Abstract
Frailty exhibits diverse influences on health-related outcomes and represents a surrogate of increased susceptibility to harmful injuries. Patients with chronic kidney disease (CKD) are at a higher risk of accelerated biologic aging, and, in this population, the concept of frailty emerges as an instrumental measurement of physiologic reserves. However, a comprehensive description of known independent contributors to, and risk associates of, frailty in these patients remain unavailable. In the present review, original studies up to 28 February 2019 that assessed frailty in patients with all stages of CKD were retrieved and reviewed, with results extracted and summarized. By pooling 62 original investigations, 58.1% and 49.1% used cohort and cross-sectional designs, respectively. Dialysis-dependent end-stage renal disease patients (n = 39; 62.9%) were the most commonly examined population, followed by those with nondialysis CKD (n = 12; 19.4%) and those receiving renal transplantation (n = 11; 17.7%). Contributors to frailty in CKD patients included sociodemographic factors, smoking, CKD severity, organ-specific comorbidities, depression, hypoalbuminemia, and low testosterone levels. Conversely, the development of frailty was potentially associated with the emergence of cardiometabolic, musculoskeletal, and cerebral complications; mental distress; and a higher risk of subsequent functional and quality-of-life impairment. Moreover, frailty in CKD patients increased healthcare utilization and consistently elevated mortality among affected ones. Based on the multitude of contributors to frailty and its diverse health influences, a multifaceted approach to manage CKD patients with frailty is needed, and its potential influences on outcomes besides mortality need to be considered.
Keywords: chronic kidney disease, dialysis, end-stage renal disease, frailty, kidney transplantation, outcome, risk factors
Frailty: an ever-evolving concept with pleiotropic influences
Since the inception of the concept of frailty in the 1950s, frailty has been found to be prevalent among geriatric population and exhibits substantial influences on multiple health-related outcomes; furthermore, there has been an exponential increase in publications on frailty.1 Originally conceived to characterize the extensive vulnerability to external or endogenous insults in the elderly, frailty has vague content and ambiguous meaning; not until the operational definition of frail phenotype structuralized by Fried and colleagues in 2001 did the measurement of frailty become standardized and subject to extensive investigation.2 This status of vulnerability can stem from an individual’s demographic background, biologic illnesses with or without organ degeneration, psychologic competency, environmental features, social statuses, etc., with a cumulative and additive effect across different spectrums.3 Physical performance such as frail phenotype is also commonly used to consolidate frailty, and serves as a robust surrogate of one’s biological age.4
Regardless of the approaches used to assess frailty, the presence of frailty correlates with various detrimental outcomes among geriatric patients. A meta-analysis identified that frailty, whether measured by the frail index or frail phenotype, was associated with a higher risk of developing premature mortality, prolonged hospitalization, being institutionalized, having disability in basic or instrumental activities of daily living (ADLs), falls, fractures, cognitive impairment, and greater healthcare resource utilization.5 Similar findings have been corroborated by other.6,7 Conversely, several factors, including, but not limited to, biologic aging, genetic background, lifestyle factors, cardiovascular morbidities, and dietary and nutritional balances, play a role in the pathogenesis of frailty in the geriatric population.8,9
Frailty in patients with chronic kidney disease
The importance of frailty has also been acknowledged in patients with other chronic disorders irrespective of age, including those with chronic kidney disease (CKD) and end-stage renal disease (ESRD). The presence of frailty increases the risk of mortality in these patients, and its adverse influences in other health-related outcomes are being discovered. A previous systematic review of 30 reports focused on the relationship between functional, cognitive impairment or frailty, and adverse outcomes in patients with predialysis CKD or dialysis-dependent ESRD.10 The authors found that in these patients, functional impairment or frailty was consistently associated with a significantly higher risk of mortality or hospitalization. Another narrative review reached a similar conclusion regarding the negative effects of frailty on survival of ESRD patients.11 However, accumulating evidence suggests other frailty-related adverse effects besides mortality and hospitalization of CKD patients, although this has not been confirmed to date. A comprehensive understanding of the biology of frailty in CKD patients, including its risk factors, accompanying features, and complications, is therefore needed to facilitate the design of intervention strategies in this disproportionately affected population. In this review, we summarize evidence from the literature to answer this gap in existing knowledge.
Strategy of literature search
We used a systematic approach to identify relevant articles assessing frailty in patients with all stages of CKD in their titles or abstract using keywords, such as ‘frailty’ or ‘frail phenotype’, and ‘chronic kidney disease’, ‘renal insufficiency’, ‘chronic renal failure’, ‘end-stage renal disease’, or ‘chronic dialysis’, from databases, including PubMed, MEDLINE, and Google Scholar. Reports between 1980 and 28 February 2019 were retrieved. Inclusion criteria were original reports involving adult human subjects that examined the relationship between frailty and any types of clinical features or outcomes among the target population of CKD. Eligible studies were independently reviewed by two reviewers (P.Y.W. and C.T.C.). We excluded review articles, articles without abstract available, those that failed to measure the effects of frailty in CKD patients, or non-CKD target population (Figure 1). We further screened the abstracts and reference lists of the retrieved articles to identify additional studies that contained original data focusing on the same issue. Any discrepancy between the two reviewers was resolved by discussing with another senior author (D.C.C.). CKD (nondialysis) was mostly defined according to the estimated glomerular filtration rate based on the Modification of Diet in Renal Disease, while very few CKD cases were evaluated based on elevated serum creatinine levels. Staging of CKD, whichever available, was performed based on the Kidney Disease Improving Global Outcome criteria.12
Figure 1.
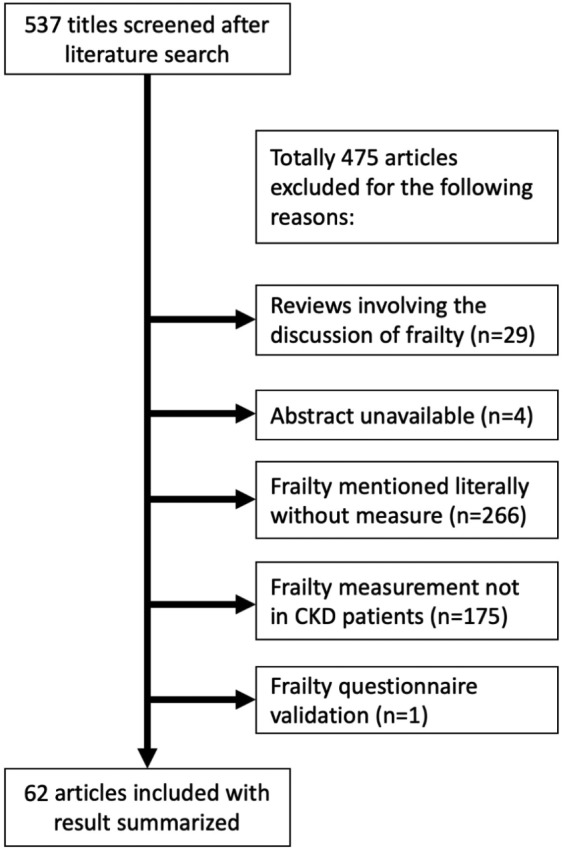
The algorithm of literature search and results retrieval.
CKD, chronic kidney disease.
We extracted the following parameters from the included studies: publication data, participants’ baseline CKD stages, method of frailty measurement, results from univariate analyses of clinical features between frail and nonfrail participants, and multivariate analyses of frail associates or complications, depending on the study design. We tabulated the study characteristics into the following categories: unadjusted risk associates of frailty, adjusted potential causes of frailty, and adjusted risks of health outcomes conferred by frailty according to the biologic relationship between frailty and clinical features that were extracted. Factors adjusted for in the multivariate analyses included at least age and gender in all studies and could further include study-specific parameters such as comorbidity, anthropometric data, and laboratory profiles.
Overview of studies addressing frailty influences in CKD patients
Our database search identified 537 articles addressing frailty and CKD in whole or in part. After an initial screening of the title and abstract, we excluded review articles, those without abstract available, those discussing frailty literally without direct measurement of frailty, and those that did not measure frailty in CKD patients (Figure 1). Overall, 62 original investigation articles with their full text (or abstract if published as conference proceedings) were finally reviewed with results extracted for summarization.13–74 We found that nearly half of these investigations were conducted in the United States (n = 28, 45.2%), followed by Taiwan (n = 7, 11.3%), Canada (n = 4, 6.5%), and Brazil (n = 4, 6.5%). Among the 62 articles, 58.1% used a cohort study design with follow up, while 41.9% had a cross-sectional design; more than half of the articles (n = 37; 59.7%) were based on single-center data, whereas others were analyzed using b multicenter registries. ESRD patients undergoing chronic hemodialysis (stage 5D) (n = 28; 45.2%) were the most common population being evaluated, followed by patients with nondialysis CKD (n = 12; 19.4%), those receiving renal transplantation (stage 5T) (n = 11; 17.7%), and ESRD patients receiving either hemodialysis or peritoneal dialysis (n = 8; 12.9%). Most retrieved studies used the Fried phenotype with or without modifications to measure frailty, whereas seven (11.3%) and four (6.5%) defined frailty according to the FRAIL scale and the Edmonton frail scale, respectively.
Among the 62 articles, 79% (n = 49) included univariate analyses of the relationship between frailty and risk features among CKD patients and 83.9% (n = 52) conducted multivariate analyses to account for influences from confounders. Most of the retrieved studies addressed frailty-related adverse complications in these patients (n = 45; 72.6%), whereas six (9.75%) evaluated potential contributors and complications in the same study; nine (14.5%) of the retrieved studies examined potential contributors to frailty only.
In the following section, we summarize findings from the 62 articles according to the role of each factor in CKD patients into four sections: unadjusted frailty associates, potential contributors to frailty (adjusted), potential modifiers of frailty course (adjusted), and health-related outcomes affected by frailty (adjusted).
Unadjusted associates of frailty in CKD patients
Existing literature examined a diverse spectrum of risk associates accompanying frailty in CKD patients (Supplementary Table), including demographic factors, anthropometric parameters, multiple types of comorbidity, psychological illnesses, physical examination parameters, nutrition, body composition details, bone mineral density, laboratory data, duration and clinical features of dialysis, residual renal function, ADL, quality of life (QoL), and functional and overall outcomes. Higher age; larger waist circumference; lower blood pressure; higher prevalence of comorbidities (heart failure, peripheral vascular disease, diabetes, and obesity); greater fat mass but less lean mass and bone mass; lower serum albumin, hemoglobin, and cholesterol levels but higher creatinine and C-reactive protein (CRP) levels; less residual renal function; worse cognitive function; lower frequency of physical activity and worse ADL; poorer nutrition and QoL; and a higher degree of healthcare utilization were consistently found in frail CKD patients compared with those in nonfrail CKD ones. However, these relationships were all unadjusted, and only some of them have been validated in multivariate analyses, as detailed in the following sections.
Potential contributors to frailty in CKD patients
After adjustment for confounders, multiple factors emerged as independent contributors to the development of frailty in CKD patients (Table 1). Sociodemographic factors, including advanced age, female gender, certain ethnicity (non-White), unemployment, lower education, and smoking, particularly age and being female, are associated with a significantly higher risk of frailty among CKD patients than among nonfrail ones. Increasing CKD severity correlates with a higher frailty risk; however, a dose-response relationship has not been consistently observed. Comorbidities such as the cardiovascular, pulmonary, and central nervous system disorders, metabolic disturbance, and musculoskeletal disorders were all significant risk factors for developing frailty in CKD patients. Among these comorbidities, endothelial dysfunction, chronic obstructive pulmonary disease, obesity, and arthritis were associated with more than two-fold risk elevation. Psychiatric impairment and disability were associated with an even higher risk of frailty (more than three-fold) relative to other contributors. Among patients undergoing chronic hemodialysis, laboratory data such as hypocreatininemia, hypoalbuminemia, and low testosterone levels, with a similar degree of risk elevation, were predictors of developing frailty in CKD patients. A summary of potential causes of frailty in CKD patients is illustrated in Figure 2.
Table 1.
Potential causes of frailty in patients with CKD reported in the literature.
| Category | Type | Risk difference (95% CI) | Patient CKD severity | Frailty assessment method | Sample size | Study | |
|---|---|---|---|---|---|---|---|
| Demographic profile | Age | Age >60 years | OR 4.0 (1.0–16.2) | Stages 3–5 | Modified Fried phenotypes | 61 | Mansur59 |
| Per year | OR 1.02 (1.01–1.03) | Stages 5D | Modified Fried phenotypes | 2275 | Johansen37 | ||
| OR 1.03 (1.01–1.04) | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| Female gender | OR 11.3 (2.3–55.6) | Stages 3–5 | Modified Fried phenotypes | 61 | Mansur59 | ||
| OR 1.55 (1.27–1.88) | Stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | |||
| OR 11.6 (1.7–79.1) | Elderly with stage 5D (HD) | Multidimensional frailty score | 46 | Lee47 | |||
| Male gender | OR 0.49 (0.39–0.62) | Stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | ||
| Non-White race | OR 1.9 (1.1–1.3) | Stages 1–4 | Modified CHS scale | 336 | Roshanravan67 | ||
| Unemployed status | OR 1.89 (1.36–2.62) | Stage 5D | Modified CHS scale | 1658 | Lee46 | ||
| Higher education level | OR 0.67 (0.49–0.91) for 7th–12th grade, 0.53 (0.35–0.82) for >12th grade | ||||||
| Lifestyle | Smoking | RR 1.18 (1.04–1.34) | Stage 5D (HD) | Fried Phenotypes | 205 | Yadla73 | |
| Anthropometric parameters | BMI | OR 1.2 (1.0–1.4) per 5 kg/m2 | Stages 1–4 | Modified CHS scale | 336 | Roshanravan67 | |
| OR 1.06 (1.02–1.1) per kg/m2 | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| OR 0.58 (0.38–0.88) per kg/m2 | Elderly with stage 5D | Multidimensional frailty score | 46 | Lee47 | |||
| Waist circumference (cm) | OR 3.84 (1.39–10.61; 3rd tertile) | Stage 5D (HD) | Fried phenotypes | 151 | Noori63 | ||
| CKD severity | Mild | OR 2.21 (1.49–3.28) | Stages 1/2 | Modified Fried phenotypes | 10,256 | Wilhelm-Leen72 | |
| OR 1.48 (1.00–2.19) | Cre >1.3 mg/dl | CHS scale | 5888 | Shlipak70 | |||
| Moderate | OR 2.48 (1.57–3.93) | Stages 3a | Modified Fried phenotypes | 10,256 | Wilhelm-Leen72 | ||
| Severe | OR 5.88 (3.40–10.16) | Stages 3b–5 | |||||
| OR 2.8 (1.3–6.3) | Stage 3b | Modified CHS scale | 336 | Roshanravan67 | |||
| OR 2.1 (1.0–4.7) | Stage 4 | ||||||
| Biological | |||||||
| Cardiovascular | Hypertension | RR 1.6 (1.26–2.04) | Stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | |
| Peripheral vascular disease | RR 1.58 (1.34–1.8) | Stage 5D (HD) | Fried phenotypes | 205 | |||
| OR 1.67 (1.16–2.41) | Stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | |||
| Left ventricular dysfunction | RR 1.18 (1.03–1.36) | Stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | ||
| Cardiac disorder (any) | OR 1.43 (1.01–1.98) | Stage 5D | Modified CHS scale | 1658 | Lee46 | ||
| Endothelial dysfunction | OR 3.86 (1.00–14.88) | Stages 3–5 | Modified Fried phenotypes | 61 | Mansur59 | ||
| Central nervous system | Cerebrovascular accident | RR 1.34 (1.19–1.5) | Stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | |
| OR 1.55 (1.05–2.29) | Stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | |||
| OR 1.85 (1.04–3.28) | Stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | |||
| OR 1.56 (1.04–2.35) | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| Pulmonary | COPD | OR 2.20 (1.20–4.03) | CKD stages 1–5 | Modified Fried phenotypes | 10,256 | Wilhelm-Leen72 | |
| Endocrinologic/ metabolic | Diabetes | OR 1.68 (1.16–2.45) | CKD stages 1–5 | Fried phenotypes | 10,256 | ||
| OR 1.35 (1.10–1.65) | Stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | |||
| OR 1.52 (1.18–1.96) | Stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | |||
| OR 1.44 (1.11–1.87) | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| Obesity | OR 6.63 (1.16–36.77) | Stages 3–5 | Modified Fried phenotypes | 61 | Mansur59 | ||
| Cancer | Cancer | OR 1.89 (1.19–2.99) | CKD stages 1–5 | Modified Fried phenotypes | 10,256 | Wilhelm-Leen72 | |
| Musculoskeletal | Arthritis | OR 3.34 (2.08–5.38) | CKD stages 1–5 | Modified Fried phenotypes | 10,256 | ||
| Body composition | Fat mass | OR 3.27 (1.17–9.09; 2nd tertile) and 4.97 (1.7–14.55; 3rd tertile) | Stage 5D (HD) | Fried phenotypes | 151 | Noori63 | |
| ECW to ICW ratio | OR 3.85 (1.18–10.50; 3rd tertile) | ||||||
| Psychiatric | Depression | OR 3.97 (2.28–6.91) | stage 5T | Fried phenotypes | 773 | Konel42 | |
| Functional status | Disability | OR 5.6 (4.12–7.62) | stage 5D | Modified CHS scale | 1658 | Lee46 | |
| Vascular access | Permanent vascular access (fistula or graft) | OR 0.71 (0.51–0.98) | stage 5D (HD) | Modified Fried phenotypes | 2275 | Johansen37 | |
| Laboratory data | Creatinine < 4 mg/dl | RR 1.46 (1.22–1.71) | stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | |
| eGFR (per 5 ml/min/1.73 m2 increase) | OR 1.44 (1.23–1.68) | stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | ||
| Albumin < 3.2 (g/dl) | OR 1.89 (1.43–2.49) | stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | ||
| Lower free testosterone, (per 50% lower) | OR 1.30 (1.03–1.58) | Male stage 5D (HD) | Fried phenotypes | 440 | Chiang26 |
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECW, extracellular water; eGFR, estimated glomerular filtration rate; HD, hemodialysis; ICW, intracellular water; OR, odds ratio; RR, relative risk.
Figure 2.
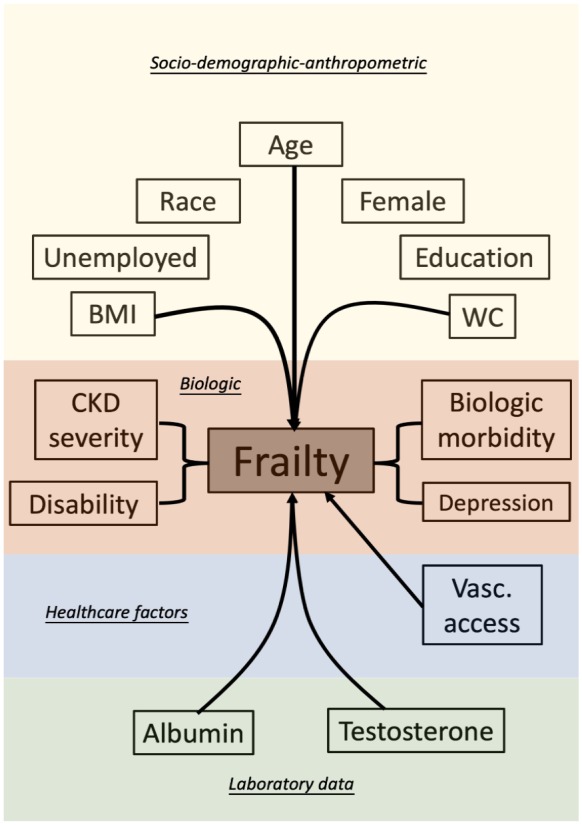
An illustrative diagram showing potential contributors to frailty in CKD patients.
BMI, body mass index; CKD, chronic kidney disease; Vasc, vascular; WC, waist circumference.
Potential modifiers of frailty courses in CKD patients
Three of the retrieved studies examined factors that modified the course of frailty in CKD patients (Table 2).26,28,40 Johansen and colleagues revealed that diabetes mellitus, certain ethnicity, and higher interleuin-6 (IL-6) levels were associated with worsening frailty over a 2-year follow-up period among chronic dialysis patients, whereas higher serum albumin levels were associated with improving frailty. Chiang and colleagues reported that a baseline lower free testosterone level predicted the risk of developing frailty over 1 year among male dialysis patients. In contrast, in renal transplant patients, Chu and colleagues found that an African-American origin was associated with improved frailty after transplantation, whereas diabetes and longer dialysis period predicted having persistent frailty despite transplantation.
Table 2.
Potential modifiers of frailty trajectories in patients with CKD reported in the literature.
| Category | Type | Risk difference (95% CI) | Patient CKD severity | Frailty assessment method | Sample size | Ref |
|---|---|---|---|---|---|---|
| Ethnicity | Hispanic | Frailty scores increase 0.6 (0–1.1) per year | stage 5D (HD) | Fried phenotypes | 762 | Johansen40 |
| Black | Frail to nonfrail after transplantation [RRR 1.98 (1.07–3.67)] | stage 5T | Fried phenotypes | 569 | Chu28 | |
| Biological | ||||||
| Endocrinologic/metabolic | Diabetes | Remain frail after transplantation [RRR 2.56 (1.22–5.39)] | stage 5T | Fried phenotypes | 569 | Chu28 |
| Frailty scores increase 0.7 (0.3–1.0) per year | stage 5D (HD) | Fried phenotypes | 762 | Johansen40 | ||
| Laboratory data | IL-6 | Frailty scores increase 0.3 (0.1–0.4) per year | ||||
| Serum Albumin Concentrations (g/dl) | Frailty scores decrease 1.1 (0.7–1.5) per g/dl | |||||
| Low free testosterone (< 147 pmol/l) | Developing Frailty over 12 months (OR 1.56, 1.04–2.33) | Male stage 5D (HD) | Fried phenotypes | 440 | Chiang26 | |
| Dialysis course | Time of dialysis (year) | Frail to nonfrail after transplantation [RRR 0.88 (0.78–1)] | Stage 5T | Fried phenotypes | 569 | Chu28 |
| Healthcare utilization | ||||||
| Hospitalization | Hospitalization during past year | Frailty scores increase 0.6 (0.3–0.8) per year | Stage 5D (HD) | Fried phenotypes | 762 | Johansen40 |
CI, confidence interval; CKD, chronic kidney disease; HD, hemodialysis; IL-6, interleukin-6; OR, odds ratio; RRR, relative risk reduction.
Established health-related complications owing to frailty in CKD patients
After confounder adjustment, frailty remained associated with multiple adverse complications in CKD patients, including disorders involving the cardiac, musculoskeletal, metabolic, and central nervous system; mental distress; impaired functional status; increased fall risk; poorer QoL; greater utilization of healthcare resources (hospitalization, emergency visits, re-admission, longer length of stay, and total medical visits); and a higher mortality than nonfrail CKD one patient (Table 3). Specifically, frailty correlated independently with abnormal cardiac conduction, lower lean and bone mass but higher adiposity, increased fracture risk, and worsened cognitive function. Interestingly, in patients undergoing chronic dialysis, frailty conferred a 2.6-fold higher risk of vascular access failure compared with nonfrail patients.23 In addition, among renal transplant recipients, frailty significantly increased the risk of subsequent graft loss; those with frailty were more likely to have immunosuppressive dose reduction than nonfrail ones.42,54 Among the spectrum of frailty-related complications in CKD patients, the risk for having sarcopenia was the highest [odds ratio (OR) 12.2],41 followed by any ADL impairment (OR 11.3)43 and renal allograft failure (OR 6.2).42 The risk for fall in frail CKD patients was consistent among existing studies (differences in risk, 1.6 to 3),32,44,50,73 and a similar degree of risk increase was noted with regard to the endpoint of hospitalization-related events.17,37,46,51,73
Table 3.
Confounder-adjusted risk of complications resulting from frailty in CKD patients.
| Category | Type | HR/OR, risk difference (95% CI), or values in F versus NF groups | Patient CKD severity | Frailty assessment method | Sample size | Study | |
|---|---|---|---|---|---|---|---|
| Biological | |||||||
| Cardiovascular | QRS duration | β = −0.29, t = −2.03, p = 0.048 | stage 5D (HD) | EFS | 41 | Chao19 | |
| β = −0.27, t = −1.84, p = 0.05 | FRAIL scale | ||||||
| Musculoskeletal | Vertebral compression fracture (any) | OR 1.8 per FRAIL score (p = 0.01) | stage 5D (HD) | FRAIL Scale | 43 | Chao21 | |
| Cognitive function | 3MS scores | At baseline | −2.37 (–4.21 to −0.53) compared with NF | stage 5D (HD) | Fried phenotypes | 324 | McAdams-DeMarco53 |
| 1-year | −2.80 (–5.37 to −0.24) compared with NF | ||||||
| Pretransplant | −1.8 compared with NF | stage 5T | Fried phenotypes | 665 | Chu27 | ||
| 1–4 years post-transplant | −0.04 per year (–0.06 to −0.01) | ||||||
| TMT-A | At baseline | 12.08 (4.73–19.43) compared with NF | stage 5D (HD) | Fried phenotypes | 324 | McAdams-DeMarco53 | |
| TMT-B | At baseline | 33.15 (9.88–56.42) compared with NF | |||||
| Body composition | Lean mass | Lower lean mass over cephalic, trunk, and 4 extremities than NF group | Stage 5D (HD) | FRAIL scale | 44 | Chao24 | |
| BMD at 1 year | Stage 5D (HD) | FRAIL Scale | 43 | Chao25 | |||
| Total | ß = −0.53, t = −3.27, p < 0.01 | ||||||
| L1 | ß = −0.4, t = −2.18, p = 0.04 | ||||||
| L4 | ß = −0.39, t = −2.1, p = 0.046 | ||||||
| Femoral neck | ß = −0.5, t = −2.96, p < 0.01 | ||||||
| Average L-spine areas | |||||||
| 1 year of follow up | β = −0.48, t = −2.84, p < 0.01 | ||||||
| Interval changes | β = −0.5, t = −3.02, p < 0.01 | ||||||
| Interval changes in L-spine Z-score percentages | β = −0.45, t = −2.11, p = 0.049 | ||||||
| QUS parameters | |||||||
| SOS | 1487.8 versus 1537.8 (female) 1493.7 versus 1542.2 (male) |
Stage 5D (HD) | CHS scale | 214 | Yoneki74 | ||
| BUA | 86.2 versus 100.7 (female) 93.8 versus 107.8 (male) |
||||||
| Stiffness index | 54.0 versus 77.7 (female) 60.9 versus 83.6 (male) |
||||||
| Muscles | |||||||
| Quadriceps muscle area | r = –30.28, p = 0.02 | Stage 5D (HD) | Performance-based frailty | 80 | Delgado31 | ||
| Appendicular SMI | Lower in Frail group (adjusted p < 0.05) | Stages 1–5 | EFS | 41 | Adame Perez13 | ||
| Appendicular fat percentage | Stage 5D (HD) | FRAIL scale scores | 44 | Chao24 | |||
| Left/Right lower extremity | β = 0.34, t = 2.32; p = 0.03 (left); β = 0.3, t = 2.05; p = 0.048 (right) | ||||||
| Left/Right upper extremity | β = 0.37, t = 2.66; p = 0.01 (left); β = 0.43, t = 3.09; p < 0.01 (right) | ||||||
| Sarcopenia | OR 12.2 (2.27–65.5) | Stage 5D (PD) | Clinical Frailty Scale | 119 | Kamijo41 | ||
| Functional status | Physical functioning | Lower in Frail group (adjusted p = 0.004) | Stages 1–5 | EFS | 41 | Adame Perez13 | |
| Need assistance in ADL | OR 1.93 (1.01–3.68) for prefrail OR 11.32 (5.49–23.32) for frail |
Stage 5D (HD) | Modified Fried phenotypes | 742 | Kutner43 | ||
| Barthel index scores | OR 0.89 (0.86–0.93) | Stage 5D | Clinical Frailty Scale | 251 | Iyasere36 | ||
| Psychological | |||||||
| Delirium | Post-transplantation delirium | OR 2.05 (1.02–4.13) | Stage 5T | Fried phenotypes | 893 | Haugen35 | |
| Distress | Self-reported distress thermometer | β = 0.35 (0.12–0.58), t = 3.0, p = 0.003 | Stage 5D (HD) | Canadian frailty score | 382 | Camilleri18 | |
| Anxiety/depression | Hospital anxiety and depression scale | OR 1.21 (1.11–1.31) | Stage 5D | Clinical Frailty Scale | 251 | Iyasere36 | |
| Fall | Any fall | HR 2.1 (1.21–3.92) | Stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | |
| OR 2.39 (1.22–4.71) | Stage 5D (HD) | Modified Fried phenotype | 762 | Kutner44 | |||
| Increased numbers of falls | HR 3.09 (1.38–6.90) | Stage 5D (HD) | Modified Fried phenotype | 95 | McAdams-DeMarco50 | ||
| Time to first fall | HR 1.60 (1.16–2.20) | Stage 5D (HD) | Self-reported frailty | 1646 | Delgado32 | ||
| Quality of Life | KDQoL | ||||||
| Physical health | 33.7 versus 40.7 | Stage 5D (HD) | Fried phenotypes | 151 | Noori63 | ||
| Effects of disease | 51.6 versus 66.8 | ||||||
| KDQoL short form | |||||||
| Physical component | Difference −6.31 (–8.16 to −4.46) | Stage 5T | Fried phenotypes | 443 | McAdams-DeMarco58 | ||
| Physical functioning | Difference −14.17 (–18.58 to −9.76) | ||||||
| Role limitations | Difference −15.37 (–22.96 to −7.78) | ||||||
| Bodily pain | Difference −9.45 (–14.33 to −4.57) | ||||||
| General health | Difference −11.76 (–15.94 to −7.59) | ||||||
| Emotional well-being | Difference −3.05 (–6.01 to −0.09) | ||||||
| Social functioning | Difference −6.19 (–10.98 to −1.41) | ||||||
| Energy | Difference −11.66 (–16.3 to −7.03) | ||||||
| Kidney disease-specific HRQoL | Difference −6.53 (–9.17 to −3.89) | ||||||
| Symptoms | Difference −5.5 (–8.2 to −2.79) | ||||||
| Effects | Difference −7.69 (–11.66 to −3.72) | ||||||
| Burden | Difference −10.19 (–15.94 to −4.44) | ||||||
| Cognitive function | Difference −5.51 (–9 to −2.02) | ||||||
| Social interaction | Difference −4.7 (–7.85 to −1.56) | ||||||
| Sleep | Difference −6.29 (–10.56 to −2.02) | ||||||
| Social support | Difference −5.69 (–9.92 to −1.47) | ||||||
| HRQoL | |||||||
| Fair/poor HRQoL at follow-up | OR 2.79 (1.32–5.90) | Stage 5D | Fried phenotypes | 233 | McAdams-DeMarco56 | ||
| Worse HRQoL after follow-up | RR 2.91 (1.08–7.80) | ||||||
| SF-36 | |||||||
| Physical components | Lower in Frail group (adjusted p = 0.002) | Stages 1–5 | EFS | 41 | Adame Perez13 | ||
| β = –0.566, t = –8.792, p < 0.001 | Stage 2–4 | Modified Fried phenotypes | 168 | Lee45 | |||
| Mean difference −1.12 (–1.47 to −0.76) | Stages 3–5 | Modified Fried phenotypes | 61 | Mansur48 | |||
| Mental components | Mean difference −0.75 (–1.4 to −0.16) | ||||||
| β = –0.485, t = –6.709, p < 0.001 | Stage 2–4 | Modified Fried phenotypes | 168 | Lee45 | |||
| SF-12 | |||||||
| Lower MCS | OR 0.94 (0.91–0.97) | Stage 5D | Clinical Frailty Scale | 251 | Iyasere36 | ||
| Lower PCS | OR 0.88 (0.84–0.91) | ||||||
| Symptom scores (high) | OR 1.23 (1.13–1.34) | ||||||
| KDQoL-SF scores 3 months after transplant | Stage 5T | Fried phenotypes | 443 | McAdams-DeMarco58 | |||
| Physical HRQoL | 0.34/month versus 1.35/month | ||||||
| Kidney disease-specific HRQoL | 2.41/month versus 3.75 points/month | ||||||
| Effects | 4.01/month versus 7.1/month | ||||||
| Cognitive function | 1.28/month versus 2.88/month | ||||||
| Social interaction | −0.57/month versus 1.18/month | ||||||
| Graft Loss | Risk of graft loss in depressive patients | HR 6.20 (1.67−22.95) | Stage 5T | Fried phenotypes | 773 | Konel42 | |
| Immunosuppressant use | MMF dose reduction | HR 1.29 (1.01–1.66) | Stage 5T | Modified Fried phenotypes | 525 | McAdams-DeMarco54 | |
| Dialysis access survival | Access failure | HR 2.63 (1.03–6.71) | Stage 5D (HD) | FRAIL scale | 51 | Chao23 | |
| Health-care utilization | Hospitalization or mortality | HR 1.56 (1.36–1.79) | Stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | |
| Hospitalization | HR 2.06 (1.18–3.58) | Stage 5D (HD) | Fried phenotypes | 205 | Yadla73 | ||
| HR 1.83 (1.41–2.37) | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| HR 1.43 (1.00–2.03) | Stage 5D (HD) | Fried phenotypes | 146 | McAdams-DeMarco51 | |||
| Number of all-cause hospitalizations | β = 0.29, p < 0.0001 | Stage 5D (PD) | In-house frailty questionnaire | 193 | Ng62 | ||
| Number of cardiovascular hospitalizations | β = 0.37, p < 0.0001 | ||||||
| Time to first hospitalization | HR 1.26 (1.09–1.45) | Stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | ||
| Early Hospital Readmission | RR 1.59 (1.17–2.17) | Stage 5T | Fried phenotypes | 383 | McAdams-DeMarco52 | ||
| Longer LOS | |||||||
| LOS (days) | RR 1.15 (1.03–1.29) | Stage 5T | Fried phenotypes | 589 | McAdams-DeMarco57 | ||
| >2 weeks | OR 1.57 (1.06–2.33) | Stage 5 | Fried phenotypes | 569 | Chu28 | ||
| OR 2.02 (1.20–3.40) for increased frail category; OR 1.92 (1.13–3.25) for increased frail scores | |||||||
| In depressive patients | RR 1.88 (1.70–2.08) | Stage 5T | Fried phenotypes | 773 | Konel42 | ||
| Hospitalization frequency | Higher in Frail group (adjusted p < 0.001) | Stages 1–5 | EFS | 41 | Adame Perez13 | ||
| Emergency department visit frequency | Higher in Frail group (adjusted p = 0.002) | ||||||
| Total medical visit frequency | Higher in Frail group (adjusted p = 0.001) | ||||||
| Mortality | Overall mortality | HR 2.17 (1.01–4.65) after transplantation | stage 5T | Fried phenotypes | 537 | McAdams-DeMarco55 | |
| HR 2.0 (1.5–2.7) | stages 1–5 | Modified Fried phenotypes | 10,256 | Wilhelm-Leen72 | |||
| HR 1.57 (1.25–1.97) | stage 5D (incident) | Modified Fried phenotypes | 1576 | Bao17 | |||
| HR 2.24 (1.60–3.15) | Stage 5D | Modified Fried phenotypes | 2275 | Johansen37 | |||
| HR 1.22 (1.04–1.43) | Stage 5D | Clinical frailty scale | 390 | Alfaadhel14 | |||
| HR 4.28 (1.22–14.98) | Stages 4/5 | PRISMA questionnaire & TUGT | 104 | Ali15 | |||
| HR 9.83 (1.80–53.7) | Stage 5D (PD) | Clinical frailty scale | 119 | Kamijo41 | |||
| HR 2.60 (1.04–6.49) | Stage 5D (HD) | Fried phenotypes | 146 | McAdams-DeMarco51 | |||
| HR 2.08 (1.04–4.16) | Stage 5D | Modified CHS scale | 1658 | Lee46 | |||
| HR 1.78 (1.15–2.8) for performance-based frailty; HR 1.66 (1.06–2.6) for self-reported frailty; HR 1.95 (1.19–3.2) for both definition positivity | Stage 5D (HD) | Modified Fried phenotypes and self-reported frailty | 771 | Johansen39 | |||
| HR 1.66 (1.03–2.67) in general; HR 3.77 (1.10–12.92) in general obesity; HR 2.38 (1.17–4.82) in abdominal obesity | Stage 5D (HD) | Fried phenotypes | 370 | Fitzpatrick34 | |||
| HR 2.43 (1.48–3.99) | Stage 5D and 5T from ANCA vasculitis | Inability to walk without help | 425 | Romeu66 | |||
| HR 1.93 (1.58–2.36) | Stage 5D and 5T from MM or amyloidosis | Inability to walk without help | 1462 | Decourt30 | |||
| In depressive patients | HR 2.62 (1.03−6.70) | Stage 5T | Fried phenotypes | 773 | Konel42 | ||
| Modify the association between comorbidity and mortality | HR 0.75 (0.44–1.29) in F group versus 1.66 (1.17–2.35) in NF group | Stage 5 | Fried phenotypes | 2086 | Perez13 | ||
| HR 1.93 (1.58–2.36) | Stage 5D and 5T from MM/ amyloidosis | Inability to walk without help | 1462 | Decourt30 | |||
| Post-transplant mortality | HR 2.27 (1.11–4.65) for increased frail category; OR 2.36 (1.12–4.99) for increased frail scores | Stage 5T | Fried phenotypes | 569 | Chu28 | ||
| Composite | Mortality or dialysis | HR 2.5 (1.4–4.4) | Stages 1–4 | Modified CHS scale | 336 | Roshanravan67 | |
| Mortality or cardiovascular hospitalization | HR 23.58 (1.61–346.03) | Elderly with stage 5D (HD) | Multidimensional frailty score | 46 | Lee47 | ||
| 30-day post-transplant complications | β = 13.31 (5.72–20.89), p = 0.0007 | Stage 5T | Groningen Frailty Indicator | 150 | Schopmeyer69 |
3-MS, Modified Mini-Mental State; ADL, activity of daily living; BMD, bone mineral density; BUA, broadband ultrasound attenuation; CHS, Cardiovascular Health Study; CI, confidence interval; CKD, chronic kidney disease; EFS, Edmonton frail scale; HD, hemodialysis; HR, hazard ratio; HRQoL, health-related quality of life; LOS, length of stay; KDQOL-SF, Kidney disease quality of life instrument – short form; MCS, mental component score; MMF, mycophenolate mofetil; NF, nonfrail; OR, odds ratio; PCS, physical component score; PD, peritoneal dialysis; QUS, quantitative ultrasound; RR, relative risk; SGA, standardized global assessment; SMI, skeletal muscle index; SOS, speed of sound; TMT, trail making test.
The relationship between frailty and mortality in CKD patients has been repeatedly examined in the literature (Table 3). Frailty is predictive of a higher risk of mortality in CKD patients across stages from early CKD to chronic dialysis or stage 5T patients, and the hazard ratios (HRs) ranged between 1.22 and 9.83 compared with nonfrail CKD patients, with most studies deriving a HR between 2 and 3. One study reported an exceptionally higher risk of mortality related to frailty (OR 9.83)41; however, this likely resulted from the modest case number, the frailty measurement approach (clinical frailty scale), and the population they examined (peritoneal dialysis). We also noted that the mortality risk conferred by frailty did not increase linearly with higher CKD severity based on the literature search results; however, mortality risk increased substantially among elderly compared with others.47 This suggests that chronologic aging substantially enhances the adverse influence of frailty in CKD patients who already have accelerated biologic aging.
A brief summary of frailty-related adverse health-related outcomes is illustrated in Figure 3.
Figure 3.
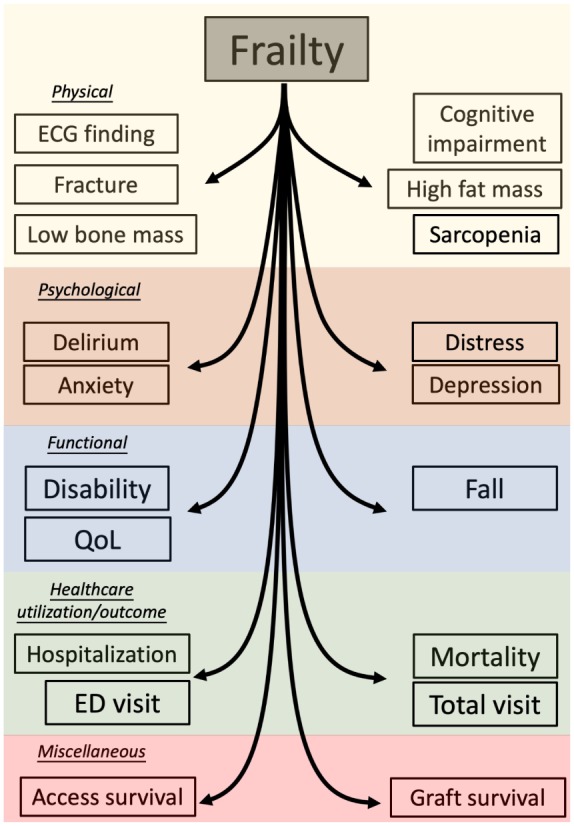
An illustrative diagram showing potential complications of frailty in CKD patients.
CKD, chronic kidney disease; ECG, electrocardiogram; ED, emergency department; QoL, quality of life.
Reciprocal relationship between frailty and clinical features in CKD patients
Several features have been examined both as contributors to and complications of frailty in CKD patients, with potential biologic plausibility. These risk features associated with frailty included hypoalbuminemia, higher fat mass, depression, and having a disability (Tables 1 and 3). In addition, it is interesting to note that having permanent vascular access (fistula or graft) is predictive of a lower frailty risk, whereas frail patents were at a higher risk of access failure among chronic dialysis patients.23,37 Similarly, musculoskeletal disorders such as arthritis were independent causes of frailty in CKD patients, whereas frailty in CKD patients might contribute to a higher risk of fractures.21,72
Serum albumin level has long been considered a composite indicator for nutritional status, inflammatory status, and possibly beyond, exhibiting a strong outcome-predictive ability in diverse clinical settings.75 It is plausible that nutritional impairment contributes to an increased risk of frailty; conversely, the physical limitation imposed by frailty may further compromise nutrient-seeking ability and cause protein-energy malnutrition in affected individuals with CKD. Alternatively, it can be that subclinical inflammation or cytokine interplay stays at the core of this albumin-frailty connection.76 Dysfunctional muscle and fat tissues with resultant metabolic defects, such as insulin resistance, are potential contributors to frailty and sarcopenia, and frailty can adversely affect eating behavior and lean mass building.77 This vicious cycle is expected to perpetuate itself in CKD patients who are already at risk of deranged homeostasis with negative body composition alterations. Psychiatric disorders, particularly depression, suppress one’s appetite and decrease oral intake; moreover, frail individuals have poorer QoL and an increased risk of mood disorders. This bidirectional relationship between depression and frailty has been affirmed in older adults,78 and likely still holds true in CKD patients. Disability and frailty frequently overlap in older adults, and crosstalk between these two adverse phenotypes exists and both independently contribute and act synergistically to an increased risk of mortality among elderly and possibly CKD patients as well.79
Factors that exhibit an opposite relationship with frailty in CKD patients
Among the retrieved reports, body mass index (BMI) exhibited an inverse relationship with the risk of frailty depending on the population being examined. Greater body BMI increases the probability of frailty in CKD patients regardless of stages,46,67 but decreases the risk in one study involving elderly patients undergoing dialysis47 (Table 1). A similar scenario has been reported by other studies involving the elderly,80,81 and may be explained partially by the close association between better nutritional status and higher BMI in geriatric patients but not in the general population. It may be worthwhile to note that interventions directed toward reducing BMI can have differential influences in general CKD patients and in older ones.
Nonindependent risk features for frailty
The prevalence and values of many clinical features differed significantly between CKD patients with and without frailty (Supplementary Table); however, their relationship with frailty disappears after confounder adjustment. These factors include multimorbidity, blood pressure, individual morbidities such as osteoporosis and viral infection, and many laboratory parameters ranging from electrolytes (phosphate), hemogram (hemoglobin), lipid profile, and hormonal panel (parathyroid hormone or vitamin D). In addition, care modality, dialysis modality or duration, dialysis clearance, or several nutritional measurement parameters (standard global assessment, mini-nutritional assessment, and malnutrition-inflammation scores) were similarly neutral regarding their relationship to frailty after accounting for other variables in CKD and ESRD patients. It is possible that these factors are surrogates of other vital pathogenic players of frailty, such as serum albumin, cardiovascular morbidities, CKD severities, and residual renal function (Table 1). It will be more appropriate for researchers to account for these instrumental variables that contribute deeply to the development of frailty in subsequent studies aiming to examine frailty risk factors.
Implications for subsequent studies involving frailty in CKD patients
Understanding the risk factors and complications of frailty can be of importance in CKD population from both clinical and public health perspectives. Previous reviews and meta-analyses placed much emphasis on the adverse influences on survival conferred by frailty in CKD patients10,11; however, emerging studies hint at the diverse organ and functional influences posed by frailty. In addition, there are reports suggesting that frailty significantly modifies the association between other risk features and mortality.82 Researchers are in the process of devising strategies to combat frailty in CKD patients, especially those with advanced CKD and dialysis-dependent ESRD.83 With the information summarized in this review, we can gain more insight into the beneficial influences of frailty-targeted interventions besides mortality or hospitalization alone. Moreover, by targeting independent risk associates of frailty before or near its onset in CKD patients, we can more efficiently identify upstream etiologies amenable for reducing frailty, paving the way toward outcome improvement in the future. However, we should still remember that only some of the relationships that we described are causal because 41.9% of studies were cross-sectional in nature, precluding overinferences. More than half are single-center studies, and there may be center-specific frailty features that are not generalizable to other populations. Nonetheless, we believe that this comprehensive summarization of existing literature can facilitate the design of subsequent frailty studies in CKD patients.
Summary and conclusion
We conducted an extensive literature search and retrieved 62 reports that addressed the risk associates or complications of frailty in CKD patients. We found that more than half of these studies focused on dialysis-dependent ESRD patients, while only one-fifth of these studies examined those with nondialysis CKD or renal transplantation. Fried phenotype with or without modifications was the most common approach for measuring frailty in CKD patients, followed by FRAIL scale and Edmonton frail scale. Contributors to frailty in CKD patients include sociodemographic factors, smoking, higher CKD severity, several organ-specific comorbidities, depression, disability, hypoalbuminemia, and low testosterone levels. The development of frailty is independently associated with subsequent complications in CKD patients, including cardiometabolic, musculoskeletal, and cerebral disorders; mental distress; functional and QoL impairment; excessive healthcare consumption; and higher risk of mortality. Considering these wide array of frailty-related detrimental influences, frailty-reducing therapies are expected to produce a plethora of benefits in CKD patients. Further intervention studies are awaited to answer this unmet clinical need.
Supplemental Material
Supplemental material, Supplementary_Table_3 for Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review by Patrick Yihong Wu, Chia-Ter Chao, Ding-Cheng Chan, Jenq-Wen Huang and Kuan-Yu Hung in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: Study design: CTC, PYH; Data analysis: PYH, CTC, DCC; Article drafting: PYH, CTC, JWH, KYH; All authors approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The study is financially sponsored by National Taiwan University Hospital, National Taiwan University Hospital Beihu branch, and Ministry of Science and Technology, Taiwan (MOST 108-2314-B-002-055-).
Sponsor’s role: The sponsors have no role in the study design, data collection, analysis, and result interpretation of this study.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Chia-Ter Chao  https://orcid.org/0000-0003-2892-7986
https://orcid.org/0000-0003-2892-7986
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Patrick Yihong Wu, School of Medicine, National Taiwan University, Taipei.
Chia-Ter Chao, Department of Medicine, National Taiwan University Hospital BeiHu Branch, College of Medicine, National Taiwan University, Taipei, Geriatric and Community Medicine Research Center, National Taiwan University Hospital BeiHu Branch, Taipei, Graduate Institute of Toxicology, National Taiwan University, NO.87, Nei-Jiang Street, WanHua District, 108 Taipei, Taiwan.
Ding-Cheng Chan, Department of Medicine, National Taiwan University Hospital ChuTung Branch, Hsin-Chu County; Department of Geriatrics and Gerontology, National Taiwan University Hospital, Taipei.
Jenq-Wen Huang, Nephrology Division, Department of Internal Medicine, National Taiwan University Hospital, Taipei.
Kuan-Yu Hung, Nephrology Division, Department of Internal Medicine, National Taiwan University Hospital, Taipei; Department of Internal Medicine, National Taiwan University Hospital HsinChu branch, HsinChu County.
Reference
- 1. Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med 2017; 33: 293–303. [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M57. [DOI] [PubMed] [Google Scholar]
- 3. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cesari M. Role of gait speed in the assessment of older patients. JAMA 2011; 305: 93–94. [DOI] [PubMed] [Google Scholar]
- 5. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016; 17: 1163.e1–e17. [DOI] [PubMed] [Google Scholar]
- 6. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc 2015; 16: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 7. Kojima G. Frailty as a predictor of disabilities among community-dwelling. Older people: a systematic review and meta-analysis. Disabil Rehabil 2017; 39: 1897–1908. [DOI] [PubMed] [Google Scholar]
- 8. Stewart R. Cardiovascular disease and frailty: what are the mechanistic. Links? Clin Chem 2019; 65: 80. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell SJ, Mitchell GJ, Mitchell JR. Modulation of frailty syndrome by diet: a review of evidence from mouse studies. Mechanisms of Ageing and Development 2019; 180: 82–88. [DOI] [PubMed] [Google Scholar]
- 10. Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-A systematic review. Clin J Am Soc Nephrol 2016; 11: 1624–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sy J, Johansen KL. The impact of frailty on outcomes in dialysis. Curr Opin Nephrol Hypertens 2017; 26: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–735. [DOI] [PubMed] [Google Scholar]
- 13. Adame Perez SI, Senior PA, Field CJ, et al. Frailty, health-related quality of life, cognition, depression, vitamin D and health-care utilization in an ambulatory adult population with type 1 or type 2 diabetes mellitus and chronic kidney disease: a cross-sectional analysis. Can J Diabetes 2019; 43: 90–97. [DOI] [PubMed] [Google Scholar]
- 14. Alfaadhel TA, Soroka SD, Kiberd BA, et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015; 10: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali H, Abdelaziz T, Abdelaal F, et al. Assessment of prevalence and clinical outcome of frailty in an elderly predialysis cohort using simple tools. Saudi J Kidney Dis Transpl 2018; 29: 63–70. [DOI] [PubMed] [Google Scholar]
- 16. Bancu I, Graterol F, Bonal J, et al. Frail patient in hemodialysis: a new challenge in nephrology-incidence in our area, barcelones nord and maresme. J Aging Res 2017; 2017: 7624139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao Y, Dalrymple L, Chertow GM, et al. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 2012; 172: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camilleri S, Chong S, Tangvoraphonkchai K, et al. Effect of self-reported distress thermometer score on the maximal handgrip and pinch strength measurements in hemodialysis patients. Nutr Clin Pract 2017; 32: 682–686. [DOI] [PubMed] [Google Scholar]
- 19. Chao CT, Huang JW. Frailty severity is significantly associated with electrocardiographic QRS duration in chronic dialysis patients. PeerJ 2015; 3: e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chao CT, Hsu YH, Chang PY, et al. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology 2015; 20: 321–328. [DOI] [PubMed] [Google Scholar]
- 21. Chao CT, Chiang CK, Huang JW, et al. Effect of frail phenotype on bone mass and vertebral compression fracture in individuals undergoing dialysis. J Am Geriatr Soc 2016; 64: e19–e21. [DOI] [PubMed] [Google Scholar]
- 22. Chao CT, Lai HJ, Tsai HB, et al. Frail phenotype is associated with distinct quantitative electroencephalographic findings among end-stage renal disease patients: an observational study. BMC Geriatr 2017; 17: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chao CT, Chiang CK, Huang JW, et al. Self-reported frailty among end-stage renal disease patients: a potential predictor of dialysis access outcomes. Nephrology 2017; 22: 333–334. [DOI] [PubMed] [Google Scholar]
- 24. Chao CT, Chan DC, Huang JW. Frail phenotype might be associated with higher appendicular but not truncal fat among end-stage renal disease patients. J Pain Symptom Manage 2017; 53: e1–e4. [DOI] [PubMed] [Google Scholar]
- 25. Chao CT, Huang JW, Chan DC. Frail phenotype might herald bone health worsening among end-stage renal disease patients. PeerJ 2017; 5: e3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiang JM, Kaysen GA, Segal M, et al. Low testosterone is associated with frailty, muscle wasting and physical dysfunction among men receiving hemodialysis: a longitudinal analysis. Nephrol Dial Transplant 2018; 34: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu NM, Gross AL, Shaffer AA, et al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol 2019; 30: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chu NM, Deng A, Ying H, et al. Dynamic frailty before kidney transplantation—time of measurement matters. Transplantation 2019; 103: 1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Sousa Meira A, Batista M, Maria de Pina Pereira R, et al. Frailty in elderly patients with chronic kidney disease under conservative treatment. Rev Rene 2016; 17: 386–392. [Google Scholar]
- 30. Decourt A, Gondouin B, Delaroziere JC, et al. Trends in survival and renal recovery in patients with multiple myeloma or light-chain amyloidosis on chronic dialysis. Clin J Am Soc Nephrol 2016; 11: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delgado C, Doyle JW, Johansen KL. Association of frailty with body composition among patients on hemodialysis. J Ren Nutr 2013; 23: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delgado C, Shieh S, Grimes B, et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol 2015; 42: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Demircioglu DT. The association of vitamin D levels and the frailty phenotype among non-geriatric dialysis patients: a cross-sectional study. Clinics 2018; 73: e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fitzpatrick J, Sozio SM, Jaar BG, et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients: the predictors of arrhythmic and cardiovascular risk in end stage renal disease study. Nephrol Dial Transplant 2018; 34: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haugen CE, Mountford A, Warsame F, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol 2018; 29: 1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iyasere OU, Brown EA, Johansson L, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 2016; 11: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol 2007; 18: 2960–2967. [DOI] [PubMed] [Google Scholar]
- 38. Johansen KL, Painter P, Delgado C, et al. Characterization of physical activity and sitting time among patients on hemodialysis using a new physical activity instrument. J Ren Nutr 2015; 25: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johansen KL, Dalrymple LS, Glidden D, et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 2016; 11: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansen KL, Dalrymple LS, Delgado C, et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamijo Y, Kanda E, Ishibashi Y, et al. Sarcopenia and frailty in PD: impact on mortality, malnutrition, and inflammation. Perit Dial Int 2018; 38: 447–454. [DOI] [PubMed] [Google Scholar]
- 42. Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clin Transplant 2018; 32: e13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kutner NG, Zhang R, Allman RM, et al. Correlates of ADL difficulty in a large hemodialysis cohort. Hemodial Int 2014; 18: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kutner NG, Zhang R, Huang Y, et al. Falls among hemodialysis patients: potential opportunities for prevention? Clin Kidney J 2014; 7: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee SJ, Son H, Shin SK. Influence of frailty on health-related quality of life in pre-dialysis patients with chronic kidney disease in Korea: a cross-sectional study. Health Qual Life Outcomes 2015; 13: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee SY, Yang DH, Hwang E, et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr 2017; 27: 106–112. [DOI] [PubMed] [Google Scholar]
- 47. Lee SW, Lee A, Yu MY, et al. Is frailty a modifiable risk factor of future adverse outcomes in elderly patients with incident end-stage renal disease? J Korean Med Sci 2017; 32: 1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mansur HN, Colugnati FAB, Grincenkov FRdS, et al. Frailty and quality of life: a cross-sectional study of Brazilian patients with pre-dialysis chronic kidney disease. Health Qual Life Outcomes 2014; 12: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Margiotta E, Caldiroli L, Vettoretti S, et al. Gut microbiota composition and frailty in elderly patients with chronic kidney disease (abstract). Nephrol Dial Transplant 2018; 33: abstract SuO004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013; 14: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McAdams-DeMarco MA, Law A, Salter M, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61: 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant 2013; 13: 2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol 2015; 10: 2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 2015; 99: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McAdams-DeMarco MA, Law A, King E, et al. . Frailty and mortality in kidney transplant recipients. Am J Transplant 2015; 15: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McAdams-DeMarco MA, Ying H, Olorundare I, et al. Frailty and health-related quality of life in end stage renal disease patients of all ages. J Frailty Aging 2016; 5: 174–179. [PMC free article] [PubMed] [Google Scholar]
- 57. McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg 2017; 266: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and postkidney transplant health-related quality of life. Transplantation 2018; 102: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mansur HN, Damasceno VdO, Bastos MG. Prevalência da fragilidade entre os pacientes com doença renal crônica em tratamento conservador e em diálise. Brazilian J Nephrol 2012; 34: 153–160. [DOI] [PubMed] [Google Scholar]
- 60. Meulendijks FG, Hamaker ME, Boereboom FTJ, et al. Groningen frailty indicator in older patients with end-stage renal disease. Ren Fail 2015; 37: 1419–1424. [DOI] [PubMed] [Google Scholar]
- 61. Moffatt H, Moorhouse P, Mallery L, et al. Using the frailty assessment for care planning tool (FACT) to screen elderly chronic kidney disease patients for frailty: the nurse experience. Clin Interv Aging 2018; 13: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ng JKC, Kwan BCH, Chow KM, et al. Frailty in Chinese peritoneal dialysis patients: prevalence and prognostic significance. Kidney Blood Press Res 2016; 41: 736–745. [DOI] [PubMed] [Google Scholar]
- 63. Noori N, Sharma Parpia A, Lakhani R, et al. Frailty and the quality of life in hemodialysis patients: the importance of waist circumference. J Ren Nutr 2018; 28: 101–109. [DOI] [PubMed] [Google Scholar]
- 64. Orlandi FdS, Gesualdo GD. Assessment of the frailty level of elderly people with chronic kidney disease undergoing hemodialysis. Acta Paulista de Enfermagem 2014; 27: 29–34. [Google Scholar]
- 65. Pérez Fernández M, Martínez Miguel P, Ying H, et al. Comorbidity, frailty, and waitlist mortality among kidney transplant candidates of all ages. Am J Nephrol 2019; 49: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Romeu M, Couchoud C, Delarozière JC, et al. Survival of patients with ANCA-associated vasculitis on chronic dialysis: data from the French REIN registry from 2002 to 2011. QJM 2014; 107: 545–555. [DOI] [PubMed] [Google Scholar]
- 67. Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 2012; 60: 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sales C, Tavares R, Amado L, et al. Anxiety and depression in end stage renal disease patients and its association with clinical and laboratory data. Nephrol Dial Transplant 2017; 32: abstract SP651. [Google Scholar]
- 69. Schopmeyer L, El Moumni M, Nieuwenhuijs-Moeke GJ, et al. Frailty has a significant influence on postoperative complications after kidney transplantation—a prospective study on short-term outcomes. Transpl Int 2019; 32: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004; 43: 861–867. [DOI] [PubMed] [Google Scholar]
- 71. Szeto CC, Chan GCK, Ng JKC, et al. Depression and physical frailty have additive effect on the nutritional status and clinical outcome of Chinese peritoneal dialysis. Kidney Blood Press Res 2018; 43: 914–923. [DOI] [PubMed] [Google Scholar]
- 72. Wilhelm-Leen ER, Hall YN, Tamura MK, et al. Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. Am J Med 2009; 122: 664–671.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yadla M, John J, Mummadi M. A study of clinical assessment of frailty in patients on maintenance hemodialysis supported by cashless government scheme. Saudi J Kidney Dis Transpl 2017; 28: 15–22. [DOI] [PubMed] [Google Scholar]
- 74. Yoneki K, Kitagawa J, Hoshi K, et al. Association between frailty and bone loss in patients undergoing maintenance hemodialysis. J Bone Miner Metab 2019; 37: 81–89. [DOI] [PubMed] [Google Scholar]
- 75. Kim S, McClave SA, Martindale RG, et al. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg 2017; 83: 1220–1227. [DOI] [PubMed] [Google Scholar]
- 76. Marzetti E, Picca A, Marini F, et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty “cytokinome” at its core. Exp Gerontol 2019; 122: 129–138. [DOI] [PubMed] [Google Scholar]
- 77. Yannakoulia M, Ntanasi E, Anastasiou CA, et al. Frailty and nutrition: from epidemiological and clinical evidence to potential mechanisms. Metabolism 2017; 68: 64–76. [DOI] [PubMed] [Google Scholar]
- 78. Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev 2017; 36: 78–87. [DOI] [PubMed] [Google Scholar]
- 79. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc 2018; 19: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 80. Chao CT, Wu VC, Tsai HB, et al. Impact of body mass on outcomes of geriatric postoperative acute kidney injury patients. Shock 2014; 41: 400–405. [DOI] [PubMed] [Google Scholar]
- 81. Zhang S, Tomata Y, Tanji F, et al. The relationship between body mass index and disability-free survival in elderly Japanese: the Ohsaki Cohort 2006 study. Int J Obes (Lond) Epub ahead of print 2 April 2019. DOI: 10.1038/s41366-019-0359-3. [DOI] [PubMed] [Google Scholar]
- 82. Van Hateren KJJ, Hendriks SH, Groenier KH, et al. Frailty and the relationship between blood pressure and mortality in elderly patients with type 2 diabetes (Zwolle Outpatient Diabetes project Integrating Available Care-34). J Hypertens 2015; 33: 1162–1166. [DOI] [PubMed] [Google Scholar]
- 83. Sheshadri A, Johansen KL. Prehabilitation for the frail patient approaching ESRD. Semin Nephrol 2017; 37: 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table_3 for Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review by Patrick Yihong Wu, Chia-Ter Chao, Ding-Cheng Chan, Jenq-Wen Huang and Kuan-Yu Hung in Therapeutic Advances in Chronic Disease


