Abstract
Objectives
To address the need for long-term lasmiditan data, the GLADIATOR study evaluated the safety (primary) and efficacy (secondary) of lasmiditan for the intermittent, acute treatment of migraine attacks for up to 1 year.
Methods
In this prospective, randomized, open-label, Phase 3 study, patients who had completed either of two single-attack studies were offered the opportunity to be randomized 1:1 to lasmiditan 100 mg or 200 mg. Patients were asked to use lasmiditan as the first treatment for each new migraine attack of at least moderate severity. Assessments occurred at baseline and at prespecified time increments up to 48 hours after each dose of study drug using an electronic diary, and safety was assessed throughout the study. Migraine Disability Assessment (MIDAS) was assessed at each visit.
Results
As of the cut-off date for this interim analysis (6 March 2018), 1978 patients had received ≥ 1 lasmiditan dose and treated 19,058 migraine attacks. Overall, treatment-emergent adverse events (TEAEs) were similar to those in the single-attack studies and included dizziness (18.6%), somnolence (8.5%), and paresthesia (6.8%). The frequency of TEAEs generally decreased with subsequent attacks. No treatment-related serious adverse events and no cardiovascular TEAEs potentially due to vasoconstriction were observed. For both lasmiditan doses, efficacy measures were generally consistent over study quarters and treated attacks. Overall, across all treated attacks at 2 hours post-dose, pain freedom was observed in 26.9% of the attacks treated with lasmiditan 100 mg and 32.4% of the attacks treated with lasmiditan 200 mg. MIDAS total scores decreased over time.
Conclusions
The interim results of this long-term study showed intermittent lasmiditan (100 mg and 200 mg) to be generally well tolerated and efficacious for the acute treatment of migraine over a 1-year period.
Trial registration number: NCT02565186; https://clinicaltrials.gov/ct2/show/NCT02565186
Keywords: Episodic migraine, serotonin, 5-HT1F agonist, ditan, MIDAS
Introduction
In a study by the American Migraine Prevalence and Prevention (AMPP), 40.7% of patients with episodic migraine were shown to have one or more significant unmet needs (1). Within this cohort, the most frequent complaints were headache-related disability (47%) and dissatisfaction with current medications (37%). The current standard of care for the acute treatment of migraine attacks is the drug class known as triptans; however, it has been estimated that approximately 25% to 40% of patients with migraine have suboptimal response to triptans (2,3). Triptans primarily target 5-hydroxytryptamine (5-HT) receptor subtypes 1B and 1D (5-HT1B/1D), and their mechanism is thought to involve cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release (4–7). However, because 5-HT1B receptors are also located on endothelial cells, triptans can cause vasoconstriction and rare cardiovascular (CV) events (4,5,7). Accordingly, triptans are considered contraindicated in patients with CV disease, thus limiting the population of patients with migraine who may safely use this class of acute agents (3,8,9).
Lasmiditan (LY573144, also formerly known as COL-144; Eli Lilly and Company, Indianapolis, IN) is the first “ditan” to reach Phase 3 development and is a centrally penetrant, highly selective 5-HT1F receptor agonist (10). Evidence suggests that lasmiditan exerts its therapeutic effects in the acute treatment of migraine by decreasing neuropeptide release and inhibiting pain pathways, including those in the trigeminal nerve and ganglion (3,5,10,11). The functional selectivity of lasmiditan for the 5-HT1F receptor versus the 5-HT1B receptor, the receptor thought to be responsible for vasoconstrictive effects, is > 440-fold, and thus lasmiditan lacks the vasoconstrictor activity inherent with triptans (3,5,10). Because migraine symptoms may be associated with activation of the trigeminal nerve and additional sites in the brain and brainstem, a neural rather than vascular approach may be feasible for acute treatment of migraine (7,12).
Two placebo-controlled Phase 3 studies of lasmiditan for the acute treatment of migraine have been completed, SAMURAI (n = 1856; ClinicalTrials.gov identifier: NCT02439320) and SPARTAN (n = 2583; NCT02605174) (13,14). Both studies were similarly designed, with participants randomized to treat a single migraine attack with oral lasmiditan (50 mg [SPARTAN only], 100 mg, or 200 mg) or placebo in double-blind fashion. For all doses of lasmiditan, the percentage of patients who achieved migraine pain freedom at 2 hours post-dose was significantly greater than for those given placebo. In addition, both studies met the key secondary endpoint of significantly more patients in the lasmiditan group than in the placebo group achieving freedom from their “most bothersome symptom” (MBS) at 2 hours post-dose. Lasmiditan was generally safe and well tolerated, with no deaths among patients who took the study drug and few serious adverse events (SAEs) reported. The most common adverse events (AEs) reported during treatment with lasmiditan were dizziness, paresthesia, somnolence, fatigue, and nausea.
The Open-label LonG-term Safety Study of LAsmiDItan in the Acute Treatment Of MigRaine (GLADIATOR) was designed to address the need for data on chronic intermittent exposure to lasmiditan. GLADIATOR is an ongoing prospective, randomized, open-label, Phase 3 study to evaluate the safety and efficacy of lasmiditan 100 mg and 200 mg for the treatment of multiple migraine attacks for up to 1 year in patients who previously completed either of the single-attack SAMURAI or SPARTAN studies. The primary objective of this study is to evaluate the safety and tolerability of long-term intermittent use of lasmiditan 100 mg and 200 mg for the acute treatment of migraine. The secondary objective is to assess the efficacy of long-term intermittent use of lasmiditan, with regard to migraine pain and MBS freedom as well as changes in migraine disability. This report provides interim safety and efficacy results from this ongoing study.
Methods
Overview of study design and selection of participants
GLADIATOR is being conducted by 199 investigators at 199 study sites in the United States (US), United Kingdom, and Germany. The first patient was enrolled on 07 October 2015, and the data cut-off for this interim analysis was 06 March 2018. The study is being conducted in compliance with the International Council for Harmonisation (ICH) principles of Good Clinical Practice. Before the initiation of GLADIATOR, ethics review boards approved the study protocol and the informed consent form. The study is registered at ClinicalTrials.gov (identifier: NCT02565186).
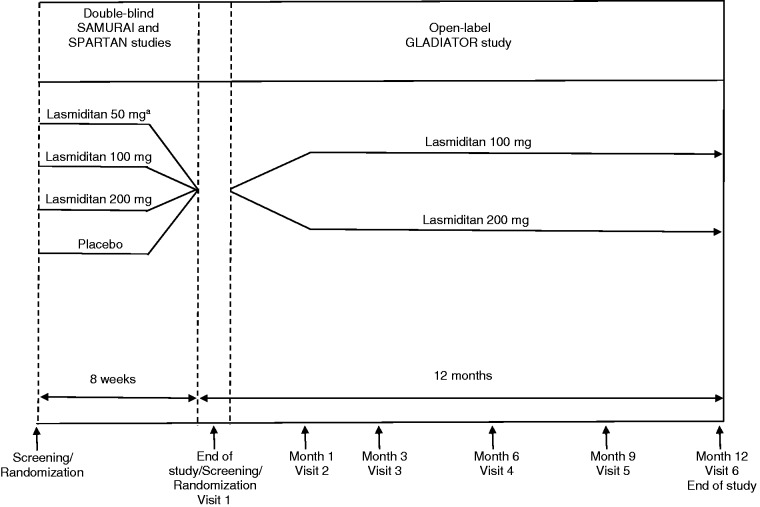
Patients who had completed either SAMURAI or SPARTAN single-attack lasmiditan studies were offered the opportunity to participate in GLADIATOR (Figure 1). The study designs of the single-attack studies have been previously described in detail elsewhere (13,14). Briefly, eligible patients met International Headache Society (ICHD 2nd edition) diagnostic criterion 1.1 or 1.2.1 for migraine with or without aura (15) and had at least moderate migraine disability, defined as a Migraine Disability Assessment (MIDAS) score of ≥ 11, and had episodic migraine (three to eight migraine attacks per month, < 15 headache days per month). In addition, these studies did not exclude older patients or patients with CV risk factors, as defined by American College of Cardiology/American Heart Association guidelines (16–18), and SPARTAN did not exclude patients with known coronary artery disease (CAD), clinically significant arrhythmia, or uncontrolled hypertension; these patients were excluded in SAMURAI.
Figure 1.
GLADIATOR study design. aLasmiditan 50 mg was included only in the SPARTAN study.
Patients who were non-compliant with electronic diary (e-diary) recordings or dosing requirements during the parent study or who had initiated or changed concomitant migraine preventive medications since completing the parent study were not eligible for participation. Additional exclusion criteria included any medical condition or clinical laboratory test that the investigator thought made the patient unsuitable for the study; imminent risk of suicide; and pregnancy, breastfeeding, or unwillingness to use a highly effective form of contraception. Because dizziness, somnolence, and fatigue had been observed with lasmiditan treatment, patients were to be advised not to drive or operate machinery for 12 hours after taking lasmiditan. All participants provided written informed consent and indicated that they were willing to complete an e-diary.
Interventions
Patients were randomized 1:1 to treatment with lasmiditan 100 mg or 200 mg and were stratified (yes or no) at randomization for use of concomitant migraine preventive medications. Because there was a lack of availability of the 100-mg dose in Europe, all patients enrolled in GLADIATOR at European study sites were assigned to lasmiditan 200 mg.
Patients were instructed to use lasmiditan as the first treatment for each new migraine attack within 4 hours of pain onset, provided that the pain severity was moderate to severe and was not improving. If the migraine attack pain did not respond at 2 hours or the patient became pain free within 2 hours, but the pain recurred, a second dose of lasmiditan was allowed up to 24 hours after the first dose as long as no other migraine medication had been used. Alternatively, patients were allowed to take their own medication for rescue or recurrence; however, triptans, ergots, opioids, and barbiturates were not allowed within 24 hours of lasmiditan administration.
Data collection and study endpoints
GLADIATOR Screening/Visit 1 was permitted to be the same day as the end-of-study visit of the parent study. During Visit 1, any changes or updates to the patient’s medical or migraine history since enrolling in the parent study were noted, and baseline assessments were performed as needed, according to the protocol. All patients were trained on the use of the e-diary and completed the MIDAS questionnaire, which is a validated patient-reported questionnaire designed to assess headache-related disability over a 3-month period (see Supplemental Material) (19,20). The MIDAS questionnaire has demonstrated reliability and validity, such that scores correlate with clinical judgment on the need for medical care (21).
During the 12-month treatment period, patients were instructed to treat migraine attacks with their assigned lasmiditan dose on an outpatient basis. The e-diary included daily questions “How are you feeling today?” and “Did you take any medication that you do not usually take (and had not told the study nurse about)?” Additionally, at 0.5, 1, 2, 4, 24, and 48 hours after dosing with lasmiditan, patients were asked the question “Do you feel anything unusual since you took the study medication that you have not felt with a migraine before?” The primary endpoints of safety and tolerability were assessed via AEs and changes from baseline in physical and laboratory examinations, electrocardiograms (ECGs), and vital signs (during clinical visits, as described below). If the patient recorded feeling not well on the daily e-diary entry or felt something unusual after taking lasmiditan for a migraine attack, the study site was to follow up with a phone call to ascertain whether an AE (coded by Medical Dictionary for Regulatory Activities [MedDRA], Version 21.0) had occurred. A treatment-emergent AE (TEAE) was defined as an AE that started or worsened within 48 hours after the last dose (either the first or the second dose) of lasmiditan. The investigator assessed whether or not an AE was related to lasmiditan treatment.
For each migraine attack, patients were asked to record in their e-diary the date and time that the migraine pain started and when the pain first became moderate or severe, the date and time of taking the first dose of lasmiditan, and their response to the first dose of lasmiditan over the next 48 hours. For the efficacy endpoints, patients were asked to record migraine headache pain, using a 4-point IHS pain severity rating scale (none, mild pain, moderate pain, and severe pain) at 0 (pre-dose), 0.5, 1, 2, 4, 24, and 48 hours post-dose (22). Although patients were instructed to dose with lasmiditan when their pain was moderate or severe, a small number of patients dosed when the pain was mild. Pain relief was defined as experiencing moderate or severe pain at baseline that became mild or none at the summarized time point or mild pain at baseline that became none at the summarized time point for each treated attack. Pain freedom was defined as a reduction in pain severity from mild, moderate, or severe at baseline to none at the summarized time point. In addition, patients were asked to record the presence or absence of nausea, vomiting, phonophobia, and photophobia at baseline, 0.5, 1, 2, 4, 24, and 48 hours, and, at baseline, were to select which of the accompanying symptoms (nausea, phonophobia, or photophobia) was the MBS. MBS freedom was defined as the absence of the selected MBS at subsequent time points. Use of rescue or recurrence medication, including medications other than the study drug, was also recorded in the e-diary. If patients used lasmiditan for rescue or recurrence, they were to record responses in the e-diary up to 48 hours after the second dose. Other assessments included change from baseline in MIDAS total score and number of days with headache over the past 3 months as well as the number of migraine attacks treated with lasmiditan over the course of the study.
Clinic visits occurred at 1, 3, 6, 9, and 12 months (visits 2 through 6). At each visit, a brief physical examination based on AEs was performed, vital signs were collected, a urine pregnancy test was administered for women of childbearing potential, and patients completed the MIDAS questionnaire. Laboratory tests and a 12-lead ECG were collected at baseline if more than 2 weeks had elapsed since the end-of-study visit in the parent study. At 1, 6, and 12 months, blood and urine samples were collected for laboratory analyses, which included biochemistry, hematology, urinalysis, and lipid profiles. An ECG was obtained at 6 and 12 months.
Patients could voluntarily withdraw from the study or could be removed from the study at the discretion of the investigator or sponsor at any time, with the primary reason for discontinuation noted.
Statistical methods
The study sample size was chosen to provide a long-term safety database in accordance with ICH, US Food and Drug Administration (FDA), and the Committee for Medicinal Products for Human Use (CHMP) guidelines. The sample size aimed to achieve ≥ 300 patients enrolled to treat on average ≥ 2 migraine attacks per month for 6 months and ≥ 100 patients to treat on average ≥ 2 migraine attacks per month for 12 months.
All safety analyses were performed on the safety population, which included all randomized patients who used ≥ 1 dose of lasmiditan. As prespecified, the pain freedom and MBS freedom analyses were conducted on the modified intent-to-treat (mITT) population, and other efficacy analyses were performed on the intent-to-treat (ITT) population. The ITT population at the patient level included all randomized patients who used ≥ 1 dose of lasmiditan and had any post-dose pain severity or symptom assessments for ≥ 1 migraine attack and at the treated attack level included a migraine attack treated with ≥ 1 dose of lasmiditan and having any post-dose pain severity or symptom assessments. The mITT population at the patient level included all ITT patients who used ≥1 dose of lasmiditan to treat a migraine attack within 4 hours of onset for ≥1 migraine attack during the study and at the treated attack level included all ITT migraine attacks treated within 4 hours of onset. Treated migraine attacks that did not have a pain severity rating at baseline or were reported with a severity of “none” were excluded from the pain freedom and pain relief analyses.
Continuous variables were summarized using descriptive statistics (i.e. n [number of patients with available data], mean, standard deviation [SD], and interquartile range [IQR]). Categorical variables were summarized using counts and percentages. A subgroup analysis was conducted to compare TEAEs among patients with and without CV risk factors (hypertension, hypercholesterolemia, smoking, obesity, diabetes mellitus, family history of CAD, men over 40 years of age, and postmenopausal women) in the safety population using Fisher’s exact test. Post-hoc analyses were conducted to compare the lasmiditan 100 mg group with the 200 mg group for treated migraine attacks achieving pain freedom, MBS freedom, and pain relief at 2 hours post-dose overall using a one-sided test from a logistic regression model with treatment group and background use of medication to reduce the frequency of migraines as covariates. Subgroup analyses of TEAEs and efficacy measures were conducted for migraine attacks 1 to 5 only for those patients who treated ≥ 5 migraine attacks. MIDAS score changes were modeled using a mixed model repeated measures analysis using the MIDAS patient population, which includes patients with MIDAS score data at baseline and post-baseline assessments, regardless of whether or not they took lasmiditan. The number of lasmiditan-treated migraine attacks over time on a per-patient basis was evaluated using data by quarter of study participation. Statistical analyses were performed using SAS (Version 9.4 or higher; SAS Institute, Cary, NC).
Results
Patient characteristics and disposition
Baseline demographic and disease characteristics were generally well balanced between the treatment groups (Table 1). In the overall safety population, the mean age of the patients was 43.2 years (range, 18–79 years) and 85.3% were women. The majority of the patients were white (78.4%), 17.6% were black, and 19.9% were of Hispanic or Latino origin, which is similar to the distribution found in the overall US population (23). A family history of CAD was reported by 29.8% of the patients, and 82.0% had ≥1 CV risk factor. Overall, 24.2% of patients were taking ≥1 CV medication and 22.1% of the patients were taking a migraine preventive medication. Medical history events were similar between treatment groups (Supplemental Table 1). Twenty-two percent had a history of depression, 19.0% anxiety, and 12.2% insomnia.
Table 1.
Baseline patient characteristics (safety population).
| Characteristic | Lasmiditan 100 mg n = 963 | Lasmiditan 200 mg n = 1015 | All patients n = 1978 |
|---|---|---|---|
| Age, mean (SD), y | 42.7 (12.3) | 43.8 (12.5) | 43.2 (12.4) |
| Sex, n (%) | |||
| Female | 822 (85.4) | 866 (85.3) | 1688 (85.3) |
| Male | 141 (14.6) | 149 (14.7) | 290 (14.7) |
| Race, n (%) | |||
| White | 746 (77.5) | 805 (79.3) | 1551 (78.4) |
| Black/African American | 181 (18.8) | 168 (16.6) | 349 (17.6) |
| Asian | 7 (0.7) | 6 (0.6) | 13 (0.7) |
| American Indian/Alaska Native | 3 (0.3) | 8 (0.8) | 11 (0.6) |
| Native Hawaiian/Pacific Islander | 4 (0.4) | 6 (0.6) | 10 (0.5) |
| Other | 11 (1.1) | 9 (0.9) | 20 (1.0) |
| Multiple | 11 (1.1) | 13 (1.3) | 24 (1.2) |
| Hispanic or Latino | 186 (19.3) | 208 (20.5) | 394 (19.9) |
| Body mass index, mean (SD), kg/m2 | 31.2 (8.2) | 31.0 (8.2) | 31.1 (8.2) |
| Current smoker, n (%) | 133 (13.8) | 128 (12.6) | 261 (13.2) |
| Family history of CAD, n (%) | 296 (30.7) | 293 (28.9) | 589 (29.8) |
| CV risk factorsa, n (%) | |||
| ≥1 | 805 (83.6) | 816 (80.4) | 1621 (82.0) |
| ≥2 | 480 (49.8) | 500 (49.3) | 980 (49.5) |
| Use of ≥ 1 CV medicationb, n (%) | 232 (24.1) | 246 (24.2) | 478 (24.2) |
| Parent study, n (%) | |||
| SAMURAI | 373 (38.7) | 332 (32.7) | 705 (35.6) |
| SPARTAN | 590 (61.3) | 683 (67.3) | 1273 (64.4) |
| Duration of migraine history, mean (SD), y | 18.8 (12.7) | 18.9 (13.1) | 18.8 (12.9) |
| Number of migraine attacks/month in past 3 months, mean (SD) | 5.3 (1.8) | 5.2 (1.8) | 5.2 (1.8) |
| Experienced migraine with aura, n (%) | 349 (36.2) | 351 (34.6) | 700 (35.4) |
| Use of migraine preventive medications, n (%) | 212 (22.0) | 226 (22.3) | 438 (22.1) |
| MIDAS total scorec,d, mean (IQR) | 29.4 (15,36) | 28.9 (15,35) | |
| Number of days with headached, mean (IQR) | 15.5 (8,20) | 15.5 (8,20) |
ATC: anatomical therapeutic chemical; CAD: coronary artery disease; CV: cardiovascular; IQR: interquartile range; MIDAS: Migraine Disability Assessment; SD: standard deviation; WHO: World Health Organization.
Hypertension, hypercholesterolemia, smoking, obesity, diabetes mellitus, family history of CAD, men over 40 years of age, and postmenopausal women.
Cardiac therapy, antihypertensives, diuretics, peripheral vasodilators, vasoprotectives, beta-blocking agents, calcium channel blockers, agents acting on the renin-angiotensin system, lipid-modifying agents, antithrombotic agents based on WHO Drug ATC2 codes.
Calculated as the sum of the answers to the five questions on the MIDAS questionnaire (0–5 = little or no disability; 6–10 = mild disability; 11–20 = moderate disability; ≥21 = severe disability).
In the past 3 months.
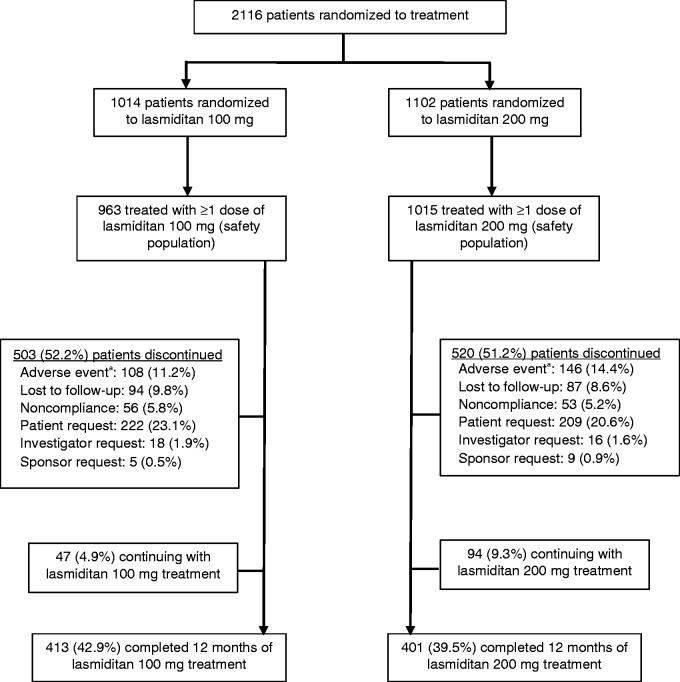
Detailed patient disposition and the flow of participants through the study are shown in Figure 2. Of the 2116 patients who were randomized, 1978 patients received ≥ 1 dose of lasmiditan (safety population) and treated 19,058 migraine attacks. At the time of the data cut-off for this interim analysis, 814 (41.2%) patients in the safety population had completed all 12 months of the study and 141 (7.1%) patients were continuing treatment. The median duration of time in study was 288 days (IQR, 98–363 days; n = 1834). Of the 51.7% of patients who discontinued, the most common reason for discontinuation was patient request (21.8%), followed by an AE (12.8%) and lost to follow-up (9.2%).
Figure 2.
Flow of study participants.
aIn the safety population, common adverse events leading to discontinuation (>1%) in the lasmiditan 100 mg group included dizziness (26 patients) and somnolence (14 patients) and in the 200 mg group included dizziness (44 patients), paresthesia (19 patients), fatigue (17 patients), nausea (13 patients), and somnolence (11 patients).
Migraine attack characteristics
For all treated migraine attacks that had ≥ 1 post-dose efficacy assessment (ITT population, n = 17,295), treatment was initiated within a mean of 1.2 hours (SD = 2.6) of the start of migraine pain, with 65.4% of the attacks treated when pain was of moderate intensity and 33.1% when pain was severe (Table 2). Baseline photophobia was reported in 75.5% of the attacks. The MBS was photophobia for 54.4% of the attacks that recorded an MBS, phonophobia for 23.8%, and nausea for 21.8%. A small percentage of attacks were treated with medications other than lasmiditan before the 2-hour assessment (3.3%) and after the 2-hour assessment (8.2%). Thirty-seven percent of attacks were treated with a second dose of lasmiditan, primarily for rescue (90.5%). Of the attacks that achieved pain freedom at 2 hours post-dose, 6.1% were treated with a second dose of lasmiditan for recurrence. For the remaining 4.8% of attacks treated with a second lasmiditan dose, data were unavailable to determine whether the second dose was for rescue or for recurrence. For the mITT population (n = 16,777 attacks treated with ≥ 1 dose of lasmiditan within 4 hours of onset), a total of 17.1% of all treated migraine attacks that achieved pain freedom at 2 hours had recurrence of pain up to 48 hours post-dose. Of all treated migraine attacks that were pain-free at 2 hours, 10.1% of those in the lasmiditan 100 mg group and 8.7% of those in the lasmiditan 200 mg group were treated with either a second dose of lasmiditan or with other medication. Among patients who were in the study ≥ 30 days, one patient in the 100 mg group and two patients in the 200 mg group treated an average of ≥ 6 migraine attacks in a 30-day period. No patient treated an average of ≥ 7 migraine attacks in a 30-day period.
Table 2.
Characteristics of treated migraine attacks (ITT population).
| Characteristic | Lasmiditan 100 mg n = 8782 | Lasmiditan 200 mg n = 8513 | All treated migraine attacks n = 17295 |
|---|---|---|---|
| Time to dosing from migraine pain start, mean (SD), h | 1.2 (2.4) | 1.3 (2.8) | 1.2 (2.6) |
| Baseline migraine severity, n (%) | |||
| Severe | 2872 (32.7) | 2846 (33.4) | 5718 (33.1) |
| Moderate | 5762 (65.6) | 5546 (65.1) | 11308 (65.4) |
| Mild | 141 (1.6) | 115 (1.4) | 256 (1.5) |
| None | 7 (0.1) | 6 (0.1) | 13 (0.1) |
| Baseline symptoms, n (%) | |||
| Nausea | 3527 (40.2) | 3188 (37.4) | 6715 (38.8) |
| Phonophobia | 5609 (63.9) | 4988 (58.6) | 10597 (61.3) |
| Photophobia | 6741 (76.8) | 6322 (74.3) | 13063 (75.5) |
| Vomiting | 275 (3.1) | 302 (3.5) | 577 (3.3) |
| None | 792 (9.0) | 962 (11.3) | 1754 (10.1) |
| Baseline MBS, n | 7987 | 7550 | 15537 |
| Nausea, n (%) | 1710 (21.4) | 1683 (22.3) | 3393 (21.8) |
| Phonophobia, n (%) | 1970 (24.7) | 1726 (22.9) | 3696 (23.8) |
| Photophobia, n (%) | 4307 (53.9) | 4141 (54.9) | 8448 (54.4) |
| Other medications taken to treat migraine, n (%) | |||
| Yes, before 2-h assessment | 274 (3.1) | 293 (3.4) | 567 (3.3) |
| Yes, after 2-h assessment | 841 (9.6) | 584 (6.9) | 1425 (8.2) |
| Number of attacks treated with a second dose, n (%) | 3627 (41.3) | 2776 (32.6) | 6403 (37.0) |
| Number of attacks treated with second dose for rescue, n (%) | 3317 (91.5) | 2476 (89.2) | 5793 (90.5) |
| Number of attacks pain free at 2 ha,b, n (%) | 2298 (26.9) | 2665 (32.4) | 4963 (29.6) |
| Number of attacks treated with second dose for recurrence, n (%) | 152 (6.6) | 150 (5.6) | 302 (6.1) |
| Total number of treated migraine attacks during the entire study, mean (SD)c | 9.3 (9.4) | 8.0 (9.0) | 9.0 (9.2) |
| Number of doses taken per treated migraine attack, mean (SD) | 1.4 (0.5) | 1.3 (0.5) | 1.4 (0.5) |
ITT: intent-to-treat; MBS: most bothersome symptom; SD: standard deviation.
Pain freedom is defined as a reduction in pain severity from mild, moderate, or severe at baseline to none at 2 hours post-dose. Patients were assumed to not have achieved pain freedom at 2 hours if they did not have an associated pain severity rating at the 2-hour time point, took rescue medication within the first 2 hours, or used alternative medication prior to the study drug to treat the migraine attack.
Percentages calculated using the number of treated migraine attacks with mild, moderate, or severe headache pain at baseline as denominator: n = 8532 for lasmiditan 100 mg; n = 8232 for lasmiditan 200 mg; n = 16,764 overall.
Percentages calculated using the number of patients with treated migraine attacks that met the ITT criteria as denominator: n = 941 for lasmiditan 100 mg; n = 990 for lasmiditan 200 mg; n = 1931 overall.
Safety
A total of 962 patients (48.6%) reported ≥ 1 TEAE during the study (Table 3). Fewer patients in the lasmiditan 100 mg group (45.1%) than in the 200 mg group (52.0%) reported ≥ 1 TEAE. Of the patients who reported ≥ 1 TEAE, 86.0% reported a TEAE that was considered by the investigator to be reasonably or possibly related to lasmiditan treatment. Nine patients (0.5%) reported 13 treatment-emergent SAEs (100 mg group: limb abscess [n = 1], carbuncle [n = 1], cellulitis [n = 1]; 200 mg group: bradycardia and sinus node dysfunction [n = 1], gastritis {reported twice by the same patient}, sinusitis, and acute cholecystitis [n = 1], urinary tract infection [n = 1], lumbar spinal stenosis [n = 1], recurrent thyroid cancer [n = 1], nephrolithiasis [n = 1]). No treatment-emergent SAE occurred in more than one patient, and none were considered by the investigator to be related to lasmiditan. No deaths were reported during the study.
Table 3.
Summary of adverse events (safety population).
| Type of eventa | Lasmiditan 100 mg n = 963 |
Lasmiditan 200 mg n = 1015 |
All patients n = 1978 |
|||
|---|---|---|---|---|---|---|
| Events | n (%) | Events | n (%) | Events | n (%) | |
| Any AE | 2368 | 636 (66.0) | 3438 | 731 (72.0) | 5806 | 1367 (69.1) |
| Relatedb AEs | 1293 | 398 (41.3) | 2224 | 523 (51.5) | 3517 | 921 (46.6) |
| Any TEAEc | 1337 | 434 (45.1) | 2219 | 528 (52.0) | 3556 | 962 (48.6) |
| Relatedb TEAEs | 1157 | 361 (37.5) | 2010 | 466 (45.9) | 3167 | 827 (41.8) |
| Any SAE | 37 | 28 (2.9) | 43 | 32 (3.2) | 80 | 60 (3.0) |
| Treatment-emergentc SAEs | 3 | 3 (0.3) | 10 | 6 (0.6) | 13 | 9 (0.5) |
| Relatedb SAEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
AE: adverse event; SAE: serious adverse event; MedDRA: Medical Dictionary for Regulatory Activities; TEAE: treatment-emergent adverse event.
AEs were coded using MedDRA, Version 21.0.
“Related” events are those recorded by the study investigator as “Reasonably or Possibly Related” or those with a missing relationship.
An AE that started or worsened within 48 hours after the last dose (either the first or the second dose) of lasmiditan was considered treatment-emergent.
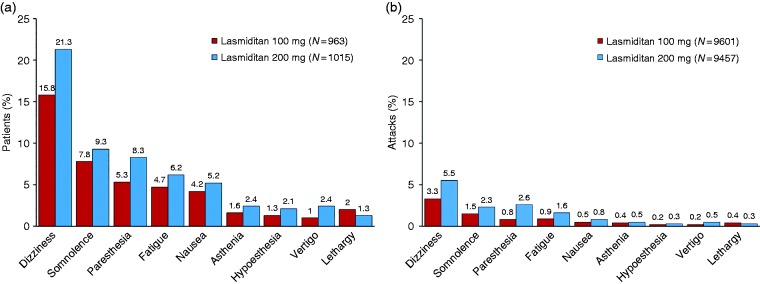
Most TEAEs were central nervous system (CNS) in nature, with the most frequently reported event being dizziness (Figure 3). The TEAEs were generally of mild to moderate severity. In a subset of patients who treated ≥ 5 attacks (n = 1126), in general, the incidence of TEAEs decreased across treated attacks 1 to 5 both overall (Figure 4) and for the most frequently reported TEAEs (Supplemental Table 2).
Figure 3.
Frequently reported treatment-emergent adverse events (a) by percentage of patients and (b) by percentage of attacks (safety population).
AE: adverse event; MedDRA: Medical Dictionary for Regulatory Activities.
Note: Reported by ≥ 2% of patients in either treatment group. An AE that started or worsened within 48 hours after the last dose (either the first or the second dose) of lasmiditan was considered treatment-emergent. AEs were coded using MedDRA Version 21.0.
Figure 4.
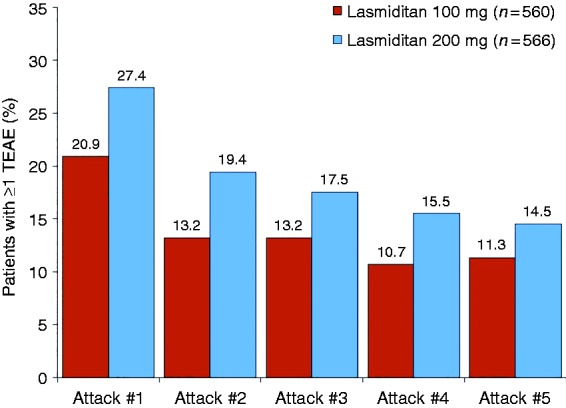
Treatment-emergent adverse events by migraine attack in patients who treated ≥ 5 attacks (safety population).
TEAE: treatment-emergent adverse event.
Because most TEAEs were CNS in nature, data were medically reviewed for patients experiencing a CNS TEAE in temporal association with accidents and/or injuries. Of note, one patient had a treatment-emergent road traffic accident and sciatica reported on the same date. Follow-up information from the site revealed that the accident led to sciatica. Dosing in this case was 2 days prior to the road traffic accident (the patient was driving the vehicle; concomitant medications included lithium and quetiapine). In addition, although the common TEAEs associated with lasmiditan are indicative of CNS drug effects and some of these CNS-related TEAEs can be associated with the potential for abuse, there were no instances of abuse of lasmiditan reported and no evidence of lasmiditan misuse, abuse, or diversion.
Of the patients in the safety population, 12.8% discontinued due to an AE (11.2% of the 100 mg group, 14.4% of the 200 mg group) (Figure 2). Dizziness was the most common AE leading to discontinuation (2.7% of the 100 mg group, 4.3% of the 200 mg group). Patients who discontinued due to dizziness were more likely to do so after the first or second treated migraine attack than after subsequent treated attacks.
No CV TEAEs potentially due to vasoconstriction (e.g. angina pectoris, uncontrolled hypertension, ischemic stroke) were observed. The incidence and type of AEs (TEAEs or other AEs) reported were similar for patients with and without CV risk factors (data not shown).
Overall, no pattern of clinically meaningful changes from baseline were observed in laboratory parameters, vital signs, or ECGs (data not shown).
Efficacy
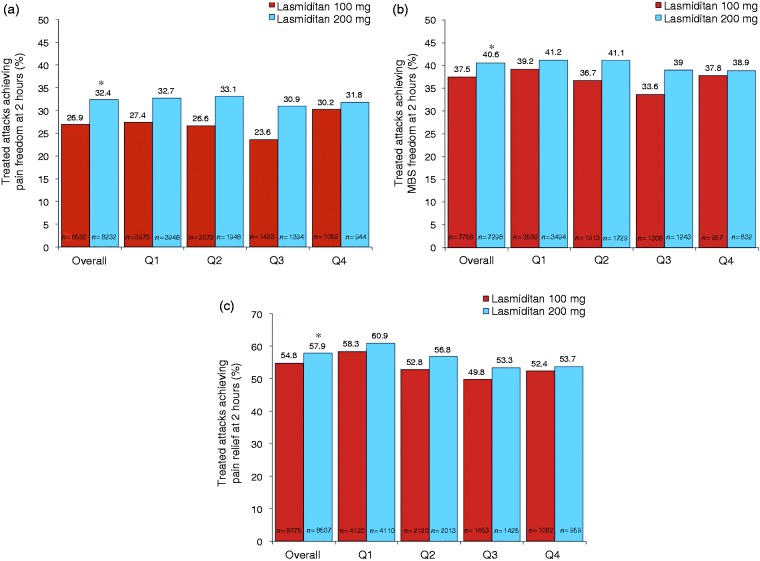
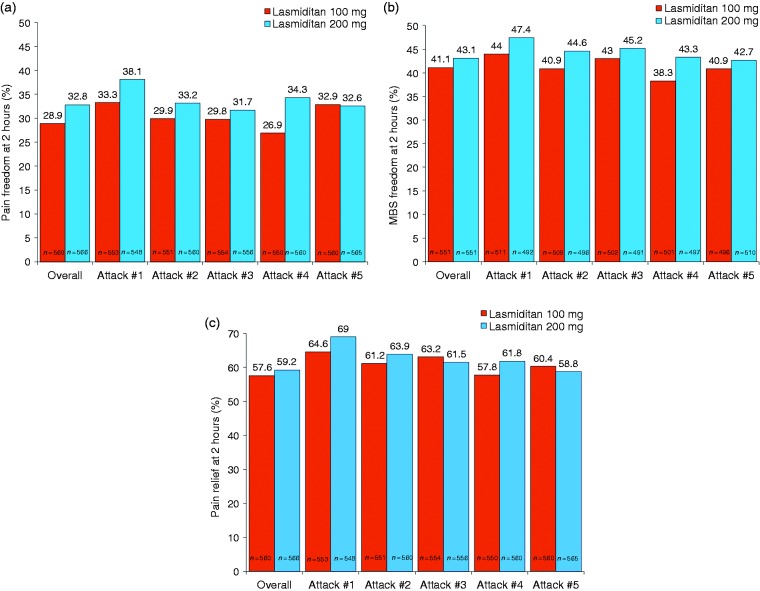
Overall, across all treated attacks at 2 hours post-dose, pain freedom was observed in 29.6% of attacks, MBS freedom in 39.0%, and pain relief in 56.3%, with significantly higher percentages observed in the 200 mg group than in the 100 mg group (all comparisons, p < 0.001) (Figure 5).The percentage of attacks achieving pain freedom, MBS freedom, and pain relief at 2 hours post-dose was similar across quarters. Results were similar for completers (data not shown). In a subgroup analysis, the percentage of patients achieving pain freedom, MBS freedom, and pain relief at 2 hours post-dose was consistent across treated attacks 1 to 5 in patients who treated ≥ 5 attacks (Figure 6).
Figure 5.
Treated migraine attacks achieving (a) pain freedom, (b) most bothersome symptom freedom, and (c) pain relief at 2 hours post-dose overall and by quarter.
ITT: intent-to-treat; MBS: most bothersome symptom; mITT: modified intent-to-treat; Q1: quarter 1 (months 0–3); Q2: quarter 2 (months 3–6); Q3: quarter 3 (months 6–9); Q4: quarter 4 (months 9–12).
Note: Pain freedom is defined as a reduction in pain severity from mild, moderate, or severe at baseline to none at 2 hours post-dose. MBS freedom is defined as the absence of the associated symptom of migraine (nausea, phonophobia, or photophobia) at 2 hours post-dose that was identified pre-dose as the MBS. Pain relief is defined as experiencing moderate or severe pain at baseline that becomes mild or none at 2 hours post-dose or mild pain at baseline that becomes none at 2 hours post-dose for each treated attack. Patients who recorded that no symptoms were present at baseline were excluded from the MBS analysis. Patients were assumed to not have achieved pain freedom at 2 hours if they did not have an associated pain severity rating at the 2-hour time point, took rescue medication within the first 2 hours, or used alternative medication prior to the study drug to treat the migraine attack. The pain relief analysis was conducted on the ITT population; the pain freedom and MBS freedom analyses were conducted on the mITT population. *p < 0.001, 100 mg versus 200 mg (based on a one-sided test from a logistic regression model with treatment group and background use of medication to reduce the frequency of migraines as covariates).
Figure 6.
Patients achieving (a) pain freedom, (b) most bothersome symptom freedom, and (c) pain relief at 2 hours post-dose by migraine attack in patients who treated ≥ 5 attacks (ITT population).
ITT: intent-to-treat; MBS: most bothersome symptom.
Note: Pain freedom is defined as a reduction in pain severity from mild, moderate, or severe at baseline to none at 2 hours post-dose. MBS freedom is defined as the absence of the associated symptom of migraine (nausea, phonophobia, or photophobia) at 2 hours post-dose that was identified pre-dose as the MBS. Pain relief is defined as experiencing moderate or severe pain at baseline that becomes mild or none at 2 hours post-dose or mild pain at baseline that becomes none at 2 hours post-dose for each treated attack. Patients who recorded that no symptoms were present at baseline were excluded from the MBS analysis. Patients were assumed to not have achieved pain freedom at 2 hours if they did not have an associated pain severity rating at the 2-hour time point, took rescue medication within the first 2 hours, or used alternative medication prior to the study drug to treat the migraine attack.
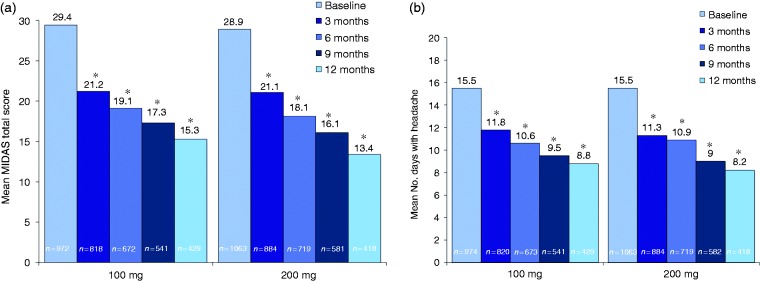
The mean baseline MIDAS total score of approximately 29 indicated that, on average, patients had severe migraine disability (Table 1). Mean MIDAS total scores and number of days with headache over the past 3 months decreased significantly from baseline to 3, 6, 9, and 12 months for both the lasmiditan 100 mg and 200 mg treatment groups (all comparisons, p < 0.001 vs. baseline), with no significant differences between dose groups (Figure 7). For the safety population, the number of migraine attacks treated with lasmiditan on a per-patient basis by quarter of study participation decreased across the study quarters for both the 100 mg and 200 mg groups (Figure 8).
Figure 7.
Change over time in (a) MIDAS total score and (b) days with headache in the past 3 months (MIDAS population).
MIDAS: migraine disability assessment. MIDAS total score was calculated as the sum of the answers to the five questions on the MIDAS questionnaire (0–5 = little or no disability; 6–10 = mild disability; 11–20 = moderate disability; ≥ 21 = severe disability). *p < 0.001 vs. baseline (mixed model for repeated measures); no significant differences were observed between the lasmiditan doses.
Figure 8.
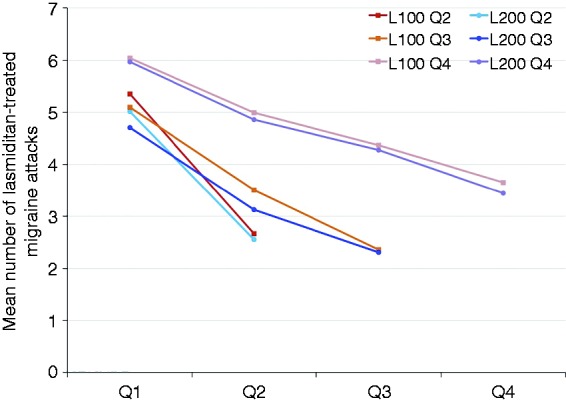
Lasmiditan-treated migraine attacks by quarter in patients with a maximum of two, three, and four quarters of data available (safety population).
L100: lasmiditan 100 mg; L200: lasmiditan 200 mg; Q1: quarter 1 (months 0–3); Q2: quarter 2 (months 3–6); Q3: quarter 3 (months 6–9); Q4: quarter 4 (months 9–12). The figure includes data for patients who had two quarters, three quarters, and four quarters of data available.
Discussion
The GLADIATOR study is an ongoing, prospective, randomized, open-label Phase 3 study of lasmiditan 100 mg or 200 mg taken intermittently as needed for up to 1 year in patients with migraine who previously completed one of the randomized, placebo-controlled, double-blind, Phase 3 single-attack studies, SAMURAI or SPARTAN. The interim safety and efficacy results of this study are consistent with those of the single-attack studies, which showed a favorable safety profile and a benefit of lasmiditan for reducing both the pain and most bothersome symptoms of migraine attacks (13,14). Analyses of TEAEs over time generally showed a decrease in the incidence of these events with subsequent treated migraine attacks. Consistent efficacy at a population level over time was observed with long-term intermittent lasmiditan treatment.
No new serious safety findings were observed, with no deaths occurring and no other trends with regard to SAEs reported during treatment with lasmiditan for up to 1 year. Laboratory parameters, vital signs, and ECG assessments did not show any clinically meaningful changes. Moreover, lasmiditan was shown to have an acceptable safety profile during treatment for up to a year in a population of patients with CV risk factors, which is consistent with the non-vasoconstrictive mechanism of lasmiditan (24) and is important for patients for whom triptans are contraindicated due to their vasoconstrictive properties.
With regard to TEAEs, the incidence and types of events observed were similar to those in the single-attack studies, with the most commonly reported events being CNS in nature (13,14). Most events were of mild to moderate severity, and the most frequently reported TEAEs generally appeared to be dose dependent. GLADIATOR extended the safety findings of the single-attack studies by showing that, in general, at the population level and in a subgroup of patients treating ≥ 5 migraine attacks, the frequency of TEAEs decreased with subsequent treated attacks. In addition, despite the most frequently reported TEAEs being CNS in nature, there were no reports of accidents or injuries resulting from a CNS TEAE and no evidence of lasmiditan misuse, abuse, or diversion reported during long-term intermittent treatment.
Because there was little opportunity to discontinue in the single-attack lasmiditan studies, GLADIATOR is the first study to evaluate the tolerability of lasmiditan with regard to AEs leading to discontinuation. The most common AE leading to discontinuation was dizziness, which was also the most common TEAE reported during lasmiditan treatment. Approximately 17% of the patients reporting dizziness at any time in the study (not necessarily treatment-emergent) discontinued due to this event. In addition, patients who discontinued due to dizziness were more likely to do so after the first or second treated attack than after subsequent treated attacks. When tolerability of the 100 mg versus the 200 mg dose group is considered, including dose dependency of TEAEs and discontinuations due to AEs, dose adjustment (which was not permitted in this study) may help improve tolerability and subsequent adherence to lasmiditan treatment.
The overall rate of discontinuation in GLADIATOR was 51.7% for patients taking ≥ 1 dose of lasmiditan. Although differences in study designs must be considered, this rate of discontinuation is higher than that observed in a randomized, double-blind, 12-month study of telcagepant versus rizatriptan, in which 42.4% and 35.1% of patients discontinued, respectively (25). Earlier long-term studies of zolmitriptan (26) and almotriptan (27) had rates of discontinuation similar to those for the telcagepant versus rizatriptan trial (25). The most common reason for discontinuation in the present study was “patient request,” which accounted for 42.1% of the discontinuations. To better understand discontinuations, patients were asked to provide comments regarding why they discontinued. Medical review of all comments suggested that some patients discontinued because of lack of efficacy or dislike of investigational product, dislike of e-diary requirements, relocation, scheduling conflicts, reduction in the frequency or lack of migraine attacks, and initiation of prohibited medication or medication reducing frequency of migraine attacks. It should also be noted that this trial included study design features that are unusual for an open-label extension trial that might have influenced persistence, including the following: A 12-hour driving restriction after lasmiditan dosing, a requirement that patients wait for moderate to severe headache pain prior to dosing, daily e-diary entry requirement, baseline and six post-baseline assessments for each migraine attack, and randomization to either 100 mg or 200 mg lasmiditan without an opportunity to titrate dose to optimize benefits and risks. The long-term persistence to lasmiditan during more real-world use would be of interest.
Although this study was designed primarily to evaluate the long-term safety of lasmiditan, efficacy was also assessed as a secondary endpoint, and the efficacy results observed in GLADIATOR were similar to those observed in the single-attack studies (13,14). Because no dose adjustments were made over the course of GLADIATOR participation, the efficacy dose-response relationships observed between the 100-mg and 200-mg doses in the single-attack studies were also confirmed. The present study adds data showing that lasmiditan was effective when taken for more than one attack, and, notably, very few of the attacks that were pain-free at 2 hours post-dose were treated with a second lasmiditan dose for recurrence (6.1%). Similar efficacy outcomes were observed at the population level across all four quarters of the study and in the first five treated attacks in patients who treated ≥ 5 attacks. The finding that efficacy outcomes were relatively constant over study quarters is consistent with long-term trials of triptans, which generally show stable efficacy, rather than improvement, over time (25–27). Finally, MIDAS total score and number of days with headache in the past 3 months both decreased during intermittent treatment with lasmiditan. Given that the MIDAS questionnaire estimates productive time lost in the preceding 3 months due to the disabling effect of migraine, the decreases in MIDAS total scores in the present study are clinically relevant. The number of migraine attacks treated with lasmiditan per patient per quarter similarly decreased over time. The decrease in MIDAS total scores, number of days with headache, and patient frequency of treating migraine attacks with lasmiditan over time merit further investigation.
Some limitations exist with regard to the interpretation of results from this study. First, this was an open-label study that lacked a placebo control for comparison of both safety and efficacy results. Second, there was a high rate of patient dropout, with study design and burden likely being contributing factors. Additionally, patients taking placebo or 50 mg, 100 mg, or 200 mg lasmiditan during the double-blind parent studies were randomized to receive 100 mg or 200 mg lasmiditan regardless of prior response and tolerability, which could also have led to discontinuations for both efficacy and safety reasons. Third, patients did not necessarily use lasmiditan to treat all of their migraine attacks; for example, if their migraine pain did not rise above mild severity. Fourth, because the primary objective of this long-term study was to assess the safety of lasmiditan for treatment of multiple attacks, we did not collect efficacy information associated with alternative acute treatment. Fifth, assessments at scheduled visits of laboratory parameters, vital signs, and ECGs were not necessarily conducted in temporal proximity to the dosing of lasmiditan. Finally, the results of this study may not be fully generalizable to all patients with migraine.
In conclusion, the interim safety and efficacy results of this long-term lasmiditan study were consistent with those observed in the single-attack lasmiditan studies (13,14). Lasmiditan had a favorable long-term safety profile, with no new safety issues identified, and, in general, the frequency of TEAEs appeared to decrease with repeated dosing. The positive effects of lasmiditan treatment on pain freedom, MBS freedom, and pain relief were similar by quarter for up to 1 year. Further research may be useful in elucidating the potential of lasmiditan for influencing migraine frequency and severity.
Abbreviations
AMPP: American Migraine Prevalence and Prevention; 5-HT: 5-hydroxytryptamine; CV; Cardiovascular; MBS: Most bothersome symptom; SAE: Serious adverse event; AE: Adverse event; US: United States; ICH: International Council for Harmonisation; IHS: International Headache Society; MIDAS: Migraine Disability Assessment; CAD: Coronary artery disease; ECG: Electrocardiogram; MedDRA: Medical Dictionary for Regulatory Activities; TEAE: Treatment-emergent adverse event; FDA: US Food and Drug Administration; CHMP: Committee for Medicinal Products for Human Use; mITT: Modified intent-to-treat; ITT: Intent-to-treat; SD: Standard deviation; IQR: Interquartile range; CNS: Central nervous system.
Clinical implications
This study adds long-term, repeated intermittent dosing data to the current evidence that lasmiditan is generally safe and well tolerated for the acute treatment of migraine attacks.
Lasmiditan demonstrated consistent efficacy at a population level by quarter for the acute treatment of migraine attacks for up to 1 year.
Patients treated with lasmiditan showed a decrease in migraine disability. Further research may be useful in elucidating the potential of lasmiditan to influence migraine frequency and severity.
Supplemental Material
Supplemental material, CEP864132 Supplemental material criteria for Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study) by Jan Lewis Brandes, Suzanne Klise, John H Krege, Michael Case, Rashna Khanna, Raghavendra Vasudeva, Joel Raskin, Eric M Pearlman and David Kudrow in Cephalalgia
Supplemental Material
Supplemental material, CEP864132 Supplemental material tables for Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study) by Jan Lewis Brandes, Suzanne Klise, John H Krege, Michael Case, Rashna Khanna, Raghavendra Vasudeva, Joel Raskin, Eric M Pearlman and David Kudrow in Cephalalgia
Acknowledgments
Writing support for the development of this manuscript was provided by Cindy C Taylor at Synchrogenix, a Certara company, and funded by Eli Lilly and Company. Draft development and revisions were under the direction of the authors in accordance with the International Committee of Medical Journal Editors (ICMJE) criteria for authorship.
Author contributions
All authors were involved in the analysis and interpretation of the data and critically revised manuscript drafts for intellectual content. In addition, JLB and DK conducted the study as principal investigators at their respective sites, and MC conducted statistical analyses.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SK, JHK, MC, RK, RV, JR, and EMP are employees and minor shareholders of Eli Lilly and Company. DK has received research grants from Eli Lilly and Company, Amgen, Allergan, Teva, Alder, Biohaven, Roche, and Zosano and has served on advisory boards for Amgen/Novartis, Eli Lilly and Company, Alder, and Biohaven. JLB has received research grants from Allergan, Teva, Amgen, Alder, Biohaven, Zosano, Colucid, and Eli Lilly and Company and has served on advisory boards/lecturer for Eli Lilly and Company, Teva, Amgen, Promius, Supernus, Valeant, and Avanir.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The GLADIATOR study was sponsored and supported by Eli Lilly and Company.
Previous presentation
Some results of this trial were presented at the 17th Biennial Migraine Trust International Symposium (MTIS), September 6–9, 2018, London, UK; American Academy of Neurology (AAN) Annual Meeting, 4–11 May 2019, Philadelphia, PA, USA (28,29).
References
- 1.Lipton RB, Buse DC, Serrano D, et al. Examination of unmet treatment needs among persons with episodic migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2013; 53: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 2.Viana M, Genazzani AA, Terrazzino S, et al. Triptan nonresponders: Do they exist and who are they? Cephalalgia 2013; 33: 891–896. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Beltran E, Labastida-Ramirez A, Villalon CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther 2018; 186: 88–97. [DOI] [PubMed] [Google Scholar]
- 4.Negro A, Koverech A, Martelletti P. Serotonin receptor agonists in the acute treatment of migraine: A review on their therapeutic potential. J Pain Res 2018; 11: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuter U, Israel H, Neeb L. The pharmacological profile and clinical prospects of the oral 5-HT1F receptor agonist lasmiditan in the acute treatment of migraine. Ther Adv Neurol Disord 2015; 8: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goadsby PJ. The vascular theory of migraine – a great story wrecked by the facts. Brain 2009; 132: 6–7. [DOI] [PubMed] [Google Scholar]
- 7.Mitsikostas DD, Tfelt-Hansen P. Targeting to 5-HT1F receptor subtype for migraine treatment: Lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem 2012; 12: 241–249. [DOI] [PubMed] [Google Scholar]
- 8.Dodick D, Lipton RB, Martin V, et al. Consensus statement: Cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache 2004; 44: 414–425. [DOI] [PubMed] [Google Scholar]
- 9.Tajti J, Majlath Z, Szok D, et al. Drug safety in acute migraine treatment. Expert Opin Drug Saf 2015; 14: 891–909. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 2010; 30: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 11.Capi M, de Andres F, Lionetto L, et al. Lasmiditan for the treatment of migraine. Expert Opin Investig Drugs 2017; 26: 227–234. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wietecha LA, Kuca B, Asafu-Adjei J, et al. Phase 3 studies (SAMURAI, SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine. Neurology 2018; 90(Suppl 15): S50.008–S50.008. [Google Scholar]
- 14.Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018; 91: e2222–e2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24: S9–S160. [DOI] [PubMed]
- 16.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S49–S73. [DOI] [PubMed] [Google Scholar]
- 17.American Heart Association. Understanding blood pressure readings: Know your numbers, https://www.heart.org/HEARTORG/Conditions/HighBloodPressure/KnowYourNumbers/Understanding-Blood-Pressure-Readings_UCM_301764_Article.jsp (2017, accessed 5 December 2018).
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 19.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001; 56: S20–S28. [DOI] [PubMed] [Google Scholar]
- 20.Stewart WF, Lipton RB, Kolodner K, et al. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999; 19: 107–114. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WF, Lipton RB, Kolodner KB, et al. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000; 88: 41–52. [DOI] [PubMed] [Google Scholar]
- 22.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau. Quick facts: United States, https://www.census.gov/quickfacts/fact/table/US/PST045217 (2010, accessed 3 December 2018).
- 24.Oswald JC, Schuster NM. Lasmiditan for the treatment of acute migraine: A review and potential role in clinical practice. J Pain Res 2018; 11: 2221–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor KM, Aurora SK, Loeys T, et al. Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache 2011; 51: 73–84. [DOI] [PubMed] [Google Scholar]
- 26.The International 311C90 Long-term Study Group. The long-term tolerability and efficacy of oral zolmitriptan (Zomig, 311C90) in the acute treatment of migraine. An international study. Headache 1998; 38: 173–183. [PubMed] [Google Scholar]
- 27.Pascual J, Falk R, Docekal R, et al. Tolerability and efficacy of almotriptan in the long-term treatment of migraine. Eur Neurol 2001; 45: 206–213. [DOI] [PubMed] [Google Scholar]
- 28.Lipton RB, Lombard L, Ruff DD, et al. Disability improvements over 12 months with lasmiditan for acute treatment of migraine: Interim analysis of migraine disability assessment (MIDAS) scale changes in the GLADIATOR study. Cephalalgia 2018; 38(Suppl 1): 141–142. [Google Scholar]
- 29.Brandes J, Kudrow D, Klise S, et al. Long-term safety and efficacy of lasmiditan for acute treatment of migraine over a one-year period: Interim results of an open-label phase 3 study (GLADIATOR). Neurology 2019; 92(Suppl 15): P1.10–21–P1.10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CEP864132 Supplemental material criteria for Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study) by Jan Lewis Brandes, Suzanne Klise, John H Krege, Michael Case, Rashna Khanna, Raghavendra Vasudeva, Joel Raskin, Eric M Pearlman and David Kudrow in Cephalalgia
Supplemental material, CEP864132 Supplemental material tables for Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study) by Jan Lewis Brandes, Suzanne Klise, John H Krege, Michael Case, Rashna Khanna, Raghavendra Vasudeva, Joel Raskin, Eric M Pearlman and David Kudrow in Cephalalgia