Abstract
Purpose
HER2 + breast cancer (BC) is an aggressive subtype with high rates of brain metastases (BCBM). Two-thirds of HER2 + BCBM demonstrate activation of the PI3K/mTOR pathway driving resistance to anti-HER2 therapy. This phase II study evaluated everolimus (E), a brain-permeable mTOR inhibitor, trastuzumab (T), and vinorelbine (V) in patients with HER2 + BCBM.
Patients and methods
Eligible patients had progressive HER2 + BCBM. The primary endpoint was intracranial response rate (RR); secondary objectives were CNS clinical benefit rate (CBR), extracranial RR, time to progression (TTP), overall survival (OS), and targeted sequencing of tumors from enrolled patients. A two-stage design distinguished intracranial RR of 5% versus 20%.
Results
32 patients were evaluable for toxicity, 26 for efficacy. Intracranial RR was 4% (1 PR). CNS CBR at 6 mos was 27%; at 3 mos 65%. Median intracranial TTP was 3.9 mos (95% CI 2.2–5). OS was 12.2 mos (95% CI 0.6–20.2). Grade 3–4 toxicities included neutropenia (41%), anemia (16%), and stomatitis (16%). Mutations in TP53 and PIK3CA were common in BCBM. Mutations in the PI3K/mTOR pathway were not associated with response. ERBB2 amplification was higher in BCBM compared to primary BC; ERBB2 amplification in the primary BC trended toward worse OS.
Conclusion
While intracranial RR to ETV was low in HER2 + BCBM patients, one-third achieved CNS CBR; TTP/OS was similar to historical control. No new toxicity signals were observed. Further analysis of the genomic underpinnings of BCBM to identify tractable prognostic and/or predictive biomarkers is warranted.
Clinical Trial:
().
Keywords: Breast cancer; Brain metastases; Metastases; PI3K, MEK; Targeted therapy
Introduction
HER2 + breast cancer (BC) is characterized by protein overexpression or gene amplification of human epidermal growth factor receptor 2 (HER2, ERBB2) [1]. Up to 30% of patients with advanced HER2 + breast cancer experience intracranial recurrence [2]. The precise reason for this increased incidence is unclear, but likely related to reduced ability of HER2-directed, monoclonal antibodies, trastu-zumab or pertuzumab, to cross the blood brain barrier and inherent genomic changes causing an increased propensity for HER2 + cells to seed the central nervous system (CNS) [3, 4]. While the advent of HER2-targeted therapies has improved the survival of patients with HER2 + BC brain metastases (BM), survival remains less than 2 years [5–7]. Novel, brain-permeable therapies targeting HER2 and inherent resistance pathways to more optimally treat HER2 + BCBM are needed.
Radiation therapy remains a mainstay to treat BCBM; neurosurgical resection is generally reserved for solitary lesions or for large, space-occupying lesions [8]. Current systemic treatment options for patients with progressive HER2 + BCBM include the HER1/2-targeting, small molecule inhibitor lapatinib, with or without capecitabine [9]. The LANDSCAPE study, evaluating lapatinib/capecitabine in radiation therapy-naïve patients, showed an intracranial response rate (RR) of 67% by volumetrics and progression-free survival (PFS) of 5.5 months [10]. The irreversible HER1/2 tyrosine kinase inhibitor, neratinib, plus capecitabine yielded intracranial RR of 49% by volumetrics and a similar PFS of 5.5 months. Treatment with neratinib/capecitabine results in notable toxicity, with grade 3 diarrhea in 32% of patients. Several case reports further illustrated durable intracranial responses to trastuzumab emtansine [11, 12].
While HER2 + BCBM have illustrated respectable response to HER2-directed therapy, responses are not durable, and patients from the aforementioned studies progressed within 6 months. The phosphoinositide-3-kinase/ mammalian target of rapamycin (PI3K/mTOR) pathway has been implicated as a driver of metastasis in HER2 + BC and resistance to HER2-directed therapies [13, 14]. Hyperactivation of PI3K/mTOR after trastuzumab treatment [15] correlates with poor OS and increased metastasis to the brain [15]. BCBM have enrichment of PTEN loss and increased PI3K signaling [16–19]. Thus, inhibition of the PI3K/mTOR pathway, combined with HER2-directed therapy, may yield more sustained responses for patients with advanced HER2 + BCBM.
Everolimus is a brain-permeable, small molecule inhibitor of mTOR complex 1 (mTORC1). Everolimus was approved in the United States in 2012 for the treatment of refractory sub-ependymal giant cell astrocytomas, intracranial neoplasms in patients with mutations in TSC1 or TSC2 resulting in activation of mTOR [20]. The addition of everolimus to vinorelbine and trastuzumab for patients with metastatic HER2 + BC without BM yielded modest, yet significant, improvements in progression-free survival favoring the everolimus arm (7 versus 5.78 months, Hazard Ratio (HR) 0.78, p = 0.0067) [14]. To this end, we designed a phase II, open-label, single-arm study evaluating the efficacy and safety of everolimus plus vinorelbine and trastuzumab among patients with progressive HER2 + BCBM, with correlative DNA sequencing of primary and metastatic tumors.
Patients and methods
Patient population
Eligible patients, enrolled 9/9/2011–5/11/2016, had histologically confirmed HER2+ (3 + or amplified by fluorescence in-situ hybridization) breast adenocarcinoma with progressive and/or new BCBM ≥ 5 mm by gadolinium-enhanced magnetic resonance imaging (MRI). Receipt of prior intracranial radiation therapy was allowed, but not required. Additional inclusion criteria included age > 21 years, ECOG performance status of 0–2, life expectancy > 12 weeks, and adequate organ function. Concurrent dexamethasone was allowed if stable or decreasing dose ≥ 7 days.
Exclusion criteria included prior mTOR inhibition, intracranial hemorrhage, impending herniation, leptomeningeal disease, cardiac disease/dysfunction, or pregnancy/ breastfeeding, or HIV-positivity. Hepatitis B and C testing was required of high-risk patients. If sero-positive for hepatitis B, lamivudine prophylaxis was initiated 12 weeks prior to everolimus. If hepatitis C positive, close monitoring of liver function was required. All patients provided written informed consent, and the study was institutional review board approved (No. (NCT01305941).
Study design
This was an open-label, single-arm, phase II study (Fig. 1). The primary endpoint was intracranial response rate (RR) as defined via modified response evaluation criteria in solid tumors (RECIST) criteria [21]. Secondary objectives included intracranial RR by MacDonald criteria [22], time to progression (TTP), extracranial RR, progression-free survival (PFS), overall survival (OS), safety/tolerability as per NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [23], and targeted DNA sequencing [24].
Fig. 1.
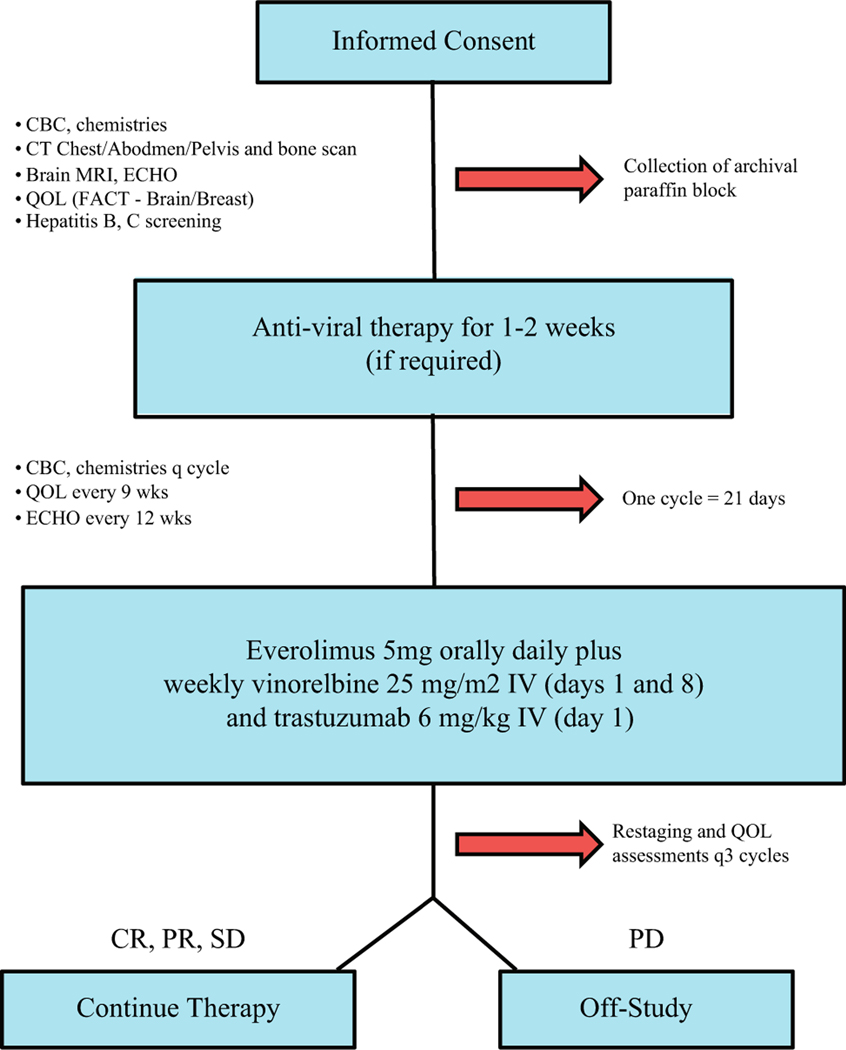
Consort diagram of LCCC 1025: vinorelbine, trastuzumab, and everolimus in HER2 + breast cancer brain metastases
Study treatment
Eligible patients received everolimus 5 mg PO daily as two 2.5-mg tablets, weekly vinorelbine 25 mg/m2 intravenously (IV) (days 1, 8), and trastuzumab 2 mg/kg IV (days 1, 15) on a 21-day cycle. Vinorelbine was initially dosed on days 1, 8, and 15, but day 15 was removed due to neutropenia following accrual of 13 subjects (October 14, 2013).
Safety assessments
Adverse events were assessed every 3 weeks and graded according to the NCI CTCAE Version 4.0 [23].
Efficacy assessments
Response assessment was obtained every 9 weeks. Intracranial response rate (RR) was evaluated using modified RECIST criteria with brain MRI [21]. An intracranial response was defined as either a complete response (CR) or a partial response (PR) (30% decrease in the sum of the longest diameter (LD) of target lesions AND an absolute decrease of 5 mm in at least one target lesion). Clinical Benefit was defined as a CR, PR, or stable intracranial disease, reported as sustained for ≥ 3 months with no evidence of extracranial PD [9, 25]. Progressive disease (PD) intracranially was defined as a 20% increase in the sum LD of target lesions AND an absolute increase of 5 mm in at least one target lesion OR the appearance of one or more new lesions of at least 6 mm in size. Stable disease (SD) in the CNS did not meet criteria for either PR or PD.
Extracranial disease was assessed via a serial computed tomography of the chest/abdomen/pelvis and a nuclear bone scan (if bone metastases on baseline imaging). Extracranial disease status was determined using RECIST 1.1 criteria [25].
Health-related quality of life
Participants’ health-related quality of life (HRQL) was assessed using the Functional Assessment of Cancer Therapy, General (FACT-G) along with the Brain Tumor and Breast Cancer Additional Concerns Subscales (www.facit.org) [26–28]. HRQL questionnaires were administered during the pre-study evaluation, every 9 weeks during treatment, at the time of progression, and at 60-day follow-up.
DNA sequencing
Formal-fixed, paraffin-embedded primary and/or metastatic tumors from enrolled patients were collected. A hematoxylin and eosin (H&E) slide from each case was reviewed by the study pathologist (NP, CRM) to map tumor content and location. DNA sequencing was performed according to the UNCSeq protocol [24]. Briefly, 750 ng DNA library was target-captured with 250–800 genes from UNCSeq V7, V7.1, or V8 (eTable 1). Quality libraries were sequenced on the Illumina NextSeq500 (3/pool, NextSeq v2 300 cycle, v2 High-Output Flowcells, 2 × 100 paired-end).
DNA sequencing mapping
Sequencing reads were mapped to the reference genome hs37d5 with added viral contigs by BWA 0.7.9a [29] and samtools 0.1.19–44428 cd [30], sorted with biobambam 2.0.33 [31], filtered and quality controlled with BEDTools 2.0.15 [32], realigned with ABRA 0.96 [33], and analyzed for further quality metrics with PicardTools 1.92 [34]. Germline calls were made with Freebayes v0.9.15–1-g076a2a2 [35] and ISAAC 1.0.4 [36]. For tumors with a matched normal, somatic calls were made with STRELKA [37] by comparing the matched tumor and normal. If matched normal was unavailable, 20 normal samples from the same targeted capture version of UNCSeq were used to compare for somatic variance calling. Somatic variants were filtered and annotated with SNPSift 1.3.4 [38], SNPEff [39], COSMIC v10250210 [40], dbSNP 132 [41], ExAC 0.3 [42], and Oncotator 1.9.0 [43]. All sequenced patient samples are including in the 1025 dataset in dbGaP.
DNA mutation and copy number analyses
Mutations previously reported in COSMIC ≥ 10 times or with an ExAC population frequency < 1 × 10– 5 were kept for further analyses; mutations with more frequent prevalence in the population, but not reported in COSMIC, were discarded. All mutations in HLA genes were removed due tothe high degree of genetic diversity in the population. Mutation plotting was performed with GenVisR [44]. SynthEx [45] was applied to the mapped DNA sequencing data using KNN=4 using previously sequenced UNCSeq [24] normals. Pearson correlation was calculated with R v.3.3.2.
Statistical analyses
A two-stage design [46] was planned to test the null hypothesis response rate of 5% against a one-sided alternative. After evaluating 11 patients for response in the first stage, the trial would be terminated if no patient had a CR, PR, or Clinical Benefit (CR+PR + SD ≥ 3) [9, 21]. If at least one patient met these criteria, an additional 17 patients would be enrolled during the second stage for a total of 28. At the end of the trial, if the total number of responses (CR+PR) is 4 or more, the combination would be considered promising. This trial design yielded a type-I error rate of at most 0.05. The power was at least 80% if the true response rate (response = CR+PR) was equal to 0.2 and for any value of Clinical Benefit Rate (CBR). Fisher’s Exact test was used to compare intracranial RR by HR status. The Kaplan Meier method estimated time to intracranial progression (TTP) and overall survival (OS), with both measurements starting at time of treatment initiation. The association of changes in QOL measures from baseline to 9 weeks with clinical benefit at 12 weeks was evaluated using Wilcoxon Rank Sum tests.
Results
Patient characteristics
Forty-one patients were enrolled, of which 9 were consented and not treated (7 were deemed ineligible, 1 non-compliant with screening, and 1 withdrew consent prior to treatment). Thirty-two patients were evaluable for toxicity; 26 patients were evaluable for efficacy. Enrollment was halted at 26 evaluable patients as there was only 1 PR (in stage I); 4 responses were needed to reject the null hypothesis which was unattainable.
Patient demographics and prior treatments are outlined in Table 1. Median age was 53 years (28–70). Most patients (84%) were white; 16% black. 41% of patients were diagnosed with stage IV BC de novo; median time since diagnosis of BCBM prior to study enrollment was 1.12 years (0.4–6.5).
Table 1.
Patient demographics and prior treatment information
| Demographics and clinical data | |
|---|---|
| Median age, years (range) | 53 (28–70) |
| Race, White | 26/31 (84%) |
| Black/other | 5/31 (16%) |
| Stage at breast cancer diagnosis, 0–III | 19 (59%) |
| IV | 13 (41%) |
| Median Time since first brain metastases (years) | 1.12 (0.4–6.5) |
| Prior systemic chemotherapy (metastatic) | 30 (94%) |
| Prior metastatic lines, # (range) | 2 (0–7) |
| Prior anti-HER2 (metastatic) | 30 (94%) |
| Trastuzumab | 29/30 (97%) |
| Lapatinib | 22/30 (73%) |
| Pertuzumab | 12/30 (40%) |
| TDM-1 | 8/30 (27%) |
| Prior CNS local therapy | |
| Surgery | 10/31 (32%) |
| Any CNS RT | 31/32 (97%) |
| WBRT | 22/32 (69%) |
| SRS | 17/32 (53%) |
| Median time since CNS RT (days) (n = 31) | 237 (IQR 135–392) (range: 12–1911) |
| CNS RT within 12 weeks of starting treatment | 2 (6%) |
| Recursive partitioning analysis score (1, 2, or 3) | 10 (31%), 21 (65%), 3 (3%) |
| Steroid use at baseline | 10 (31%) |
| Extracranial disease at enrollment | 20 (63%) |
Most patients received systemic therapy in the metastatic setting; median prior lines of therapy was 2 (0–7). Prior anti-HER2 therapies included 97% trastuzumab, 73% lapatinib, 40% pertuzumab, and 27% trastuzumab emtansine. Local therapy directly to the CNS included neurosurgery (32%), whole brain radiation therapy (69%), and stereotactic radiosurgery (53%). One patient had not received local therapy to the brain and was neurologically stable.
Recursive partitioning analysis (RPA) scores [47], a prognostic model for patients with BCBM, were 1 (31%), 2 (65%), and 3 (3%). Approximately one-third of patients were on steroids and two-thirds had extracranial disease at time of enrollment.
Toxicity and dose intensity
Study design is outlined in Fig. 1. Everolimus plus vinorel-bine and trastuzumab was generally well tolerated with no new safety signals (Fig. 2a). The most common grade 1–2 toxicities included oral mucositis (50%), constipation (31%), fatigue (28%), anemia (16%), elevated ALT (19%), diarrhea (22%), anorexia (22%), elevated AST (19%), thrombocytopenia (19%), acneiform rash (16%), and peripheral neuropathy (16%).
Fig. 2.
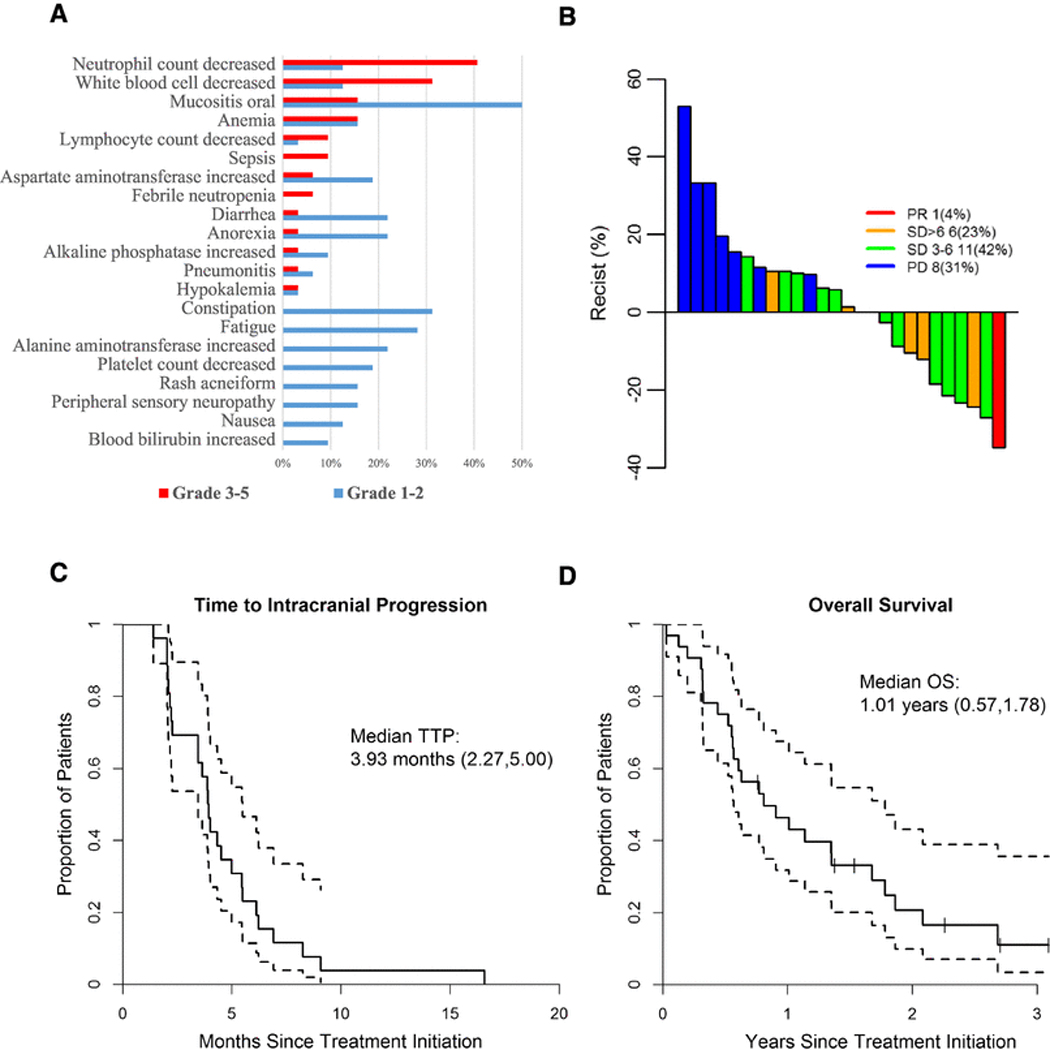
Clinical outcomes and survival. a Most prevalent toxicities (All grades) in response to everolimus, vinorelbine, trastuzumab therapy. Grade 1 and 2 toxicities are presented in blue; Grade 3–5 toxicities are presented in red. b Waterfall plot of intracranial objective response rates by modified RECIST criteria. c Median time toprogression (TTP) and d median overall survival (OS) in response to everolimus, vinorelbine, trastuzumab among patients with progressive or new brain metastases arising from HER2 positive breast cancer
The most common grade 3–4 toxicities included neutropenia (41%), leukopenia (31%), oral mucositis (16%), and anemia (16%). Of note, steroid mouth rinse was not mandated as the results of the SWISH study were not reported until 2016 [48].
Efficacy
Objective response rate
Among 26 patients evaluable for efficacy, the intracranial RR by modified RECIST was 4% (0 complete responses (CR); 1 partial response (PR); Table 2 and Fig. 2b). Seventeen additional patients (65%) had stable disease (SD) as best response. Clinical benefit rate (CBR) for ≥ 6 months was 27% and for ≥ 3 months was 69%. Extracranial RR for 12 patients was 42% PR and 50% SD.
Table 2.
Summary of objective response rates (best response observed at any time)
| Summary of objective response rates | Intracranial (n = 26) N (%) |
Extrac- ranial (n = 12) N (%) |
|---|---|---|
| Complete response | 0 (0%) | 0 (0%) |
| Partial response | 1 (4%) | 5 (42%) |
| Stable disease | 17 (65%) | 6 (50%) |
| Progressive disease | 18 (69%) | 1 (8%) |
| Clinical benefit rate (CR or PR + SD ≥ 6 months) | 7 (27%) | – |
| Clinical benefit rate (CR or PR + SD ≥ 3 months) | 8 (69%) | – |
Intracranial RR were not significantly different by hormone receptor (HR) status (p = 0.26). For those with ER/ PR negative, HER2 + BC (n = 15), intracranial RR were PR 6.7%, SD for ≥ 6 months 27%, SD for 3–6 months 27%, and PD 40%. For those with ER and/or PR positive, HER2 + BC (n = 11), intracranial RR were PR 0%, SD for ≥ 6 months 18%Clinical benefit rat, SD for 3–6 months 64%, and PD 18%.
Intracranial RR was also evaluated by bi-dimensional MacDonald criteria: 0% CR, 8% with > 50% decrease, 54% with decrease ≤ 50% and < 25% increase, and 38% with > 25% increase in intracranial lesions, eFigure 1.
Time to intracranial progression
The median TTP was 3.93 months (95% CI 2.27–5.00; Fig. 2c). There was no difference in TTP by HR status: HR– 3.88 months versus HR + 4.01 months (95% CI 2.11–5.46 versus 95% CI 2.04–5.49, respectively; eFigure 2A). Only 4 patients had extracranial progression prior to intracranial progression; however, the estimate for median time to first (extra- or intra-) progression is the same (3.93 CI 2.27–4.34).
Overall survival
OS for evaluable patients was 1.01 years (95% CI 0.57–1.78; Fig. 2d). OS was numerically, but not significantly, longer for those with HR+ (1.78 years, 95% CI 0.55–2.69), compared to those with HR– BC (0.63 years, 95% CI 0.32–1.35, p = 0.14; eFigure 2B).
Health-related quality of life
Of the 32 patients evaluable for toxicity, 20 completed baseline QOL, and 11 completed both baseline and 9-week QOL assessment. On a scale of 0–108, median baseline FACT-G scores were 76 (range 54–105). Change from baseline to 9-week follow-up was – 10.5. Median baseline Brain Cancer subscale results (range 0–92) were 63 (range 46–88), with change from baseline to 9 weeks of – 1.0. Median Breast Cancer subscale results (range 0–40) were 27 (range 17–35), with change from baseline to 9 weeks of 1.0 (eTable 2).
Tumor sequencing and correlative endpoints
Hypothesis-generating targeted DNA sequencing was completed on 23 samples from 20 patients: 12 primaries, 11 metastases (6 BCBM, 3 lymph nodes (LN), 2 liver metastases), with 3 matched primary/metastasis pairs (2 primary/ brain, 1 primary/LN) (diagram, eFigure 3). The most commonly mutated gene was TP53 (87%, 20/23), followed by PI3KCA (35%, 8/23, Fig. 3a), with 5/8 being the canonical His1074Arg PIK3CA mutation. Given that the mechanism of action of everolimus targets PI3K/mTOR signaling, we next examined alterations in the PI3K/mTOR pathway: 68% (15/23) of tumors had at least one alteration in the pathway, with 35% (8/23) of tumors having > 1 (Fig. 3B). PI3K/ mTOR mutations were not associated with clinical response, and globally these genes illustrated copy number loss (bottom row, Fig. 3b).
Fig. 3.
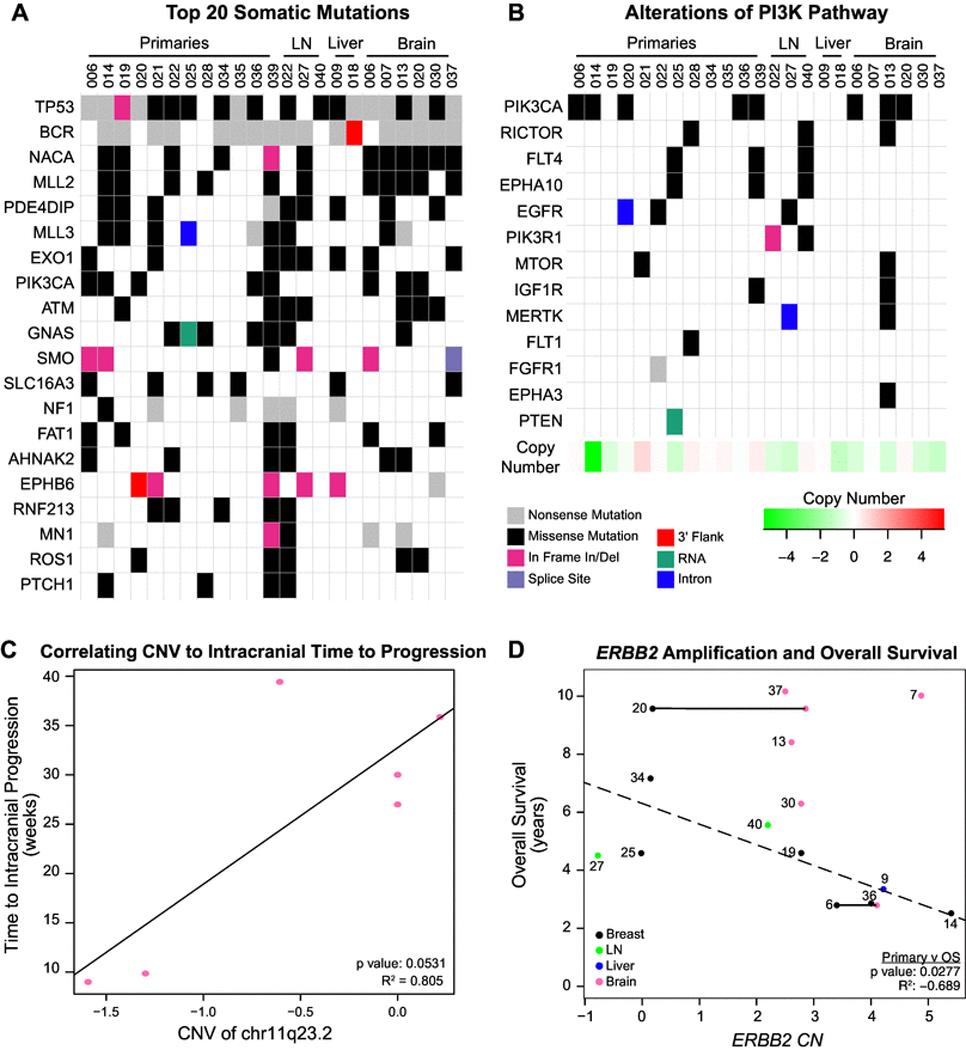
Targeted DNA sequencing mutations and copy number alterations. a Significantly mutated genes (SMGs) across all available tissues, b mutations in the PI3K/mTOR pathway, c HER2 Copy Number Variations (CNVs) versus Intracranial Time to Progression in brain metastases, d HER2 CNVs versus Overall Survival across all available tissues. Dotted line is the correlation based on the primary tumors only. Horizontal lines indicate tissues from matched pairs (n = 2)
We next examined intracranial TTP in the context of BCBM copy number alterations. Interestingly, loss of chr11q23.2 was associated with shortened time to intracranial progression (p = 0.051, R2 = 0.805, Fig. 3c). Genes at this copy number segment include some involved in neuronal processes and tumor progression: KMT2A [49, 50], ARCN1 [51], PHLDB1 [51, 52], DDX6 [52, 53], CXCR5 [54, 55], ABCG4 [56–58], and NLRX1 [59, 60].
Lastly, evaluation of ERBB2 amplification across our dataset demonstrated considerable heterogeneity. 19/23 tumors had copy number gain, while one primary without ERBB2 CN amplification contained a previously reported K755S Tyr-kinase domain mutation [40]. Interestingly, ERBB2 amplification increased in BCBM compared to matched primaries (Fig. 3D, horizontal line). HER2 amplification in the primary (black dots) BC negatively trends with OS from original BC diagnosis (Fig. 3d, p = 0.085, R2=−0.39).
Discussion
This study investigated the efficacy of mTOR inhibition using brain-penetrant everolimus in combination with standard anti-HER2 therapy, vinorelbine and trastuzumab, in patients with progressive HER2 + breast cancer brain metastases (BCBM). While intracranial response rate (RR) to combination therapy was only 4%, intracranial clinical benefit was observed at 3 (69%) and 6 months (27%). Intracranial time to progression (TTP) was nearly 4 months for this heavily pretreated patient population. Median survival was > 1 year with average time from BCBM diagnosis to study participation also > 1 year. Thus, enrolled patients lived > 2 years with HER2 + BCBM, consistent with current literature [61–63]. Quality of life scores were not adversely impacted by treatment with this regimen; however, improvements in quality of life were not observed in the responding patients.
Clinically, everolimus has been evaluated across various BC subtypes. In HR+, HER2− metastatic BC, the addition of everolimus to aromatase inhibition yielded significant improvements in PFS that led to FDA approval [64]. Moreover, the addition of everolimus to standard adjuvant endocrine therapy is being examined in a large phase III clinical trial (NCT01674140). In HER2 + metastatic BC, the addition of everolimus to vinorelbine and trastuzumab yielded improvements in PFS (7 versus 5.78 months, p = 0.0067) [14]. Coupling the known brain-permeable property of everolimus with the high incidence of BCBM in HER2 + BC, our study and others are examining everolimus in the setting of CNS relapse. Results anticipated from the phase 1b/2 study of lapatinib, everolimus, and capecitabine in trastuzumab-pretreated HER2 + BCBM (NCT01783756) will continue to contextualize our findings.
Our exploratory correlative sequencing results in primary and metastatic HER2 + BC demonstrate frequent alterations in TP53 and throughout the PI3KCA pathway. We identified TP53 alterations in 83% of HER2-positive primaries and 100% of BCBM, similar to previous reports [65, 66]. Increased HER2-amplification in the BCBMs was observed, including a relative increase in the BCBM in two matched pairs, and is consistent with previous reports [67, 68]. Further characterization of chr11q23.2 with RNAseq is needed to understand dynamic gene expression localized to this segment of the genome, many of which are involved in neuronal processes [49–60]. Overall, our data support prior findings of genetic heterogeneity in metastatic HER2 + BCBM amid a background of TP53 mutations and ERBB2 amplification.
The present study must be interpreted considering its limitations. The sample size is small, particularly for robust correlative findings, thus is exploratory and hypothesisgenerating. Future studies with larger patient populations, prospective sample collection, and parallel RNA and DNA sequencing could more efficiently identify potential correlations between genetic alterations, gene expression, and clinical outcomes, and better define the evolution of genetic changes within patients over the course of their disease.
In closing, while the combination of everolimus, vinorelbine, and trastuzumab did not meet our pre-specified intracranial response endpoint, TTP and OS were similar to prior studies in a heavily pretreated patient population with vanishingly few additional clinical options. Moreover, this combination showed an acceptable toxicity profile. Further evaluation of the molecular profile of primary and/or metastatic tumors from patients with HER2 + BCBM should be investigated to correlate genomic alterations with clinical response. If able to enrich for responders using a strategic biomarker approach, comparison of this combination to current standard of care in the setting of HER2 + BCBM is warranted.
Supplementary Material
Acknowledgements
The authors thank the patients and their families who participated in this trial. We also thank the following cores and groups at UNC-Chapel Hill for their assistance with this correlative study: Tissue Procurement Facility, Translational Pathology Laboratory, Translational Genomics Laboratory, and Lineberger Bioinformatics Group. We also appreciate Erin Laurie’s administrative support.
Funding This study was funded by the Novartis Pharmaceuticals, Damon Runyon Research Foundation (CKA), National Institute of Health K23 (CKA), National Institute of Health F30-CA200345 (MBS), and University of North Carolina Network Group Integrated Translational Science Center award (NCI 5U10CA181009).
Footnotes
Data Availability The datasets generated during and/or analyzed during the current study will be made available in the dbGAP repository.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10549-018-4852-5) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of interest Carey K. Anders is an uncompensated consultant/advisory board member for Novartis, Sanofi, toBBB, Angiochem, Merrimack, Lily, Genentech, Nektar, and Kadmon, receives unrelated research funding from Novartis, Sanofi, toBBB, Angiochem, Merrimack, PUMA, Lily, Merck, Oncothyreon, Cascadian, Nektar, and Tesaro, and receives honoraria for UptoDate and Jones and Bartlett Publishing. Rita Nanda is a consultant/advisory board member for AstraZeneca, Celgene, Genentech, Merck, Pfizer, Puma, and Syndax. Nikita Shah is a consultant for Novartis. The other authors declare they have no conflicts of interest.
Ethical approval This manuscript complies with all current laws of the country in which they were performed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained by all individual participants included in the study.
Research involving human and animal participants This article does not contain any studies with animals performed by any of the authors.
References
- 1.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JMS, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138:241–256. 10.5858/arpa.2013-0953-SA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977. 10.1002/cncr.11436 [DOI] [PubMed] [Google Scholar]
- 3.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS (2008) Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. JNCI J Natl Cancer Inst 100:1092–1103. 10.1093/jnci/djn216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain SM, Baselga J, Miles D, Im Y-H, Quah C, Lee LF, Cortés J (2014) Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol 25:1116–1121. 10.1093/annonc/mdu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niwinska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 21:942–948. 10.1093/annonc/mdp407 [DOI] [PubMed] [Google Scholar]
- 6.Niwinska A, Murawska M, Pogoda K (2010) Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer 116:4238–4247. 10.1002/cncr.25391 [DOI] [PubMed] [Google Scholar]
- 7.Mounsey LA, Deal AM, Keith KC, Benbow JM, Shachar SS, Zagar T, Dees EC, Carey LA, Ewend MG, Anders CK (2017) Changing natural history of HER2–Positive breast cancer metastatic to the brain in the era of new targeted therapies. Clin Breast Cancer. [DOI] [PubMed] [Google Scholar]
- 8.Zagar TM, Van Swearingen AED, Kaidar-Person O, Ewend MG, Anders CK (2016) Multidisciplinary management of breast cancer brain metastases. Oncology 30:923–933 [PubMed] [Google Scholar]
- 9.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, Roche H, Liu MC, Greil R, Ciruelos E, Loibl S, Gori S, Wardley A, Yardley D, Brufsky A, Blum JL, Rubin SD, Dharan B, Steplewski K, Zembryki D, Oliva C, Roychowdhury D, Paoletti P, Winer EP (2009) Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 15:1452–1459. 10.1158/1078-0432.CCR-08-1080 [DOI] [PubMed] [Google Scholar]
- 10.Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga J-Y, Gonçalves A, Leheurteur M, Domont J, Gutierrez M, Curé H, Ferrero J-M, Labbe-Devilliers C (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71. 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 11.Keith KC, Lee Y, Ewend MG, Zagar TM, Anders CK (2016) Activity of Trastuzumab-Emtansine (Tdm1) in Her2-positive breast cancer brain metastases: a case series. Cancer Treat Commun 7:43–46. 10.1016/).ctrc.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalsi R, Feigenberg S, Kwok Y, Tkaczuk K, Mehta M, Chum-sri S (2015) Brain metastasis and response to ado-trastuzumab emtansine: a case report and literature review. Clin Breast Cancer 15:e163–6. 10.1016/j.clbc.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 13.Wilks ST (2015) Potential of overcoming resistance to HER2− targeted therapies through the PI3K/Akt/mTOR pathway. Breast 24:548–555. 10.1016/j.breast.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 14.André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, Yap Y-S, Papai Z, Lang I, Armstrong A, Lerzo G, White M, Shen K, Litton J, Chen D, Zhang Y, Ali S, Taran T, Gianni L (2014) Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 15:580–591. 10.1016/1470-2045(14)70138-X [DOI] [PubMed] [Google Scholar]
- 15.Gallardo A, Lerma E, Escuin D, Tibau A, Muñoz J, Ojeda B, Barnadas A, Adrover E, Sánchez-Tejada L, Giner D, Ortiz-Martínez F, Peiró G (2012) Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer 106:1367–1373. 10.1038/bjc.2012.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wikman H, Lamszus K, Detels N, Uslar L, Wrage M, Benner C, Hohensee I, Ylstra B, Eylmann K, Zapatka M, Sauter G, Kemming D, Glatzel M, Müller V, Westphal M, Pantel K (2012) Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res BCR 14:R49 10.1186/bcr3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing J, Greshock J, Holbrook JD, Gilmartin A, Zhang X, McNeil E, Conway T, Moy C, Laquerre S, Bachman K, Wooster R, Degenhardt Y (2012) Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther 11:720–729. 10.1158/1535-7163.MCT-11-0505 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527:100–104. 10.1038/nature15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamo B, Deal AM, Burrows E, Geradts J, Hamilton E, Blackwell KL, Livasy C, Fritchie K, Prat A, Harrell JC, Ewend MG, Carey LA, Miller CR, Anders CK (2011) Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res 13:R125 10.1186/bcr3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, Curatolo P, de Vries PJ, Whittemore VH, Thiele EA, Ford JP, Shah G, Cauwel H, Lebwohl D, Sahmoud T, Jozwiak S (2013) Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381:125–132. 10.1016/S0140-6736(12)61134-9 [DOI] [PubMed] [Google Scholar]
- 21.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, Harris GJ, Bullitt E, Van den Abbeele AD, Henson JW, Li X, Gelman R, Burstein HJ, Kasparian E, Kirsch DG, Crawford A, Hochberg F, Winer EP (2008) Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26:1993–1999. 10.1200/JCO.2007.12.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald DR, Cascino TL, Schold SC, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280. 10.1200/JCO.1990.8.7.1277 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute (2009) Common Terminology Criteria for Adverse Events v 4.0. Available from https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.Pdf
- 24.Zhao X, Wang A, Walter V, Patel NM, Eberhard DA, Hayward MC, Salazar AH, Jo H, Soloway MG, Wilkerson MD, Parker JS, Yin X, Zhang G, Siegel MB, Rosson GB, Earp HS, Sharpless NE, Gulley ML, Weck KE, Hayes DN, Moschos SJ (2015) Combined targeted DNA sequencing in non-small cell lung cancer (NSCLC) using UNCseq and NGScopy, and RNA sequencing using UNC-qeR for the detection of genetic aberrations in NSCLC. PLoS ONE 10:e0129280 10.1371/journal.pone.0129280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986. 10.1200/JCO.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 27.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA (1995) The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75:1151–1161 [DOI] [PubMed] [Google Scholar]
- 28.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579. 10.1200/JC0.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 29.Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tischler G, Leonard S (2014) biobambam: tools for read pair collation based algorithms on BAM files. Source Code Biol Med 9:13 10.1186/1751-0473-9-13 [DOI] [Google Scholar]
- 32.Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mose LE, Wilkerson MD, Hayes DN, Perou CM, Parker JS (2014) ABRA: improved coding indel detection via assembly-based realignment. Bioinformatics 30:2813–2815. 10.1093/bioinformatics/btu376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard. In: Picard. http://broadinstitute.github.io/picard Accessed 9 Nov 2017
- 35.Garrison E, Marth G (2012) Haplotype-based variant detection from short-read sequencing. ArXiv12073907 Q-Bio [Google Scholar]
- 36.Baier H, Schultz J (2014) ISAAC - InterSpecies Analysing Application using Containers. BMC Bioinform 15:18 10.1186/1471-2105-15-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders CT, Wong WSW, Swamy S, Becq J, Murray LJ, Cheetham RK (2012) Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28:1811–1817. 10.1093/bioinformatics/bts271 [DOI] [PubMed] [Google Scholar]
- 38.Cingolani P, Patel VM, Coon M, Nguyen T, Land SJ, Ruden DM, Lu X (2012) Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Front Genet 3:35 10.3389/fgene.2012.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6:80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, Stefancsik R, Harsha B, Kok CY, Jia M, Jubb H, Sondka Z, Thompson S, De T, Campbell PJ (2017) COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45:D777–D783. 10.1093/nar/gkw1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine. Database of Single Nucleotide Polymorphisms (dbSNP). http://www.ncbi.nlm.nih.gov/SNP/
- 42.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won H-H, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Consortium EA (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536(7616):285 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, Meyerson M, Getz G (2015) Oncotator: cancer variant annotation tool. Hum Mutat 36:E2423–2429. 10.1002/humu.22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skidmore ZL, Wagner AH, Lesurf R, Campbell KM, Kunisaki J, Griffith OL, Griffith M (2016) GenVisR: genomic visualizations in R. Bioinformatics 32:3012–3014. 10.1093/bioinformatics/btw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva GO, Siegel MB, Mose LE, Parker JS, Sun W, Perou CM, Chen M (2017) SynthEx: a synthetic-normal-based DNA sequencing tool for copy number alteration detection and tumor heterogeneity profiling. Genome Biol 18:66 10.1186/s13059-017-1193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanova A, Deal AM (2016) Two-stage design for phase II oncology trials with relaxed futility stopping. Stat Interface 9:93–98. 10.4310/SII.2016.v9.n1.a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaspar LE, Scott C, Murray K, Curran W (2000) Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys 47:1001–1006 [DOI] [PubMed] [Google Scholar]
- 48.Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, Dhillon N, Hwang LC, Nangia C, Mayer IA, Meiller TF, Chambers MS, Sweetman RW, Sabo JR, Litton JK (2017) Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 18:654–662. 10.1016/S1470-2045(17)30109-2 [DOI] [PubMed] [Google Scholar]
- 49.Huang Y-C, Lin S-J, Shih H-Y, Chou C-H, Chu H-H, Chiu C-C, Yuh C-H, Yeh T-H, Cheng Y-C (2017) Epigenetic regulation of NOTCH1 and NOTCH3 by KMT2A inhibits glioma proliferation. Oncotarget 8:63110–63120. 10.18632/oncotarget.18668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y-C, Shih H-Y, Lin S-J, Chiu C-C, Ma T-L, Yeh T-H, Cheng Y-C (2015) The epigenetic factor Kmt2a/Mll1 regulates neural progenitor proliferation and neuronal and glial differentiation. Dev Neurobiol 75:452–462. 10.1002/dneu.22235 [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Sun B, Zhao Y, Song X, Fan W, Zhou K, Zhou L, Mao Y, Lu D (2012) Fine mapping of a region of chromosome 11q23.3 reveals independent locus associated with risk of glioma. PLoS ONE 7:e52864 10.1371/journal.pone.0052864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baskin R, Woods NT, Mendoza-Fandino G, Forsyth P, Egan KM, Monteiro ANA (2015) Functional analysis of the 11q23.3 glioma susceptibility locus implicates PHLDB1 and DDX6 in glioma susceptibility. Sci Rep 5:17367 10.1038/srep17367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YJ, Kang W, Kim SH, Sa JK, Kim N, Paddison PJ, Kim M, Joo KM, Hwang Y-I, Nam D-H (2016) Involvement of DDX6 gene in radio- and chemoresistance in glioblastoma. Int J Oncol 48:1053–1062. 10.3892/ijo.2016.3328 [DOI] [PubMed] [Google Scholar]
- 54.Weiss N, Deboux C, Chaverot N, Miller F, Baron-Van Evercooren A, Couraud P-O, Cazaubon S (2010) IL8 and CXCL13 are potent chemokines for the recruitment of human neural precursor cells across brain endothelial cells. J Neuroimmunol 223:131–134. 10.1016/j.jneuroim.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 55.Brunn A, Montesinos-Rongen M, Strack A, Reifenberger G, Mawrin C, Schaller C, Deckert M (2007) Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol (Berl) 114:271–276. 10.1007/s00401-007-0258-x [DOI] [PubMed] [Google Scholar]
- 56.Tarr PT, Edwards PA (2008) ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J Lipid Res 49:169–182. 10.1194/jlr.M700364-JLR200 [DOI] [PubMed] [Google Scholar]
- 57.Kim WS, Weickert CS, Garner B (2008) Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem 104:1145–1166. 10.1111/j.1471-4159.2007.05099.x [DOI] [PubMed] [Google Scholar]
- 58.Hegyi Z, Homolya L (2016) Functional cooperativity between ABCG4 and ABCG1 isoforms. PLoS ONE 11:e0156516 10.1371/journal.pone.0156516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theus MH, Brickler T, Meza AL, Coutermarsh-Ott S, Hazy A, Gris D, Allen IC (2017) Loss of NLRX1 exacerbates neural tissue damage and NF-kB signaling following brain injury. J Immunol 1950 199:3547–3558. 10.4049/jimmunol.1700251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imbeault E, Mahvelati TM, Braun R, Gris P, Gris D (2014) Nlrx1 regulates neuronal cell death. Mol Brain 7:90 10.1186/s13041-014-0090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mounsey L, Deal AM, Benbow JM, Keith K, Zagar TM, Dees EC, Carey LA, Ewend M, Anders CK (2016) A changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new, targeted therapies. Clin Breast Cancer 18(1):29–37 [DOI] [PubMed] [Google Scholar]
- 62.Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Chao ST, Barnett GH, Murphy ES, Vogelbaum MA, Angelov L, Peereboom DM, Suh JH (2017) Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer 123:2283–2293. 10.1002/cncr.30616 [DOI] [PubMed] [Google Scholar]
- 63.Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT (2017) Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer 74:17–25. 10.1016/).ejca.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 64.Yardley DA, Noguchi S, Pritchard KI, Burris HA, Baselga J, Gnant M, Hortobagyi GN, Campone M, Pistilli B, Piccart M, Melichar B, Petrakova K, Arena FP, Erdkamp F, Harb WA, Feng W, Cahana A, Taran T, Lebwohl D, Rugo HS (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30:870–884. 10.1007/s12325-013-0060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo Nigro C, Vivenza D, Monteverde M, Lattanzio L, Gojis O, Garrone O, Comino A, Merlano M, Quinlan PR, Syed N, Purdie CA, Thompson A, Palmieri C, Crook T (2012) High frequency of complex TP53 mutations in CNS metastases from breast cancer. Br J Cancer 106:397–404. 10.1038/bjc.2011.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saunus JM, Quinn MC, Patch A-M, Pearson JV, Bailey PJ, Nones K, McCart Reed AE, Miller D, Wilson PJ, Al-Ejeh F, Mariasega-ram M, Lau Q, Withers T, Jeffree RL, Reid LE, Da Silva L, Matsika A, Niland CM, Cummings MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Anderson MJ, Fink JL, Holmes O, Kazakoff S, Leonard C, Newell F, Taylor D, Waddell N, Wood S, Xu Q, Kassahn KS, Narayanan V, Taib NA, Teo S-H, Chow YP, kConFab, Jat PS, Brandner S, Flanagan AM, Khanna KK, Chenevix-Trench G, Grimmond SM, Simpson PT, Waddell N, Lakhani SR (2015) Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol 237:363–378. 10.1002/path.4583 [DOI] [PubMed] [Google Scholar]
- 67.Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R, Hamilton RL, Chmielecki J, Puhalla SL, Davidson NE, Oesterreich S, Brufsky AM, Young L, Lee AV (2016) Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 10.1001/jamaoncol.2016.5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park SH, McKenna A, Chevalier A, Rosenberg M, Barker FG 2nd, Gill CM, Van Hummelen P, Thorner AR, Johnson BE, Hoang MP, Choueiri TK, Signoretti S, Sougnez C, Rabin MS, Lin NU, Winer EP, Stemmer-Rachamimov A, Meyerson M, Garraway L, Gabriel S, Lander ES, Beroukhim R, Batchelor TT, Baselga J, Louis DN, Getz G, Hahn WC (2015) Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. 10.1158/2159-8290.CD-15-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


