Abstract
Objective:
To describe available models of HIV and noncommunicable disease (NCD) care integration in sub-Saharan Africa (SSA).
Design:
Narrative review of published articles describing various models of HIV and NCD care integration in SSA.
Results:
We identified five models of care integration across various SSA countries. These were integrated community-based screening for HIV and NCDs in the general population;screening for NCDs and NCD risk factors among HIV patients enrolled in care;integration of HIV and NCD care within clinics;differentiated care for patients with HIV and/or NCDs;and population healthcare for all. We illustrated these models with descriptive case studies highlighting the lessons learned and evidence gaps from the various models.
Conclusion:
Leveraging existing HIV infrastructure for NCD care is feasible with various approaches possible depending on available program capacity. Process and clinical outcomes for existing models of care integration are not yet described but are urgently required to further advise policy decisions on HIV/NCD care integration.
Keywords: HIV, integration, noncommunicable, service delivery
Introduction
Since 2003, significant global investment has facilitated the establishment of HIV care in sub-Saharan Africa (SSA). Consequently, people living with HIV (PLHIV) are living longer, while AIDS-related mortality is declining [1]. Additionally, this infrastructure has strengthened SSA health systems overall through monitoring and evaluation, medications procurement, and task-shifting to provide chronic disease care [2]. As PLHIV initiate and remain on antiretroviral therapy (ART), aging places them at risk of developing noncommunicable diseases (NCDs) [3] compounded by the ongoing epidemiological transition in SSA triggering an increase in NCDs [4–6]. As HIV transitions to a chronic disease, the existing HIV clinical tools, strategies, and systems can be leveraged to tackle NCD prevention and care [7,8]. Innovative models of care responsive to the emerging NCD threat are needed to preserve the attained gains among PLHIV while also informing NCD care for the general population [9].
Prior reviews have described HIV/NCD integration in low-income and middle-income countries (LMICs) [10,11]. We specifically focus on models of care integration in SSA, where HIV prevalence and incidence is highest [12]. Integrated care is defined here as the coordination, co-location, or simultaneous delivery of HIV and NCD services to patients who need it, when they need it [13,14]. Using case studies, we highlight available baseline structures (existing HIV platform) and key added elements (modifications made onto the existing platform) enabling HIV/NCD integration. We also discuss the lessons learned, evidence gaps, and propose a way forward to facilitate efficient HIV/NCD integration.
Methods
We searched PubMed using the keywords ‘HIV’, ‘AIDS’, ‘noncommunicable diseases’, ‘diabetes mellitus’, ‘hypertension’, ‘cervical cancer’, ‘depression’, ‘integration’, ‘leverage’, and their related terms. Eligible studies were included if they described models of integration for HIV/ NCD in SSA across the care continuum. We screened reference lists of retrieved articles to identify additional articles. We did not limit our search by date but we excluded articles that were not in English. For case studies, we sought examples from the retrieved articles which most comprehensively described the implementation process, baseline structures, added elements and outcomes. We also reached out to authors of the articles selected as case studies to elicit updates and seek clarification.
Models of HIV/noncommunicable disease care integration
Five case studies of integrated HIV/NCD models were identified: community-based integrated HIV/NCD screening in the general population, screening for NCDs and their risk factors among PLHIV, integrated care of HIV/NCD in healthcare facilities, differentiated care for stable HIV/NCD, and population health for all patients with any need. These examples are summarized in Table 1 and illustrated in Figure 1.
Table 1.
Case studies for integrated models of care for HIV and noncommunicable diseases in sub-Sahara Africa.
| Screening (S)/Referral | |||||||
|---|---|---|---|---|---|---|---|
| Author, year [ref no] | Country | Disease condition | Population | Brief summary | Highlighted challenges/barriers | Highlighted facilitators/successes | (R)/Care (C) |
| Case study 1. Integrated HIV and NCD screening and referral | |||||||
| Chamie et al. 2012 [15] | Uganda | HIV, TB, Malaria, DM, HTN |
General population | Community based high-throughput screening and referral campaign leveraging HIV testing to include DM and HTN screening conducted in a rural district for 4343 persons over 5 days. |
Referral to different health facilities for HIV and NCD care Insufficient linkage to care rates Low participation by male gender |
Early engagement with local government and community mobilization efforts maximized buy in and improved participation Low incremental cost of leveraging HIV screening infrastructure for NCD |
S & R for both HIV and NCD |
| Participation time long despite use of POC diagnostics |
screening Enhanced referral strategy for HIV patients with low CD4 led to higher linkage rates |
||||||
| Case study 2. Screening for NCDs and their risk factors among PLHIV enrolled in care | |||||||
| Kagaruki et al. 2014 [16] |
Tanzania | HIV, DM, HTN | PLHIV | Clinic based cross-sectional evaluation for NCDs and their risk factors in a cohort of enrolled PLHIV |
Need for a return visit to obtain fasting blood samples led to dropouts Low participation by male gender |
Utilizing existing medical personnel to perform screening required a short period of training Using local workforce addressed language barrier concerns |
S & R for PLHIV with NCD or NCD risk factors |
| Case study 3. Leveraging HIV platforms to work toward comprehensive primary care in Malawi | |||||||
| Wroe et al. 2015 [17] | Malawi | HIV, TB, DM, HTN, Asthma, Epilepsy, Heart failure, Malnutrition |
Patients with any chronic disease |
Implementation of decentralized integrated chronic care clinics in a rural district |
Initial increase in workload for nurses following integration Long patient time in clinic of approximately 2 h |
Decentralized care worked to enhance follow-up Integrated data collection designed to increase ease of patient management by minimizing redundancy Clear patient flow and health provider roles improved efficiency Private-public partnership to supplement funding for NCD drugs |
S & C for HIV and/or NCD |
| Patel et al. 2018 [18] | Malawi | HIV, HTN | Stable patients with HIV |
Demonstration project of the integration on HIV and HTN care and treatment in HIV service delivery clinics |
Scalability limited by availability and accessibility of medications |
Evidence-based, standardized protocols Task-shifting Procurement of medications by clinic HTN module for EMRS |
S &C for HIV and HTN |
| Case study 4. Differentiated care for HIV/NCD | |||||||
| Khabala et al. 2015 [19] |
Kenya | HIV, DM, HTN | Stable patients with HIV, DM or HTN |
Retrospective evaluation of a cohort of medication adherence clubs for stable patients with HIV and/or NCDs in an urban informal community |
No clinical outcomes described | Daily health talks to patients in clinic led to voluntary participation in MACs Having time flexibility improved patient attendance Protocols to standardize care provision ensured high adherence to nurse led consultations |
C for stable patients with HIV, HTN or DM |
| Case study 5. Population health | |||||||
| Pastakia et al. 2016 [20] Vedanthan et al. 2015 [21] Mercer et al. 2018 [22] |
Kenya | All | General population | Integrated population health model leveraging HIV care infrastructure in community-based care delivery through task shifting coupled with a robust referral network, electronic medical record system, supply chain strengthening, food and income security programs, and affordable health insurance, to deliver HIV/NCD and MNCH services to the Western Kenya population. |
Implementation process challenges |
Widespread consultation with program partners and key stakeholders to foster common vision |
S, R and C for all |
DM, diabetes mellitus; EMRS, electronic medical records system; HTN, hypertension; NCD, noncommunicable disease; PLHIV, people living with HIV; POC, point of care; TB, tuberculosis.
Fig. 1.
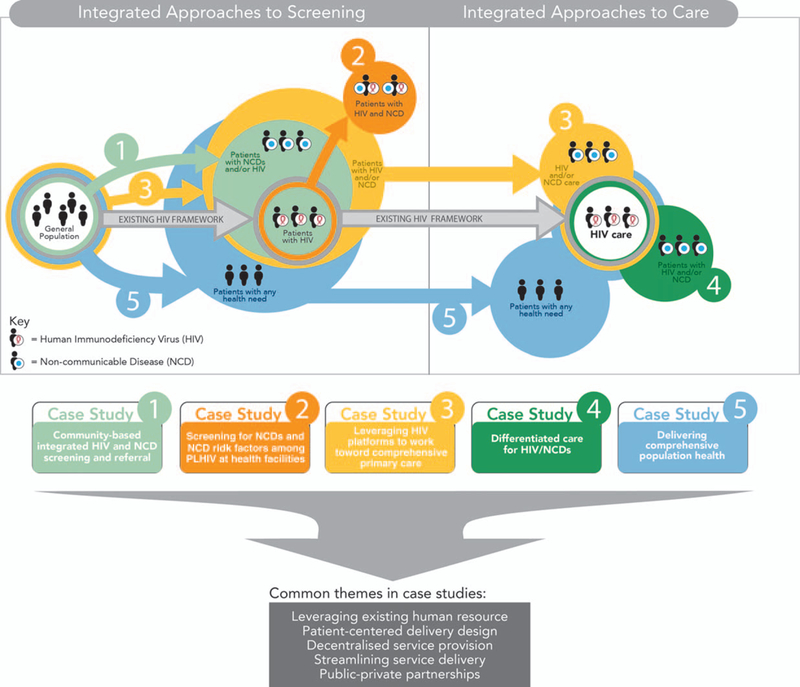
Models of care for HIV/NCD integration in sub-Sahara Africa.
Integrated approaches to screening
Screening for HIV has been a key strategy for HIV programs to improve case-finding aimed at ensuring 90% of PLHIV know their status by 2020 [23]. Case-finding has taken on many forms including facility-based, community-based, and home-based screening [24].
Community-based integrated HIV and noncommunicable disease screening and referral
Expanding on existing community-based screening programs, this model provides a multidisease screening package for HIV and NCDs in the general population. Human resources employed in various capacities such as community mobilization, logistics coordination, testing, and counseling is required, with the addition of screening kits for rapid testing in the field [15,17,25–31].
Case Study 1: Leveraging community-based HIV testing campaigns for noncommunicable diseases in Uganda
Chamie et al. [15] evaluated the feasibility and diagnostic yield of integrating community-based HIV and NCD screening and referral in three well known community locations in a rural district. Local village governments were engaged prior to the campaign to plan for community mobilization, disseminate information, and maximize participation. Local healthcare workers trained and experienced in HIV counseling and testing conducted multidisease screenings for HIV, hypertension (HTN), and diabetes mellitus (DM) and held questionnaire-based interviews with community members. Patients screened positive for any disease were referred to nearby public health facilities. Most testing was done in the field using POC equipment while a central lab provided support for tuberculosis (TB) testing.
NCD screening required the addition of a point-of-care (POC) screening device for random blood glucose measurement and blood pressure machines with varying sized cuffs. The associated incremental cost of this was $2.41 per participant, onto a base cost of $26.69.
Among adults living in the catchment area, 74% participated with more of the female population (95%) reached compared with males (52%). Researchers found that 46% ofpatients who screened positive for HIV were newly diagnosed, while 65 and 23% of patients screened positive for HTN and DM, respectively, were newly diagnosed.
A key challenge was referral to different centers for care of HIV and NCDs because NCD care was only available at a center ~20 km away. Overall, a quarter of the target population did not take part in the screening campaign. Despite a high-throughput design, participants spent a median of 95 min at the campaign, longer for HIV- infected participants, which may have deterred participation. Linkage to care within 3 months was higher for NCDs but still suboptimal, at 39, 43, and 61% for HIV HTN, and DM patients respectively. A follow-up study among patients diagnosed with HTN included an intervention package (short counseling session after diagnosis, referral appointment within 30 days, and oneway transport cost facilitation) resulting in 83% linkage to care [29]. Tracing of patients not linking within 6 months revealed that the asymptomatic nature of HTN, as well as transport cost and inconvenience were significant barriers to linkage.
Screening for noncommunicable diseases and noncommunicable disease risk factors among people living with HIV at health facilities
Leveraging established HIV clinic infrastructure, this model targets PLHIV engaged in care and screens them for NCDs and NCD risk factors, tackling the problem of dual HIV/NCD burden [16–18,32–40].
Case study 2: Screening for noncommunicable diseases and their risk factors in people living with HIV enrolled in care in Tanzania
Kagaruki et al. [16] evaluated the magnitude and risk factors of DM and HTN among PLHIV in two Tanzania regions (one rural and one urban). They screened PLHIV in 12 HIV care and treatment centrers (CTCs) for DM, HTN, and NCD risk factors such as high low-density lipoprotein (LDL), low high-density lipoprotein (HDL), suboptimal intake of fruits and vegetables, lack of participation in vigorous activity, body mass index 25 kg/m2 at least and abnormal waist circumference. Screening utilized a structured tool adopted from the WHO STEPs survey available in both English and the local language [41]. Qualified medical personnel were responsible for data collection, anthropometric measurements, and blood sample collection, following a 3-day training.
Of the 671 PLHIV participants, HTN and DM was prevalent in 26.0 and 4.2%, respectively. Prevalence of NCD risk factors were low HDL (72%), suboptimal fruit and vegetable consumption (70%), poor participation in vigorous activity (48%), abnormal waist circumference (47%), and high LDL (43%). All patients received health education based on their screening findings and a paper copy of their results. For patients with NCDs or NCD risk factors, study personnel communicated their information to the patient’s specific CTC for further workup and management.
Key challenges were failure to return for drawing of fasting blood samples despite assurance of compensation for transport, and low participation by male PLHIV (30%) and ART-naive PLHIV (47%) in the NCD screening who cited lack of time to participate. Linkage-to-care rates and follow-up clinical outcomes for PLHIV with NCDs or NCD risk factors were unavailable as this was a research study.
Integrated approaches to care of HIV/ noncommunicable disease patients
External funding for NCD care in LMICs remains low, estimated at 1.3% of total development assistance for health in 2015 [42]. Given the large investment in HIV care in SSA, leveraging HIV infrastructure represents a key opportunity to provide NCD care to large populations without needing to replicate health service streams. We identified several examples of integrated HIV and NCD screening, treatment, and follow-up, which we divided into two distinct models: integrated HIV and NCD care within chronic disease care [17] and HIV care [18] clinics, and differentiated care for stable HIV and/or NCD patients in medication adherence clubs [19].
Case Study 3: Leveraging HIV platforms to work toward comprehensive primary care in Malawi
Wroe et al. described the implementation of decentralized integrated chronic care clinics (IC3) in rural Malawi where patients with HIV and/or NCDs receive care for chronic disease [17]. The IC3 model follows the original patient flow designed for HIV care beginning with screening (both community-based and facility-based) and referral for follow-up of any and all chronic conditions. Patients receive education on common conditions before seeing clinicians trained in managing HIV and/or NCDs. Finally, patients collect their medicines and are assigned a new appointment date. Defaulter tracing occurs for patients lost to follow-up using protocols originally designed for PLHIV.
In the district where the effort was described, 7100 PLHIV received decentralized HIV care across two hospitals and 11 health centers. Prior to IC3, only two centers provided NCD care with limited success, including a high loss to follow-up rate related to patients either having to travel long distances for NCD care or requiring different clinic visits for patients with comorbid HIV/NCD. In response, IC3 was launched and decentralized to all 13 facilities in the district. To overcome human resource constraints, existing HIV-trained personnel were converted into integrated chronic disease management providers through classroom training and continuous on-the-job mentorship and supervision on NCD screening and treatment. Patient data management was re-designed for more comprehensive and integrated data collection via creation of master files for each patient that held all information instead of having multiple files on the different disease states.
A clear patient flow system was a key element in ensuring enhanced efficiency in the IC3 without imposing significant increases in workload for the care providers. An assessment of each health worker’s role in the IC3 identified a straining workload on the nurses, which was solved through task re-distribution to improve efficiency (Dr Emily Wroe, personal communication, 2017) [17].
As of May 2015, 6781 patients on ART and 721 patients with NCDs (HTN, DM, epilepsy, or asthma) were receiving care in IC3. Among the NCD patients, 109 (15%) were PLHIV Medications were provided free of charge which necessitated additional funding for NCD drugs to be provided by the implementing partner. Although clinical outcomes are yet to be described, a detailed toolkit for implementers of integrated HIV/ NCD care is available online [43,44].
Additional experience in integrated HIV/NCD care comes from Patel et al. [18] who described an effort to integrate HTN management at the Lighthouse Trust HIV service delivery sites in Malawi using a health system strengthening approach [45] utilizing evidence-based tools and practices, and continuous training of healthcare workers to improve clinical outcomes. An HTN-specific electronic medical record module was designed for monitoring and evaluation ofboth clinical and programmatic outcomes. A phased approach was used to implement HTN screening to ensure that clinic wait times and staff workload were not increased. At 12 months, of 29 359 individuals screened, 11% were newly diagnosed with HTN, and 85% of those with hypertension received treatment per standardized protocols. Among persons with mild and moderate HTN, blood pressure (BP) control rates were 38 and 30% after 6 months of treatment, respectively. Notably, adherence to ART was not affected by the additional burden of antihypertensive medications. Cost-effectiveness analyses are underway. The main challenge to scale-up of this program was noted as variability in accessibility and availability of antihypertensive medications because Malawi experiences frequent stockouts.
Integration of differentiated care for HIV/ noncommunicable disease
Differentiated care is care delivery that is customized to the individual patient’s needs [46] allowing for longer periods between clinic follow-up among stable HIV patients, leading to a reduced burden on both the patient and care provider. The benefits of differentiated care for PLHIV can be extended to HIV/NCD integrated care as demonstrated by the following case study.
Case study 4: Differentiated care for HIV/ noncommunicable diseases in Kenya
Khabala et al. [19] described an integrated model encompassing principles of differentiated care for patients with HIV, HTN or DM in a low-income population in Kenya. Groups of 25–35 stable patients with HIV HTN and/or DM were established. To be eligible to join, HTN and DM patients had to be at least 25 years of age, been on medications more than 6 months, and have a BP less than 150/100 and/or HbA1C less than 8%. PLHIV had to be at least 25 years of age, on ART for more than 1 year, have an undetectable viral load, a CD4 count more than 200, and not be in active WHO Stage 3/4 disease. Patients were informed that groups would have a mix of HIV and NCD patients but disclosure of their disease state was voluntary. The groups met quarterly in a dedicated space at the health center for a nurse-led session of approximately 2 h where they were assessed for clinical stability and received health education and drug refills. Usual clinic follow-up would occur annually, if a patient developed complications, or if they no longer met above stability criteria.
Over 1 year, 1432 patients across 47 MACs had 109 meetings and 2208 consultations (nurse-led weight and BP assessment, and ordering of routine blood tests). There was high adherence (99%) to recommended BP checks, weight checks, and blood tests by the nurse, with an overall low loss to follow-up (3.5%) or need for referral back to a clinic (2%) among members. Clinical outcomes are yet to be described.
Integrated comprehensive care for all
Early experiences with providing HIV care identified significant socioeconomic determinants of health in PLHIV in low-income settings such as food insecurity and undernutrition, and cost and logistical barriers to chronic care [47,48]. Similar determinants affect outcomes in NCDs and may be more important as few programs fully fund NCD care, often requiring user-fees for diagnostic tests, consultations, and drug purchases [20]. A population health model that builds on the HIV care infrastructure and lessons learned potentially represents the apex of integrated care [49].
Case Study 5: Delivering population health in Western Kenya
In Western Kenya, care of PLHIV has provided important experiences in delivering chronic care to SSA populations [41,50]. Several principles learned from these experiences have been leveraged and adapted to deliver NCD care [21]: home-based and community-based screening to improve NCD case-finding [51], task-shifting and decentralizing chronic care delivery to improve linkage and retention [50,52,53], using portable electronic medical records to streamline documentation [54,55], backing-up the Ministry of Health (MOH) medicines supply chain to ensure consistent availability [56], integrating income-generation and microfinance activities into care delivery to address socioeconomic barriers to health [20,52], and provision of affordable health insurance to minimize out-of-pocket costs [22].
A population health model grounded on the above principles has been developed to provide decentralized comprehensive care, including HIV/NCD, to the smallest administrative unit of the population (village) via community groups. These groups double both as convenient healthcare delivery points where clinical staff provide a variety of services, and as microfinance groups that provide low-interest loans and financial advice to members [22]. Collaboration with the Kenyan National Hospital Insurance Fund (NHIF) ensures that healthcare costs remain affordable to the majority of the population as enrollees are only required to make a monthly contribution of ~$5/month. Revolving fund pharmacies (RFPs) which utilize money collected from drug sales to cover drug re-stocking, have been established to protect against drug stock outs in government facilities [56], and a referral network has been built for patients who need to see higher cadres of staff. Clinical and process outcomes for the population health model are yet to be described.
Discussion
Designing a responsive continuity of care model for HIV provides vital lessons that can be leveraged to address the growing NCD burden that threatens aging PLHIV and the general SSA population [7,57,58]. HIV/NCD care integration is a feasible approach to health system strengthening in SSA, and the case studies described above demonstrate a variety of HIV/NCD integration models which programs can adopt and adapt based on available resources, needs and priorities. Several common themes from the reviewed models are worth highlighting, with lessons learned and prevailing research gaps summarized in Table 2 [15–22].
Table 2.
Lessons learned from integrated HIV/noncommunicable disease screening and care programs and prevailing research gaps.
| Integrated HIV/NCD model |
Lessons learned | Research gaps |
|---|---|---|
| Integrated HIV/NCD | Multidisease screening campaigns address shared | What is the cost-effectiveness of community based |
| Screening | barriers to chronic disease diagnosis and normalize | integrated screening? |
| HIV screening | What is the cost-effectiveness of facility based NCD | |
| Training existing healthcare personnel to perform | screening in PLHIV? | |
| screening activities is a feasible approach to expand | How can case finding be optimized in integrated HIV/ | |
| HIV/NCD screening | NCD screening programs? | |
| Equipping primary healthcare facilities with HIV/NCD | What are the clinical outcomes of early HIV/NCD | |
| care capacity may improve linkage following | diagnosis following screening? | |
| screening programs | What are the linkage and retention in care rates among | |
| Programmatic changes requiring regular screening for | PLHIV diagnosed with NCD or NCD risk factors? | |
| NCD and their risk factors among PLHIV required | How can integrated HIV/NCD screening models be | |
| Targeted health promotion required for PLHIV engaged | effectively scaled up? | |
| in care regarding NCD and their risk factors | How can integrated HIV/NCD screening models be made sustainable? |
|
| Integrated chronic | Transforming HIV care personnel into integrated chronic | What is the cost-effectiveness of various models of |
| disease care | care providers feasible solution for human resource | integrated HIV/NCD care? |
| shortage | What are the clinical outcomes for integrated HIV/NCD | |
| Clear patient flow coupled with clear provider roles | care? | |
| critical to minimizing clinic workload and | What is the effect of integration on stigma for HIV or | |
| streamlining patient care | NCD patients receiving care in integrated settings? | |
| Care protocols support task redistribution by | What is the effect of NCD integration on retention in care | |
| standardizing care procedures | for PLHIV? | |
| Adopting and adapting defaulter tracing strategies from | How can integrated HIV/NCD screening models be | |
| HIV care to NCD follow up can improve retention | effectively scaled up? | |
| Including programs to address socioeconomic barriers to | How can integrated chronic disease care models be | |
| access can incentivize retention in care | made sustainable? | |
DM, diabetes mellitus;HTN, hypertension; NCD, noncommunicable disease;PLHIV, people living with HIV.
Leveraging existing human resources is a key strategy to address shortfalls in human resources for health (HRH) [59] and was a common theme across our reviewed models. Use of existing medical personnel to conduct HIV/NCD screening and referral minimized training needs [15,16], task redistribution streamlined integrated HIV/NCD care delivery [17,19], and continued training and mentorship enabled transformation of HIV care providers into integrated chronic care providers to address staff shortages [17]. As SSA countries grapple with addressing the emerging NCD threat, a key intervention to consider is the creation of curricula to produce integrated chronic care providers instead of specific staff dedicated only to HIV or NCD roles [59].
Integrated HIV/NCD screening and NCD screening among PLHIV while feasible, required linkage and retention in care to reap the full benefits of early diagnosis. Linkage to care was poor following the community based integrated screening campaign described above but was noted to be higher in patients with NCDs compared to HIV [15]. Whilst sensitization on the need for care among diagnosed patients following screening may improve linkage [29], decentralized, integrated HIV/NCD care, particularly in remote areas, enhances the benefits of screening programs through linkage and retention [15]. In Kenya, integrated HIV/NCD care resulted in high retention rates [19] and was implemented to curb a high loss to follow-up associated with seeking care in distant facilities in Malawi [17]. Decentralization aims to bring care closer to patients to minimize logistical barriers to access, while reducing congestion at ‘central’ health facilities [20].
Streamlining service delivery is an important strategy in optimizing patient-centered care [60] and was key to delivering decentralized integrated care. This was made possible by clarifying individual health worker roles to promote staff buy-in, identify systemic challenges, and reduce strife among workers [17]. In addition, use of integrated files and health information technology tools minimized data management redundancies and improved efficiency, while providing a platform for monitoring patient and program progress [44,55]. Integrated MAC groups decentralized care by leveraging differentiated HIV care aspects [47,61] to provide flexible follow-up and medication refills to stable HIV and/or NCD patients, leading to improved patient satisfaction and decreasing the burden on the health system [19].
Public-private partnerships (PPP) between government agencies, health ministries, nongovernmental agencies (NGOs), federal programs, and academic partners were crucial to the success of integrated HIV/NCD models. Engagement with local government ensured community health needs were met to improve participation in a multidisease screening campaign in Uganda [15] while NGOs augmented government efforts through supplemental funding for personnel and NCD medication in Malawi and Kenya to ensure uninterrupted care [17,56]. Indeed, reviewed case studies all relied on a HIV platform established through PPP, to deliver integrated screening and/or care. Beyond ensuring interventions meet specific population health needs and facilitating implementation within existing regulatory frameworks, PPP are crucial in promoting sustainability of donor funded programs beyond funding cycles by incorporating successful programs into health policy [62].
Designing patient-centered quality care results in a higher likelihood of patient uptake and was a common theme in the reviewed models. Holding screening campaigns in three popular community locations improved participation in Uganda [15] while decentralized care and efficient clinic patient flow systems aimed to address logistical barriers for patients seeking HIV/NCD care in Malawi [17]. Integrated MAC group meetings in Kenya utilized flexible timing to improve patient convenience [19], and microfinance groups were established to address economic barriers to access and incentivize retention in care [20,52]. Delivering quality chronic care to low-income populations with limited capacity to pay remains a significant global health challenge [63]. Several programs have overcome this by providing HIV and/or NCD care free of charge to patients [18]. However, long-term sustainability of this model of service delivery remains a concern. Imposing user-fees for care services and medicines may promote sustainability but represents a significant barrier to sustained access in low-income populations [64,65]. Microfinance groups, income-generation programs and low-cost health insurance are several programs underway in Western Kenya to minimize the economic impact of receiving NCD care, for which, unlike HIV care, patients often pay out-ofpocket [49,50,52]. Ultimately, countrywide adoption of universal healthcare coverage, which has been on the SSA agenda since the late 1970s [49,66,67], is needed.
Our review has several limitations. First, it was not systematic as we aimed to describe existing models of HIV/NCD care integration, adopting a wide search strategy that was not amenable to the narrow research question requirements of a systematic review. Second, although we attempted to make contact with author leads of the case studies, we were unable to conduct interviews with all of them, possibly missing out on recent updates regarding available models. Third, case study descriptions only highlight key components in relation to HIV/NCD integration as described in the retrieved literature and author interviews and may miss out on other key programmatic aspects. Therefore, the summary here is a reflection of current efforts and may not capture ongoing HIV and/or NCD care activities that have not been published. In addition, overlap exists across the care cascade and extends to the described case studies, description of program activities under a specific model is meant only to highlight key components of the integration model, and not to imply that these are the only components of integration within the program. Finally, our review was primarily confined to discussing different typologies of HIV/NCD integration, rather than how such models are taken to scale [68]. Further research is warranted to determine how and where these models can be implemented successfully.
Nevertheless, our review illustrates that HIV/NCD care integration in SSA is feasible, with several models available that country programs can adopt and adapt based on available resources, needs, and priorities. Integration models are in their infancy, as exemplified by the general lack of clinical and process outcomes data, and a lack of cost-effectiveness data from the various models. These are urgently required to further inform implementers and policy makers as SSA continues to strengthen its health system in order to cope with the emerging health and economic threat posed by NCDs [58,69,70].
Conclusion
Leveraging the existing HIV infrastructure to provide NCD care to the SSA population is feasible. Several models of care integration have been described in SSA with important lessons that will aid context-specific replication in other SSA settings. Future efforts will need to factor in descriptions of process metrics and cost- effectiveness to further guide implementation, as well as clinical outcomes to aid decision makers.
Acknowledgements
All authors were involved in the conception and design of this review. B.N., S.V and S.P. drafted the original manuscript. All authors critically reviewed and edited the first draft and each successive version and provided final approval for submission.
Source of support: this article as part of the Research to Guide Practice: Enhancing HIV/AIDS Platform to Address Non-Communicable Diseases in sub-Saharan Africa was supported by the U.S. National Institutes of Health Fogarty International Center.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Conflicts of interest
All authors have no conflicts of interest to disclose.
References
- 1.PEPFAR. 2017 Annual Report to Congress. In;2017.
- 2.Cappuccio FP, Micah FB, Emmett L, Kerry SM, Antwi S, Martin-Peprah R, et al. Prevalence, detection, management, and control of hypertension in Ashanti, West Africa. Hypertension 2004:43. [DOI] [PubMed] [Google Scholar]
- 3.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unwin N, Alberti KGMM Chronic noncommunicable diseases. Ann Trop Med Parasitol 2006;100:455–464. [DOI] [PubMed] [Google Scholar]
- 7.Palma AM, Rabkin M, Nuwagaba-Biribonwoha H, Bongomin P, Lukhele N, Dlamini X, et al. Can the success of HIV scale-up advance the global chronic NCD agenda? Glob Heart 2016; 11 :403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabkin M, Melaku Z, Bruce K, Reja A, Koler A, Tadesse Y, et al. Strengthening health systems for chronic care: leveraging HIV programs to support diabetes services in Ethiopia and Swazi-land. J Trop Med 2012;2012:137460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens B, Van Damme W, Raleigh B, Gupta J, Khem S, Soy Ty K, et al. Offering integrated care for HIV/AIDS, diabetes and hypertension within chronic disease clinics in Cambodia. Bull World Health Organ 2007;85:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haregu TN, Setswe G, Elliott J, Oldenburg B. National responses to HIV/AIDS and non-communicable diseases in developing countries: analysis of strategic parallels and differences. J Public Health Res 2014;3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy M, Ojikutu B, Andrian S, Sohng E, Minior T, Hirschhorn LR. Noncommunicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Health 2017;22:926–937. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS report on the global AIDS epidemic 2013. UNAIDS; 2013. Available at: http://www.unaids.org/en/resources/documents/2013/20130923_UNAIDS_Global_Report_2013
- 13.World Health Organization. Integrated Health Services – What and Why? World Health 0rganization;2008. Available at: http://www.who.int/healthsystems/technicaLbrief_final.pdf [Google Scholar]
- 14.Vorkoper S, Kupfer L, Anand N, Patel P, Beecroft B, Tierney WM, et al. Building on the HIV chronic care platform to address non-communicable diseases in sub-Saharan Africa: a research agenda. AIDS 2018;32 (Suppl 1):S107–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging rapid community-based HIV testing campaigns for noncommunicable diseases in rural Uganda. PLoS One 2012;7:e43400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, et al. Magnitude and risk factors of noncommunicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health 2014;14:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wroe EB, Kalanga N, Mailosi B, Mwalwanda S, Kachimanga C, Nyangulu K, et al. Leveraging HIV platforms to work toward comprehensive primary care in rural Malawi: the Integrated Chronic Care Clinic. Healthcare 2015;3:270–276. [DOI] [PubMed] [Google Scholar]
- 18.Rabkin M, Mutiti A, Chung C, Zhang Y, Wei Y, El-Sadr WM. Missed opportunities to address cardiovascular disease risk factors amongst adults attending an urban HIV clinic in South Africa. PLoS One 2015;10:e0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khabala KB, Edwards JK, Baruani B, Sirengo M, Musembi P, Kosgei RJ, et al. Medication Adherence Clubs: a potential solution to managing large numbers of stable patients with multiple chronic diseases in informal settlements. Trop Med Int Health 2015;20:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastakia SD, Manyara SM, Vedanthan R, Kamano JH, Menya D, Andama B, et al. Impact of Bridging Income Generation with Group Integrated Care (BIGPIC) on Hypertension and Diabetes in Rural Western Kenya. J Gen Intern Med 2017;32:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vedanthan R, Kamano JH, Bloomfield GS, Manji I, Pastakia S, Kimaiyo SN. Engaging the entire care cascade in Western Kenya: a model to achieve the cardiovascular disease second-ary prevention roadmap goals. Glob Heart 2015;10:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer T, et al. Leveraging the power of partnerships: spreading the vision for a population health care delivery model in western Kenya. Globalization and Health 2018;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ending AIDS. Progress towards the 90–90-90 targets. UNAIDS. Joint United Nations Programme on HIV/AIDS. UNAIDS; 2017.
- 24.Marum E, Taegtmeyer M, Parekh B, Mugo N, Lembariti S, Phiri M, et al. What took you so long?’ The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr 2012;60 (Suppl 3):S63–S69. [DOI] [PubMed] [Google Scholar]
- 25.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population- wide HIV testing in rural east Africa: an observational study. Lancet HIV 2016;3:e111–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLOS ONE 2014; 9:e84317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, et al. Linkage to HIV, TB and noncommunicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One 2013;8:e80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P, et al. High prevalence of hypertension and of risk factors for noncommunicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med 2015;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotwani P, Balzer L, Kwarisiima D, Clark TD, Kabami J, Byonanebye D, et al. Evaluating linkage to care for hypertension after community-based screening in rural Uganda. Trop Med Int Health 2014;19:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotwani P, Kwarisiima D, Clark TD, Kabami J, Geng EH, Jain V, et al. Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study. BMC Public Health 2013;13:1151–11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, et al. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLOS ONE 2016;11 :e0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong C, Liu E, Okuma J, Spiegelman D, Guerino C, Njelekela M, et al. Dyslipidemia in an HIV-positive antiretroviral treatment-naive population in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr 2011;57:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekolo CE, Nguena MB, Ewane L, Bekoule PS, Kollo B. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health 2014;14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health 2016;16:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julius H, Basu D, Ricci E, Wing J, Basu JK, Pocaterra D, et al. The burden of metabolic diseases amongst HIV positive patients on HAART attending The Johannesburg Hospital. Curr HIV Res 2011;9:247–252. [DOI] [PubMed] [Google Scholar]
- 36.Magodoro IM, Esterhuizen TM, Chivese T. A cross-sectional, facility based study of comorbid noncommunicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes 2016;9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in South-western Uganda. AIDS Patient Care STDS 2016;30:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Njelekela M, Muhihi A, Aveika A, Spiegelman D, Hawkins C, Armstrong C, et al. Prevalence of hypertension and its asso-ciated risk factors among 34,111 HAART naive HIV-infected adults in Dar es Salaam, Tanzania. Int J Hypertens 2016; 2016:5958382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nsagha DS, Weledji EP, Assob NJ,Njunda LA, Tanue EA, Kibu OD, et al. Highly active antiretroviral therapy and dyslipidemia in people living with HIV/AIDS in Fako Division, South West Region of Cameroon. BMC Cardiovasc Disord 2015;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semu H, Zack RM, Liu E, Hertzmark E, Spiegelman D, Sztam K, et al. Prevalence and risk factors for overweight and obesity among HIV-infected adults in Dar es Salaam, Tanzania. J Int Assoc Provid AIDS Care 2016;15:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. The STEPS instrument and support materials. Available at: http://www.who.int/chp/steps/instrument/en/. [Accessed 07 September 2017]
- 42.Institute for Health Metrics and Evaluation (IHME). Financing Global Health 2015: Development assistance steady on the path to new Global Goals. Seattle, WA: IHME, 2016. [Google Scholar]
- 43.Banda L, Dunbar E, Chihana T, Jumbe A, Kachimanga C, Kerr L, et al. Integrated Care Cascade Toolkit. An guide to screening, treatment and follow-up for HIV and NCDs. Partners in Health.Neno District Council Health Sector;2017. [Google Scholar]
- 44.Integrated Care Cascade Toolkit 2017. [cited 9 October 2017]; Available from: https://www.pih.org/practitioner-resource/integrated-care-cascade-toolkit.
- 45.WHO. Everybody business: strengthening health systems to improve health outcomes: WHO’s framework for action. 2007. Available from: http://www.who.int/healthsystems/strategy/everybodys_business.pdf.
- 46.Connor MD, Thorogood M, Casserly B, Dobson C, Warlow CP. Prevalence of stroke survivors in rural South Africa: results from the Southern Africa Stroke Prevention Initiative (SASPI) Agincourt field site. Stroke 2004:35. [DOI] [PubMed] [Google Scholar]
- 47.Differentiated care. Available from: http://www.differentiatedcare.org/. [Accessed 13 April 2017].
- 48.Sunguya BF, Poudel KC, Otsuka K, Yasuoka J, Mlunde LB, Urassa DP, et al. Undernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enough. BMC Public Health 2011; 11:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization, 2005, Resolution WHA 58.33 Sustainable health financing, universal coverage and social health insurance. Fifty-Eighth Session of the World Health Assembly;World Health Organization. 2011, Resolution WHA 64.9. Sustainable health financing structures and universal coverage. Sixty-Fourth Session of the World Health Assembly; Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA58-REC1/english/Resolutions.pdf. [Google Scholar]
- 50.Einterz RM, Kimaiyo S, Mengech HN, Khwa-Otsyula BO, Esamai F, Quigley F, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med 2007;82:812–818. [DOI] [PubMed] [Google Scholar]
- 51.Pastakia SD, Ali SM, Kamano JH, Akwanalo CO, Ndege SK, Buckwalter VL, et al. Screening for diabetes and hypertension in a rural low income setting in western Kenya utilizing home-based and community-based strategies. Global Health 2013; 9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vedanthan R, Kamano JH, Lee H, Andama B, Bloomfield GS, DeLong AK, et al. Bridging income generation with group integrated care for cardiovascular risk reduction: Rationale and design of the BIGPIC study. Am Heart J 2017; 188:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vedanthan R, Kamano JH, Horowitz CR, Ascheim D, Velazquez EJ, Kimaiyo S, et al. Nurse management of hypertension in rural western Kenya: implementation research to optimize delivery. Ann Glob Health 2014;80:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mamlin BW, Biondich PG, Wolfe BA, Fraser H, Jazayeri D, Allen C, et al. Cooking up an open source emr for developing countries: OpenMRS – a recipe for successful collaboration. AMIA Annu Symp Proc 2006;2006:529–533. [PMC free article] [PubMed] [Google Scholar]
- 55.Mamlin BW, Biondich PG, Wolfe BA, Fraser H, Jazayeri D, Allen C, et al. Cooking up an open source EMR for developing countries: openMRS – a recipe for successful collaboration. In AMIA Annual Symposium Proceedings 2006;2006:529–533. [PMC free article] [PubMed] [Google Scholar]
- 56.Manji I, Manyara SM, Jakait B, Ogallo W, Hagedorn IC, Lukas S, et al. The revolving fund pharmacy model: backing up the Ministry of Health supply chain in western Kenya. Int J Pharm Pract 2016;24:358–366. [DOI] [PubMed] [Google Scholar]
- 57.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel P, Rose C, Collins PY, Nuche-Berenguer B, Sahasrabuddhe VV, Peprah E, et al. Noncommunicable Diseases among Per-sons Living with HIV in Low- and Middle-Income Countries: A Systematic Review of Cardiovascular Disease, Cervical Cancer, Depression, and Diabetes. AIDS 2018;32:00–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabkin M, de Pinho H, Michaels-Strasser S, Naitore D, Rawat A, Topp SM. Strengthening the health workforce to support in-tegration of HIV and noncommunicable disease services in sub-Saharan Africa. AIDS 2018;32 (Suppl 1):S47–S54. [DOI] [PubMed] [Google Scholar]
- 60.Institute of Medicine Committee on Quality of Healthcare in A. In: crossing the quality Chasm: a new health system for the 21st century. Washington, DC: National Academies Press;2001. [PubMed] [Google Scholar]
- 61.A Toolkit for Health Facilities. Differentiated Care For HIV and Tuberculosis. November 2015 [Google Scholar]
- 62.Johnson M, Wilkinson J, Gardner A, Kupfer LE, Kimaiyo S, Von Zinkernagel D. Global partnerships to support noncommunicable disease care in low and middle-income countries: lessons from HIV/AIDS. AIDS 2018;32 (Suppl 1):S75–S82. [DOI] [PubMed] [Google Scholar]
- 63.Lee ES, Vedanthan R, Jeemon P, Kamano JH, Kudesia P, Rajan V, et al. Quality improvement for cardiovascular disease care in low- and middle-income countries: a systematic review. PLoS One 2016;11:e0157036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagarde M, Palmer N. The impact of user fees on access to health services in low- and middle-income countries. Cochrane Database Syst Rev (4):2011:CD009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson SI, Wroe EB, Dunbar EL, Mukherjee J, Squire SB, Nazimera L, et al. The impact of user fees on health services utilization and infectious disease diagnoses in Neno District, Malawi: a longitudinal, quasi-experimental study. BMC Health Services Research 2016;16:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Declaration of Alma-Ata. International Conference on Primary Healthcare. Almaty, Kazakhstan;1978. Available at: http://www.who.int/publications/almaata_declaration_en.pdf
- 67.Health in 2015 from MDGs to SDGs. World Health Organization; 2015. [cited 9 January 2017] Available at: http://apps.who.int/iris/bitstream/handle/10665/200009/9789241565110_eng.pdf;jsessionid=44281DDD9D73E455555A50825F297E8A?sequence=1.
- 68.Kruk ME, Yamey G, Angell SY, Beith A, Cotlear D, Guanais F, et al. Transforming global health by improving the science of scale-up. PLOS Biol 2016;14:e1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kemp CG, Weiner BJ, Sherr KH, Kupfer LE, Cherutich PK, Wilson D, et al. Implementation science for integration of HIV and non-communicable disease services in sub-Saharan Africa: a systematic review. AIDS 2018;32 (Suppl 1):S93–S105. [DOI] [PubMed] [Google Scholar]
- 70.Nugent R, Barnabas RV, Golovaty I, Osetinsky B, Roberts DA, Bisson C, et al. Costs and cost-effectiveness of HIV/NCD integration in Africa: from theory to practice. AIDS 2018; 32 (Suppl 1):S83–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]


