Abstract
The best known form of oxygen therapy is hyperbaric oxygen (HBO) therapy, which increases both concentration and atmospheric pressure. HBO supports tissue regeneration and is indicated in an increasing number of pathologies. Less known but still showing some promising effects is normobaric oxygen (NBO) therapy, which provides some advantages over HBO including eliminating barotrauma risk, increased ease of administration and a significant cost reduction. However, still little is known about differences and similarities in treatment effects between HBO and NBO. Therefore we tested whether NBO induces a biological response comparable to HBO with a focus on stem progenitor cell mobilization and changes in serum cytokine concentration. We randomly assigned Sprague-Dawley rats into an NBO treatment group (n = 6), and a room air control group (n = 6). The NBO treatment group was exposed to 42% oxygen for 2 hours a day for 10 days. The room air group was concurrently kept at 20.9% oxygen. The frequency and number of stem progenitor cells in peripheral blood were analyzed by flow cytometry. Plasma cytokine expression was analyzed by cytokine array enzyme linked immunosorbent assay. All analyses were performed 24 hours after the final exposure to control for transient post treatment effects. The NBO treatment group showed an increase in circulating CD133+/CD45+ stem progenitor cell frequency and number compared to the room air control group. This rise was largely caused by CD34– stem progenitor cells (CD133+/CD34–/CD45+) without changes in the CD34+ population. The plasma cytokine levels tested were mostly unchanged with the exception of tumor necrosis factor-α which showed a decrease 24 hours after the last NBO exposure. These findings support our hypothesis that NBO induces a biological response similar to HBO, affecting serum stem progenitor cell populations and tumor necrosis factor-α concentration. The study was approved by Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin, Madison, WI, USA (approval No. M005439) on June 28, 2016.
Keywords: hyperbaric oxygen, intermittent hyperoxia, inflammation, stem cell, CD133, TNF, leptin, MIP-1α
INTRODUCTION
Hyperbaric oxygen (HBO) therapy is U.S. Food and Drug Administration (FDA) approved for pathologies including select wounds, thermal burns, and delayed radiation injury with emerging research suggesting promise in other pathologies.1,2,3 HBO promotes neovascularization, modulates the inflammatory system, and promotes stem cell recruitment resulting in accelerated tissue regeneration.1,3,4,5 Treatments involve repetitive exposures (i.e., intermittent hyperoxia, IH), of up to 60 sessions, of high concentrations of oxygen, together with increased atmospheric pressure of up to 3.0 atmospheres absolute (ATA; 1 ATA = 760 mmHg/101.325 kPa). HBO has a direct effect on activated endothelial cells in vitro including downregulating genes involved in adhesion, angiogenesis, inflammation and oxidative stress but upregulating angiogenin, which promotes both angiogenesis and nitric oxide production.6 Moreover, exposure of endothelial cells to HBO enhances capillary tube formation and oxidative stress resistance.7 However, the extent to which high pressure in HBO treatments is critical for therapeutic effect is not entirely known, and at least one in vitro experiment showed that HBO at 1.5 ATA induces a stronger anti-inflammatory response when compared to 2.4 ATA.8
The effectiveness of HBO therapy may depend on some key factors including treatment dose selection (oxygen tension and duration) and patient pathology.9,10 The mechanism of HBO treatment includes the production of reactive oxygen species, which results in a large number of responses including growth factor production, stem progenitor cell (S/PC) mobilization, and reduced inflammation.11,12,13 The increased atmospheric pressure reduces gas volume, which is instrumental in ameliorating pathological conditions such as arterial gas embolism and decompression sickness. However, these are not the majority of patients presenting in a clinical HBO setting. In addition, HBO treatment requires an expensive hyperbaric chamber, trained staff, and additional safety considerations. IH can also be delivered by exclusively increasing the oxygen concentration without changing the atmospheric pressure, referred to as normobaric oxygen (NBO). At sea level the partial pressure of oxygen is about 150 mmHg (1 mmHg = 0.1333 kPa) depending on the humidity. The resulting arterial partial pressure (PaO2) is 75–100 mmHg. At 100% O2 the transcutaneous oxygen pressure raises from 150 mmHg up to 470 mmHg.14 Moreover, this single dose of oxygen increases the expression of erythropoietin in the serum of patients,14,15 increases reactive oxygen species formation followed by an increased production of glutathione.15,16 However, it is unknown if NBO promotes S/PC mobilization or modulates cytokine production similar to HBO therapy.4,17,18,19,20,21 Therefore, the goal of this study was to examine the efficacy of NBO focusing on mobilization of S/PCs and the expression of serum cytokines. We hypothesized that NBO would show biological effects similar to those previously reported for HBO.8,18
MATERIALS AND METHODS
Animals
Twelve 10-week-old male Sprague-Dawley rats were randomly divided into two groups, a control group (n = 6) and a NBO treatment group (n = 6). Chow and water were provided ad libitum. Housing was temperature controlled at 23°C with a 12-hour/12-hour light-dark cycle. All experiments and procedures were approved by Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin, Madison, WI, USA (approval No. M005439) on June 28, 2016.
IH exposure
Animals were exposed to NBO or room air (normoxia) in clear polypropylene exposure chambers (Coy Laboratories, Grass Lake, MI, USA). The treatment group was exposed for 2 hours daily for a total of 10 hours over 5 days to an inhaled partial pressure of oxygen (PIO2) = 300 mmHg (42% O2 at 1 ATM). The oxygen concentration was monitored and maintained continuously during the NBO exposure. Control animals were concurrently exposed in identical chambers with the doors opened to room air.
Sample collection
Samples were collected 24 hours after the final 2-hour hyperoxia exposure. Animals were anesthetized with isoflurane and 8–10 mL of blood was drawn from the inferior vena cava using a 21-gauge needle and a heparinized 10 mL syringe. Blood was transferred to tubes and centrifuged at 2000 r/min for 10 minutes. Plasma was collected, flash frozen in liquid Nitrogen, and then stored at –80°C until further analysis. Red blood cells were lysed using ammonium chloride. The remaining cells were washed twice with phosphate buffer saline and the cell number was determined using a Beckman Coulter Z1 Particle Counter (Beckman Coulter Life Sciences, Indianapolis IN, USA). 1 × 106 cells were used for each control and sample staining for flow cytometry.
Flow cytometry
1 × 106 cells in 100 µL flow buffer (phosphate buffer saline 1% albumin) were used for each staining. 5 to 10 µL antibody was added to each tube, mixed, and incubated for 30 minutes in the dark at 4°C. Then cells were washed twice and then fixed with 4% formaldehyde for 30 minutes, washed with flow buffer and stored at 4°C until analyzed the following day. The following antibodies were used: anti-CD34-PE (Abcam, Cambridge, MA, USA), anti-CD45-APC (eBioscience, Grand Island, NY, USA), and anti-CD133-DyLight 488 (Novus, Littleton, CO, USA). For live and dead cell discrimination, Ghost-Dye-V450 was used (Tonbo Bioscience, San Diego, CA, USA).22
Flow cytometry was performed on a BD LSRII (BD Biosciences, San Jose, CA, USA) using DIVA software (BD Biosciences). Samples were analyzed using FlowJo software (FlowJo, Ashland, OR, USA). Lymphocytes were gated by forward and side scatter (Figure 1A), doublets were excluded (Figure 1B), and live cells were selected for further analysis (Figure 1C). CD45 positive cells were selected (Figure 1D) for further analysis of the expression of CD34 and CD133 (Figure 1E and F).
Figure 1.
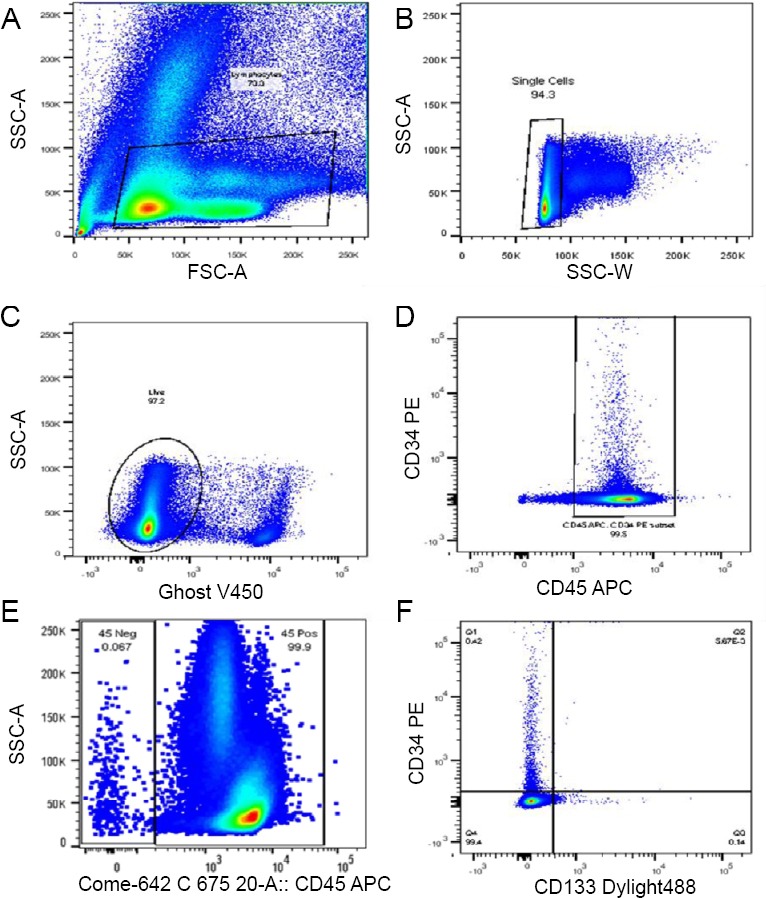
Flow cytometry gating strategy.
Note: (A–F) Lymphocytes were gated by forward and side scatter (A), doublets were excluded (B), and live cells were selected for further analysis (C). CD45 positive cells were selected (D) for further analysis of the expression of CD34 and CD133 (E, F).
Enzyme linked immunosorbent assay
We determined the expression of selected cytokines using the Signosis Rat Cytokine enzyme linked immunosorbent assay Plate Array I (Signosis Inc., Santa Clara, CA, USA). Samples were thawed and immediately prepared for enzyme linked immunosorbent assay per manufacturer’s instructions. Cytokine concentration was determined according to manufacturer’s instructions using a microplate reader at 450 nm within 30 minutes.23
The cytokines measured in this experiment include tumor necrosis factor (TNF)-α, vascular endothelial growth factor, fibroblast growth factor-β, interferon-γ, leptin, monocyte chemotactic protein-1, stem cell factor, macrophage inflammatory protein (MIP)-1α, interleukins-1α, -1β, -5, -6, -15, -10, Rantes, and transforming growth factor-β.
Statistical analysis
All statistics were calculated using Graph Pad Prism version 6.07 (GraphPad Software, San Diego, CA, USA). All comparisons between the control group and the NBO group were performed using the non-parametric Mann–Whitney U test with a P of < 0.05 to indicate a difference between the groups.
RESULTS
Cytokine expression in IH exposed rats
As previously described six rats were exposed to normoxia (150 mmHg PIO2) and six rats to NBO at 300 mmHg PIO2. Data from all study animals were used to determine the effect of NBO on cytokine expression. Our results revealed a significant decrease in TNF-α expression (Figure 2A). We noted possible trends in two other cytokines, a decrease in MIP-1α (P = 0.07; Figure 2B) and a decrease in Leptin (P = 0.09; Figure 2C). Complete results from all cytokines tested are included in Figure 2D.
Figure 2.
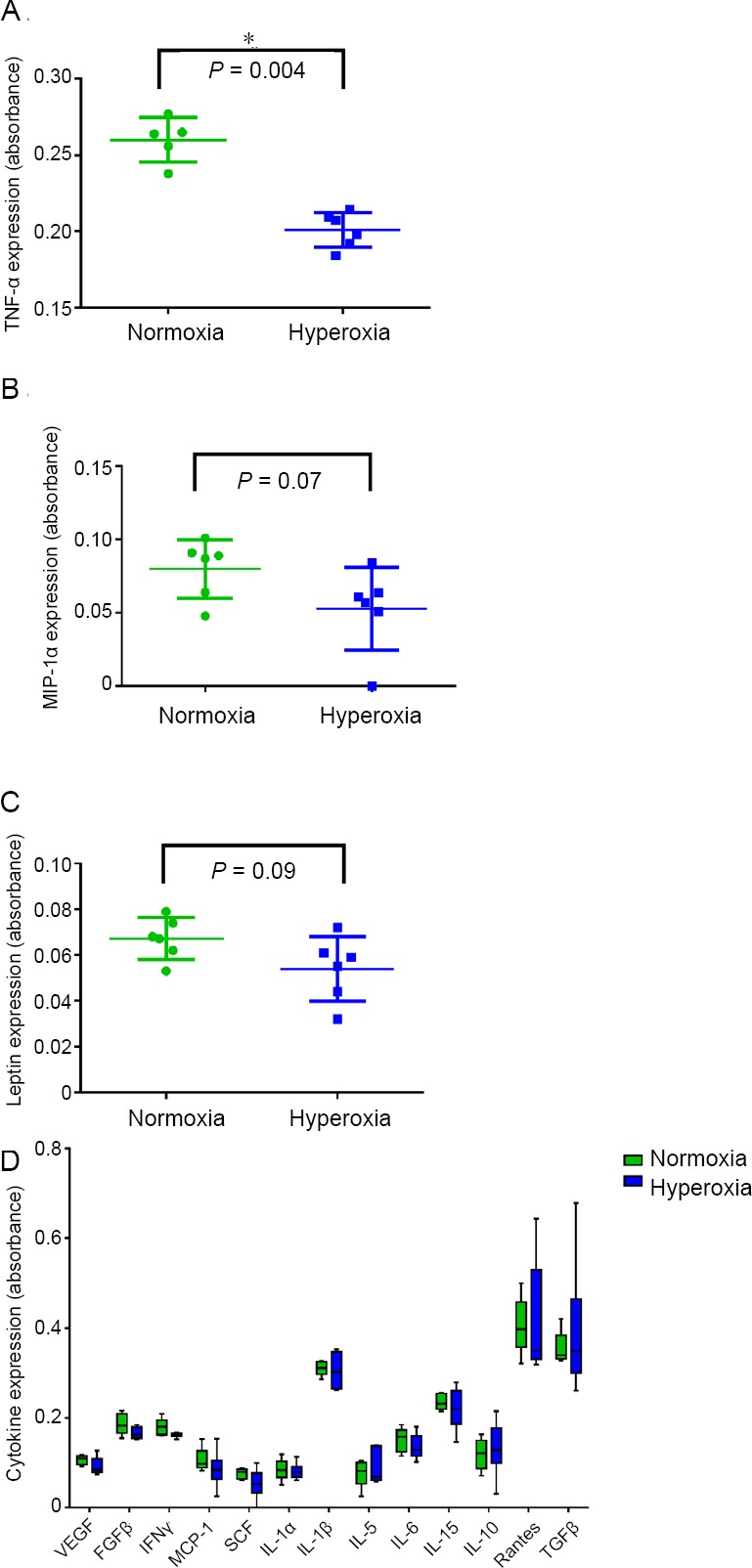
Cytokine expression in intermittent hyperoxia exposed rats.
Note: (A) Tumor necrosis factor (TNF)-α; (B) macrophage inflammatory protein (MIP)-1α; (C) leptin; (D) Other cytokines. Data are expressed as the mean ± SD. *P < 0.05 (non-parametric Mann Whitney test). VEGF: Vascular endothelial growth factor; FGF: fibroblast growth factor; IFN: interferon; MCP-1: monocyte chemotactic protein-1; SCF: stem cell factor; IL: interleukin; TGF: transforming growth factor.
Increased frequency of CD133+ S/PCs after IH exposure
The frequency of CD133+/CD45+ and CD133+/CD45+/CD34- S/PCs in venous blood was significantly increased in NBO animals compared to controls (P = 0.046 and P = 0.009 respectively; Figure 3) whereas there was no difference in the frequency of CD34+/CD45+ or CD34+/CDCD133+/CD45+ S/PCs between the two groups.
Figure 3.
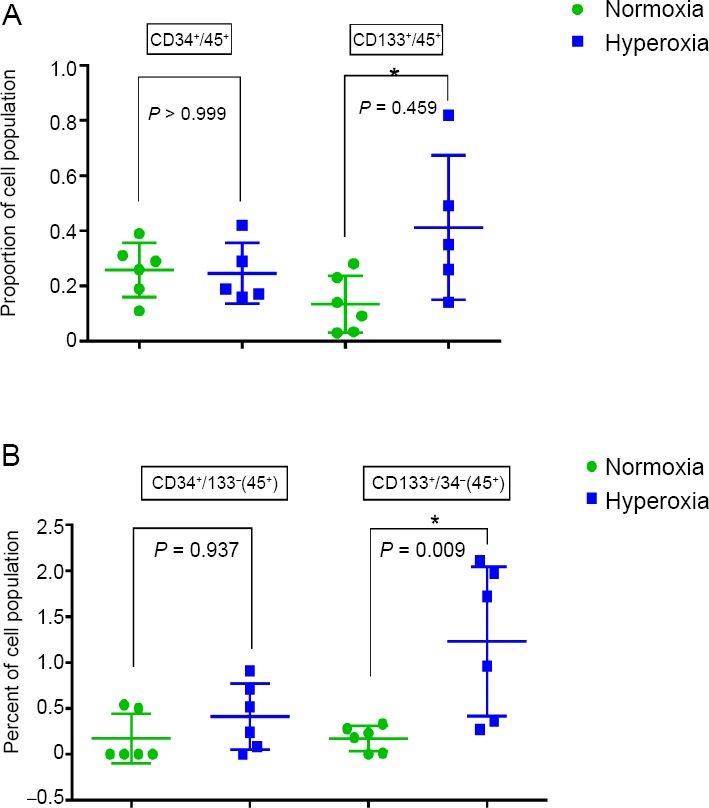
Frequency of CD133+ stem progenitor cells after intermittent hyperoxia exposure detected by flow cytometry.
Note: (A) CD34+/CD45+ and CD133+/CD45+ stem progenitor cells; (B) CD34+/ CD133-/CD45+ and CD133+/CD34+/CD45+ stem progenitor cells. Data are expressed as the mean ± SD. *P < 0.05 (non-parametric Mann–Whitney U test).
DISCUSSION
The present study assessed whether repeated NBO exposures induce biological responses that have been previously observed with HBO therapy, including S/PC mobilization and inhibition of TNF-α expression. The main finding of the study is that in adult rats, daily 2-hour exposures to NBO (for a total of 10 hours) mobilizes S/PCs and reduces serum TNF-α concentration supporting our hypothesis.
The field of therapeutic IH is dominated by the use of HBO, and HBO therapy is approved by the FDA to treat 15 indications.24 Additionally, although not FDA approved for these indications, studies appear to suggest that HBO therapy may ameliorate other conditions including myocardial infarction,25 hip fractures,26,27,28,29,30 stroke,3,31,32,33,34,35,36,37 peri-surgical healing,38,39,40,41,42 and traumatic brain injury.3,35,43,44,45,46
It is a long standing practice to use up to 1.4 ATA of hyperbaric air resulting in the alveoli PaO2 of 209 mmHg as a sham in HBO research.47,48,49,50,51,52 However, the rational for the use of hyperbaric air as a sham remains only partially elucidated because of lack of data defining an exact “dose” (determined by tension and duration).53 In this experiment we used a PaO2 of 300 mmHg which resulted in biologic activity, suggesting that the PaO2 used as a sham in HBO research could in fact induce a treatment effect. Further, the results suggest that increased pressure may not be required to elicit an effect. NBO therapy increases the transcutaneous oxygen concentration from 150 to 470 mmHg during a single treatment resulting in a biological response with increased erythropoietin levels.14,15 This is in accordance with our study showing biologic activity resulting in changes in S/PC mobilization and inflammatory cytokine levels.
Similar to the uncertainty of the O2 tension required to achieve a biological and regenerative response in HBO, the tension of O2 required in NBO is also not clear. The intermittent exposure to HBO PIO2 of 1473 mmHg and higher mobilizes CD34+ S/PCs in humans. Our findings are similar demonstrating that NBO with a PIO2 of 300 mmHg induces the mobilization of S/PCs (CD133+) and decreases expression of TNF-α in rats.4,18,54,55 Another study investigating two oxygen doses, PIO2 of 1473 mmHg and PIO2 of 1777 mmHg, found a direct correlation between oxygen tensions and S/PC mobilization, suggesting a dose relationship, but little is known about the effect of IH using a PIO2 ≤ 1473 mmHg.4 We used 10 treatment sessions of two hours each and achieved a visible response with 42% oxygen. This is in agreement with the observation that exposure above 40% oxygen induced an increase in derivatives of oxygen metabolites in the blood of exposed rats.56 However, more studies are required to optimize the treatment schedule for specific injury or disease models. The choice of treatment dose in this research project is based on previous research in mice using 100% oxygen at 2.8 ATA for one 90-minute treatment and another cohort for two 90-minute treatments.18 They found significant increases in S/PC mobilization in both groups and a larger increase in the two-treatment group over the single-treatment group, suggesting a dose effect. Given the likelihood of a dose effect in the current experiment provided by repeated exposure to NBO, we utilized 42% oxygen in the treatment condition because it was double the FIO2 compared to room air with a relatively small increase in oxygen tension. This is comparable to the small increase in oxygen utilized as a sham in previous studies investigating mild traumatic brain injury and post-traumatic stress disorder.47,48,49,50
Whereas the best tension and duration of oxygen treatment for various pathologies needs to be established, a common finding is the mobilization of S/PCs. We focused on CD133+ S/PC’s based on a study by Nakanishi et al.57 showing that CD133+ S/PC supported the neovascularization of skin graft. In addition, CD133+ S/PCs differentiated into endothelial and myogenic lineages in a rat model of muscle injury58 and circulating CD133+ cells enhance angiogenesis, astrogliosis, axon growth and functional recovery in a mouse spinal cord injury model.59 Other tissue specific stem cells may be important for the effect of HBO and NBO treatment, a topic that needs more research.
One of the major effects of HBO treatment is the control of inflammation. This is in part achieved by the increase in reactive oxygen species production associated with oxygen therapy which play central roles in coordinating cell signaling and anti-oxidant protective pathways.12 Somewhat unexpected is the observed downregulation of TNF-α with NBO treatment in our study. This downregulation is unlikely to be explained by a reduction in existing inflammation given that the animals were healthy and no injury model was tested. One possibility is that NBO induced an inflammatory response which was downregulated within 24 hours after treatment to a level that is below basal level.8
Our study demonstrated that IH using NBO at much lower PIO2 pressure than previously tested shows a biological response with S/PC mobilization and changes in cytokine expression similar to HBO. Future research examining oxygen/dose relationship is needed to further elucidate the biological effect of various doses of IH, and ascertain differences between concentration and pressure, along with establishing basal active levels of IH. In addition, future studies will be needed to test for efficacy in an injury model. The significance of this study is twofold. First, relatively small increases of IH yield a measurable change in S/PC mobilization and pro-inflammatory cytokine expression in an animal model. Second, the use of relatively small doses in IH as a sham in oxygen therapy research should be further investigated to determine if it is a sham or a small dose treatment.
Acknowledgements
The authors thank the University of Wisconsin Carbone Cancer Center Flow Cytometry lab staff for their help acquiring and analyzing data. They also thank Dr. Kara Goss (Division of Allergy, Pulmonary and Critical Care, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA), Dr. Vivek Balasubrumanium (Department of Pediatrics, University of Wisconsin Madison, Madison, WI, USA), Dr. Drew Watson (Department of Orthopedics and Rehabilitation, University of Wisconsin-Madison, Madison, WI, USA), Dr. Aleksey Sobakin (Department of Pediatrics, University of Wisconsin, Madison, WI, USA), Dr. Donata Oertel (Department of Neuroscience, School of Medicine and Public Health, Madison, WI, USA) and Dr. Barb Bendlin (Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA) for scientific insight and guidance and Dr. Barb Bendlin and J. E. MacLaughlin (O2 Clinics Hyperbaric Oxygen Outpatient Clinic, Madison, WI, USA) for manuscript review.
Footnotes
Conflicts of interest
Some of these data were presented as a poster and a 10-minute talk at the 2018 Undersea Hyperbaric Medical Society’s Annual Science Meeting and the International Hyperbaric Medical Associations 2018 Meeting.
Financial support
This work was supported by funding from the DOD, N00024-17-C-4318 (Eldridge), NIH-NHLBI R01 HL115061 (to MWE).
Institutional review board statement
The study was approved by Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin, Madison, WI, USA (approval No. M005439) on June 28, 2016.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Funding: This work was supported by funding from the DOD, N00024-17-C-4318 (Eldridge), NIH-NHLBI R01 HL115061 (to MWE).
REFERENCES
- 1.Lam G, Fontaine R, Ross FL, Chiu ES. Hyperbaric oxygen therapy: exploring the clinical evidence. Adv Skin Wound Care. 2017;30:181–190. doi: 10.1097/01.ASW.0000513089.75457.22. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D, Luo S, Xu W, Hu J, Lin S, Wang N. Efficacy and safety of hyperbaric oxygen therapy used in patients with diabetic foot: a meta-analysis of randomized clinical trials. Clin Ther. 2017;39:2088–2094. doi: 10.1016/j.clinthera.2017.08.014. e2. [DOI] [PubMed] [Google Scholar]
- 3.Tal S, Hadanny A, Sasson E, Suzin G, Efrati S. Hyperbaric oxygen therapy can induce angiogenesis and regeneration of nerve fibers in traumatic brain injury patients. Front Hum Neurosci. 2017;11:508. doi: 10.3389/fnhum.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyboer M, 3rd, Milovanova TN, Wojcik S, et al. CD34+/CD45-dim stem cell mobilization by hyperbaric oxygen - changes with oxygen dosage. Stem Cell Res. 2014;12:638–645. doi: 10.1016/j.scr.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Zhao D, Diao M, et al. Hyperbaric oxygen treatments attenuate the neutrophil-to-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2015;153:606–612. doi: 10.1177/0194599815589072. [DOI] [PubMed] [Google Scholar]
- 6.Kendall AC, Whatmore JL, Harries LW, Winyard PG, Smerdon GR, Eggleton P. Changes in inflammatory gene expression induced by hyperbaric oxygen treatment in human endothelial cells under chronic wound conditions. Exp Cell Res. 2012;318:207–216. doi: 10.1016/j.yexcr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Thom SR, Milovanova TN, Yang M, et al. Vasculogenic stem cell mobilization and wound recruitment in diabetic patients: increased cell number and intracellular regulatory protein content associated with hyperbaric oxygen therapy. Wound Repair Regen. 2011;19:149–161. doi: 10.1111/j.1524-475X.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall AC, Whatmore JL, Harries LW, Winyard PG, Eggleton P, Smerdon GR. Different oxygen treatment pressures alter inflammatory gene expression in human endothelial cells. Undersea Hyperb Med. 2013;40:115–123. [PubMed] [Google Scholar]
- 9.Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36:1961–1966. doi: 10.2337/dc12-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ennis WJ, Huang ET, Gordon H. Impact of hyperbaric oxygen on more advanced wagner grades 3 and 4 diabetic foot ulcers: matching therapy to specific wound conditions. Adv Wound Care (New Rochelle) 2018;7:397–407. doi: 10.1089/wound.2018.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:247–252. [PubMed] [Google Scholar]
- 12.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danesh-Sani SA, Shariati-Sarabi Z, Feiz MR. Comprehensive review of hyperbaric oxygen therapy. J Craniofac Surg. 2012;23:e483–491. doi: 10.1097/SCS.0b013e3182668777. [DOI] [PubMed] [Google Scholar]
- 14.Balestra C, Germonpre P, Poortmans JR, Marroni A. Serum erythropoietin levels in healthy humans after a short period of normobaric and hyperbaric oxygen breathing: the “normobaric oxygen paradox”. J Appl Physiol (1985) 2006;100:512–518. doi: 10.1152/japplphysiol.00964.2005. [DOI] [PubMed] [Google Scholar]
- 15.Donati A, Damiani E, Zuccari S, et al. Effects of short-term hyperoxia on erythropoietin levels and microcirculation in critically Ill patients: a prospective observational pilot study. BMC Anesthesiol. 2017;17:49. doi: 10.1186/s12871-017-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deuchar GA, van Kralingen JC, Work LM, et al. Preclinical validation of the therapeutic potential of glasgow oxygen level dependent (GOLD) technology: a theranostic for acute stroke. Transl Stroke Res. 2018 doi: 10.1007/s12975-018-0679-y. doi: 10.1007/s12975-018-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thom SR, Hampton M, Troiano MA, et al. Measurements of CD34+/CD45-dim stem cells predict healing of diabetic neuropathic Wwounds. Diabetes. 2016;65:486–497. doi: 10.2337/db15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 19.Fosen KM, Thom SR. Hyperbaric oxygen, vasculogenic stem cells, and wound healing. Antioxid Redox Signal. 2014;21:1634–1647. doi: 10.1089/ars.2014.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu M, Xue Y, Liang W, Zhang Y, Zhang Z. Protection mechanism of early hyperbaric oxygen therapy in rats with permanent cerebral ischemia. J Phys Ther Sci. 2015;27:3271–3274. doi: 10.1589/jpts.27.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu QH, Zhang PX, Liu Y, Liu W, Yin N. Hyperbaric oxygen preconditioning protects the lung against acute pancreatitis induced injury via attenuating inflammation and oxidative stress in a nitric oxide dependent manner. Biochem Biophys Res Commun. 2016;478:93–100. doi: 10.1016/j.bbrc.2016.07.087. [DOI] [PubMed] [Google Scholar]
- 22.Wu N, Zhang X, Du S, Chen D, Che R. Upregulation of miR-335 ameliorates myocardial ischemia reperfusion injury via targeting hypoxia inducible factor 1-alpha subunit inhibitor. Am J Transl Res. 2018;10:4082–4094. [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Wu C, Wu W, et al. Study of serum CD147 level in patients with transient ischemic attack and CD147 expression in atherosclerotic plaque. J Cardiovasc Transl Res. 2018;11:285–291. doi: 10.1007/s12265-018-9817-x. [DOI] [PubMed] [Google Scholar]
- 24.UHMS; Indications for Hyperbaric Oxygen Therapy. https://wwwuhmsorg/resources/hbo-indicationshtml . [Google Scholar]
- 25.Dotsenko EA, Nikulina NV, Salivonchik DP, Lappo OG, Gritsuk AI, Bastron AS. Low doses of hyperbaric oxygenation effectively decrease the size of necrotic zone in rats with experimental myocardial infarction. Bull Exp Biol Med. 2015;158:732–734. doi: 10.1007/s10517-015-2849-1. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Wen L, Shang F, Wu J, Sui K, Ding Y. Endothelial progenitors enhanced the osteogenic capacities of mesenchymal stem cells in vitro and in a rat alveolar bone defect model. Arch Oral Biol. 2016;68:123–130. doi: 10.1016/j.archoralbio.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Muhonen A, Haaparanta M, Gronroos T, et al. Osteoblastic activity and neoangiogenesis in distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int J Oral Maxillofac Surg. 2004;33:173–178. doi: 10.1054/ijom.2003.0489. [DOI] [PubMed] [Google Scholar]
- 28.Sawai T, Niimi A, Takahashi H, Ueda M. Histologic study of the effect of hyperbaric oxygen therapy on autogenous free bone grafts. J Oral Maxillofac Surg. 1996;54:975–981. doi: 10.1016/s0278-2391(96)90396-1. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Cheng X, Ma W, Chen C. Effects of hyperbaric oxygen therapy on open tibial fractures in rabbits after transient seawater immersion. Undersea Hyperb Med. 2017;44:235–242. doi: 10.22462/5.6.2017.4. [DOI] [PubMed] [Google Scholar]
- 30.Al Hadi H, Smerdon GR, Fox SW. Hyperbaric oxygen therapy accelerates osteoblast differentiation and promotes bone formation. J Dent. 2015;43:382–388. doi: 10.1016/j.jdent.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Hadanny A, Golan H, Fishlev G, et al. Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage. Restor Neurol Neurosci. 2015;33:471–486. doi: 10.3233/RNN-150517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boussi-Gross R, Golan H, Volkov O, et al. Improvement of memory impairments in poststroke patients by hyperbaric oxygen therapy. Neuropsychology. 2015;29:610–621. doi: 10.1037/neu0000149. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JH, Lo T, Mychaskiw G, Colohan A. Mechanisms of hyperbaric oxygen and neuroprotection in stroke. Pathophysiology. 2005;12:63–77. doi: 10.1016/j.pathophys.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Stocchetti N, Taccone FS, Citerio G, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care. 2015;19:186. doi: 10.1186/s13054-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efrati S, Ben-Jacob E. Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Rev Neurother. 2014;14:233–236. doi: 10.1586/14737175.2014.884928. [DOI] [PubMed] [Google Scholar]
- 36.Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43:21–28. [PubMed] [Google Scholar]
- 37.Efrati S, Fishlev G, Bechor Y, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients--randomized, prospective trial. PLoS One. 2013;8:e53716. doi: 10.1371/journal.pone.0053716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niyibizi E, Kembi GE, Lae C, Pignel R, Sologashvili T. Delayed hyperbaric oxygen therapy for air emboli after open heart surgery: case report and review of a success story. J Cardiothorac Surg. 2016;11:167. doi: 10.1186/s13019-016-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Dong H, Chen M, et al. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Inanmaz ME, Kose KC, Isik C, Atmaca H, Basar H. Can hyperbaric oxygen be used to prevent deep infections in neuro-muscular scoliosis surgery? BMC Surg. 2014;14:85. doi: 10.1186/1471-2482-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dequanter D, Jacobs D, Shahla M, Paulus P, Aubert C, Lothaire P. The effect of hyperbaric oxygen therapy on treatment of wound complications after oral, pharyngeal and laryngeal salvage surgery. Undersea Hyperb Med. 2013;40:381–385. [PubMed] [Google Scholar]
- 42.Allen MW, Golembe E, Gorenstein S, Butler GJ. Protective effects of hyperbaric oxygen therapy (HBO2) in cardiac care--A proposal to conduct a study into the effects of hyperbaric pre-conditioning in elective coronary artery bypass graft surgery (CABG) Undersea Hyperb Med. 2015;42:107–114. [PubMed] [Google Scholar]
- 43.Boussi-Gross R, Golan H, Fishlev G, et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury - randomized prospective trial. PLoS One. 2013;8:e79995. doi: 10.1371/journal.pone.0079995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harch PG, Andrews SR, Fogarty EF, et al. A phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder. J Neurotrauma. 2012;29:168–185. doi: 10.1089/neu.2011.1895. [DOI] [PubMed] [Google Scholar]
- 45.Hu SL, Hu R, Li F, et al. Hyperbaric oxygen preconditioning protects against traumatic brain injury at high altitude. Acta Neurochir Suppl. 2008;105:191–196. doi: 10.1007/978-3-211-09469-3_37. [DOI] [PubMed] [Google Scholar]
- 46.Zhou BC, Liu LJ, Liu B. Neuroprotection of hyperbaric oxygen therapy in sub-acute traumatic brain injury: not by immediately improving cerebral oxygen saturation and oxygen partial pressure. Neural Regen Res. 2016;11:1445–1449. doi: 10.4103/1673-5374.191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf G, Cifu D, Baugh L, Carne W, Profenna L. The effect of hyperbaric oxygen on symptoms after mild traumatic brain injury. J Neurotrauma. 2012;29:2606–2612. doi: 10.1089/neu.2012.2549. [DOI] [PubMed] [Google Scholar]
- 48.Walker WC, Franke LM, Cifu DX, Hart BB. Randomized, sham-controlled, feasibility trial of hyperbaric oxygen for service members with postconcussion syndrome: cognitive and psychomotor outcomes 1 week postintervention. Neurorehabil Neural Repair. 2014;28:420–432. doi: 10.1177/1545968313516869. [DOI] [PubMed] [Google Scholar]
- 49.Cifu DX, Walker WC, West SL, et al. Hyperbaric oxygen for blast-related postconcussion syndrome: three-month outcomes. Ann Neurol. 2014;75:277–286. doi: 10.1002/ana.24067. [DOI] [PubMed] [Google Scholar]
- 50.Cifu DX, Hart BB, West SL, Walker W, Carne W. The effect of hyperbaric oxygen on persistent postconcussion symptoms. J Head Trauma Rehabil. 2014;29:11–20. doi: 10.1097/HTR.0b013e3182a6aaf0. [DOI] [PubMed] [Google Scholar]
- 51.Miller RS, Weaver LK, Brenner LA. Hyperbaric oxygen treatment for persistent postconcussion symptoms--reply. JAMA Intern Med. 2015;175:1240–1241. doi: 10.1001/jamainternmed.2015.1048. [DOI] [PubMed] [Google Scholar]
- 52.Hoge CW, Jonas WB. Hyperbaric oxygen treatment for persistent postconcussion symptoms--reply. JAMA Intern Med. 2015;175:1241. doi: 10.1001/jamainternmed.2015.1051. [DOI] [PubMed] [Google Scholar]
- 53.Fife CE, Hopf H. Discussion. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):142S–143S. doi: 10.1097/PRS.0b013e3181fb5443. [DOI] [PubMed] [Google Scholar]
- 54.Milovanova TN, Bhopale VM, Sorokina EM, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol Cell Biol. 2008;28:6248–6261. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milovanova TN, Bhopale VM, Sorokina EM, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol (1985) 2009;106:711–728. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagatomo F, Fujino H, Kondo H, Ishihara A. Oxygen concentration-dependent oxidative stress levels in rats. Oxid Med Cell Longev. 2012;2012:381763. doi: 10.1155/2012/381763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanishi M, Ishikawa M, Sunagawa T, Yokota K, Asahara T, Ochi M. The effects of CD133-positive cells to a nonvascularized fasciocutaneous free graft in the rat model. Ann Plast Surg. 2009;63:331–335. doi: 10.1097/SAP.0b013e3181934951. [DOI] [PubMed] [Google Scholar]
- 58.Shi M, Ishikawa M, Kamei N, et al. Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived CD133-positive cells. Stem Cells. 2009;27:949–960. doi: 10.1002/stem.4. [DOI] [PubMed] [Google Scholar]
- 59.Kamei N, Kwon SM, Alev C, et al. Ex-vivo expanded human blood-derived CD133+ cells promote repair of injured spinal cord. J Neurol Sci. 2013;328:41–50. doi: 10.1016/j.jns.2013.02.013. [DOI] [PubMed] [Google Scholar]


