Abstract
Background
Previously published three-month placebo-controlled and one-year open-label clinical trial data have provided information on the efficacy and safety of erenumab.
Methods
Interim analysis was undertaken from an ongoing five-year open-label treatment phase after all patients completed three years in the open-label treatment phase or discontinued the study. Adult patients with episodic migraine enrolled in the open-label treatment phase initially received 70 mg erenumab monthly. A protocol amendment increased the dosage to 140 mg monthly to assess long-term safety of the higher dose. Safety and tolerability were assessed by monitoring adverse events, electrocardiograms, laboratory assessments, and vital signs.
Results
Of 383 patients enrolled in the open-label treatment phase, at data cutoff 235 (61.3%) remained in the study, all received 140 mg for ≥1 year. Median (Q1, Q3) exposure (70 or 140 mg) for all patients enrolled was 3.2 (1.3, 3.4) years. The most frequent adverse events (≥4.0/100 patient-years) were reported as viral upper respiratory tract infection, sinusitis, influenza, and back pain. Exposure-adjusted serious adverse event rates were 4.2/100 patient-years. There was no increase in cardiovascular events over time.
Conclusions
In this long-term study of a CGRP-receptor antibody, erenumab was found to be safe and well-tolerated with a spectrum and rate of adverse events consistent with shorter-term placebo-controlled studies.
Trial registration
ClinicalTrials.gov NCT01952574
Keywords: Erenumab, safety, migraine
Introduction
Migraine is a long-lasting disorder whose attack frequency fluctuates in the individual patient (1,2). Some patients need to be on preventive therapy for many years, requiring that treatments have favorable long-term risk benefit profiles (1,2). Multiple studies have provided clinical evidence on the efficacy and safety of investigational monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) receptor (3–7) or the ligand itself (8–10), but to date there have been no studies assessing long-term tolerability and safety of these treatments beyond one year.
Erenumab (in the US, erenumab-aooe) is a fully human anti-CGRP receptor monoclonal antibody approved in the US and EU for migraine prevention (11). Across four registrational studies, the safety of erenumab has been evaluated in 2537 patients with migraine, representing 2310 patient-years of exposure (11). One study has an ongoing five-year open-label extension (6). Among these studies, the most common adverse events (incidence ≥ 3% for either 70 mg or 140 mg and more often than placebo) were injection site reactions and constipation (11). Here, we present extended interim safety data after over 3 years of a 5-year open-label erenumab treatment of patients with episodic migraine who completed a 12-week double blind treatment phase. Efficacy and safety results from the double-blind treatment phase (6) and a preplanned 1-year open-label interim analysis (12) have been previously published. Data from this interim safety analysis have been presented in abstract form (13).
Methods
Study design
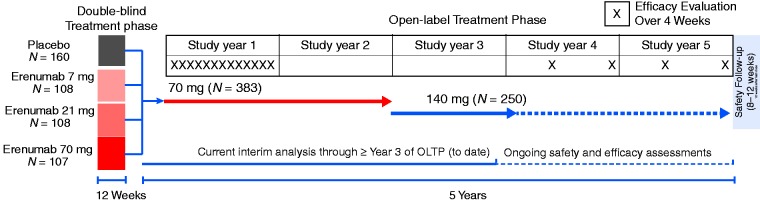
The 12-week double-blind treatment phase (DBTP) for this study (6) included patients with episodic migraine (EM) who received placebo or erenumab at 7 mg, 21 mg, or 70 mg administered subcutaneously (SC) monthly. Patients who entered the open-label treatment phase (OLTP) initially received erenumab at 70 mg SC monthly (Figure 1). A protocol amendment increased the dosage to 140 mg monthly to assess long-term safety of the higher dose. This was a preplanned safety analysis of data from all patients who completed at least three years in the OLTP or discontinued the study during the OLTP before the data cutoff date. No new efficacy data are reported here because efficacy endpoints were not assessed during years 2 and 3 of OLTP (Figure 1).
Figure 1.
Study design.
Patients
Eligibility criteria for enrollment in the parent study have been previously reported (6). In brief, key inclusion criteria included age ≥ 18 and ≤ 60 years with history of migraine based on International Classification of Headache Disorders second edition (ICHD-2) (14) for ≥ 12 months prior to screening, with at least 4 and ≤ 14 migraine days per month and < 15 headache (migraine and non-migraine) days per month. To be eligible to continue in the OLTP, patients had to complete the double-blind treatment phase, not discontinue the investigational product early, and continue to provide informed consent; continuation of treatment had to be considered appropriate by the investigator.
Study outcomes
Safety and tolerability were assessed by monitoring adverse events (AEs), electrocardiograms, laboratory assessments, and blood pressure/heart rate and other vital signs. AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 20.0, and severity was graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Dose level was classified based on the dose at which the AE occurred. Cardiovascular and cerebrovascular adverse events were identified based on a search strategy composed of events from a Standardized MedDRA Query (SMQ) for ischemic central nervous system vascular conditions and ischemic heart disease, and an internal validated search strategy for peripheral arterial disease.
We compared incidence rates to those observed during the DBTP and to those expected in the patient population based on an analysis of secondary data from the MarketScan® Research Databases in migraine patients with an age and sex distribution comparable to this study to estimate incidence rates of cardiovascular and cerebrovascular events. This retrospective cohort study (n = 741,007) included adult patients with migraine, ages 18–65 years, identified from January 2010 through December 2011 using a combination of ICD-9 diagnosis codes (34i6.xx) and prescription claims for acute migraine medications (e.g. triptans or ergots) and followed through September 30, 2015 for cardiovascular and cerebrovascular events in view of the fact that CGRP can mediate vasodilation and given that the background rates of vascular disease are increased in individuals with migraine (15–17). Patients were required to have at least 12 months of continuous enrollment in MarketScan® to be included, and no cardiovascular or cerebrovascular events in the one year prior to the start of follow-up; the one-year cutoff was selected to be consistent with the inclusion/exclusion criteria of the erenumab double-blind clinical trials (4–7,12). Cardiovascular events were defined as diagnoses of myocardial ischemia, acute myocardial infarction, unstable angina, Prinzmetal angina, coronary revascularization, and other and unspecified angina pectoris; cerebrovascular events were defined as ischemic stroke, transient ischemic attack (TIA), other acute cerebrovascular events, and other ischemic cerebrovascular events. Patient incidence rates (per 100 person-years) of new cardiovascular or cerebrovascular events (along with 95% confidence intervals, CI) were estimated based on person counts within a particular event category and corresponding person-time. A patient with multiple cardiovascular or cerebrovascular events was only counted once within the category.
Statistical considerations
All patients who received at least one dose of erenumab in the OLTP were included in the analysis. AEs are summarized as exposure-adjusted patient incidence rates, defined as the total number of patients who reported that event in a given time period of follow-up divided by total patient-years of exposure in that period. Total patient-years of exposure was defined as the sum of the duration of exposure from first erenumab dose to the earliest of end-of-study date, data cutoff date, or first report of event across all patients during the OLTP. Change from baseline values for safety endpoints were calculated based on OLTP baseline. OLTP baseline was defined as the last non-missing measurement for the endpoint of interest taken before the first dose of OLTP treatment.
Standard protocol approvals, registrations, and patient consents
This trial is registered with ClinicalTrials.gov (NCT #01952574). All procedures were approved by institutional review boards at all participating sites. Patients provided written informed consent. Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing
Results
Patients
The open-label treatment phase (OLTP) enrolled 383 patients. The demographics and baseline disease characteristics of these patients are presented in Table 1. After a median of 2.0 years exposure to 70 mg monthly in the OLTP, all remaining 250 patients increased the dose to 140 mg monthly following a protocol amendment. At the time of this interim analysis, 236 patients were continuing in the study (Table 2). Median (Q1, Q3) erenumab exposure (70 or 140 mg) for all patients enrolled was 3.2 (1.3, 3.4) years and median exposure to 140 mg was 1.2 (1.1, 1.3) years. One hundred and thirty-two patients discontinued erenumab treatment prior to the dose increase and 14 discontinued erenumab treatment while receiving 140 mg after the dose increase (Table 2). Very few discontinuations were due to adverse events or lack of efficacy and most were driven by patient request (Table 2); however, further detail on reasons for patient request were not captured, such that additional information cannot be provided for these patients.
Table 1.
Demographics and clinical characteristics at baseline of the parent study for patients who entered the OLTPa.
| All patients (n = 383) | |
|---|---|
| Age, mean years (SD) | 41.3 (10.9) |
| Sex, n female (%) | 303 (79) |
| Race, n white (%) | 354 (92) |
| Age at migraine onset, mean years (SD) | 20.9 (11.3) |
| Duration of disease, mean years (SD) | 20.9 (11.9) |
| History of migraine with aura, n (%) | 137 (36) |
| Monthly migraine days, mean (SD) | 8.7 (2.7) |
| Monthly headache days, mean (SD) | 9.8 (2.7) |
| Monthly migraine-specific medication daysb, mean (SD) | 4.3 (3.7) |
| Prior prophylactic history, n (%) | |
| Naïve | 214 (56) |
| Prior use | 169 (44) |
| Treatment failurec | 138 (36) |
| Other | 31 (8) |
Baseline was prior to the parent study double-blind phase.
Migraine-specific medications were triptans and ergot amine-derivative. Two hundred and fifty-nine patients (68%) received triptans and four (1%) patients received ergotamine derivatives during the baseline period.
Treatment failure included discontinuation due to lack of efficacy and/or side effects.
Q1, first quartile; Q3, third quartile; SD, standard deviation.
Table 2.
Patient disposition during the OLTP at time of interim analysis.
| Erenumab 70 mg n (%) | Erenumab 140 mg* n (%) | |
|---|---|---|
| Received erenumab during the OLTP | 383 (100.0) | 250 (100.0) |
| Continuing open-label erenumab 140 mg | NA | 236 (94.4) |
| Discontinued open-label erenumab | 132 (34.5) | 14 (5.6) |
| Ineligibility determined | 1 (0.3) | 0 (0.0) |
| Protocol deviation | 1 (0.3) | 0 (0.0) |
| Non-compliance | 6 (1.6) | 0 (0.0) |
| Adverse event† | 16 (4.2) | 1 (0.4) |
| Patient request | 68 (17.8) | 8 (3.2) |
| Decision by sponsor | 1 (0.3) | 1 (0.4) |
| Lost to follow up | 13 (3.4) | 1 (0.4) |
| Death | 1 (0.3)‡ | 0 (0.0) |
| Requirement for alternative therapy | 3 (0.8) | 1 (0.4) |
| Pregnancy | 5 (1.3) | 0 (0.0) |
| Lack of efficacy | 12 (3.1) | 0 (0.0) |
| Other | 5 (1.3) | 2 (0.8) |
OLTP: open-label treatment phase.
Following an amendment, patients were required to increase erenumab dose to 140 mg.
†Adverse events leading to discontinuation are detailed in Table 3.
‡Fatality, previously reported, due to arteriosclerosis, occurring in patient with prior history of hypertension and left anterior hemiblock (ECG), who on autopsy showed evidence of severe coronary artery disease and presence of cardiac stimulants (liver tissue) – considered not related to the investigational product by the investigator (12).
Safety
Adverse events
The exposure-adjusted AE patient incidence rate was 132.0 per 100 patient-years (142.0 prior to and 128.1 following dosage increase). The most frequent AEs ( ≥ 4.0 per 100 patient-years) were reported as viral upper respiratory tract infection, upper respiratory tract infection, sinusitis, influenza, and back pain (Table 3). Based on pooled results from four completed placebo-controlled trials (3–5,7), none of these events have been found to be increased in erenumab-treated patients relative to placebo, and in fact these events were similar to the common events occurring in the double-blind placebo-controlled portion of this and other studies. Furthermore, the exposure-adjusted patient incidence rates of AE during the OLTP were lower than the placebo incidence rates from the DBTP. Sixteen patients discontinued erenumab treatment due to AEs; one patient experienced an AE during the DBTP that led to treatment discontinuation during the OLTP (Table 4). None of the AEs leading to treatment discontinuation occurred in more than one patient except for rash and depression, which were each reported in two patients (Table 4). The types and nature of AEs and the patient incidence rates and severity (usually non-serious and ≤ Grade 2) were comparable with previous observations and did not reveal any new safety concerns.
Table 3.
Exposure-adjusted patient incidence rates of adverse events.
| Double-blind treatment phase |
Open-label treatment phase |
||||
|---|---|---|---|---|---|
| Placebo (n = 153) n (r) | Erenumab 70 mg (n = 106) n (r) | Erenumab 70 mg (n = 383) n (r) | Erenumab 140 mg (n = 250) n (r) | Erenumab 70/140 mg (n = 383) n (r) | |
| All AEs | 82 (350.1) | 57 (326.2) | 323 (142.0) | 179 (128.1) | 335 (132.0) |
| Grade ≥ 2 | 37 (117.1) | 23 (98.0) | 245 (66.9) | 133 (70.2) | 273 (63.6) |
| Grade ≥ 3 | 2 (5.3) | 3 (11.5) | 55 (8.8) | 18 (6.3) | 67 (7.7) |
| Serious AEs | 0 (0.0) | 1 (3.8) | 29 (4.4) | 14 (4.9) | 39 (4.2) |
| AEs leading to discontinuation of IP | 2 (5.3) | 3 (11.5) | 15 (2.2) | 1 (0.3) | 16 (1.6) |
| Fatal* | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Most frequent AEs (>4.0/100 patient-years) | |||||
| Viral upper respiratory tract infection | 12 (33.9) | 6 (22.3) | 85 (14.8) | 40 (15.1) | 100 (12.9) |
| Upper respiratory tract infection | 3 (8.0) | 3 (11.4) | 52 (8.3) | 29 (10.6) | 62 (7.2) |
| Sinusitis | 2 (5.3) | 2 (7.5) | 30 (4.6) | 15 (5.3) | 42 (4.6) |
| Influenza | 5 (13.5) | 1 (3.8) | 36 (5.5) | 7 (2.4) | 38 (4.2) |
| Back pain | 4 (10.8) | 1 (3.8) | 30 (4.6) | 10 (3.5) | 38 (4.2) |
| Serious AEs occurring in >1 patient | |||||
| Adjustment disorder | 1 (0.1) | 1 (0.3) | 2 (0.2) | ||
| Syncope | 2 (0.3) | 0 (0.0) | 2 (0.2) | ||
| Uterine leiomyoma | 1 (0.1) | 1 (0.3) | 2 (0.2) | ||
| Breast cancer | 2 (0.3) | 0 (0.0) | 2 (0.2) | ||
AEs: adverse events; IP: investigational product; n: number of patients reporting at least one occurrence of event; r: exposure-adjusted patient incidence rate per 100 patient-years (n/e*100).
Fatality, previously reported, due to arteriosclerosis, occurring in patient with prior history of hypertension and left anterior hemiblock (ECG), who on autopsy showed evidence of severe coronary artery disease and presence of cardiac stimulants (liver tissue) – considered not related to the investigational product by the investigator.
Note: Each column includes adverse events that occurred while receiving the open-label erenumab at that dose level.
Table 4.
Exposure-adjusted patient incidence rates of adverse events leading to discontinuation of erenumab during the OLTP.
| Erenumab 70 mg (n = 383) n (r) | Erenumab 140 mg (n = 250) n (r) | Erenumab 70/140 mg (n = 383) n (r) | |
|---|---|---|---|
| AEs leading to discontinuation of investigational product | 15 (2.2) | 1 (0.3) | 16 (1.6) |
| Rash | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Depression | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Pancreatic cyst | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Febrile convulsion | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Breast cancer | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Dyspnoea exertional | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Oedema peripheral | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Biliary cirrhosis primary | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Headache | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Invasive lobular breast carcinoma | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Syncope | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Myocardial ischaemia | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Influenza-like illness | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Suicide attempt | 0 (0.0) | 1 (0.3) | 1 (0.1) |
| Lung adenocarcinoma stage III | 1 (0.1) | 0 (0.0) | 1 (0.1) |
n: number of patients reporting at least one occurrence of event; r: exposure-adjusted patient incidence rate per 100 patient-years (n/e*100).
Serious adverse events
Exposure-adjusted serious AE (SAE) patient incidence rates were 4.2 per 100 patient-years; the exposure-adjusted patient incidence rates in the OLTP were similar to the placebo rates from the DBTP (Table 3). SAEs were in general isolated events without a clear treatment-related pattern. The most frequent SAEs (occurring in two patients each) were adjustment disorder, syncope, uterine leiomyoma, and breast cancer. One death, which occurred during the first year of the OLTP at 70 mg, was confounded by comorbidities, as previously reported (12).
Cardiovascular and cerebrovascular safety
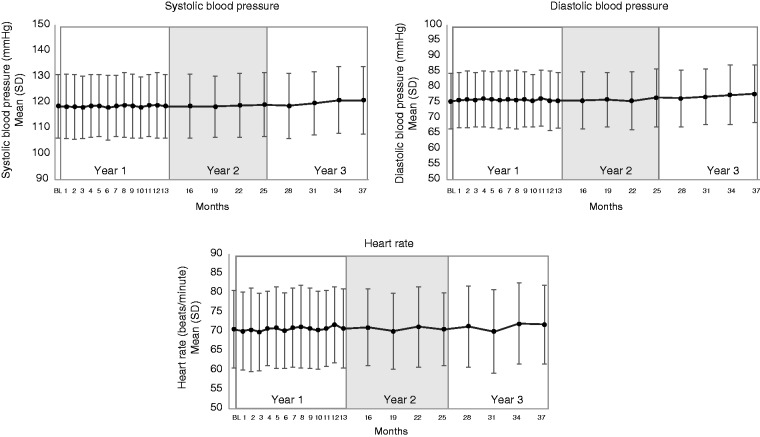
There were only two patients reporting a cardiovascular event in the OLTP, and no patient reported a cerebrovascular event (Table 5). An event of myocardial ischemia was reported during the first year of the OLTP (12) based on results of an exercise treadmill test confounded by sumatriptan administration 4 hours prior to the event. Since then, one additional event was reported, which was increased blood creatine phosphokinase MB that was noted during an evaluation for myocarditis based on symptoms of feeling listless and tired, and intermittent inability to take a full breath after having severe laryngitis for two weeks. This event was adjudicated by an independent clinical endpoint committee as not being an event of myocardial infarction or hospitalization for unstable angina. Given the lack of a placebo arm, a large claims database of migraine patients was analyzed to establish background rates for cardiovascular and cerebrovascular events. The background patient incidence rate of cardiovascular events was 0.42 per 100 person-years and 0.59 per 100 person-years for cerebrovascular events, which are both higher than the exposure-adjusted cardiovascular and cerebrovascular patient incidence rates in this long-term interim analysis (0.2 per 100 patient-years, and 0.0 per 100 patient-years, respectively) (Table 6). There were no meaningful changes in systolic/diastolic blood pressure or heart rate up to ∼3.3 years of follow-up (Table 7 and Figure 2).
Table 5.
Exposure-adjusted patient rates of cardio- and cerebrovascular disorder events during the OLTP.
| Erenumab 70 mg (n = 383) n (r) | Erenumab 140 mg (n = 250) n (r) | Erenumab 70/140 mg (n = 383) n (r) | |
|---|---|---|---|
| Cardiovascular adverse events | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Ischemic heart disease | 2 (0.3) | 0 (0.0) | 2 (0.2) |
| Cardiac disorders | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Myocardial ischemia | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Investigations | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Blood creatine phosphokinase MB increased | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Cerebrovascular adverse events | 0 (0.0) | 0 (0.0) | 0 (0.0) |
n: number of patients reporting at least one occurrence of event; r: exposure-adjusted rate per 100 patient-years.
Note: Table based on the following search criteria: Ischemic central nervous system vascular conditions SMQ (narrow), ischemic heart disease SMQ (narrow) and peripheral arterial disease (PAD) AMQ (narrow). Coded using MedDRA version 20.0.
Table 6.
Incidence of cardiovascular and cerebrovascular events in persons with migraine.
| # Patients with event (n = 741,007 persons) | Person-years (PY) of follow-up | Rate (95% CI) per 100 PY | |
|---|---|---|---|
| Cardiovascular events | 8,250 | 1,981,899 | 0.42 (0.41, 0.43) |
| Cerebrovascular events | 11,591 | 1,978,926 | 0.59 (0.58, 0.60) |
Data are incidence rates from MarketScan® database of adult patients with migraine identified from January 2010 through December 2011 followed through 30 September, 2015.
Table 7.
Blood pressure and heart rate at Year 3 during the OLTP.
| Erenumab 70/140 mg (n = 240) | |
|---|---|
| Systolic blood pressure, mmHg | |
| OLTP baseline | 118.4 (12.3) |
| Year 3 | 120.7 (13.1) |
| Patients with increase from OLTP baseline of ≥20 mmHg, n (%) | |
| <120 mmHg at Year 3 | 2 (0.8) |
| 120–129 mmHg at Year 3 | 7 (2.9) |
| 130–139 mmHg at Year 3 | 6 (2.5) |
| ≥140 mmHg at Year 3 | 10 (4.2) |
| Diastolic blood pressure, mmHg | |
| OLTP baseline | 74.9 (9.5) |
| Year 3 | 77.7 (9.3) |
| Patients with increase from OLTP baseline of ≥10 mmHg, n (%) | |
| <80 mmHg at Year 3 | 11 (4.6) |
| 80–99 mmHg at Year 3 | 23 (9.6) |
| ≥100 mmHg at Year 3 | 18 (7.5) |
| Heart rate, beats/min | |
| OLTP baseline | 70.1 (9.6) |
| Year 3 | 71.7 (10.2) |
Note: Data represent mean (SD) unless otherwise indicated.
n: number of patients with observed results at Year 3.
Figure 2.
Blood pressure and heart rate over time during the OLTP.
BL: Open-label treatment phase baseline; study month: four weeks.
Liver function
There were no cases of suspected Hy's law (alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 3 × upper limit of normal (ULN) and total bilirubin > 2 × ULN and alkaline phosphatase (ALP) < 2 × ULN) predictive of potential for drug-related hepatotoxicity (Table 8). There were eight patients with ALT or AST increases greater than three times the upper limit of normal (only one with ALT greater than five times the upper limit of normal) over this long-term observation period, corresponding to 1.8% and 0.8% of patients receiving 70 mg and 140 mg (Table 8). Among the eight patients with ALT or AST > 3 ×ULN, one had ALT and AST decreased to >1 ×ULN to ≤ 3 × ULN and the other seven decreased to < 1 × ULN by the latest assessment. The patients with total bilirubin > 2 × ULN had total bilirubin reduced to > 1 × ULN to ≤ 1.5 × ULN by the latest assessment.
Table 8.
Incidence of liver function test abnormalities during the OLTP.
| Erenumab 70 mg (n = 383) n (%) | Erenumab 140 mg (n = 250) n (%) | |
|---|---|---|
| ALT or AST > 3 × ULN* | 7 (1.8) | 2 (0.8) |
| Total bilirubin > 2 × ULN | 0 (0.0) | 1 (0.4) |
| ALT or AST > 3 × ULN and total bilirubin > 2 × ULN and ALP < 2 × ULN | 0 (0.0) | 0 (0.0) |
ULN: Upper limit of normal; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase.
Only one case was > 5 × ULN for ALT in erenumab 140 mg group.
Anti-drug antibodies
No patients in any group had pre-existing antibodies prior to the first erenumab dose. Among 400 subjects with postbaseline results after receiving 70 mg or 140 mg during DBTP or OLTP, 38 (9.5%) developed non-neutralizing binding antibodies, 29 of whom had a transient response (negative result at last time point tested), and three (0.8%) developed neutralizing antibodies, two of whom had a transient response. Since the interim analysis after 52 weeks of the OLTP, only two patients have newly developed a positive non-neutralizing binding antibody, both of which were transient.
Discussion
This three-year interim safety update of a five-year long-term open label study confirms the favorable tolerability and safety profile of erenumab seen in short-term and one-year OLE trials (3,4,6,7,12). This publication represents the longest-term data for a CGRP pathway-targeted therapy to date and allows for an assessment of the risk-benefit profile of erenumab treatment over 3 years of treatment. The safety profile of erenumab remained in line with that observed during the DBTP, with no increase in the rate of AEs over time or following the dose increase, and no evidence of cardiovascular or cerebrovascular risk.
Safety and tolerability profiles during the OLTP (total exposure 961 patient-years) were similar to those observed for erenumab 70 mg in the double-blind parent study (total exposure 23.9 patient-years) and overall were similar to placebo (34.1 patient-years) in the parent study. Although constipation was identified as a common AE in the pooled analysis of the double-blind studies (11), patient incidence rates for constipation were low in this OLTP (1.8 per 100 patient-years) and there were no cases of treatment discontinuation due to constipation. During the placebo-controlled studies, constipation events were mild and tended to dissipate over time, so it is unsurprising that it is not observed over longer treatment.
Retention rates in long-term clinical trials provide an indication of the long-term efficacy and tolerability of therapies, and hence are of particular importance in chronic conditions. Of 383 patients who entered into the OLTP, patient retention rates at year 3 were relatively high (62%), highlighting the favorable long-term tolerability profile of erenumab and patient satisfaction with treatment, in contrast to those observed in long-term studies with other migraine preventatives (18). In the 8-month open-label extension of the pivotal topiramate trials, 29% of participants withdrew and of those withdrawing 42% withdrew due to an adverse event (18). The observed discontinuation rate due to reported lack of efficacy or adverse events with erenumab in this analysis was low (<5%). This is in contrast to the high discontinuation rates for commonly used migraine prophylactics (18–20).
In short-term phase 1 and phase 2 clinical trials of erenumab (including the double-blind treatment period of the study reported here), there was a low incidence of binding and neutralizing anti-erenumab antibodies. The incidence of anti-erenumab antibodies remained low throughout the OLTP, and only two patients developed positive non-neutralizing binding antibodies since the 52-week interim analysis of the OLTP, indicating that late development of anti-erenumab antibodies occurs infrequently. The development of anti-erenumab antibodies has not been associated with any clinical finding or safety events.
Long-term safety assessments are often complicated by lack of placebo comparison, which makes it difficult to distinguish spontaneously occurring AEs or laboratory abnormalities from those due to treatment. In the absence of a placebo comparison, context can be added to events observed during open-label treatment by comparing with background rates observed in real-world settings. Given that CGRP can mediate vasodilation, at least in theory inhibition of CGRP's effects might pose a vascular risk, though this has not been observed in short-term placebo-controlled erenumab studies (3–7). Previously published epidemiological studies have shown an increased risk of cardiovascular and cerebrovascular events in patients with migraine compared to those without migraine, particularly among patients who experience migraine with aura (15–17). Overall, migraine is significantly associated with myocardial infarction (Odds Ratio (OR) = 2.2, 95% CI = 1.7–2.8) and stroke (OR = 1.5, 95% CI = 1.2–2.1) (21). In the Nurses' Health Study II, adjusted for CV risk factors, the hazard ratios (95% CIs) for women with migraine were 1.50 (1.33–1.69) for major CV events, 1.39 (1.18–1.64) for MI, 1.62 (1.37–1.92) for stroke, and 1.37 (1.02–1.83) for CV mortality when compared to study participants without migraine (22). In women, migraine with aura was the second strongest single contributor to major cardiovascular disease risk with an incidence rate of 7.9 per 1000 women per year (17). Therefore, in order to contextualize cardiovascular/cerebrovascular patient incidence rates in our study, a cohort of patients with migraine from the MarketScan® claims database of 741,007 persons over a similar follow-up period as this interim analysis was analyzed to establish background patient incidence rates. The cardiovascular background rates (0.42/100 person-years) were higher than those observed in the OLTP of this study (0.2/100 patient-years), consistent with the preclinical (23) and clinical observations (24,25) to date that erenumab does not increase the risk of adverse cardiovascular/cerebrovascular events.
The frequency of elevated ALT or AST > 3 × ULN during the OLTP with 3 years of exposure was low given the long duration of follow-up. For each of these cases, elevation was < 5 × ULN. For context, the frequency of ALT or AST elevation > 3 × ULN over 12 weeks in the pooled placebo groups of four completed studies (3,4,6,7) was 0.5%. Given the much longer period of observation in the OLTP, the low level ( > 3 × but < 5 × ) of AST or ALT elevation in few patients supports that long-term treatment with erenumab does not increase liver function tests. More importantly there were no cases of Hy's law, which predicts the potential for severe liver toxicity.
In this three-plus years, long-term study of an antibody targeting the CGRP receptor, erenumab was found to be safe and well-tolerated with a spectrum and rate of AEs consistent with shorter-term placebo-controlled studies. The favorable tolerability and safety profile, and the low discontinuation rates, suggest that adherence is favorable with erenumab and may result in positive long-term outcomes.
Acknowledgements
This study was fully funded by Amgen Inc. Erenumab is co-developed in partnership with Amgen Inc. and Novartis. Jon Nilsen (Amgen Inc.) provided medical writing support for this manuscript.
Clinical implications
Erenumab (in the US, erenumab-aooe) is a fully human anti-CGRP receptor monoclonal antibody approved in the US and EU for migraine prevention.
In this three-year, long-term study, erenumab was found to be safe and well-tolerated with a spectrum and rate of adverse events consistent with shorter-term placebo-controlled studies.
The favorable tolerability and safety profile, and the low discontinuation rates, suggest that adherence is favorable with erenumab and may result in positive long-term outcomes.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MA reports research grants from Lundbeck Foundation, Research Foundation of the Capital Region of Copenhagen, and Novo Nordisk Foundation; consulting from Allergan, Amgen Inc., Alder, Eli Lilly, Novartis and Teva. PJG reports grants and personal fees from Amgen and Eli-Lilly and Company, and personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc., Dr Reddy's Laboratories, Electrocore LLC, eNeura, Novartis, Scion, Teva Pharmaceuticals, and Trigemina Inc., and personal fees from MedicoLegal work, NEJM Journal Watch, Up-to-Date, Oxford University Press, and Wolters Kluwer; and a patent Magnetic stimulation for headache assigned to eNeura without fee. UR reports consulting fees, speaking/teaching fees, and/or research grants – Allergan, Amgen, Autonomic Technologies, Eli Lilly and Co, Medscape, Novartis, CoLucid, StreaMedUp, Teva. SS reports consultant/ad board fees: Alder Biopharmaceuticals; Allergan, Inc.; Amgen; Avanir Pharmaceuticals, Inc.; Curelator, Inc.; Dr. Reddy’s Laboratories; eNeura Inc.; electroCore Medical, LLC; Lilly USA, LLC; Medscape, LLC.; NINDS; Supernus Pharmaceuticals, Inc.; Teva Pharmaceuticals; Theranica; and Trigemina, Inc. DWD reports the following conflicts: Personal fees: Amgen, Association of Translational Medicine, University Health Network, Daniel Edelman Inc., Autonomic technologies, Axsome, Aural Analytics, Allergan, Alder, Biohaven, Charleston Laboratories, Dr Reddy’s Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, University of British Columbia, Zosano, ZP Opco, Foresite Capital, Oppenheimer. CME fees or royalty payments: Healthlogix, Medicom Worldwide, Medlogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, Academy for Continued Healthcare Learning, Universal meeting management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, Wolters Kluwer Health; Stock options: Aural analytics, Healint, Theranica, Second Opinon/Mobile Health, Epien, GBS/Nocira, Matterhorn/Ontologics, King-Devick Technologies. Consulting without fee: Aural Analytics, Healint, Second Opinion/Mobile Health, Epien; Board of Directors: Epien, Matterhorn/Ontologics, King-Devick Technologies. Patent: 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee. Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, International Headache Society, Canadian Headache Society. Other: Use agreement through employer: Myndshft. JK reports employee and stock/stock options: Novartis Pharma AG. GR, FZ, SC, and DM report: Employees and stock/stock options, Amgen Inc.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Amgen Inc.
References
- 1.Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: Implications for diagnosis, treatment and clinical trial design. J Headache Pain 2017; 18: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 3.Dodick DW, Ashina M, Brandes JL, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018; 38: 1026–1037. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123–2132. [DOI] [PubMed] [Google Scholar]
- 5.Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018. 392: 2280–2287. . [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390. [DOI] [PubMed] [Google Scholar]
- 7.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017; 16: 425–434. [DOI] [PubMed] [Google Scholar]
- 8.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Escandon R, Bronson M, et al. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia 2014; 34: 483–492. [DOI] [PubMed] [Google Scholar]
- 10.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 11.Amgen Inc. Aimovig (erenumab-aooe) (US package insert). Thousand Oaks, CA: Amgen Inc., 2018.
- 12.Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology 2017; 89: 1237–1243. [DOI] [PubMed] [Google Scholar]
- 13.Ashina M, Goadsby P, Silberstein S, et al. Long-term safety and tolerability of erenumab: Three-plus year results from an ongoing open-label extension study in episodic migraine. Headache 2018; 58: 18–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. ICHD-II Classification: Parts 1–3: Primary, secondary and other. Cephalalgia 2004; 24: 23–136. .14687009 [Google Scholar]
- 15.Buse DC, Reed ML, Fanning KM, et al. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2017; 57: 31–44. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1,152,407 subjects. BMJ Open 2018; 8: e020498 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schurks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ 2009; 339: b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapoport A, Mauskop A, Diener HC, et al. Long-term migraine prevention with topiramate: Open-label extension of pivotal trials. Headache 2006; 46: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 19.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second international burden of migraine study (IBMS-II). Headache 2013; 53: 644–555. [DOI] [PubMed] [Google Scholar]
- 20.Silberstein SD, Neto W, Schmitt J, et al. Topiramate in migraine prevention: Results of a large controlled trial. Arch Neurol 2004; 61: 490–495. [DOI] [PubMed] [Google Scholar]
- 21.Buse DC, Manack A, Serrano D, et al. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010; 81: 428–432. [DOI] [PubMed] [Google Scholar]
- 22.Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: Prospective cohort study. BMJ 2016; 353: i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Beltran E, Labastida A, de Vries R, et al. Effects of AMG 334 on human isolated coronary artery (abstract). Cephalalgia 2016; 36(S): 41 . [Google Scholar]
- 24.Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache 2018; 58: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Hoon J, Van Hecken A, Vandermeulen C, et al. Phase 1, randomized, parallel-group, double-blind, placebo-controlled trial to evaluate the effects of erenumab (AMG 334) and concomitant sumatriptan on blood pressure in healthy volunteers. Cephalalgia 2019; 39: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]