Abstract
Background
Meta-analyses of clinical trial data have identified clinically relevant gender differences in the efficacy of smoking cessation pharmacotherapy. It is unclear whether these findings are generalizable to smokers quitting in real-world contexts.
Methods
Using Tobacco Use Supplement to the Current Population Survey (TUS-CPS) 2010–2011 cross-sectional data, we generated propensity score matched samples of smokers who quit either unassisted by medication, using only varenicline, or using only transdermal nicotine patch (TNP). We used generalized estimating equations to estimate gender differences in the comparative effectiveness of these cessation options for achieving 30-days of abstinence, adjusting for potential confounders.
Results
When stratified by gender, TNP was significantly more effective than unassisted quit attempts for men (OR=1.37; 95%CI=1.02,1.83; p=0.03), but not for women (OR=0.96; 95%CI =0.71,1.31; p=0.82). Varenicline was significantly more effective than unassisted quit attempts for women (OR=1.63; 95%CI=1.16, 2.31; p=0.005), but not men (OR=1.35; 95%CI=0.94,1.96; p=0.11). Varenicline was also more effective than TNP for women (OR=1.51; 95%CI=0.12,2.05; p=0.007) but not men (OR=0.92; 95%CI=0.65,1.31; p=0.64). A significant gender by medication interaction was found only for the comparison of varenicline to TNP (OR=1.64; 95%CI=1.04,2.61; p=0.04).
Conclusions
Findings for varenicline vs. TNP were consistent with clinical trial data, showing greater differences in effectiveness for women compared to men. Results lend support to the generalizability of clinical trial findings, highlighting the importance of considering gender when offering treatment for smoking cessation.
Keywords: gender, smoking, cessation, varenicline, patch, effectiveness
1. INTRODUCTION
Cigarette smoking continues to affect a large portion of U.S. adults (Jamal, 2016), despite recent declines in smoking, and is therefore poised to adversely affect public health for the considerable future. However, declines in smoking suggest that more and more smokers are interested in quitting, and so it is important to continue exploring and improving upon the ability to aid smokers in their quit attempts. A large portion of those attempting to quit smoking use at least one of the medications approved by the U.S. Food and Drug Administration (nicotine replacement therapy (NRT), varenicline, or bupropion) as smoking cessation aids (Smith et al., 2015).
1.1. Sociopharmacology of tobacco addiction
Leventhal (2016) proposed the Sociopharmacological framework for the study of nicotine dependence and its treatment in the context of tobacco health disparities. In the model, psychopharmacological stimuli and resulting acute psychopharmacological effects are influenced by (and also influence) an individual’s disparity group membership and related contextual factors, resulting in greater or lesser burden of tobacco use and tobacco-related morbidity and mortality. Study of the sociopharmacology of tobacco dependence and treatment holds great promise for reducing tobacco-related health disparities. Cross-cutting methodologies can be used to study the sociopharmacology of tobacco dependence by examining interactions between social/contextual factors and pharmacology-related variables using both observational and experimental study designs.
1.2. Gender disparities and tobacco use
Women have not traditionally been a focus of tobacco disparity research (Smith et al., 2014a; Smith et al., 2016a) perhaps in part because historically and throughout the world men have been more likely to smoke than women. Yet, in several countries smoking among men is on the decline while smoking among women is increasing, particularly among young women and among those with social and economic disadvantages (Amos et al., 2012). Further, across countries researchers have found that in any given quit attempt women are less likely to successfully quit smoking than men (Smith et al., 2016a). Factors underlying this difference may cover the full social-ecological spectrum of individual and contextual variables. For example, there are gender differences in the relationship between negative affect and tobacco use (Perkins and Karelitz, 2015), and women tend to be more strongly influenced by tobacco use cues than men (Carpenter et al., 2014). There are also important differences in hepatic metabolism for women compared to men (Ilic et al., 2013; Lamba et al., 2003), and these differences have been hypothesized to result in gender differences in smoking cessation medication response (Smith et al., 2016b). Women are much more likely than men to experience both sexual victimization and harassment (Breiding et al., 2014), both of which are associated with substance use (Kristman-Valente et al., 2013; Neville et al., 2014; Smith et al., 2014b). With regard to the U.S., there is a well-document gender gap in income and poverty (DeNavas-Walt, 2015; Institute for Women’s Policy Research, 2015) and evidence suggests financial distress may be more strongly related to difficulty with quitting smoking among women compared to men (McKee et al., 2003). Particular sub-groups of women
1.3. Gender and smoking cessation pharmacotherapy clinical efficacy
Given the existence of gender inequalities in the U.S. and elsewhere, accompanied by gender differences in smoking cessation, Leventhal’s Sociopharmacological model provides a fitting framework for the study of gender differences in the effectiveness of smoking cessation pharmacotherapy. Previous research has built empirical support for this notion, finding important gender differences in the clinical efficacy of smoking cessation medications (McKee et al., 2015; Perkins and Scott, 2008; Scharf and Shiffman, 2004). In a recently published meta-analysis of over 30 clinical trials Smith et al. (2016) demonstrated that the relative benefit of varenicline over transdermal nicotine patch (TNP) and bupropion was significantly greater among women compared to men. Although varenicline demonstrated equivalent efficacy for women and men, among women TNP and bupropion were substantially less efficacious than varenicline, while among men there was no significant difference in the clinical efficacy of the three medications.
1.4. Gender and smoking cessation pharmacotherapy real-world effectiveness
Clinical trial participants represent a relatively homogenous sample of cigarette smokers who are attempting to quit in highly regulated and unique contexts, making study of generalizability to real-world medication effectiveness critical. A number of investigations have documented the real-world effectiveness of smoking cessation medications (e.g. (Brose et al., 2013; Prado et al., 2011; Ucar et al., 2014)); however gender differences in real-world effectiveness are understudied. Walker et al. (2016) studied such differences using data from a national quit service in the United Kingdom, Quit-5. Their findings demonstrated that women were less likely than men to achieve 12-week abstinence, and that the relative advantage of varenicline over NRT was significantly greater for women compared to men. It is notable that this gender difference in effectiveness is consistent with efficacy results from clinical trial data (Smith et al., 2016b). Given that Walker et al. (2016) utilized data from health records, the authors were limited in their ability to adjust for potentially relevant confounders. There is a need to test hypotheses related to gender differences in smoking cessation medication effectiveness using data with availability of a range of potential confounders. Addressing this gap has the potential to help inform clinical decisions when considering smoking cessation pharmacotherapy options, improving personalized treatment of nicotine independence and smoking cessation outcomes for both women and men.
1.5. Study aims and hypotheses
We conducted a comparative effectiveness investigation utilizing cross-sectional observational population data from the United States (U.S.), in order to compare the relative effectiveness of using TNP, varenicline only, or no medication during quit attempts for women versus men. In concordance with recommended practice, we utilized both propensity score matching and regression adjustment techniques to estimate medication effectiveness comparisons. We hypothesized that previous meta-analytic clinical trial findings would extend to real-world contexts, as supported by prior evidence (Walker et al., 2016), whereby the advantage of varenicline over TNP would be greater for women compared to men. Specifically, that women and men would demonstrate similar effectiveness for varenicline, but that TNP would be less effective for women as compared to men.
2. MATERIAL AND METHODS
Data were analyzed from the 2010–2011 Tobacco Use Supplement of the Current Population Survey, collected by the U.S. Census Bureau and sponsored by the National Cancer Institute (U.S. Department of Commerce, 2012). We included in our sample those who were smoking 12 months prior to their interview and who made at least one quit attempt during since that time, regardless of the success of the outcome (n = 7,906). We defined those who were smoking 12 months prior to the interview as persons who smoked at least 100 cigarettes in their lifetime and either a) were smoking ‘all days’ or ‘some days’ at the time of the interview (current smokers) and reported smoking for at least 1 year, or b) were not smoking at the time of the survey, but retrospectively reported they had been smoking ‘all days’ or ‘some days’ when asked, “Around this time 12 MONTHS AGO, were you smoking cigarettes every day, some days, or not at all?”. Quit attempters were defined as meeting the criteria above for smoking 12 months ago and either a) among current smokers, reported having made at least one attempt to quit smoking that lasted at least one day during the previous 12 months, or b) were former smokers at the time of the interview who had at least 30 days of abstinence. Former smokers who had achieved less than 30 days of abstinence were removed from analyses.
2.1. Measures
2.1.1. Smoking cessation outcome
Our smoking cessation outcome was having achieved at least 30-days of abstinence from smoking. When considering our smoking cessation outcome, we were constrained by the cross-sectional study design. For example, ideally, we would have considered a more extended time period of abstinence (e.g., 6 months); however, we had to take into account the inverse relationships between our time period for smoking abstinence and both statistical power and the potential for recall bias. We considered 30-day abstinence as providing adequate balance among these three factors; i.e., a balance between having achieved abstinence for a meaningful period of time, statistical power, and accuracy of recall.
2.1.2. Treatment
Current smokers who reported a quit attempt during the previous 12 months were asked about medication use during their previous quit attempt. Medications included NRT (including a question about specific formulation; e.g., oral, transdermal), bupropion, and varenicline. We originally considered conducting pairwise comparisons between each of the following categories: no medication, TNP only, oral NRT only, bupropion only, varenicline only, combination NRT only, combination bupropion plus NRT only, and combination varenicline plus NRT only. However, sample sizes were prohibitively small for some groups, particularly when employing propensity score matching. For example, after employing the propensity score matching procedure detailed below (see Statistical analyses) for the comparison of bupropion vs. varenicline, the sample sizes of women and men in the bupropion treatment group were 64 and 38, respectively. We ultimately limited our pairwise comparisons among the following treatments: no treatment, TNP only, and varenicline only. Throughout this manuscript we use the term ‘unassisted’ quit attempt to mean pharmacologically unassisted, with potential use of non-pharmacological cessation aids.
2.1.3. Potential confounder variables
We selected a range of potential confounder variables from those available in the TUS-CPS data, using Leventhal’s Sociopharmacological model of tobacco use as a guide (Leventhal, 2016). Based on recommended best practices for effectiveness research using observational data (Berger et al., 2009; Cox et al., 2009; Johnson et al., 2009), our goal was to be over inclusive of potential confounders and then use data driven approaches to make our final selection of confounders for modeling (see Statistical analysis). Consequently we did not impose any strict criteria of variables having shown prior empirical evidence of affecting smoking cessation; rather, we grouped potential confounders into two general categories based on Leventhal’s model: disparity group membership/contextual factors and psychopharmacological stimuli. Disparity group membership/contextual related factors included age, race/ethnicity, education, household income, marital status, employment status, physician or dentist advice to quit smoking during the previous 12 months, and the following alternative smoking cessation methods: quitline, counseling, utilization of smoking cessation clinic services, friend/family support, internet, self-help books, or acupuncture. Psychopharmacological stimuli include cigarettes per day 12 months prior to the survey (i.e., before the quit attempt), usual frequency of smoking 12 months prior to the survey (days of smoking per month), age of smoking initiation, and whether the individual smoked menthol cigarettes as their cigarette of choice 12 months prior to the survey.
2.2. Statistical analyses
We first described our sample, stratified by medication use within stratification by gender. Our first step in propensity score matching analyses was to identify variables most likely to be confounders. To do so, we stratified the sample by gender and examined mutually adjusted associations between all confounder variables and our dependent variable (30-day abstinence). Variables significantly associated with 30-day abstinence for both women and men were determined to be the variables most likely to confound associations, and were consequently selected for propensity score matching.
For each medication comparison, we first used the program PSMATCH2 in Stata version 13.1 to conduct 1:1 matching, stratified by gender. We conducted matching without replacement based on the log odds of propensity scores, and specified calipers of 0.05. We also considered Mahalanobis matching; however, 1:1 matching generated the most balanced groups. After propensity-score matched samples were generated for each medication comparison, we stratified by gender and tested whether our propensity score analysis generated balanced groups for the matched variables. We then combined data from genders, and tested treatment by gender interactions in relation to 30-day abstinence, using Generalized Estimating Equations with robust standard errors and exchangeable working correlation matrices. We elected to model using Generalized Estimating Equations to account for non-independence inherent in matched data. We added all potential covariates to our models in order to minimize residual confounding, and to control for differences between women and men.
3. RESULTS
3.1. Sample characteristics
Descriptive statistics the sample of women and for women stratified by medication use (prior to propensity score matching) are displayed in Table 1. Corresponding estimates for men are displayed in Table 2. Among both women and men, differences between medication use groups were generally statistically significant, due to the large sample sizes. Among women, those making unassisted quit attempts were younger, more likely to identify as a minority race/ethnicity, more likely to be never married, had lower income, were less likely to report physician or dentist advice to quit, and were less likely to use non-medication cessation aids (e.g., quitline). Those making unassisted quit attempts also smoked fewer cigarettes per day, smoked less frequently, initiated smoking at a later age, and were more likely to smoke menthol cigarettes. Differences in education and likelihood of using acupuncture/hypnosis were not statistically significant. The same general pattern of findings was found among men.
Table 1.
Sample descriptive statistics and comparison by abstinence status: Women (n=4,049)
| Overall sample [mean(SD) or %] | By treatment [mean(SD) or %] | ||||
|---|---|---|---|---|---|
| Unassisteda (n=3,148) | TNP only (n=468) | Varenicline only (n=433) | p-valueb | ||
| Disparity group membership/contextual factors | |||||
| Age | 42.65 (14.54) | 41.55 (14.72) | 45.89 (13.66) | 47.62 (12.36) | <.001 |
| Race/ethnicity | <.001 | ||||
| White, non-Hispanic | 74.67 | 72.12 | 79.65 | 88.84 | |
| Black, non-Hispanic | 12.21 | 13.55 | 9.77 | 4.59 | |
| Hispanic | 8.85 | 10.10 | 5.14 | 3.24 | |
| Other | 4.27 | 4.23 | 5.44 | 3.34 | |
| Educationc | 8.57 (2.07) | 8.55 (2.07) | 8.59 (2.15) | 8.70 (1.99) | .47 |
| Household incomed | 8.77 (4.06) | 8.64 (4.00) | 8.82 (4.15) | 9.67 (4.24) | <.001 |
| Marital status | <.001 | ||||
| Married | 40.96 | 39.21 | 41.90 | 53.45 | |
| Widowed/separated/divorced | 31.10 | 29.43 | 40.45 | 33.71 | |
| Never married | 27.94 | 31.36 | 17.66 | 12.84 | |
| Employment status | .004 | ||||
| Employed, part/fulltime | 53.20 | 54.07 | 49.55 | 50.48 | |
| Unemployed | 10.76 | 11.47 | 7.92 | 8.37 | |
| Not in labor force | 36.04 | 34.46 | 42.53 | 41.15 | |
| Physician or dentist advice to quit smoking during the previous 12 months | 56.04 | 52.28 | 63.32 | 76.91 | <.001 |
| Smoking cessation methods | |||||
| Quitline | 2.46 | 1.00 | 9.74 | 5.68 | <.001 |
| Counseling | 1.91 | 1.03 | 6.59 | 3.63 | <.001 |
| Smoking cessation clinic | 1.40 | 0.73 | 4.18 | 3.48 | <.001 |
| Friend/family support | 31.77 | 28.32 | 42.40 | 46.71 | <.001 |
| Internet | 1.35 | 1.05 | 1.96 | 2.92 | .01 |
| Self-help books | 4.58 | 3.01 | 11.02 | 9.56 | <.001 |
| Acupuncture/hypnosis | 1.31 | 1.16 | 1.69 | 2.00 | .34 |
| Psychopharmacological stimuli | |||||
| Cigarettes per day 12 months prior | 11.95 (7.63) | 11.01 (7.31) | 14.98 (7.96) | 15.85 (7.73) | <.001 |
| Usual frequency of smoking 12 months prior (days per month) | 26.21 (7.35) | 25.65 (7.73) | 27.96 (5.70) | 28.49 (4.61) | <.001 |
| Age of smoking initiation | 18.51 (5.28) | 18.68 (5.38) | 17.99 (5.35) | 17.81 (4.14) | <.001 |
| Menthol cigarette use 12 months prior | 36.20 | 37.63 | 33.27 | 28.38 | .003 |
Note. TNP = Transdermal nicotine patch
Refers to pharmacologically unassisted quit attempts. Could have used non-pharmacological smoking cessation aids.
All p-values account for the survey design. For categorical variables, p-value from Pearson chi-square. For continuous variables, p-value from one-way ANOVA.
Education responses ranged from 0 (less than 1st grade) to 15 (doctorate degree). Means were between 8 (high school diploma/GED) and 9 (some college but no degree).
Household income responses ranged from 1 (less than $5,000) to 16 ($150,000 or more). Means ranged between 8 ($25,000 to $29,999) and 10 ($35,000 to $39,999).
Table 2.
Sample descriptive statistics and comparison by abstinence status: Men (n=3,857)
| Overall sample [mean(SD) or %] | By abstinence status [mean(SD) or %] | ||||
|---|---|---|---|---|---|
| Unassisteda (n=3,146) | TNP only (n=416) | Varenicline only (n=295) | p-valueb | ||
| Disparity group membership/contextual factors | |||||
| Age | 41.74 (13.98) | 40.71 (13.94) | 45.72 (13.51) | 47.87 (12.59) | <.001 |
| Race/ethnicity | <.001 | ||||
| White, non-Hispanic | 69.87 | 68.09 | 75.00 | 82.99 | |
| Black, non-Hispanic | 12.95 | 13.65 | 12.04 | 6.12 | |
| Hispanic | 10.93 | 11.80 | 8.19 | 4.95 | |
| Other | 6.25 | 6.46 | 4.77 | 5.95 | |
| Educationc | 8.56 (2.10) | 8.51 (2.10) | 8.76 (2.01) | 8.77 (2.15) | .05 |
| Household incomed | 9.40 (3.76) | 9.25 (3.75) | 9.61 (3.76) | 10.80 (3.65) | <.001 |
| Marital status | <.001 | ||||
| Married | 42.05 | 40.22 | 41.37 | 64.22 | |
| Widowed/separated/divorced | 23.55 | 22.90 | 30.30 | 21.22 | |
| Never married | 34.40 | 36.88 | 28.33 | 14.56 | |
| Employment status | <.001 | ||||
| Employed, part/fulltime | 63.70 | 64.19 | 60.81 | 62.23 | |
| Unemployed | 13.25 | 14.16 | 9.57 | 8.20 | |
| Not in labor force | 23.05 | 21.66 | 29.62 | 29.57 | |
| Physician or dentist advice to quit smoking during the previous 12 months | 44.39 | 40.10 | 58.18 | 73.48 | <.001 |
| Smoking cessation methods | |||||
| Quitline | 1.73 | .67 | 8.08 | 4.73 | <.001 |
| Counseling | 1.43 | .74 | 6.40 | 2.17 | <.001 |
| Smoking cessation clinic | 1.32 | .94 | 3.85 | 2.06 | <.001 |
| Friend/family support | 27.74 | 25.47 | 35.49 | 42.69 | <.001 |
| Internet | 1.23 | .73 | 3.60 | 3.64 | <.001 |
| Self-help books | 3.22 | 2.14 | 9.36 | 6.68 | <.001 |
| Acupuncture/hypnosis | .65 | .50 | 1.65 | .99 | |
| Psychopharmacological stimuli | |||||
| Cigarettes per day 12 months prior | 13.51 (8.35) | 12.71 (8.08) | 16.92 (8.40) | 17.65 (9.08) | <.001 |
| Usual frequency of smoking 12 months prior (days per month) | 25.99 (7.31) | 25.47 (7.66) | 28.65 (4.05) | 27.98 (5.30) | <.001 |
| Age of smoking initiation | 17.91 (4.67) | 17.99 (4.66) | 17.76 (4.97) | 17.23 (4.31) | .04 |
| Menthol cigarette use 12 months prior | 26.30 | 27.21 | 24.14 | 18.98 | .004 .02 |
Note. TNP = Transdermal nicotine patch
Refers to pharmacologically unassisted quit attempts. Could have used non-pharmacological smoking cessation aids.
All p-values account for the survey design. For categorical variables, p-value from Pearson chi-square. For continuous variables, p-value from one-way ANOVA.
Education responses ranged from 0 (less than 1st grade) to 15 (doctorate degree). Means were between 8 (high school diploma/GED) and 9 (some college but no degree).
Household income responses ranged from 1 (less than $5,000) to 16 ($150,000 or more). Means ranged between 8 ($25,000 to $29,999) and 10 ($35,000 to $39,999).
3.2. Propensity score matching
As a first step in propensity score matching, we examined associations between all potential matching variables and 30-day abstinence, stratified by gender. Associations were estimated using logistic regression, adjusting for all potential covariates. Variables associated with abstinence for both women and men were used for propensity score matching. Results of this preliminary modeling step are displayed online only in supplementary Table S1. Three variables emerged as statistically significant for both women and men: education, household income, and usual frequency of smoking.
We then conducted propensity score matching, stratified by gender, as described above in the methods section. Descriptive statistics and comparisons between medication groups for all comparisons, stratified by gender, are displayed online only in supplementary Table S2–Table S4. Samples were successfully matched on education, household income, and usual frequency of smoking. There remained significant differences between medication groups for several other covariates, all of which were accounted for using generalized estimating equations modeling using statistical adjustment (see below).
3.3. Medication effectiveness comparisons using propensity score matched samples
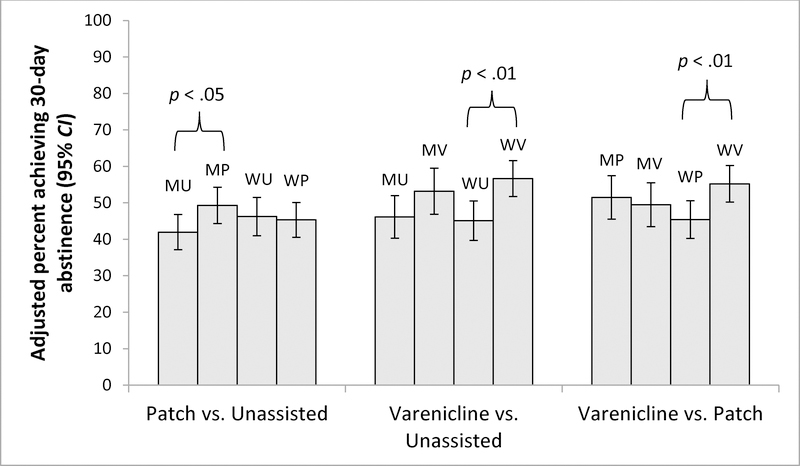
Results for each medication comparison using the propensity score matched samples and adjusting for all covariates (to account for potential confounding of gender comparisons, and residual confounding of treatment comparisons after propensity score matching) are presented in Figure 1. When stratified by gender, TNP was significantly more effective than unassisted quit attempts for men (OR = 1.37; 95% CI = 1.02, 1.83; p = 0.03), but not for women (OR = 0.96; 95% CI = 0.71, 1.31; p = 0.82). Varenicline was significantly more effective than unassisted quit attempts for women (OR = 1.63; 95% CI = 1.16, 2.31; p = 0.005), but not men (OR = 1.35; 95% CI = 0.94, 1.96; p = 0.11). Varenicline was also more effective than TNP for women (OR = 1.51; 95% CI = 1.12, 2.05; p = 0.007) but not men (OR = 0.92; 95% CI = 0.65, 1.31; p = 0.64). A significant gender by medication interaction was found only for the comparison of varenicline to TNP (OR = 1.64; 95% CI = 1.04, 2.61; p = 0.04; stratified ORs provided above), while the interactions for TNP vs. unassisted quit attempt (OR = 0.70; 95% CI = 0.47, 1.05; p = 0.09) and for varenicline vs. unassisted quit attempt (OR = 1.21; 95% CI = .77, 1.90; p = 0.41) were not significant.
Figure 1. Propensity score analysis results, comparing 30-day abstinence by medication.
MU = men, unassisted; MP = men, transdermal nicotine patch (TNP); MV = men, varenicline. WU = women, unassisted; WP = women, TNP, WV = women, varenicline. Unassisted refers to pharmacologically unassisted quit attempts; respondents could have used non-pharmacological smoking cessation aids. Samples were matched on education, household income, and usual frequency of smoking. Odds ratio estimates were calculated using generalized estimating equations, adjusting for age, education, household income, race/ethnicity, marital status, employment status, cigarettes per day, frequency of smoking, age started smoking, whether received physician advice to quit, the use of other cessation aids (quitline, counseling, cessation clinic services, friend/family support, internet, self-help book, acupuncture/hypnosis) and menthol cigarette smoking. For TNP vs. unassisted, the gender by treatment group interaction was non-significant (p =0.09). For varenicline vs. unassisted, the gender by treatment group interaction was non-significant (p = 0.44). For varenicline vs. TNP the interaction for gender by treatment group was significant (OR= 1.64; 95% CI= 1.04, 2.61; p < .05).
4. DISCUSSION
In this observational study of gender differences in the comparative effectiveness of smoking cessation medications (varenicline only and TNP only), a significant gender difference was found when comparing varenicline to TNP. Women reporting varenicline use had 51% greater odds of reporting 30-day abstinence compared to women reporting TNP use. No differences in effectiveness of varenicline versus TNP were found among men. Stratified comparisons with unassisted quit attempts showed greater effectiveness of TNP for men but not women, and greater effectiveness of varenicline for women but not men, although neither gender by medication interaction was statistically significant.
In a recently published network meta-analysis comparing women and men on the relative differences in efficacy of placebo, TNP, and varenicline in a sample of 14,389 smokers, we found a statistically significant gender by medication interaction for the varenicline vs. TNP comparison. Varenicline was significantly more efficacious relative to TNP for women but not men (Smith et al., 2016b). A key limitation of the meta-analysis was the potential lack of external validity. The findings from the current investigation suggest this gender difference in the comparative efficacy of varenicline and TNP found in clinical trial data may extend to smokers quitting in real-world contexts. Potential mechanisms underlying these differences include gender differences in expression of CYP genes (Ilic et al., 2013) and consequently hepatic drug metabolism, and gender differences in non-pharmacological drivers of cigarette smoking (e.g., negative affect and smoking related cues) (Carpenter et al., 2014; Perkins and Karelitz, 2015), which have been demonstrated to be affected by smoking cessation pharmacotherapy. For example, research has demonstrated that smoking related cues are more strongly related to smoking behavior in women compared to men (Carpenter et al., 2014), and varenicline substantially reduces cue reactivity in smokers (Franklin et al., 2011).
It is notable that among men, varenicline was not significantly more effective than unassisted quit attempts, despite having nearly the same sized OR as TNP, which was significantly more effective than unassisted quit attempts among men (1.35 for varenicline vs. 1.37 for TNP). The lack of statistical significance suggests our study was underpowered to detect this small effect size for varenicline. The small effect size may be due to the use of a 30-day abstinence definition for smoking cessation. Thirty-day abstinence rates in this investigation were in the range of approximately 40–55%, and relative differences with baseline around 50% are expected to be small. In terms of their general size, the quit rates are comparable to other real-world effectiveness investigations using a similar time frame. For example, Walker and colleagues (2016) found that 54.9% of those using NRT and 63.6% of those using varenicline had achieved 4-week abstinence in a real-world effectiveness investigation, a time-frame that is consistent with the current investigation. Walker and colleagues (2016) did not examine unassisted quit attempts because everyone in the sample received medication, and so we are unable to draw comparisons for those making unassisted quit attempts. It is possible that the effect size for varenicline would have been larger had we used a longer time-frame for abstinence. Our study was limited in our ability to use a longer time-frame given the cross-sectional nature of our data.
These findings are clinically relevant for a number of reasons. After a preponderance of evidence documenting varenicline’s safety (e.g. the EAGLES trial (Anthenelli et al., 2016)), the FDA lifted the medication’s black box warning label in December 2016. Particularly for women who are trying to quit, it is important for physicians to be aware of this decision and to strongly consider varenicline as their first-line treatment. While TNP is often recommended as a first-line medication, there is a downside to trying this first with women. Women are less successful when using TNP, which may lead to reduced motivation to engage in another quit attempt or use pharmacotherapy to assist future quit attempts, leading to continued tobacco exposure and associated health risks. For men, the findings reinforce the potential value of TNP in a quit attempt. Both clinical trial and now this observational data seem to suggest that TNP on its own is comparably effective in comparison to varenicline when used on its own. Previous reviews, including Cochrane’s meta-analytic review of the comparative effectiveness of smoking cessation medications (Cahill et al., 2013), have pointed to varenicline as superior to TNP and other forms of NRT when used in isolation. Both our clinical trial meta-analyses as well as the current findings suggest this difference is largely driven by women. Further, our meta-analyses demonstrated little difference in efficacy between bupropion and varenicline for men, although too few smokers reported quitting with bupropion in the TUS-CPS data to conduct the comparison in the current analyses. Physicians considering a first-line treatment when prescribing to men who are trying to quit smoking might weigh the pros and cons across the possible options, including tolerability and availability.
There were relevant differences between those who made pharmacologically unassisted quit attempts and those who used medications, and between those who used varenicline and those who used TNP. Our findings reflect those recently reported by McCarthy and colleagues (2016) in their analysis of the TUS-CPS data, focusing on socioeconomic disparities among those making unassisted quit attempts. Those making unassisted quit attempts were more likely to self-identify as racial/ethnic minority and had lower income than those reporting use of smoking cessation medications. Similar differences were found between those using TNP and those using varenicline, with those using varenicline more likely to be white and reporting higher household income. It was not the purpose of this investigation to delve into the mechanisms underlying these differences; however, one can speculate that access to medications may play an important role, with access being lowest for varenicline as a prescription-only medication. Our findings suggest that especially for women of racial/ethnic minority groups and low socioeconomic status, improving access to varenicline is an important public health and tobacco treatment goal.
We consider the findings reported here to be preliminary, due to the cross-sectional nature of the data and the consideration of 30-day abstinence as opposed to a longer period of abstinence. Further, in the TUS-CPS data there is no information on dose, compliance, or adverse events. It is possible that such factors are driving gender differences, although this is unlikely given the consistency of results with clinical trial data where such factors are taken into account. Another limitation is the lack of assessment of nicotine dependence prior to quit attempts, other than information on quantity of smoking (i.e., cigarettes per day; which may not be a valid indicator of dependence), frequency of smoking (most respondents reported daily use), number of previous quit attempts, or previous use of pharmacological and non-pharmacological smoking cessation aids. The observational nature of the data and the lack of these potentially relevant variables leaves residual confounding to be a possible interpretation of the data. With population survey data, biochemical verification of smoking is typically not available and this was the case for our investigation. Recall bias is a potential issue as well, given the extended period of recall for medication use, the use of historical smoking-related variables, and the recall of our 30-day abstinence dependent variable. We did not include analyses of bupropion, forms of NRT other than TNP, or combination medication use due to limitations of sample size. Although this omission limits the clinical implications of our findings, varenicline and TNP are the two most commonly used treatments in real world contexts (as evidenced by us having sufficient sample size to conduct this investigation). A strength of the data is the extent that information about potential confounding variables were available (Dreyer et al., 2010), much more so than would be typical of a medical chart review, for example. The consistency between these survey findings and our previous clinical trial meta-analysis also support the validity of the data and our methods.
4.1. Conclusions
The influence of gender is often not considered when clinically addressing smoking cessation. However, there are a host of investigations documenting clinically relevant gender differences in relation to smoking cessation, and a growing body of empirical evidence suggesting relevant gender differences in the effectiveness of smoking cessation medications. Consistent with a previous meta-analytic review of clinical trial data (Smith et al., 2016b), the current study found a greater advantage of varenicline over TNP for women compared to men. The consideration of gender in the treatment of nicotine addiction could benefit both women and men who are attempting to quit smoking.
Supplementary Material
References
- Amos A, Greaves L, Nichter M, Bloch M, 2012. Women and tobacco: A call for including gender in tobacco control research, policy and practice. Tob Control 21, 236–243. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–2520. [DOI] [PubMed] [Google Scholar]
- Berger ML, Mamdani M, Atkins D, Johnson ML, 2009. Good research practices for comparative effectiveness research: Defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part I. Value Health 12, 1044–1052. [DOI] [PubMed] [Google Scholar]
- Breiding MJ, Smith SG, Basile KC, Walters ML, Chen J, Merrick MT, 2014. Prevalence and characteristics of sexual violence, stalking, and intimate partner violence victimization-national intimate partner and sexual violence survey, United States, 2011. Morb Mortal Wkly Rep Surveill Summ 63, 1–18. [PMC free article] [PubMed] [Google Scholar]
- Brose LS, West R, Stapleton JA, 2013. Comparison of the effectiveness of varenicline and combination nicotine replacement therapy for smoking cessation in clinical practice. Mayo Clin Proc 88, 226–233. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T, 2013. Pharmacological interventions for smoking cessation: An overview and network meta‐analysis. Cochrane Database Syst Rev, CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, LaRowe SD, McClure EA, Simonian S, Upadhyaya HP, Gray KM, 2014. Craving, cue reactivity, and stimulus control among early-stage young smokers: Effects of smoking intensity and gender. Nic Tob Res 16, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML, 2009. Good research practices for comparative effectiveness research: Approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: The International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health 12, 1053–1061. [DOI] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD, 2015. Income and Poverty in the United States: 2014 U.S. Census Bureau. Current Population Reports P60–252. Washington, DC, U.S. Government Printing Office. [Google Scholar]
- Dreyer NA, Tunis SR, Berger M, Ollendorf D, Mattox P, Gliklich R, 2010. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood) 29, 1818–1825. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR, 2011. Effects of varenicline on smoking cue–triggered neural and craving responses. Arch Gen Psychiatry 68, 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic K, Hawke RL, Thirumaran RK, Schuetz EG, Hull JH, Kashuba ADM, Stewart PW, Lindley CM, Chen M-L, 2013. The influence of sex, ethnicity, and CYP2B6 genotype on bupropion metabolism as an Index of hepatic CYP2B6 activity in humans. Drug Metab Dispos 41, 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Women’s Policy Research, 2015. Women’s median earnings as a percent of men’s median earnings, 1960–2014 (full-time, year-round workers) with projection for pay equity in 2059. http://www.iwpr.org/publications/pubs/equal-pay-projection-2059.accessed on January 25, 2016.
- Jamal A, 2016. Current cigarette smoking among adults—United States, 2005–2015. Morb Mortal Wkly Rep Surveill Summ 65, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U, 2009. Good research practices for comparative effectiveness research: Analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part III. Value Health 12, 1062–1073. [DOI] [PubMed] [Google Scholar]
- Kristman-Valente AN, Brown EC, Herrenkohl TI, 2013. Child physical and sexual abuse and cigarette smoking in adolescence and adulthood. J Adolesc Health 53, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, Fackenthal JD, Rogan PK, Ring B, Wrighton SA, Schuetz EG, 2003. Hepatic CYP2B6 expression: Gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther 307, 906–922. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, 2016. The sociopharmacology of tobacco addiction: implications for understanding health disparities. Nic Tob Res 18, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, Siahpush M, Shaikh RA, Sikora Kessler A, Tibbits M, 2016. Social disparities in unaided quit attempts among daily current and former smokers: Results from the 2010–2011 Tobacco Use Supplement to the Current Population Survey. Nic Tob Res 18, 1705–1710. [DOI] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM, 2003. Sex differences in the effects of stressful life events on changes in smoking status. Addiction 98, 847–855. [DOI] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH, 2015. Sex differences in varenicline efficacy for smoking cessation: A meta-analysis. Nic Tob Res 18, 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville FG, Goodall CA, Williams DJ, Donnelly PD, 2014. Sexual assault and harassment, perceived vulnerability, and association with alcohol use in a student population: A cross-sectional survey. Lancet 384, S56. [Google Scholar]
- Perkins KA, Karelitz JL, 2015. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nic Tob Res 17, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J, 2008. Sex differences in long-term smoking cessation rates due to nicotine patch. Nic Tob Res 10, 1245–1251. [DOI] [PubMed] [Google Scholar]
- Prado GF, Lombardi EMS, Bussacos MA, Arrabal‐Fernandes FL, Terra‐Filho M, de Paula Santos U, 2011. A real‐life study of the effectiveness of different pharmacological approaches to the treatment of smoking cessation: Re‐discussing the predictors of success. Clinics 66, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf D, Shiffman S, 2004. Are there gender differences in smoking cessation, with and without bupropion? Pooled‐and meta‐analyses of clinical trials of Bupropion SR. Addiction 99, 1462–1469. [DOI] [PubMed] [Google Scholar]
- Smith MV, Ramsay C, Mazure CM, 2014a. Understanding disparities in subpopulations of women who smoke. Current addiction reports 1, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA, 2016a. Sex/gender differences in smoking cessation: A review. Prev Med 92, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, Carpenter MJ, Hartwell K, Cummings KM, McKee SA, 2015. Gender differences in medication use and cigarette smoking cessation: Results from the International Tobacco Control Four Country Survey. Nic Tob Res 17, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Saddleson ML, Homish GG, McKee SA, Kozlowski LT, Giovino GA, 2014b. The relationship between childhood physical and emotional abuse and smoking cessation among US women and men. Psychol Addict Behav 29, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA, 2016b. Sex differences in smoking cessation pharmacotherapy comparative efficacy: A network meta-analysis. Nic Tob Res 19, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Commerce, C.B., 2012. National Cancer Institute-sponsored Tobacco Use Supplement to the Current Population Survey (2010–11). Data files and technical documentation.
- Ucar EY, Araz O, Nafiye Y, Akgun M, Meral M, Kaynar H, Saglam L, 2014. Effectiveness of pharmacologic therapies on smoking cessation success: Three year results of a smoking cessation clinic. Multidisciplinary Respiratory Medicine 9, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NJ, van Woerden HC, Kiparoglou V, Yang Y, Robinson H, Croghan E, 2016. Gender difference and effect of pharmacotherapy: findings from a smoking cessation service. BMC Public Health 16, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.