a previous PCI, or CABG.
Figure 1.
Diagram of study participant.
Angiographic definition
Unaware of the clinical data of the patients, two independent and experienced interventional cardiologists evaluated the CAG images. Each coronary lesion with at least a 50% diameter stenosis in vessels over 1.5 mm was scored and recorded with the SYNTAX score calculator (www.syntaxscore.com).
Laboratory analysis
We collected blood specimens from patients before the procedure for routine biochemical parameters. For serum C-reactive protein (CRP) and visfatin measures, the participant’s serums were collected by centrifugation in a clinical microbiology lab, transferred to cryotubes and stored at −70°C until tested. Serum visfatin levels were calculated using an enzyme-linked immunosorbent assay (ELISA; human visfatin (VF) ELISA kit, Sunred Biological Technology, Shanghai, China) according to the manufacturer’s protocol. The kit is a double-antibody sandwich ELISA for the quantitative detection of human visfatin. Absorbance optical density of each well was determined at 450 nm with a microtiter plate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA) in the 5th minute. Standard curves were prepared using Titri ELISA software. The curve was used to convert sample absorbance readings to visfatin concentration. Serum CRP levels were measured with a nephelometric method (UniCel DxC 800 System, Beckman Coulter, Fullerton, CA, USA; normal range 0–0.5).
Statistical analysis
We used a Kolmogorov–Smirnov test to determine whether the data were distributed in a normal range. Categorical data were expressed as a percentage, and continuous data were given as mean ± standard deviation or median and interquartile range. A Chi-square test was used to compare categorical variables, a Student’s t-test was used to analyze parametric data, and Mann–Whitney U-test was used to evaluate nonparametric continuous variables. Correlations between variables were tested using the Pearson correlation test for normally distributed variables and the Spearman correlation test for non-normally distributed variables. The effects of different variables on SYNTAX score were calculated in a univariate logistic regression analysis. Unadjusted (p < 0.05) scores of clinical, demographic, angiographic, laboratory data were tested by univariate analysis and independent predictors of CAD extent were determined by a multivariate logistic regression model. A p value <0.05 was considered statistically significant at a confidence interval of 95%. We used SPSS version 18.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA) for data evaluation. In addition, power of the sample size was calculated by G Power tool (Franz Faul, University of Kiel, Germany, version 3.1.9.4) with 0.005 alpha and 0.3 effect size values for a total number of 110 patients according to post hoc-type power analysis.
Results
The study population comprised 110 consecutive patients with stable angina pectoris who underwent CAG. The calculated power value was 0.9487 according to post hoc-type power analysis (the minimum required number of patients was determined as 65 for an acceptable power 0.8, 0.05 alpha and 0.3 effect size values). The mean age was 59 ± 10 years, and 54% were male. Sex was not associated with the visfatin level (male, 10.5 ± 5.8 ng/ml; female, 10.3 ± 4.3 ng/ml p = 0,834). The demographic and clinical features of both groups were similar (Table 1). However, diabetes mellitus was more common in group 2 (31% versus 10%, p = 0.009). There were no differences between the groups in terms of hemoglobin, white blood cell count, lipid profile, glomerular filtration rate, and albumin values. Serum visfatin (13.4 ± 5.2 ng/ml versus 8.6 ± 4.2 ng/ml, p < 0.001), CRP [0.71 (0.32–1.10) mg/dl versus 0.46 (0.25–0.77) mg/dl, p = 0.013] and neutrophil–lymphocyte rate (NLR) (6.1 ± 2.6 versus 5.1 ± 2.4, p = 0.033) were higher in group 2
Table 1.
Baseline clinical and laboratory characteristics of the study population and univariate analysis of the variables.
| SYNTAX score |
||||
|---|---|---|---|---|
| Variable | Overall (n = 110) | Group 1 <22 (n = 69) | Group 2 ⩾22 (n = 41) | p value |
| Age, years | 59 ± 10 | 55.7 ± 10 | 57.2 ± 13 | 0.267 |
| Sex, male % | 54 | 53 | 56 | 0.845 |
| Diabetes, % | 18 | 10 | 31 | 0.009 |
| Hypertension, % | 43 | 39 | 49 | 0.42 |
| Smoking, % | 32 | 33 | 29 | 0.679 |
| Dyslipidemia, % | 27 | 23 | 34 | 0.269 |
| Family history of CAD | 37 | 36 | 39 | 0.839 |
| Systolic blood pressure, mmHg | 122 ± 23 | 122 ± 22 | 120 ± 23 | 0.646 |
| Diastolic blood pressure, mmHg | 75 ± 17 | 74 ± 16 | 76 ± 18 | 0.534 |
| Heart rate, min | 70 (65–97) | 70 (62–85) | 74 (66–97) | 0.253 |
| Visfatin, ng/ml | 10.4 ± 5.1 | 8.6 ± 4.2 | 13.4 ± 5.2 | <0.001 |
| Albumin, g/dl | 3.3 (2.8–3.7) | 3.1 (2.8–3.7) | 3.4 (2.7–3.7) | 0.627 |
| C-reactive protein, mg/dl | 0.52 (0.29–0.86) | 0.46 (0.25–0.77) | 0.71 (0.32–1.10) | 0.013 |
| NLR | 5.5 ± 2.5 | 5.1 ± 2.4 | 6.1 ± 2.6 | 0.033 |
| Hemoglobin, g/dl | 12.9 ± 1.4 | 13.1 ± 1.4 | 12.7 ± 1.4 | 0.125 |
| White blood cell count×103/µl | 8.6 ± 2.5 | 8.3 ± 2.4 | 9.2 ± 2.7 | 0.094 |
| Platelet count, ×103/µl | 263 ± 86 | 265 ± 85 | 255 ± 87 | 0.554 |
| Total cholesterol, mg/dl | 222 ± 33 | 220 ± 36 | 225 ± 26 | 0.443 |
| LDL cholesterol, mg/dl | 153 ± 30 | 151 ± 35 | 157 ± 17 | 0.323 |
| HDL cholesterol, mg/dl | 27 ± 8 | 27.8 ± 9.2 | 26.7 ± 5.4 | 0.459 |
| Triglyceride, mg/dl | 205 ± 67 | 204 ± 62 | 206 ± 77 | 0.899 |
| GFR, ml/min | 76 ± 17 | 70 ± 16 | 76 ± 18 | 0.103 |
| EF, % | 60 ± 5 | 61 ± 5 | 59 ± 5 | 0.090 |
| Previous medications, % | ||||
| Aspirin | 30 | 29 | 31 | 0.831 |
| Statin | 19 | 23 | 17 | 0.804 |
| ACE inhibitors/ARB | 29 | 27 | 31 | 0.669 |
| β-blocker | 13 | 15 | 10 | 0.407 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; EF, ejection fraction; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NLR, neutrophil–lymphocyte ratio.
The normal cutoff value of troponin in our laboratory is <0.04 mg/l.
In the bivariate correlation analysis (Table 2), the SYNTAX score had a positive strong correlation with serum visfatin levels (r = 0.559, p < 0.001), CRP (r = 0.374, p < 0.001) and NLR (r = 0.306, p = 0.001).
Table 2.
Correlation of SYNTAX score with visfatin, CRP and neutrophil–lymphocyte ratio.
| SYNTAX score |
||
|---|---|---|
| r | p | |
| Visfatin | 0.559 | <0.001 |
| CRP | 0.374 | <0.001 |
| Neutrophil–lymphocyte ratio | 0.306 | 0.001 |
CRP, C-reactive protein.
The parameters significant with univariate analysis were tested by multivariate logistic regression analysis. Visfatin [odds ratio (OR) 1.22, 95% confidence interval (CI) 1.10–1.36; p < 0.001], CRP (OR 6.22, 95% CI 1.70–22.7; p = 0.006) and diabetes mellitus (OR 3.83, 95% CI 1.10–13.2; p = 0.034) were the independent predictors of SYNTAX score. NLR (OR 1.10, 95% CI 0.90–1.33; p = 0.329) was not detected as an independent predictor (Table 3).
Table 3.
Independent predictors of SYNTAX score (multivariate p value, OR with 95% CI).
| Parameters | p value | OR | 95% CI |
|---|---|---|---|
| Visfatin | <0.001 | 1.22 | 1.10–1.36 |
| CRP | 0.006 | 6.22 | 1.70–22.7 |
| NLR | 0.329 | 1.10 | 0.90–1.33 |
| DM | 0.034 | 3.83 | 1.10–13.2 |
CI, confidence interval; CRP, C-reactive protein; DM, diabetes mellitus; NLR, neutrophil–lymphocyte ratio; OR, odds ratio.
Discussion
The research showed that serum visfatin level was correlated with the extent and severity of CAD, and visfatin was an independent predictor of this disease. Serum CRP and NLR levels were correlated with the SYNTAX score. In addition, increased CRP and diabetes mellitus were found to be independent predictors of the extent and severity of CAD. This study is the first to show a significant correlation between increased serum visfatin level and the severity and extent of CAD in patients with stable angina pectoris.
The role of inflammation in the initiation and progression of coronary atherosclerosis, is well known.11 Visfatin (also known as pre-B cell colony growth factor) is a new proinflammatory adipokine that is expressed in visceral fat.12 Studies have shown that visfatin is involved in inflammation processes, an essential part of atherosclerosis. Serum visfatin level is correlated with serum interleukin-6 and CRP levels, suggesting that circulating visfatin may reflect inflammation status.13–15 Visfatin is localized to foam cell macrophages in unstable atherosclerotic lesions, which may play a role in plaque stability.16 Macrophages, transformed from circulating monocytes, are one of the essential cell types for atherosclerotic plaque formation. Therefore, the circulating monocyte count may be essential in clarifying atherosclerosis pathogenesis. Monocyte activation is a crucial step in the early stages of atherosclerosis. Macrophages from visceral white adipose tissue contain higher visfatin levels than mature adipocytes. Dahl and colleagues confirmed that, visfatin was expressed in symptomatic atherosclerotic carotid plaques and localized in lipid-laden areas.17,18 However, Choi and colleagues20 reported no association between visfatin level and CAD.19 A nonspecific small patient group might have affected the outcome of this study.19 Visfatin is a useful marker for the presence and severity of coronary ectasia. Haleh and colleagues showed the correlation of the extent and severity of isolated coronary artery ectasia with visfatin level.20 Yung and colleagues21 showed the relationship between increased visfatin level and CAD in patients with chronic renal failure;20 we found a positive correlation with stable CAD. In this current study, there was no control group because we wanted to find out whether visfatin level was only related to the extent and severity of CAD.
We evaluated the extent and severity of CAD with the SYNTAX score, the most popular scoring tool. The SYNTAX score is vital in determining the treatment strategy by detecting the anatomical extent of CAD. It is also an independent predictor of overall vascular mortality, cardiac death, myocardial infarction and target vessel revascularization ratio in patients with acute coronary syndrome.22,23 To characterize the various properties of atherosclerotic disease, many new cytokines that encode different stages of atherogenesis have been investigated. Thus, studies have detected the association between inflammatory-marker-mediated endothelial dysfunction and the level of atherosclerosis.11,24 Takebayashi and colleagues showed the association of visfatin with vasodilatation involved in endothelial dysfunction.25 The serum visfatin level is related to the expansion of the infarct-related artery and myocardial damage in patients with ST-elevation myocardial infarction.26 Studies detected an association between inflammatory markers and the SYNTAX score, and found a strong correlation between these markers and the extent of CAD.7,27 The role of visfatin in the inflammatory process and in the maturation of vascular smooth muscle cells28 can shed light on a possible effect of coronary atherosclerosis. Therefore, visfatin may act as a proinflammatory cytokine, play a role in chronic inflammation, and may contribute to the pathogenesis of atherosclerosis and CVD. In this current study, the association of CAD and serum visfatin level may be related to these mechanisms.
The presence and extent of CAD closely affects human health and the cost of treatment.29 The measurement of serum visfatin level, which correlates with the prevalence of atherosclerosis, as confirmed in the study, can be used to predict the severity of stable CAD.
Limitations
The study population was small. A causal relationship could not be identified due to limitations of the cross-sectional study. It is not clear whether increased visfatin is a causal factor or only a spectator in the pathogenesis of atherosclerosis in patients with CAD. To clarify the clinical role of plasma visfatin, we need more extensive studies and more extended follow-up periods to understand its mechanism in the human myocardium.
Conclusion
Serum visfatin concentration is associated with a high SYNTAX score in patients with stable CAD. Plasma visfatin level is a strong and independent predictor of SYNTAX score, and can be used as an indicator to identify patients with CAD at a high risk for an atherosclerotic burden. As a part of the inflammatory process, visfatin can be a prominent marker in determining the severity and extent of CAD.
Footnotes
Author contributions: Duman H. and Özyıldız A.G. were responsible for patient selection, conception, design, analysis and writing. Bahçeci I., Duman H., Uslu A. and Ergül E. equally contributed to data collection, critical revision and editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Hakan Duman  https://orcid.org/0000-0002-1441-7320
https://orcid.org/0000-0002-1441-7320
Contributor Information
Hakan Duman, Department of Cardiology, Faculty of Medicine, Recep Tayyip Erdoğan University, Islampaşa, Rize 53100, Turkey.
Ali Gökhan Özyıldız, Department of Cardiology, Faculty of Medicine, Recep Tayyip Erdoğan University, Rize, Turkey.
İlkay Bahçeci, Department of Medical Microbiology, Faculty of Medicine, Recep Tayyip Erdoğan University, Rize, Turkey.
Handan Duman, Ministry of Health, Family Health Center, Rize, Ankara, Turkey.
Abdulkadir Uslu, Department of Cardiology, University of Health Sciences, Kartal Kosuyolu Training and Research Hospital, Istanbul, Turkey.
Elif Ergül, Department of Cardiology, Faculty of Medicine, Recep Tayyip Erdoğan University, Rize, Turkey.
References
- 1. De Backer G. Epidemiology and prevention of cardiovascular disease: Quo vadis? Eur J Prev Cardiol 2017; 24: 768–772. [DOI] [PubMed] [Google Scholar]
- 2. Bundhun PK, Sookharee Y, Bholee A, et al. Application of the SYNTAX score in interventional cardiology: a systematic review and meta-analysis. Medicine (Baltimore) 2017; 96: e7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurtul A, Murat SN, Yarlioglues M, et al. Usefulness of serum albumin concentration to predict high coronary SYNTAX score and in-hospital mortality in patients with acute coronary syndrome. Angiology 2016; 67: 34–40. [DOI] [PubMed] [Google Scholar]
- 4. van Gaal WJ, Ponnuthurai FA, Selvanayagam J, et al. The SYNTAX score predicts peri-procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol 2009; 135: 60–65. [DOI] [PubMed] [Google Scholar]
- 5. Task Force Members; Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 6. Depre C, Wijns W, Robert AM, et al. Pathology of unstable plaque: correlation with the clinical severity of acute coronary syndromes. J Am Coll Cardiol 1997; 30: 694–702. [DOI] [PubMed] [Google Scholar]
- 7. Ucgun T, Başar C, Memişoğulları R, et al. Serum visfatin and omentin levels in slow coronary flow. Rev Port Cardiol 2014; 33: 789–794. [DOI] [PubMed] [Google Scholar]
- 8. Karabağ Y, Çağdaş M, Rencuzogullari I, et al. Relationship between C-reactive protein/albumin ratio and coronary artery disease severity in patients with stable angina pectoris. J Clin Lab Anal 2018; 32: e22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duman H, Çetin M, Durakoğlugil ME, et al. Relation of angiographic thrombus burden with severity of coronary artery disease in patients with ST segment elevation myocardial infarction. Med Sci Monit 2015; 17; 21: 3540–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtul A, Elcik D. Procalcitonin is an independent predictor for coronary atherosclerotic burden in patients with stable coronary artery disease. Int J Cardiol 2017; 236: 61–64. [DOI] [PubMed] [Google Scholar]
- 11. Ross R. Atherosclerosis an inflammatory disease. N Engl J Med 1999; 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 12. Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005; 307: 426–430. [DOI] [PubMed] [Google Scholar]
- 13. Oki K, Yamane K, Kamei N, et al. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol 2007; 67: 796–800. [DOI] [PubMed] [Google Scholar]
- 14. Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 2007; 178: 1748–1758. [DOI] [PubMed] [Google Scholar]
- 15. Yan JJ, Tang NP, Tang JJ, et al. Genetic variant in visfatin gene promoter is associated with decreased risk of coronary artery disease in a Chinese population. Clin Chim Acta 2010; 411: 26–30. [DOI] [PubMed] [Google Scholar]
- 16. Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007; 115: 972–980. [DOI] [PubMed] [Google Scholar]
- 17. Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006; 49: 744–747. [DOI] [PubMed] [Google Scholar]
- 18. Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis possible role in inflammation and plaque destabilization. Circulation. 2007; 115: 972–980. [DOI] [PubMed] [Google Scholar]
- 19. Choi KM, Lee JS, Kim EJ, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol 2008; 158: 203–207. [DOI] [PubMed] [Google Scholar]
- 20. Ashraf H, Soltani D, Sobh-Rakhshankhah A, et al. Visfatin as marker of isolated coronary artery ectasia and its severity. Cytokine 2019; 113: 216–220. [DOI] [PubMed] [Google Scholar]
- 21. Lu YC, Hsu CC, Yu TH, et al. Association between visfatin levels and coronary artery disease in patients with chronic kidney disease. Iran J Kidney Dis 2013; 7: 446–452. [PubMed] [Google Scholar]
- 22. Acet H, Ertaş F, Bilik MZ, et al. The relationship of TIMI risk index with SYNTAX and Gensini risk scores in predicting the extent and severity of coronary artery disease in patients with STEMI undergoing primary percutaneous coronary intervention. Ther Adv Cardiovasc Dis 2015; 9: 257–266. [DOI] [PubMed] [Google Scholar]
- 23. Sianos G, Morel M-A, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005; 1: 219–227. [PubMed] [Google Scholar]
- 24. Ozkaramanli Gur D, Guzel S, et al. The role of novel cytokines in inflammation: defining peripheral artery disease among patients with coronary artery disease. Vasc Med 2018; 23: 428–436. [DOI] [PubMed] [Google Scholar]
- 25. Takebayashi K, Suetsugu M, Wakabayashi S, et al. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism 2007; 56: 451–458. [DOI] [PubMed] [Google Scholar]
- 26. Yu T-H, Lu L-F, Hung W-C, et al. Circulating visfatin level at admission is associated with occlusion of the infarct-related artery in patients with acute ST-segment elevation myocardial infarction. Acta Cardiologica Sinica 2011; 27: 77–85. [Google Scholar]
- 27. Bolayır HA, Kıvrak T, Gunes H, et al. The association between serum serglycin level and coronary artery disease severity in patients with stable angina pectoris. Kardiol Pol 2018; 76: 783–790. [DOI] [PubMed] [Google Scholar]
- 28. Liu SW, Qiao SB, Yuan JS, et al. Association of plasma visfatin levels with inflammation, atherosclerosis, and acute coronary syndromes in humans. Clin Endocrinol 2009; 71: 202–207. [DOI] [PubMed] [Google Scholar]
- 29. Hayıroğlu Mİ, Çınar T, Bıçakçı B, et al. Predictors of femoral hematoma in patients undergoing elective coronary procedure: a trigonometric evaluation. Int J Cardiovasc Imaging 2018; 34: 1177–1184. [DOI] [PubMed] [Google Scholar]