Abstract
The most important of the extra-thyroidal manifestations of Graves’ disease, Graves’ orbitopathy (GO), remains a vexing clinical problem. Treatment of severe active disease has been limited to steroids or radiotherapy. In the relatively rare case where vision is threatened, emergent decompression surgery can be performed. The proptosis, motility, or cosmetic concerns associated with stable GO are commonly remedied with surgical intervention. Substantial obstacles have prevented the development of specific medical therapies for GO, in large part resulting from poor understanding of disease pathogenesis and the absence of pre-clinical animal models. Fundamental aspects of GO’s etiology have been uncovered from studies based in cell culture, extensive analysis of blood constituents, and detailed examination of orbital contents collected at the time of surgical intervention. Many of the published reports resulting from these studies are descriptive and all have failed to yield unifying concepts that integrate the anatomically divergent manifestations of Graves’ disease. This brief review covers recent findings of several research groups. While major breakthroughs continue to occur in closely related autoimmune diseases, progress in identifying the pathogenic mechanisms relevant to GO has been limited. As emerging insights into human autoimmunity becomes applied to the study of Graves’ disease, we anticipate that improved therapeutic strategies will find their way to our patients with GO.
Keywords: Autoimmune, B cells, fibrocytes, thyroid, TSH receptor
INTRODUCTION
Graves’ orbitopathy (GO), also known as thyroid-associated ophthalmopathy (TAO) or thyroid eye disease, represents the most common and vexing extra-thyroidal manifestation of Graves’ disease (GD) (1). Why the connective tissues investing the human orbit are susceptible to this autoimmune process remains an enigma. But increasing information about the peculiar phenotype exhibited by orbital fibroblasts is beginning to provide some insight into the potential pathogenic mechanisms involved in GO. An unresolved question concerns the identity of the autoantigen(s) that participates in disease initiation and progression and characterizing the mechanisms through which peripheral immune tolerance to it is lost. In this brief summery, I will attempt to review some of the recent advances made in the study of pathogenesis, characterization, and treatment of GO.
AUTOANTIGENS CURRENTLY UNDER CONSIDERATION
The autoantigens associated with GD might legitimately be parsed into two groups, those with pathogenic function and others which probably serve only as clinical markers but are unlikely to initiate or propagate disease. TSH receptor (TSHR) represents the central autoantigen in GD by virtue of its role in mediating the action of thyroid-stimulating immunoglobulins (TSI) (2, 3). Thus, it be longs in the first group. These antibodies activate TSHR and elicit the production of thyroid hormones in a manner superficially resembling that of TSH. Initial evidence for the participation of TSHR and TSI in the pathogenesis of GO was presented by Feliciello et al. (4) who reported detecting TSHR mRNA in orbital tissues from healthy individuals and those with GO. Shortly thereafter, Heufelder and colleagues found the transcript expressed in cultured orbital fibroblasts (5). Both of these early studies relied on PCR-based assays and thus even trivial levels of TSHR transcripts could be detected. Subsequently a number of reports have confirmed the presence of both TSHR mRNA and protein expressed by fibroblasts from the orbit as well as those from other connective/adipose tissue depots (6–13). The levels in adipocytes provoked to differentiation in vitro have been generally higher than those in undifferentiated fibroblasts. Thus, TSHR appears to represent a ubiquitous protein with regard to its expression in connective tissues. Whether the density or function of the receptor differs in orbit compared to any of the other connective tissues thus far examined remains to be established. In any event, the contention by some that the distribution of this receptor protein is limited to the thyroid and orbit appears to be incorrect. A more realistic view of TSHR would accommodate its emerging role in other biological processes, such as those associated with immune function (14) and adipogenesis in many different connective tissue depots (15).
At about the same time the TSHR was demonstrated in orbital tissues, Weightman et al. (16) reported that IgG collected from patients with GD (GD-IgG) could displace radiolabeled IGF-I from the surface of fibroblasts. While these early studies were rather preliminary in nature and failed to identify the determinant to which the displaced IGF-I was binding, they did suggest the possibility that IGF-I receptor (IGF-IR) or one (or more) of the IGF-I binding proteins might prove relevant to the disease. Whether the interaction between GD-IgG and the then unidentified cell surface protein resulted in cellular responses was not determined in that early communication. Many years later, Pritichard et al. reported that IGF-I and GD-IgG could bind and activate IGF-IR (17). Moreover, they found that in those fibroblasts from patients with GO, activation of IGF-IR resulted in the expression of two powerful T cell chemoattractants, interleukin (IL)-16 and regulated upon activation, normal T cell expressed (RANTES) (18). Both of these molecules have been associated with the pathogenesis of autoimmune disease (19, 20). The Akt/FRAP/mTor/p70s6k pathway mediates the induction of IL-16, effects which were blocked with the macrolide, rapamycin (18). This same group of investigators then reported that IGF-I and GD-IgG could induce the accumulation of hyaluronan by orbital fibroblast cultures (21). The studies failed to examine whether glycosaminoglycan synthesis was up-regulated or whether macromolecular turnover was decreased but given the absence of hyaluronan degradation in human fibroblasts reported previously (22), this possibility remains unlikely. That report demonstrated that human recombinant TSH failed to alter the accumulation of the glycosaminoglycan. Orbital fibroblasts from control donors failed to respond to either IGF-I or GD-IgG as did dermal fibroblast cultures.
OTHER ANTIGENS THAT MIGHT PROVE RELEVANT TO GD
A series of potential autoantigens that may play roles in orbital Graves’ disease or could serve as markers of extra-ocular muscle involvement has been reported by Wall and colleagues over the past two decades. In large part, this body of work reflects the long-held contention by that author that extraocular muscles are primarily targeted for autoimmune reactivity in GO. Among the muscle-associated antigens that have been identified include a 63kD/64 kDa protein (23), 1D antigen (23), collagen XIII (24), calsequestrin, G2s and flavoprotein (25). No forceful claims have been made by this group concerning the functional/pathogenic importance that any of these antigens might possess in GD or any other orbital pathology. Rather they appear to favor a role for some or all of these proteins as disease markers. Their experience in searching for the “important” autoantigen should serve to remind us that autoimmunity is often associated with the production of multiple autoantibodies of varying specificities. This might be explained at least in part by the phenomenon known as epitope spreading (26). Time will tell whether the study of any of these proteins can lead us to a better understanding of the development of GO. More likely, they will prove to reflect the non-specific but powerful activation of systemic immune processes, some of which might drive GD, GO, and other forms of autoimmunity. The formidable task of substantiating the relationship between these antibodies and the extraocular muscles remains. We continue to look to Dr Wall and his colleagues for further evidence that these antibodies are related to the orbit and its diseases.
DO THE FIBROBLASTS THAT PLAY A PATHOGENIC ROLE IN GO ORIGINATE FROM BONE MARROW DERIVED FIBROCYTES?
Fibroblasts in the human orbit represent a heterogeneous cell population (27). While one subset expresses the cell-surface glycoprotein, Thy-1 (CD90), another displays a Thy-1− phenotype. Human orbital fibroblasts exhibiting each phenotype can now be coupled to their potential for differentiation into either myofibroblasts expressing smooth muscle actin (Thy-1+) or those capable of developing into triglyceride-accumulating adipocytes (Thy-1−) (28, 29). Differentiation into either phenotype requires a specific set of culture conditions. For Thy-1+ fibroblasts to differentiate into myofibroblasts, the culture medium must be enriched with transforming growth factor (TGF)-β. In contrast, Thy-1− fibroblasts must be treated with prostacyclin in combination with compounds that increase intracellular cAMP levels (29). Alternatively, activation of peroxisome proliferator activator γ by the prostaglandin J2 series of molecules or by thiazolidinediones, including rosiglitazone can promote adipogenesis (27). These attributes are congruent with the concept that increased adipogenesis results in tissue expansion seen in GO. But this contention rests only on circumstantial evidence. While orbital fat expansion is known to occur in GO, whether fat cell size or fat cell number increases as part of the disease process has yet to be determined. It remains possible that an exaggerated accumulation of hyaluronan and other glycosaminoglycans in conjunction with increased mass of fat cells might account for a substantial component of the tissue expansion associated with GO.
Orbital fibroblasts from patients with TAO also display higher levels of IGF-IR than do their control counterparts (17). When treated with IGF-I, these cells exhibit a set of responses, mediated through IGF-IR, that are absent in orbital fibroblasts from healthy donors (17, 18, 21). When orbital fibroblasts are provoked to differentiate into fat cells, they express higher levels of TSHR (30, 31). The underlying pattern of cell recruitment to the orbit in GO that might account for the cellular heterogeneity found in GO is only now beginning to be identified. Could the phenotypic divergence found among fibroblasts in the orbit arise from a subset of cells trafficked there in GO? A very recent report by Douglas et al. suggests that the abundance of circulating fibrocytes in patients with GD might be elevated above those found in individuals without autoimmune disease (32). Fibrocytes derive from a monocyte lineage of bone marrow cells (33). They have been insinuated in several aspects of tissue remodeling and wound repair (34). Consistent with the findings of others, this report suggests that fibrocyte abundance in cultured peripheral blood mononuclear cells (PBMC) derived from healthy subjects is extremely rare (32). PBMC cultured from patients with GD generated substantially more frequent fibrocytes exhibiting the anticipated CD34+Col1+CXCR4+ phenotype. The authors found relatively high levels of IGF-IR on fibrocytes from patients with GD, but these are similar to those on control fibrocytes from healthy donors.
The report of Douglas et al. contained preliminary characterization of the fibrocyte phenotype in cells collected from patients with GD (32). The objective there was to identify cellular features of these cells that might set them apart from fibrocytes donated by healthy donors. An array of cellular markers was examined, including CD34, Col1, IGF-IR, LSP-1, CD31, CD14, and CXCR4. CD34+ cells were identified in situ in orbital fat from patients undergoing surgical decompression for severe GO (Fig. 1). In contrast, none of these CD34+ cells could be detected in healthy orbital tissues. Unexpectedly, CD34+Col+CD31− cells from patients with GO expressed high levels of TSHR, both in circulation and in those infiltrating diseased orbital tissues (32) (Fig. 2). Moreover, the levels of TSHR were comparable to those found on thyroid epithelial cells. Surprisingly, TSHR displayed on fibrocytes appears to be functional. When ligated with bovine TSH, the receptor mediates cellular responses by fibrocytes, including the up-regulation of IL-6 and tumor necrosis factor (TNF)-α production (32) (Fig. 3).
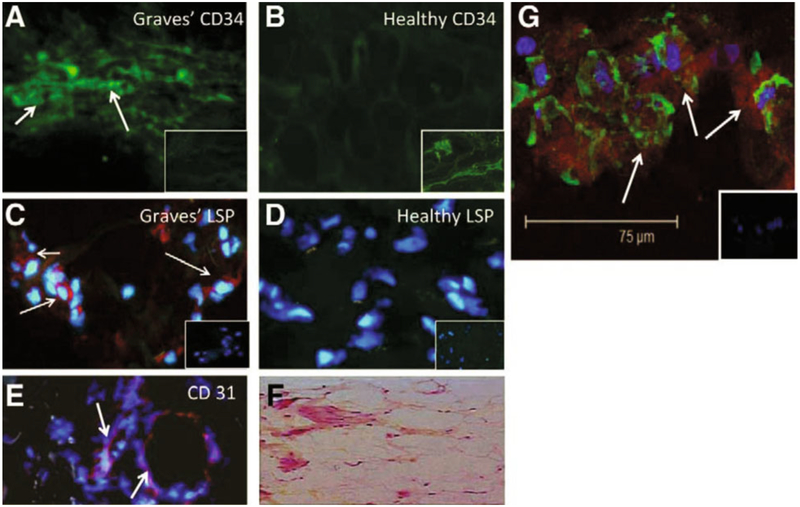
Fig. 1 -.
CD34+ LSP-1+ TSHR+ fibrocytes can be identified in the orbital tissue of patients with thyroid-associated ophthalmopathy (TAO) but are absent in tissues from healthy donors. A) CD34 expression (arrows, green FITC) in TAO-derived tissue (inset, negative control staining). B) Absent CD34 expression in healthy tissue (inset, positive staining control). C) LSP-1 expression in TAO-derived tissue [red, arrows, nuclei counterstained with DAPI (blue)] (inset negative control). D) Absence of LSP-1 expression in healthy tissue (inset negative control). E) CD31 expression in disease-derived tissue is limited to vascular endothelium (red, arrows). F) H and E stained consecutive thin-sections of the same orbital tissue (40 x). G) Fibrocytes present in orbital tissue from patients with TAO co-express CD34 and TSH receptor (TSHR). Thin sectioned tissue from a donor with TAO was stained according to procedures described in “Methods” with anti-CD34 (green) and anti-TSHR (red) antibodies. Nuclei were counterstained with DAPI (blue). Thin sections were then subjected to confocal microscopy. Inset contains a negative staining control. [Reprinted with permission from (32), copyright 2010, The Endocrine Society].
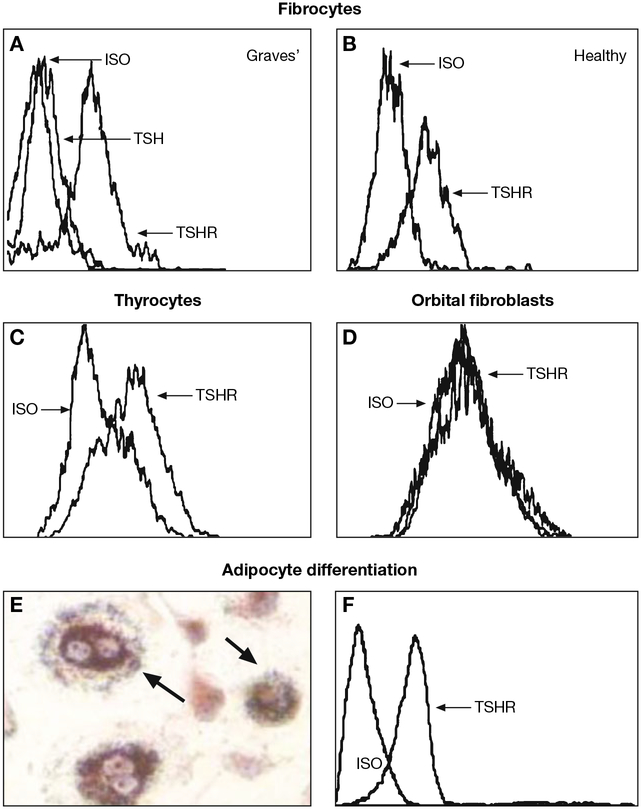
Fig. 2 -.
Fibrocytes cultivated from peripheral blood mononuclear cells express high levels of TSH receptor (TSHR) regardless of whether they derive from (A) patients with Graves’ disease (GD) or (B) from healthy donors. (C) These levels are comparable to those found on primary human thyroid epithelial cells. (D) In contrast, undifferentiated orbital fibroblasts fail to express TSHR. (E) Fibrocytes differentiated into adipocytes accumulate intracellular lipid droplets staining with Oil Red O. (F) TSHR levels on fibrocytes remain elevated following differentiation. Fibrocytes, orbital fibroblasts, and thyrocytes were cultivated. In panel A, fibrocytes were pre-incubated with bovine TSH (bTSH) (5 mU/ml) prior to staining with anti-TSHR antibodies. [Reprinted with permission from (32), Copyright 2010, The Endocrine Society].
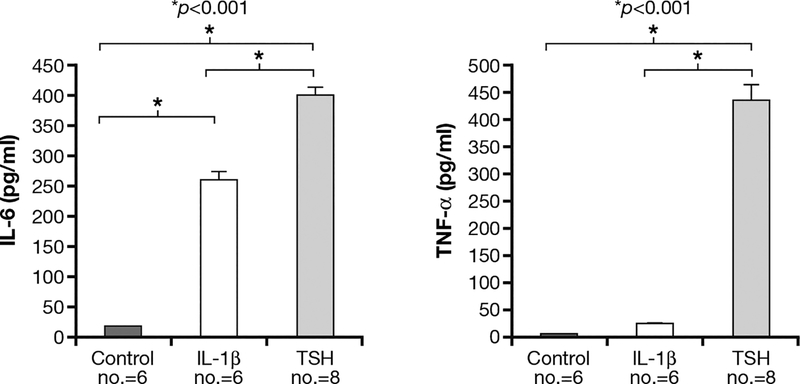
Fig. 3 -.
TSH receptor (TSHR) displayed on fibrocytes generated from peripheral blood mononuclear cells can function to initiate cytokine production. Cultured cells, in this case, from a patient with Graves’ disease, were treated with bovine TSH (bTSH) (5 mU/ml) or inter-leukin (IL)-1β (10 ng/ml) for 48 h. The medium was subjected to enzyme-linked immunososrbent assays specific for (A) IL-6 or (B) tumor necrosis factor (TNF)-α. Data are expressed as the mean±SEM of 3 replicate culture wells from a representative experiment (*p<0.001). [Reprinted with permission from (32), Copyright 2010, The Endocrine Society].
NEW INSIGHTS IN TO THE PHENOTYPE OF ORBITAL FIBROBLASTS
Several groups of investigators have focused on refining and substantiating the concept that orbital fibroblasts exhibit a novel phenotype and that these peculiarities might underlie the susceptibility of the orbit to GO. A recent study by Tomlinson et al. (35) has examined the expression and activity of 11β-hyroxysteroid dehydrogenase 1 (11β HSD-1), several cytokines, and markers of late adipogenesis in affected orbital tissues and in orbital fibroblasts from patients with GO. These various parameters were compared to unaffected controls (35). They reported that levels of transcripts encoding 11β HSD1, the glucocorticoid receptor α, TNF-α, TNF-α inducible protein, TGF-β, IL-1 receptor, and IL-6 were all significantly elevated in diseased tissues as were fatty acid-binding protein 4 and glycerol 6 phosphate dehydrogenase. The activity of 11β HSD1 as determined by the rate of cortisone conversion to cortisol was induced by TNF-α in cultured orbital fat cells. This effect was far more robust in cultures from patients with GO (35). In aggregate their findings might provide important insight into the patterns of orbital inflammation seen in GO.
With regard to the potential relationship between hyaluronan production and TSHR display on orbital fibroblasts, Zhang et al. (36) reported that orbital fibroblasts expressing TSHR with a gain of function mutation exhibit increased activities of several target genes. Among these, levels of hyaluronan synthase-1 and −2 were increased, as was the production of hyaluronan. These data suggest that, at least in contrived models of cell function, TSHR might mediate the increases in hyaluronan production associated with orbital fibroblast activation with TSHR-activating GD-IgG. The findings will need to be reconciled with earlier results demonstrating that GD-IgG might act through IGF-IR in enhancing hyaluronan production in orbital fibroblasts (21).
DO LYMPHOCYTES FROM PATIENTS WITH GD AND GO EXHIBIT A DIVERGENT PHENOTYPE?
Given the over-expression of IGF-IR by orbital fibroblasts coming from patients with GO, a broader search for cell-types that might also express elevated levels the receptor was undertaken. A disproportionately large fraction of T cells from patients with Graves’ disease in the peripheral circulation display the receptor protein (37); 48±4% of CD3+ lymphocytes from patients with GD exhibit the IGFIR+ phenotype compared to 15±3% of those from healthy donors. This increased proportion results in part from an expansion of CD45RO+ memory T cells. While the phenotypes of both CD4+ and CD8+ memory cells are skewed, naïve T cells do not appear to express IGF-IR at an increased frequency. The phenotypic skew among CD3+IGF-IR+ cells was durable and the levels of protein expression were up-regulated following CD3 complex activation. T cells harvested from affected orbital tissues reflect similar bias with regard to IGF-IR+CD3+ cells and IGFIR+CD3+CD4+ cells as those collected from the periphery (37). When treated with either GD-IgG or IGF-I, these T cells exhibit a growth advantage and resist apoptosis, suggesting possible mechanisms underlying the previously recognized T cell population expansion associated with GD and other forms of autoimmunity.
A similarly skewed population of B cells was also described in individuals with GD (38). This display exhibits durability in culture and is maintained or increased with CpG activation. It too can be found among B cells infiltrating the orbit and those circulating and appears to be independent of disease duration or treatment. From those studies, a subset of IGF-IR+ cells might prove responsible for the production of pathogenic anti-TSHR activating antibodies in GD (38). IGF-I enhanced IgG production and increased B cell expansion in cultures from patients with GD, but the growth factor exerted no such effects on B cells from healthy donors. In a study of Danish monozygotic twins discordant for GD, Douglas et al. reported that the skew toward the IGF-IR+ phenotype was present in the affected twin but was absent in the healthy sibling, suggesting that an environmental component might underlie this alteration in lymphocyte phenotype profile (39).
IMPLICATIONS OF THE ENHANCED EXPRESSION OF IGF-IR AND THE ABERRANT CELLULAR RESPONSES IT MEDIATES TO THE PATHOGENESIS OF GO
IGF-I, its receptors, and binding proteins appear to influence immune responses (40). This pathway might also mediate pathogenic signals in autoimmune diseases such as GD and regulate antibody responses. While no insight currently exists concerning the events leading to the breakdown in peripheral immune tolerance to IGFIR, its identification as an autoantigen might allow development of therapies directed at interrupting the abnormal signaling associated with disease. Should this prove to be the case, the substantial efforts made in designing drugs targeting IGF-IR for the treatment of some cancers might be applied to GD and GO.
WHAT IS THE RELATIONSHIP BETWEEN GO AND 131IODINE THYROID ABLATION?
The question of whether 131iodine ablation of thyroid in GD causes or worsens GO has consumed much discussion over the last several decades and continues to spark controversy (41, 42). The role of steroid prophylaxis too as a means of attenuating the deleterious effects of this approach to treatment of hyperthyroidism continues to generate debate. Träisk et al. compared the occurrence of GO or its worsening in individuals with GD treated with anti-thyroid medications vs those receiving 131iodine ablation (43). The authors found that 63 patients (39%) developed GO or their pre-existing ocular disease was worsened following ablation compared to 32 patients (21%) of those medically managed (p<0.001). Fifty-three of the patients developed GO de novo following ablation while 23 patients who were medically treated also did so. In contrast, a similar number of those subjects who already manifested GO prior to participation in the study experienced worsening with either treatment. The report reinforced the view that smokers were at higher risk and that smokers treated with 131iodine ablation were particularly susceptible to GO (43).
Another report from Vannucchi and colleagues retrospectively compared in 113 patients the effects of 131iodine ablation on the activation of GO (44). Eighty-three of these subjects did not receive prophylactic steroids. The remaining 30 received either oral steroids (no.=21) or intraveneous glucocorticoids (no.=9). Activation of GO occurred in 33% of those receiving steroids while only 7% of those not receiving prophylaxis did so. When compared, none of those patients receiving intravenous steroids became active while 48% of those on oral steroids developed orbital activity. The authors concluded that oral steroids failed to offer protection against activation of GO in individuals receiving 131iodine ablation (44). In contrast, the report of Lai et al. explored in a retrospective matched cohort study a total of 111 patients with either no evidence of GO or mild orbital disease treated with radioactive iodine (45). Thirty-five of these individuals were not treated with steroid prophylaxis, 28 received low-dose prednisone ranging from 0.16–0.27 mg/kg body weight, while the third group of 48 subjects received prednisone at a dose of greater than 0.3 mg/kg. Steroids were begun 1 day after the 131iodine administration and treatment continued for 6 weeks. Of the 35 patients not receiving steroids, two developed GO with clinical activity scores (CAS) of 2/7 and 3/7. None of the individuals treated with prednisone developed GO. The authors concluded that the lower steroid doses used and the shortened treatment interval were probably sufficient for the protective effects of steroids observed.
RECENT PROGRESS IN TREATMENT
Despite considerable efforts, the treatment of severe, active GO remains largely ineffective and relies on non-specific approaches such as systemic steroids and radio-therapy (46). Similarly, correction of the manifestations of chronic stable disease relies almost exclusively on surgical rehabilitation. Thus, before we indulge in self-congratulations for the current state of treatment, a more complete understanding GO will probably be necessary. We must confront the unfortunate reality that our efforts have yet to meaningfully improve therapy beyond incremental refinement of surgical techniques and steroid dosing protocols. Treatment of stable disease relies almost entirely on surgical rehabilitation of severe proptosis and compromised eye motility.
But advances in treatment of other autoimmune diseases might be applied to GO since aspects of tissue remodeling appear to be shared by many of these conditions. Anti-cytokine therapies are currently used widely in rheumatoid arthritis and have been successfully introduced into those agents comprising the standard of care (47). Use of drugs interfering with cytokine action has been discussed in the context of orbital inflammation (48), but no adequately powered studies have yet been conducted. The strong suspicion that B cells play an important role in its pathogenesis has been the rationale underlying the use of rituxiimab as a means of CD20 B cell depletion in GD. This compound was introduced to the marketplace approximately 20 years ago as an anti-neoplastic drug used in non-Hodgkin’s lymphomas and leukemia (49). More recently, it has been successfully used in patients with various forms of autoimmunity, including lupus erythematosus, psoriasis, and rheumatoid arthritis where it is generally well-tolerated and effective (50). Because of the critical role that anti-TSHR antibodies play in GD, B cell depletion makes great mechanistic sense as a therapeutic strategy. Initial studies, emanating primarily from European investigators, have focused on the possible application of rituximab to treat the thyroidal aspects of the disease (51, 52). Most of these studies have been limited in scope, uncontrolled, and unmasked. With regard to GO, this therapeutic approach has shown some early promise. Salvi et al. (53) reported that rituximab therapy resulted in an almost complete absence of lymphocytes in orbital tissues. In contrast, Nielsen et al. (54) found similar numbers of lymphocytes in a single patient following therapy compared with another who had not been treated with rituximab. Three studies examining the impact of rituximab in GD have included a total of 17 individuals with GO. In a study by El Fassi et al. (55), 2 ex-smokers were included, both of whom had been treated with orbital irradiation and steroids. In both, a dramatic reduction in CAS was noted 8 months after rituximab therapy. In another prospective study, Salvi and colleagues (56) included 9 patients, of which 7 manifested active GO. The other 2 exhibited eye lid changes. Seven months after therapy with rituximab, the mean CAS fell from a baseline of 4.7 to 1.8 with concomitant reduction in severity. Khanna et al. (57) studied 6 patients who had failed steroid therapy, all of whom presented with severe and active GO. Four of these patients had evidence of optic neuropathy. The CAS fell from a baseline of 5.3 to 1.8 after 8 weeks following therapy with rapid improvement of visual acuity within 4 weeks. The concomitant steroid therapy given to all of these patients was withdrawn without any evidence of worsening disease. A single patient with poor response to steroid therapy has also failed treatment with rituximab (58). In aggregate, this line of investigation into the potential utility of B cell depletion would seem to be a worthwhile pursuit. But, considerably more work will be required before any strong endorsement of this therapy can be made. Treatment with rituximab is certainly not without its drawbacks. The side effect profile must be carefully weighed as the decision of whether a particular patient should be treated with the agent is made. This issue of caution has been made in the past when consideration is given in the treatment of GD, given its generally good outcome (59). Moreover, the basis for clinical improvement following B cell depletion remains unresolved. In the 3 studies where it was monitored, it appears that TRAb levels decrease with treatment in GD but that the decline is similar to that found in patients with methimazole or glucocorticoids (52, 56, 60). In contrast, the recent findings by El Fassi et al. suggest that rituximab might lower the levels of thyroid stimulating antibodies as assessed by a TSHR-transfected CHO cell assay based on cAMP generation (61). It should be remembered that any benefit associated with B cell depletion might be unrelated to effects on antibody levels. The B cell has other functions besides antibody production. They represent important antigen presenting cells and express many cytokines, the functions of which are numerous and none more important than their ability to provide T cell help.
CONCLUSIONS
GO remains a troublesome and poorly understood component of GD. Absence of effective and safe therapies continues to represent a major unmet need confronting patient and clinician alike. Incremental progress continues to be made in better understanding the disease, but most of these research activities remain mired in cell culture systems using contrived experimental models in vitro. Absence of complete and robust pre-clinical models of GD continues to compromise our progress in generating insight into GO. It also impairs any effort to conduct systematic clinical testing of drug candidates. In concluding her recent review of the topic, Bahn states that “we have the means to precisely measure the effect of new therapies on quality of life and specific clinical end points.” (62). Unfortunately, many of her colleagues fail to share the optimistic view she expresses (63). An appreciable number of the most active workers in the field remain unconvinced that the current level of understanding of the pathogenesis GD will prove adequate for great advances in therapy to be made. Moreover, substantial disagreement currently exists regarding how we are critically evaluating the activity and severity of disease in our patients with GO. These deficits may preclude meaningful therapeutic trails to proceed. Rather, a critical self-inventory of what we know and, more importantly, identifying the disease aspects we have yet to grasp remains necessary. Only when the more difficult work has been completed will the laudable goal shared by all investigators in this field-improving the care of our most severely affected patients with GO-be realized.
Supplementary Material
ACKNOWLEDGMENTS
The author is grateful to Ms Jen Mironas for secretarial support. This work was supported in part by National Institutes of Health grants EY08976, EY011708, and DK063121.
REFERENCES
- 1.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the Pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev 2003, 24: 802–35. [DOI] [PubMed] [Google Scholar]
- 2.Parmentier M, Libert F, Maenhaut C, et al. Molecular cloning of the thyrotropin receptor. Science 1989, 246: 1620–2. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie JM. Humoral factors in the pathogenesis of Graves’ disease. Physiol Rev 1968, 48: 252–309. [DOI] [PubMed] [Google Scholar]
- 4.Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G.. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet 1993, 342: 337–8. [DOI] [PubMed] [Google Scholar]
- 5.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid 1993, 3: 297–300. [DOI] [PubMed] [Google Scholar]
- 6.Chang TC, Wu SL, Hsiao YL, et al. TSH and TSH receptor antibody-binding sites in fibroblasts of pretibial myxedema are related to the extracellular domain of entire TSH receptor. Clin Immunol Immunopathol 1994, 71: 113–20. [DOI] [PubMed] [Google Scholar]
- 7.Endo T, Ohno M, Kotani S, Gunji K, Onaya T. Thyrotropin receptor in non-thyroid tissues. Biochem Biophys Res Commun 1993, 190: 774–9. [DOI] [PubMed] [Google Scholar]
- 8.Cianfarani F, Baldini E, Cavalli A, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol 2010, 130: 93–101. [DOI] [PubMed] [Google Scholar]
- 9.Wu SL, Yang CS, Wang HJ, Liao CL, Chang TJ, Chang TC. Demonstration of thyrotropin receptor mRNA in orbital fat and eye muscle tissues from patients with Graves’ ophthalmopathy by in situ hybridization. J Endocrinol Invest 1999, 22: 289–95. [DOI] [PubMed] [Google Scholar]
- 10.Paschke R, Vassart G, Ludgate M. Current evidence for and against the TSH receptor being the common antigen in Graves’ disease and thyroid associated ophthalmopathy. Clin Endocrinol 1995, 42: 565–9. [DOI] [PubMed] [Google Scholar]
- 11.Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol 2000, 279: C335–40. [DOI] [PubMed] [Google Scholar]
- 12.Agretti P, Chiovato L, De Marco G, et al. Real-time PCR provides evidence for thyrotropin receptor mRNA expression in orbital as well as in extraorbital tissues. Eur J Endocrinol 2002, 147: 733–9. [DOI] [PubMed] [Google Scholar]
- 13.Haraguchi K, Shimura H, Kawaguchi A, Ikeda M, Endo T, Onaya T. Effects of thyrotropin on the proliferation and differentiation of cultured rat preadipocytes. Thyroid 1999, 9: 613–9. [DOI] [PubMed] [Google Scholar]
- 14.Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNF-α production by bone marrow cells. Blood 2003, 101: 119–23. [DOI] [PubMed] [Google Scholar]
- 15.Endo T, Ohta K, Haraguchi K, Onaya T. Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J Biol Chem 1995, 270: 10833–7. [DOI] [PubMed] [Google Scholar]
- 16.Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoanti-bodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 1993, 16: 251–7. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin Activation of T cell chemoattractant expression in fibrob- lasts from patients with Graves’ disease Is mediated through the insulin-like growth factor I receptor pathway. J Immunol 2003, 170: 6348–54. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol 2002, 168: 942–50. [DOI] [PubMed] [Google Scholar]
- 19.Center DM, Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol 1982, 128: 2563–8. [PubMed] [Google Scholar]
- 20.Simchen C, Lehmann I, Sittig D, Steinert M, Aust G. Expression and regulation of regulated on activation, normal T cells expressed and secreted in thyroid tissue patients with Graves’ disease and thyroid autonomy and in thyroid-derived cell populations. J Clin Endocrinol Metab 2000, 85: 4758–64. [DOI] [PubMed] [Google Scholar]
- 21.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab 2004, 89: 5076–80. [DOI] [PubMed] [Google Scholar]
- 22.Arbogast B, Hopwood JJ, Dorfman A. Absence of hyaluronidase in cultured human skin fibroblasts. Biochem Biophys Res Commun 1975, 67: 376–82. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZG, Wall JR, Bernard NF. Identification of antigenic epitopes of 1D antigen recognized by antibodies in the serum of patients with thyroid-associated ophthalmopathy. Clin Immunol Immunopathol 1995, 77: 193–200. [DOI] [PubMed] [Google Scholar]
- 24.Gopinath B, Ma G, Wall JR. Eye signs and serum eye muscle and collagen XIII antibodies in patients with transient and progressive thyroiditis. Thyroid 2007, 17: 1123–9. [DOI] [PubMed] [Google Scholar]
- 25.Mizokami T, Salvi M, Wall JR. Eye muscle antibodies in Graves’ ophthalmopathy: pathogenic or secondary epiphenomenon? J Endocrinol Invest 2004, 27: 221–9. [DOI] [PubMed] [Google Scholar]
- 26.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol 2002, 2: 85–95. [DOI] [PubMed] [Google Scholar]
- 27.Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2002, 87: 385–92. [DOI] [PubMed] [Google Scholar]
- 28.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol 2002, 32: 477–85. [DOI] [PubMed] [Google Scholar]
- 29.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab 1996, 81: 3428–31. [DOI] [PubMed] [Google Scholar]
- 30.Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab 1999, 84: 2557–62. [DOI] [PubMed] [Google Scholar]
- 31.Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol 2008, 181: 4397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010, 95: 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994, 1: 71–81. [PMC free article] [PubMed] [Google Scholar]
- 34.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004, 36: 598–606. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson JW, Durrani OM, Bujalska IJ, et al. The role of 11beta-hydroxysteroid dehydrogenase 1 in adippogenesis in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010, 95: 398–406. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem 284: 26447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol 2007, 178: 3281–7. [DOI] [PubMed] [Google Scholar]
- 38.Douglas RS, Naik V, Hwang CJ, et al. B Cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 2008, 181: 5768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas RS, Brix TH, Hwang CJ, Hegedus L, Smith TJ. Divergent frequencies of IGF-1 receptor-expressing blood lymphocytes in monozygotic twin pairs discordant for Graves’ disease: Evidence for a phenotypic signature ascribable to nongenetic factors. J Clin Endocrinol Metab 2009, 94: 1797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev 2010, 62: 199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tallstedt L, Lundell G, Tørring O, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med 1992, 326: 1733–8. [DOI] [PubMed] [Google Scholar]
- 42.Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med 1998, 338: 73–8. [DOI] [PubMed] [Google Scholar]
- 43.Träisk F, Tallstedt L, Abraham-Nordling M, et al. ; Thyroid Study Group of TT 96. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab 2009, 94: 3700–7. [DOI] [PubMed] [Google Scholar]
- 44.Vannucchi G, Campi I, Covelli D, et al. Graves’ orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endorcrinol Metab 2009, 94: 3381–6. [DOI] [PubMed] [Google Scholar]
- 45.Lai A, Sassi L, Compri E, et al. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab 2010, 95: 1333–7. [DOI] [PubMed] [Google Scholar]
- 46.Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and meta-analysis. J Clin Endocrinol Metab 2009, 94: 2708–16. [DOI] [PubMed] [Google Scholar]
- 47.Goldbach-Mansky R, Lipsky PE. New concepts in the treatment of rheumatoid arthritis. Annu Rev Med 2003, 54: 197–216. [DOI] [PubMed] [Google Scholar]
- 48.Kapadia MK, Rubin PA. The emerging use of TNF-alpha inhibitors in orbital inflammatory disease. Int Ophthalmol Clin 2006, 46: 165–81. [DOI] [PubMed] [Google Scholar]
- 49.Hagemeister F Rituximab for the treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2010, 70: 261–72. [DOI] [PubMed] [Google Scholar]
- 50.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 2009, 5: 433–41. [DOI] [PubMed] [Google Scholar]
- 51.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol 2006, 154: 623–32. [DOI] [PubMed] [Google Scholar]
- 52.El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedus L. B lymphocyte depletion with a monoclonal antibody rituximab in Graves’ disease: a controlled pilot study. J Clin Endocrinol Metab 2007, 92: 1769–72. [DOI] [PubMed] [Google Scholar]
- 53.Salvi M, Vannucchi G, Campi I, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol 2006, 154: 511–7. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen JF, El Fassi D, Nielsen CH, et al. Evidence of orbital B and T cell depletion after rituximab therapy in Graves’ ophthalmopathy. Acta Ophthalmologic 2009, 87: 927–9. [DOI] [PubMed] [Google Scholar]
- 55.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid 2006, 16: 709–10. [DOI] [PubMed] [Google Scholar]
- 56.Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol 2007, 156: 33–40. [DOI] [PubMed] [Google Scholar]
- 57.Khanna D, Chong KK, Afifiyan NF, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology 2010, 117: 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krassas GE, Stafilidou A, Boboridis KG. Failure of rituximab treatment in a case of severe thyroid ophthalmopathy unresponsive to steroids. Clin Endocrinol (Oxf) 2010, 72: 853–5. [DOI] [PubMed] [Google Scholar]
- 59.Smith TJ. B cell depletion in Graves’ disease: the right answer to the wrong question? J Clin Endocrinol Metab 2007, 92: 1620–2. [DOI] [PubMed] [Google Scholar]
- 60.Heemstra KA, Toes RE, Sepers J, et al. Rituximab in relapsing Graves’ disease, a phase II study. Eur J Endocrinol 2008, 159: 609–15. [DOI] [PubMed] [Google Scholar]
- 61.El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH. Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol 2009, 130: 252–8. [DOI] [PubMed] [Google Scholar]
- 62.Bahn RS. Graves’ Ophthalmopathy. N Engl J Med 2010, 362: 726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Douglas RD, Tsirbas A, Gordon M, et al. ; International Thyroid Eye Disease Society. Devleopment of criteria for evaluating clinical response in thyroid eye disease using a modified Delphi technique. Arch Ophthalmol 2009, 127: 1115–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.