Abstract
Purpose
We measure neural responses associated with form and motion processing in children with anisometropia before and after treatment with spectacles and occlusion.
Methods
In this prospective, case-control treatment study, 10 children with anisometropia and amblyopia and 16 age-matched visually normal children participated. Steady-state visual evoked potentials (VEP) were recorded from electrodes over the occipital cortex. The visual stimulus comprised a horizontal bar grating into which Vernier offsets were introduced and withdrawn periodically at 3.75 Hz. The VEP amplitude at 3.75 Hz (first harmonic [1F]) and 7.5 Hz (second harmonic [2F]) were recorded to index the sensitivity of form/position-sensitive versus motion/transient-sensitive neural populations, respectively. Response amplitude at 1F and 2F were recorded over a series of 10 logarithmically spaced offset sizes before and after treatment. Main outcome measures are VEP amplitude versus displacement functions, interocular response amplitude differences.
Results
After relaxing into spectacles (minimally-treated state), form/position-sensitive responses in the dominant/less ametropic eye of the children with anisometropia were larger and responses in the more ametropic eye were smaller than those of controls. Motion-transient responses were equal to those of controls in the less ametropic eye, but were smaller than controls in the more ametropic eye. After treatment, responses did not differ from those of controls.
Conclusions
Form and motion responses are differentially susceptible to neural deprivation via optical blur. Form responses are more plastic than motion responses in minimally-treated children with anisometropic amblyopia. Most treatment effects occurred above threshold range, suggesting some treatment effects are not detected clinically.
Keywords: amblyopia, neural plasticity, anisometropia, treatment, human
Asymmetric visual input during early development leads to functional losses in the deprived eye and to a lesser extent in the fellow eye.1–3 Much of our understanding of the mechanisms underlying such experience-dependent plasticity has come from animal models where visual input is deprived through experimental manipulations, such as lid suture, induced blur, or uncorrelated visual input as in strabismus by surgery on extraocular muscles. When visual input to one eye is degraded via lid suture or induced blur, a number of anatomic and functional changes have been observed in the visual cortex4–11 and the lateral geniculate nucleus (LGN) in some reports.6,8,9,12,13 Both manipulations remove high spatial frequency information from the retinal image in one eye, with much more dramatic effects occurring as a result of lid-suture. Both methods result in preferential shrinkage of cell bodies in the parvocellular division of the LGN and in preferential loss in parvocellular recipient layers in striate cortex. In the case of induced optical blur, the magnocellular division of the LGN and its target layers in visual cortex are relatively spared.8,14
It is relatively common for humans to experience asymmetric optical input during early visual development due to unequal refractive errors (anisometropia). Unlike the animal models, the exact timing of the visual insult is unknown and an important confounding variable in many studies of human amblyopia is that most of what we know about visual functions in human amblyopia comes from studies performed in adults long after the initial visual insult and after various treatments have already been completed. Moreover, the outcome of amblyopia treatment is variable, adding additional uncertainty.
Thus, the picture of human amblyopia derived from studies in the visually mature individuals is clouded by a mixture of the effects of the initial insult, attempts to reverse it, and assumption that no further changes have occurred between the end of treatment and the time that the research was conducted. Conversely, animal models are only approximations of the human disease because they study abruptly applied experimental manipulations, rather than naturally occurring processes. Therefore, these shortcomings make it appropriate to study developing humans rather than animals, to make the measurements before treatment has occurred and to control for the type of amblyopic insult. It also is important to use age-matched typically developing individuals rather than fellow eyes as controls.
The visual insult of anisometropic blur comprises the degradatory effect of the defocused image itself and the neural deficiency that arises as a result. The process of wearing spectacles itself reduces the optical defocus and alleviates partially the magnitude of the entire deficiency. Previous work has shown that some treatment effect occurs during the period of wearing spectacles even before occlusion/patching is undertaken,15,16 (a process initially termed spectacle adaptation16 and later referred to as refractive adaptation15), but before this occurs the visual acuity can be rather variable as judged by the fellow eye acuity initially worsening for a time.16 This period of variability usually lasts a few weeks and often is now referred to as ‘relaxing into spectacles/glasses.' Therefore, the purely neural deficit of anisometropic amblyopia was made shortly after the patient had ‘relaxed into spectacles' and although some neural treatment may, indeed, have already occurred, it will most likely be minimal.
Here we use visual evoked potentials (VEPs) to study the effects on form and motion processing caused by anisometropia soon after the removal of optical defocus. The cortical responses we measured are generated simultaneously by a single stimulus that elicits two distinctive response components, one of which is associated with fine position sensitivity or the form of the stimulus and the other with motion and/or temporal transients.17–19 The study population are children who experienced loss of high-spatial frequency input in one eye due to chronic optical defocus (anisometropia). Typically developing children of similar age act as controls. We find that form/position-specific responses are super-normal in the dominant (nondeprived) eye and are markedly subnormal in the nondominant (amblyopic/deprived) eye. Motion/transient responses, on the other hand, show no difference from normal in the dominant eye and milder losses in the nondominant eye. After a period of spectacle and occlusion treatment of the dominant eye, the form/position signal decreased in this eye and increased in the nondominant eye. After occlusion, the motion/transient signals also reduced in the dominant eye, but no significant change occurred in the nondominant eye. Thus, anisometropia creates bidirectional shifts in form responses, but only losses in motion processing. Because form and motion responses are differentially affected by the insult of anisometropic amblyopia and by treatment, it is likely they arise from separate neural populations with different developmental sensitivities.
Methods
Participants
Ten children with anisometropic amblyopia (four females, aged 5.08–6.92 years, mean 5.62, SD 0.57, median 5.43) participated in the study. Patients were considered to be anisometropic if a difference of ≥1.0 diopter sphere in the maximum anisometropic meridian existed between each eye.20,21 Patients were considered to be amblyopic if their interocular acuity differed by 0.2 logMAR or more. Patients were deemed to have relaxed into their spectacles when the acuity in the fellow/dominant eye was equal or better than that in the unaided state.22 Once relaxed into spectacles (average wear, 46 days; SD 18) study data were collected. Visual acuity was measured using the Early Treatment of Diabetic Retinopathy Study (ETDRS)-style Lea Symbols chart (catalog number 2503, Precision Vision, Woodstock, IL, USA). The Table provides a summary of the main characteristics of these participants during the course of the study.
Table.
Summary Characteristics of Participants With Amblyopia
|
Patient |
Entry Age, y |
Unaided Acuity, logMAR |
Refraction |
Spectacle Adapted Acuity, logMAR |
Spectacle Adaptation, Days |
Applied Treatment |
Treatment Duration, Days |
Outcome Acuity (logMAR) |
||||
|
Dom Eye |
Nondom Eye |
Dom Eye |
Nondom Eye |
Dom Eye |
Nondom Eye |
Dom Eye |
Nondom Eye |
|||||
| P1 | 5.20 | 0.00 | 1.00 | +1.75/+0.50 × 90 | +6.50/+0.50 × 90 | 0.00 | 0.40 | 77 | SO | 224 | ‖ | ‖ |
| P2 | 6.92 | 0.00 | 0.30 | +2.00 DS | +3.00/+0.75 × 180 | 0.10 | 0.30 | 53 | SO | 210 | −0.10 | 0.00 |
| P3 | 5.52 | 0.00 | 0.30 | +3.25/+0.75 × 70 | +5.50/+0.50 × 100 | 0.20 | 0.40 | 60 | SO | 130 | 0.20 | 0.20 |
| P4 | 5.09 | 0.10 | 0.80 | +1.50 DS | +5.25 DS | 0.10 | 0.40 | 37 | SO | 279 | 0.00 | 0.40 |
| P5 | 5.34 | 0.00 | 0.90 | +4.00 DS | +6.50/+0.50 × 90 | 0.20 | 0.70 | 42 | SO | 161 | −0.10 | 0.40 |
| P6 | 5.92 | 0.20 | 0.70 | +4.50/+1.00 × 100 | +5.25/+1.25 × 90 | 0.10 | 0.30 | 28 | SO | 120 | 0.20 | 0.30 |
| P7 | 5.08 | Unavailable* | Unavailable* | +1.00/+0.50 × 85 | +2.00/+1.00 × 100 | 0.10 | 0.40 | § | SO | ‡ | ‖ | ‖ |
| P8 | 5.32 | 0.10 | 1.00 | Plano | −4.50/−1.25 × 180 | 0.00 | 0.50 | 27 | S | † | −0.10 | 0.10 |
| P9 | 5.97 | Unavailable* | Unavailable* | −0.25 DS | +4.00/+0.50 × 90 | 0.00 | 0.80 | § | SO | 146 | 0.00 | 0.60 |
| P10 | 5.87 | Unavailable* | Unavailable* | +2.00/+0.50 × 90 | +5.75 DS | 0.00 | 0.60 | § | SO | 338 | −0.10 | 0.30 |
Patients 1, 7, and 10 did not provide Outcome VEP data. Dom, dominant; Nondom, nondominant; P, patient; SO, spectacle and occlusion treatment; S, spectacle treatment only.
Referred from external clinic immediately after spectacle prescription; therefore, unaided acuity not available.
Spectacle only treatment required of total 119 days.
Defaulted from study after providing initial data, and did not provide outcome acuity data.
Calculation not possible as first spectacle wear commenced before initial entry to research project but had not worn spectacles before this time.
Did not provide outcome acuity data.
Sixteen healthy children (eight females, age 0.6–8.6 years, mean 5.14, SD 2.38, median 5.50) acted as normal controls (normals). They had normal monocular and binocular vision and no previous history of amblyopia, patching, or intermittent strabismus. Fifteen of these children contributed data recorded under related but different stimulus conditions than used in a prior study.19
VEP testing in this study occurred for normal controls at the point of recruitment, and for children with amblyopia testing occurred immediately after full relaxation into spectacles (“initial visit”) and then again after recognition visual acuity of the nondominant eye failed to improve further after continued treatment with spectacles and occlusion therapy as needed (“outcome visit”). Local ethical committee approval was obtained and each observer provided fully informed consent. The research complied with the principles of the Declaration of Helsinki.
Stimulus Generation and Apparatus
Details of the apparatus and basic signal acquisition and processing operations have been described in detail in previously18,19 and are described here only briefly. The stimulus we used is a variation of a target introduced by Zemon and Ratliff to study nonlinear spatial interactions.23 A series of spatial offsets were introduced and withdrawn periodically at 3.75 Hz from a collinear set of bars. Evoked responses were measured as the magnitude of the offsets was swept over a range of values spanning the perceptual threshold and well beyond it. In the primary test condition, the image alternated between a perfectly collinear pattern and a pattern with an increasingly large set of offsets. We have shown previously that this pattern alternation results in an evoked response that contains even and odd harmonics of the 3.75 Hz stimulus frequency. Furthermore, we have shown that the odd harmonics and especially the first harmonic response (1F) are specific to the relative form/position of the static and moving display elements. The even harmonics and especially the second harmonic (2F) index the motion and contrast/transient responses.19 The even harmonics also may contain Vernier-related activity that is nonlinear.
The stimulus used for this study is described schematically in Figure 1 and comprised a rectangular area of 13.5° × 14°. Vernier offsets were introduced vertically within a horizontally oriented square wave grating (2 cpd). The display comprised equal height regions of moving and static bars, with the distance between offsets being 0.50°. The size of the offset was swept in logarithmic steps over a 10-second recording period divided into 1-second epochs termed ‘bins'. The sweep range began with 0.5 to 8 arc min offsets and the range was increased for patients with amblyopia who failed to produce a measurable Vernier acuity threshold for the initial sweep range. The trial began with a 1 second “prelude” whose value was the same as the first bin in the sweep. The prelude was included to eliminate the initial transient evoked response that occurs when the pattern first appears. Three to six trials were averaged for each stimulus condition.
Figure 1.
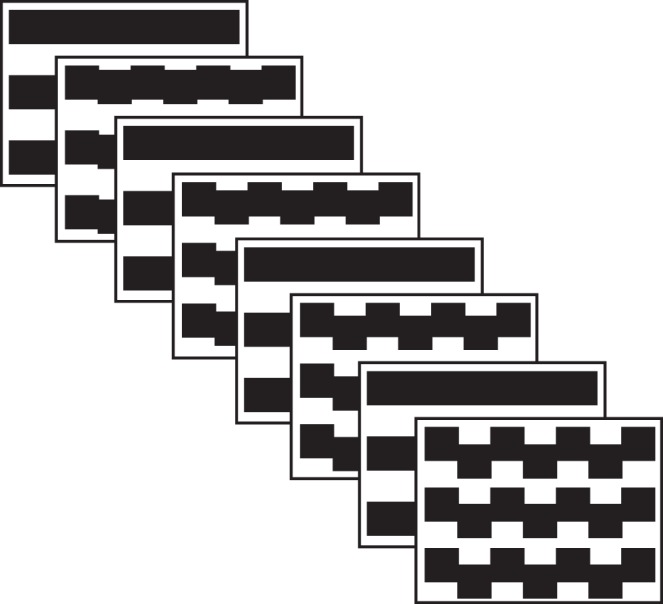
Schematic illustration of the stimulus. Vernier offsets were introduced and withdrawn from a 2 c/deg bar grating at 3.75 Hz. The magnitude of the offsets was systematically swept over 10 equal logarithmically spaced values over a period of 10 seconds.
Stimulus Schematic: VEP Recording and Statistical Analysis
The EEG was amplified by 50,000 over an amplifier pass-band of 1 to 100 Hz (−6 dB) using Grass P511 amplifiers. The sampling rate was 600 Hz (16 bits). The electrode montage consisted of Oz, O1, and O2 each referenced to Cz. Spectrum analysis was used to extract the amplitude and phase of the evoked response at the first (1F) and second (2F) harmonics of the stimulus frequency as these were the largest and most reliable response components. The absolute values of these complex spectral components at each displacement amplitude were averaged across subjects within the two groups being compared. Error bars were estimated by boot-strapping, taking the standard deviation of 5000 random resamplings of subjects with replacement within each group. The statistical significance of differences between the two groups at each displacement was evaluated by a t-test for two samples with unequal variance. For the response functions, a four-parameter descriptive function was fit to the mean of each resampling of subjects during the bootstrap procedure above. The four-parameter descriptive function for the VEP response amplitude (y) as a function of displacement (x) was:
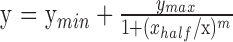 |
where parameters were estimated using the Optimization Toolbox in MATLAB (Mathworks, Natick, MA, USA).
Results
Initial Visit Evoked Responses
VEP data were collected after the patients had relaxed into their spectacles (average wear, 46 days; SD 18). At this initial VEP visit, the children with anisometropia had best-corrected visual acuities of 0.48 (SD 0.17) logMAR in the nondominant eye and 0.08 (SD 0.08) logMAR in the dominant eye. Each normal observer capable of logMAR acuity (n = 12) had a mean logMAR visual acuity of 0.05 (SD 0.11) left and 0.03 (SD 0.10) right eye and normal stereopsis on testing with Frisby Near stereo test plates. Mean stereo acuity in those capable (n = 9) was 29 (SD 22) arc sec.
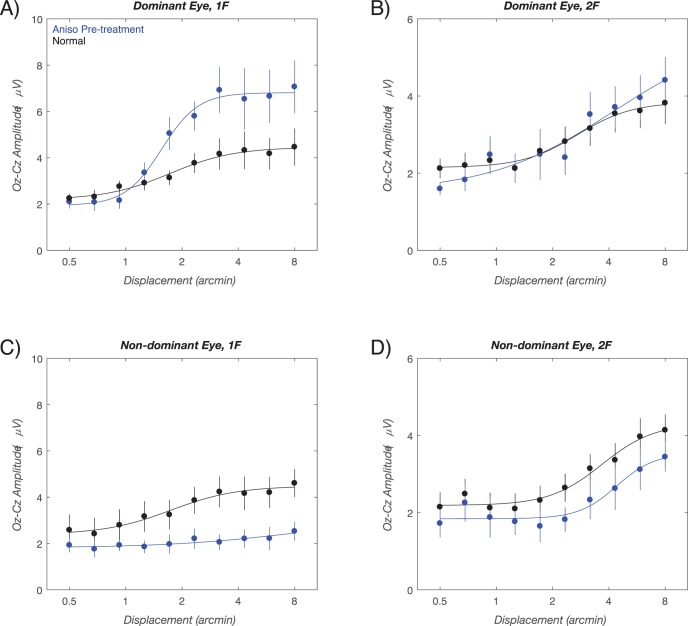
VEP response functions plotting evoked-response amplitude versus stimulus displacement are shown in Figure 2 for data collected at the initial visit. Figure 2A plots functions obtained at 1F of the stimulus frequency (position/form signal). Here the maximal response amplitudes in the dominant eyes of the children with anisometropia (blue curve, Fig. 2A) are approximately 7 μV at the largest displacements, compared to approximately 4 μV in the right eye of normal-vision, age-matched control eyes (black curve, Fig. 2A). By contrast, the simultaneously recorded second harmonic (Fig. 2B) showed no difference between the dominant eyes of the children with anisometropia and the normal-vision controls (compare blue versus black curves, respectively, in Fig. 2B).
Figure 2.
Initial visit group-average response functions. VEP amplitude versus displacement size is plotted for the dominant (A, B) and nondominant eyes (C, D) at 1F (A, C) and 2F (B, D). Data for the age-matched normal vision control children is shown in black, with data from the children with anisometropia shown in blue. The smooth curves are describing function fits. Error bars: SEM for response amplitude. See text for details.
The corresponding response functions for the nondominant eyes of the children with anisometropia are shown in Figures 2C and 2D, with data from the children with anisometropia again plotted in blue and the data from the left eyes of normal-vision, age-matched controls shown in black. The response from the nondominant (amblyopic) eyes of the children with anisometropia showed no relationship to displacement amplitude, with the amplitude measured at 1F being approximately 2 μV at all displacements (blue curve, Fig. 2C). As noted above, maximal amplitudes in the normal-vision eyes of the age-matched controls reached approximately 4 at the largest displacements (black curve Fig. 2C). By contrast, at 2F, the group response function for the nondominant eye of the children with anisometropia, curve is clearly measurable (blue curve in Fig. 2D), but the function is shifted rightward by a factor of approximately 2 from the normal-vision response functions shown in black.
Initial Visit 1F/2F Dominant/Nondominant-Eye Response Functions
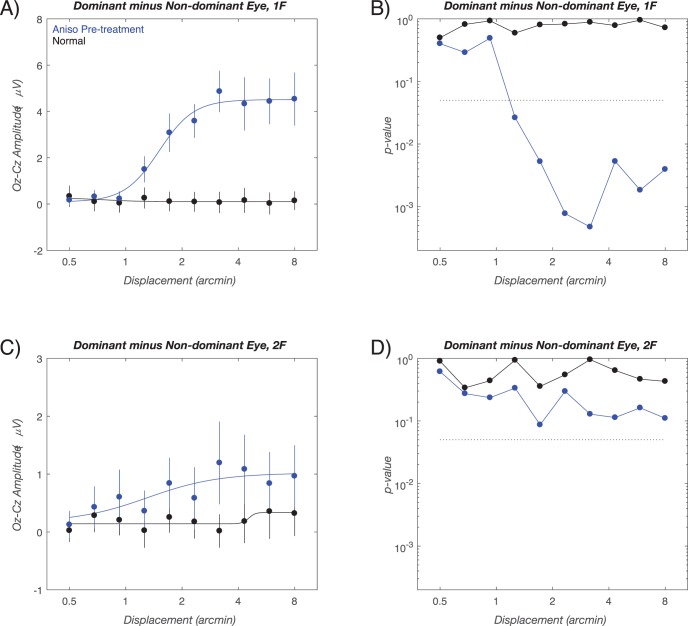
To compare response patterns between the children with anisometropia to the normal-vision age-matched controls, we first calculated response functions on the basis of ‘within-subjects' interocular differences in amplitude. Interocular differences are clinically meaningful and also control for between subject variability, making the comparison between groups more sensitive. The use of interocular differences reduced the comparison between groups to a test of the mean values over stimulus displacements. As was apparent from the dominant versus nondominant eye response curves in Figures 2A and 2C, there are large interocular differences in the children with anisometropia at 1F. These amplitude differences are shown in Figure 3A where positive values indicate a greater response in the dominant versus the nondominant eyes. For the children with anisometropia, these differences exceed a 0.05 significance threshold (dotted line) at small displacement values just larger than 1 arcmin and continue to exceed the significance threshold throughout the suprathreshold response range (blue curve in Fig. 3B). As expected, there are no significant interocular differences in the normal-vision, age-matched controls (black curve, Fig. 3B). At 2F, the magnitude of the interocular differences are smaller than those measured at 1F (compare Figs. 3C and 3A) and these differences do not exceed the P < 0.05 threshold at any point (blue curve in Fig. 3D), but there are multiple points with approximately P = 0.10 indicative of a trend toward this response being decreased relative to controls. Again, no significant interocular differences were measured in the normal-vision, age-matched control children (black curve, Fig. 3D).
Figure 3.
Initial visit group average interocular difference functions. (A) 1F data (dominant eye minus nondominant eye) for children with anisometropia (blue curve) and for normal vision, age-matched control children (black curve). The corresponding two-sample t-test significance values are shown in (B). Interocular differences in the children with anisometropia exceed a P < 0.05 criterion (dotted line) for all values above approximately 1 arcmin. Interocular differences are nonsignificant in the normal-vision control group. (C) The 2F data using the same conventions. There is a nonsignificant trend for the dominant eye to have larger signals than the nondominant eye in the children with anisometropia (D). The smooth curves in (A) and (C) are describing function fits. Error bars: SEM for response amplitude. See text for details.
Initial Visit Interocular Differences 1F/2F Response Functions and Significance Plots
The interocular differences in the children with anisometropia just shown combine the bi-directional effect of anisometropic deprivation on the dominant (increased amplitude) and nondominant eyes (decreased amplitude). To assess these effects in absolute terms relative to corresponding data from control eyes, we made cross-group comparisons separately for dominant and nondominant eyes. These comparisons are shown in Figure 4 and the raw data upon which these comparisons were made are shown in Figure 2. Response amplitudes at 1F in the dominant eyes of the children with anisometropia were larger than those of the normal-vision, age-matched controls for the three bins (between 1 and 4 arcmin) of the sweep (see Fig. 4A, blue curve). There were no measurable differences between groups in the dominant eye at this study time point for the 2F response (blue curve, Fig. 4B). In the nondominant eyes, the responses of the children with anisometropia were significantly lower than those of the normal vision, age-matched controls in last five bins of the sweep (Fig. 4C, blue curve). There were no differences between groups in the nondominant eye 2F response (Fig. 4D, blue curve).
Figure 4.
Initial and outcome visit responses for between group comparisons of response amplitude in dominant (A, B) versus nondominant eyes (C, D). Each Figure plots the result of two-sample t-tests for between group amplitude differences. Initial visit differences are plotted in blue and outcome visit differences are plotted in red. At initial visit, the dominant eyes of children with anisometropia have larger than normal amplitudes in their dominant eyes and smaller than normal amplitudes in their nondominant eyes (see Figs. 2A and 2C for the raw amplitude values that are the basis of the comparison).
Taken together, the larger than normal 1F responses in the dominant eyes and smaller than normal 1F responses in the nondominant eyes account for the large interocular differences shown in Figure 3A. By contrast with the 1F responses, the amplitude differences between groups at 2F were small and not significantly different from controls in dominant and nondominant eyes.
Significance of Between Group Differences
Outcome Visit Responses
The outcome visit recordings occurred after an average of 201 (SD 77) days of occlusion and/or spectacle wear. At this point of the treatment children with anisometropia had best corrected visual acuity of 0.29 (SD 0.20) logMAR in the nondominant eye and 0.00 (SD 0.13) logMAR in the dominant eye.
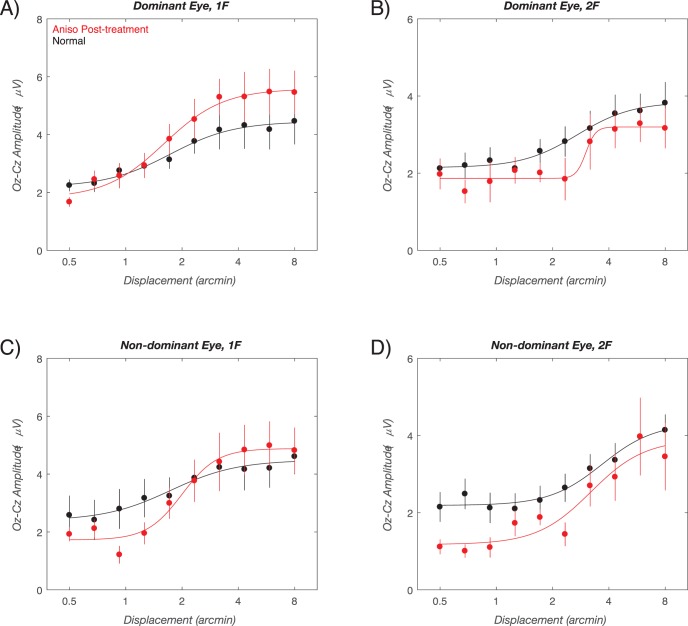
Outcome visit response functions are shown in Figure 5, with responses from the children with anisometropia shown in red and data from normal-vision, age-matched controls shown in black. Here, when visual acuity has stabilized with spectacles and patching, the dominant-eye response curve lies above that of the controls at 1F (Fig. 5A) and below controls at 2F (Fig. 5B). In the nondominant eye, the curves of the anisometropic children cross over the curve for the controls at 1F (Fig. 5C) and lie below controls at 2F (Fig. 5D).
Figure 5.
Outcome visit group-average response functions. VEP amplitude versus displacement size is plotted for the dominant (A, B) and nondominant eyes (C, D) at 1F (A, C) and 2F (B, D). Data for the age-matched normal vision control children is shown in black, with data from the children with anisometropia shown in red. The smooth curves are describing function fits. Error bars: SEM for response amplitude. See text for details.
Outcome Visit 1F/2F Dominant/Nondominant-Eye Response Functions
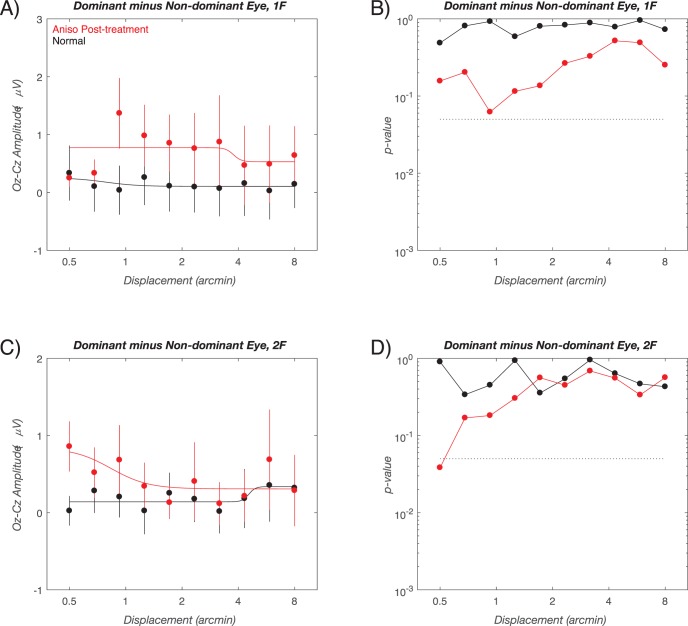
Interocular difference functions and corresponding significant plots for the data of Figure 5 are shown in Figure 6. Further treatment had the effect of reducing the interocular difference at 1F (red curves, Fig. 6A) to nonsignificant levels (Fig. 6B). The 2F interocular differences (red traces) were small (Fig. 6C) and nonsignificant (Fig. 6D).
Figure 6.
Outcome visit group average interocular difference functions. (A) 1F data (dominant eye minus nondominant eye) for children with anisometropia (red curve) and for normal vision, age-matched control children (black curve). The corresponding two-sample t-test significance values are shown in (B). Interocular differences in the children with anisometropia do not exceed a P < 0.05 criterion (dotted line). Interocular difference are nonsignificant in the normal-vision control group. (C) 2F data using the same conventions. Nine of 10 bins do not exceed significance criterion (D). The smooth curves in (A) and (C) are describing function fits. Error bars: SEM for response amplitude. See text for details.
Outcome Visit Interocular Differences 1F/2F Response Functions and Significance Plots (Fig. 6)
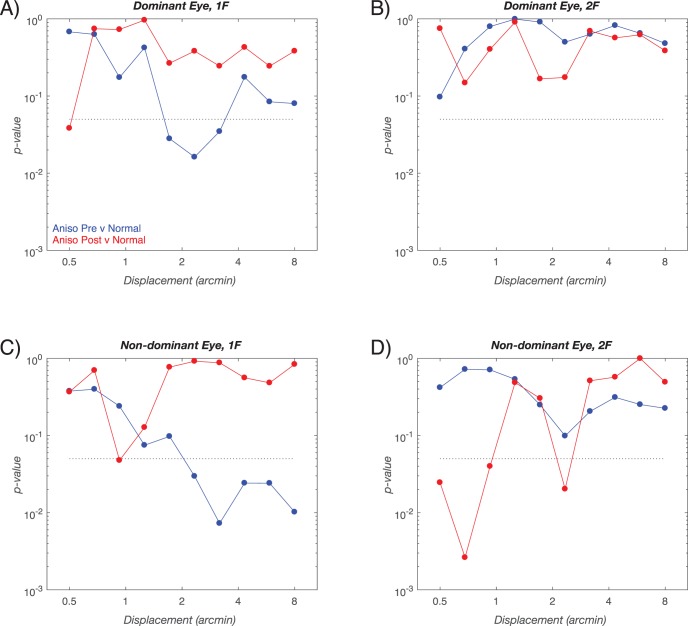
We also compared the response amplitudes for the outcome and initial visits on a bin-by-bin basis for the children with anisometropia. The formerly super-normal response at 1F in the dominant eye (e.g., the blue curve Fig. 4A) is no longer measurably different from the values recorded from the controls (shown as the red curve in Fig. 4A). This comes about because the dominant eye response at the Outcome visit lies between the value measured at the initial visit and that of the controls (compare Figs. 2A and 5A). Thus, treatment reduces the larger than normal 1F responses relative to control values. The 2F response in the dominant eye did not differ from controls either at the outcome (Fig. 4B, red curve) or initial (Fig. 4B, blue curve) visits. In the nondominant eyes of the children with anisometropia, the significantly smaller than normal response 1F has been mostly reversed post-treatment (compare nonsignificant post-treatment values along the red curve in Fig. 4C to the significant, initial visit values along the blue curve). For the 2F response, there were sporadic differences in the nondominant eyes (Fig. 4D, red curve). These differences were the result of the outcome visit responses being even smaller than those of the normal children (see Fig. 5D, red versus black curves). A similar pattern of results described in Supplementary Materials is present when the analysis is performed within the patient group, rather than in reference to the control group.
Regarding the potential outlier effect of the single youngest control subject (0.6 years old) we have made a set of analysis with this child excluded from the data set and found no change to the conclusions or statistical inferences.
Discussion
Considering the multitude of influences that can impact upon a developing visual system, it makes sense to examine the deficits related to risk factors for amblyopia such as anisometropia, as early as is practicably detectable. The existence of critical and plastic periods in neural development is widely accepted even if there is uncertainty about their exact timing24,25 and a complete understanding of the genesis and natural history of amblyopia development continues to elude the field.2 Amblyopia treatment is undertaken most effectively during a naturally sensitive period of development. Therefore, the importance of understanding the natural history of visual development in amblyopia compared to typically developing visual systems is vital.
Visual loss through blur long has been known to produce reduced behavioral acuity and contrast sensitivity in the more ametropic eye26–28 and reports exist of motion processing deficits as well.29,30 However, almost all of the published data on anisometropic amblyopia has come from studies of the developed rather than developing and the treated versus untreated visual system. The picture of the functional and neural deficits seen in “late-stage” amblyopia is likely to be confounded by additional variability caused by treatment effects and long-term adaptation effects that will have inextricably modified performance.
Here we show that at the point of relaxing into spectacles anisometropia present during early childhood has affected position-cue related responsivity of both eyes, with a reduction of responses in the previously deprived/amblyopic eye and a complementary increase in response amplitude in the nondeprived, fellow eye. 2F responses, by contrast only show a response reduction in the previously deprived eye. Measurements at the time of the initial visit are a conservative estimate of the full loss that is present before the introduction of spectacles. Before spectacle introduction, two effects can act to reduce visual responsivity—the high refractive error itself and any consequent neural deprivation effects. The process of spectacle correction/relaxing into spectacles removes the deprivation from blur and may itself have a therapeutic effect.15,16,31–33 At the outcome visit, continued treatment via spectacle correction and occlusion therapy preferentially modified the position-based responses. Taken together, our results suggested that the form system is more susceptible to abnormal visual experience due to anisometropia during early childhood than is the motion system.
The enhanced Vernier VEP response of the fellow eye found here is consistent with an early report of enhanced psychophysical Vernier acuity in adults with a history of anisometropic amblyopia34 and with two studies conducted in infancy that have found super-normal grating acuity in the nondeprived eye of patients with media opacities or strabismus. One of these studies measured grating acuity behaviorally with forced choice preferential looking and found that acuity in six infants with visual deprivation secondary to media opacities or strabismus was higher than expected in the fixating eye.35 The other study found that visual acuity measured for gratings with the VEP was higher than expected in the fellow eye of four infants with unilateral congenital cataracts or a unilateral ocular malformation.36
Studies of monocular deprivation in animal models have suggested functional connectivity of the deprived eye is first weakened by synaptic depression, followed by a potentiation of the nondeprived eye.37 If similar processes operate in human anisometropic amblyopia, the depression and potentiation mechanism involved would predict reduced 1F responses in the eye that experienced deprivation via blur and enhanced responses in the fellow eye, respectively.
Fellow eye motion sensitivity has been reported to be reduced in patients with amblyopia, but to a lesser extent than in the amblyopic eye, although few studies have included a significant number of participants with anisometropic amblyopia.3 We find that fellow eye response is of normal amplitude rather than being depressed. The 2F response reflects a mixture of contrast transient and motion-related responses and potentially nonlinear Vernier offset-related responses.18,19,38 It is likely that motion/transient responses rely more on low spatial frequency information in the stimulus than do form/position-based responses, such as those measured at 1F.39–42 This would make motion/transient responses less susceptible to deprivation by optical defocus. Alternatively, or in parallel, it is possible that the motion/transient response system has matured to a greater degree before the onset of the optical degradation than the position-based system indexed by the 1F response.43 If the latter is the case, this response might be less susceptible to deprivation due to the lessening of plasticity that typically is associated with later stages of development making this response component correspondingly less malleable by treatment. Unfortunately, the timing of the onset of the visual insult is rarely known in human studies, but a lower degree of plasticity in the motion/transient response is supported by the lack of observed plastic changes in this response with treatment.
The disparate effects of initial visual insult on these 1F and 2F response components strongly suggest that they are derived from different neural substrates. Further evidence for separate substrates comes from the differential plasticity of these two components after treatment. The position signal at 1F shows plastic changes after treatment with decreases in responsiveness of the dominant/patched eye and increases in responsiveness of the initially deprived eye. The motion signal, by contrast is little affected. Although it is possible that the even harmonics contain contributions from nonlinear mechanisms associated with the processing of Vernier offsets, this contribution—if present—is either small18 or does not share the pattern of loss that is present at 1F.
The present functional results parallel a similar pattern of loss in an animal model of anisometropic amblyopia in which V1 responsiveness in parvocellular recipient laminae of V1 was more affected than that of magnocellular recipient laminae.8,14 However, our results contrast with psychophysical work using global motion and form tasks that have found greater deficits on global motion than global form.44,45 The random dot stimuli used in global motion and form tasks differ on many dimensions from the stimuli used here and the psychophysical measures index threshold, but not suprathreshold responsivity that is measured in the VEP. Only a direct comparison of the Vernier VEP response components and corresponding global motion- and form-evoked responses could determine whether the results are, indeed, in conflict.
Our study contributed to the literature on the origins of anisometropia amblyopia in several respects. Our measure of neural activity spans threshold and suprathreshold levels and we find the largest effects at suprathreshold stimulus levels. Previous behavioral studies that have focused on threshold measures would not have detected the suprathreshold effects we observed. Suprathreshold responsiveness is highly relevant to vision under natural viewing conditions. Secondly, our paradigm provides access to form and motion/transient related activity measured simultaneously from the same stimulus. This advantage makes the comparison of deprivation and treatment effects particularly precise as there are no differences in the quality of fixation or attentional state that could modulate the results as would be the case if separate measurements were made at different times or if different stimulus parameters were used for form versus motion tasks that is commonly done in developmental studies.42,46
Our data indicated the manner in which fundamental features of visual input, such as form and motion, demonstrate differing susceptibilities to common modulations of input––in the present case visual deprivation in anisometropic amblyopia and its clinical treatment. The extension of this work to amblyopia with strabismus and also toward investigating the neural effects of nonocclusive treatments also would be of considerable clinical interest.
Supplementary Material
Acknowledgments
Previously presented at the Child and Vision Research Meeting, Coleraine, Ireland, United Kingdom, June 19–21, 2017 and as a poster presentation at annual meeting of the American Academy of Pediatric Ophthalmology and Strabismus, Washington, D.C., United States, March 18–22, 2018.
The authors thank Wesley J. Meredith for assistance with data analysis and Vladimir Y. Vildavski for VEP software development.
Supported by Guide Dogs for the Blind (OR2001-99a) and the Smith-Kettlewell Eye Research Institute. The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure: S.I. Chen, None; A. Chandna, None; S. Nicholas, None; A.M. Norcia, None
References
- 1.Levi DM. Visual processing in amblyopia: human studies. Strabismus. 2006;14:11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- 2.Wong AM. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012;47:399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Meier K, Giaschi D. Unilateral amblyopia affects two eyes: fellow eye deficits in amblyopia. Invest Ophthalmol Vis Sci. 2017;58:1779–1800. doi: 10.1167/iovs.16-20964. [DOI] [PubMed] [Google Scholar]
- 4.Crewther DP, Crewther SG. Neural site of strabismic amblyopia in cats: spatial frequency deficit in primary cortical neurons. Exp Brain Res. 1990;79:615–622. doi: 10.1007/BF00229329. [DOI] [PubMed] [Google Scholar]
- 5.Gillard-Crewther S, Crewther DP. Neural site of strabismic amblyopia in cats: X-cell acuities in the LGN. Exp Brain Res. 1988;72:503–509. doi: 10.1007/BF00250595. [DOI] [PubMed] [Google Scholar]
- 6.Kiorpes L, Boothe RG, Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS. Effects of early unilateral blur on the macaque's visual system. I. Behavioral observations. J Neurosci. 1987;7:1318–1326. doi: 10.1523/JNEUROSCI.07-05-01318.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9:480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS, Boothe RG, Kiorpes L. Effects of early unilateral blur on the macaque's visual system. II. Anatomical observations. J Neurosci. 1987;7:1327–1339. doi: 10.1523/JNEUROSCI.07-05-01327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker FH, Grigg P, von Noorden GK. Effects of visual deprivation and strabismus on the response of neurons in the visual cortex of the monkey, including studies of the striate and prestriate cortex in the normal animal. Brain Res. 1974;66:185–208. [Google Scholar]
- 10.Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- 11.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 12.Crawford ML, Pesch TW, von Noorden GK, Harwerth RS, Smith EL. Bilateral form deprivation in monkeys. Electrophysiologic and anatomic consequences. Invest Ophthalmol Vis Sci. 1991;32:2328–2336. [PubMed] [Google Scholar]
- 13.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 14.Movshon JA, Eggers HM, Gizzi MS, Hendrickson AE, Kiorpes L, Boothe RG. Effects of early unilateral blur on the macaque's visual system. III. Physiological observations. J Neurosci. 1987;7:1340–1351. doi: 10.1523/JNEUROSCI.07-05-01340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart CE, Moseley MJ, Fielder AR, Stephens DA; MOTAS Cooperative. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004;88:1552–1556. doi: 10.1136/bjo.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moseley MJ, Neufeld M, McCarry B, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmic Physiol Opt. 2002;22:296–299. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- 17.Skoczenski AM, Norcia AM. Development of VEP Vernier acuity and grating acuity in human infants. Invest Ophthalmol Vis Sci. 1999;40:2411–2417. [PubMed] [Google Scholar]
- 18.Norcia AM, Wesemann W, Manny RE. Electrophysiological correlates of vernier and relative motion mechanisms in human visual cortex. Vis Neurosci. 1999;16:1123–1131. doi: 10.1017/s0952523899166124. [DOI] [PubMed] [Google Scholar]
- 19.Chen SI, Norcia AM, Pettet MW, Chandna A. Measurement of position acuity in strabismus and amblyopia: specificity of the Vernier VEP paradigm. Invest Ophthalmol Vis Sci. 2005;46:4563–4570. doi: 10.1167/iovs.05-0792. [DOI] [PubMed] [Google Scholar]
- 20.Chandna A, Gonzalez-Martin JA, Norcia AM. Recovery of contour integration in relation to LogMAR visual acuity during treatment of amblyopia in children. Invest Ophthalmol Vis Sci. 2004;45:4016–4022. doi: 10.1167/iovs.03-0795. [DOI] [PubMed] [Google Scholar]
- 21.Morgan M, Peters HB. Calculation of anisometropic sphere and cylinder derived from the optics of ophthalmic lenses. In: Morgan MW, editor. The Optics of Ophthalmic Lenses. Chicago: Professional Press;; 1978. [Google Scholar]
- 22.Chen SI, Chandna A, Norcia AM, Pettet M, Stone D. The repeatability of best corrected acuity in normal and amblyopic children 4 to 12 years of age. Invest Ophthalmol Vis Sci. 2006;47:614–619. doi: 10.1167/iovs.05-0610. [DOI] [PubMed] [Google Scholar]
- 23.Zemon V, Ratliff F. Visual evoked potentials: evidence for lateral interactions. Proc Natl Acad Sci U S A. 1982;79:5723–5726. doi: 10.1073/pnas.79.18.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 25.Knudsen EI, Knudsen PF. Sensitive and critical periods for visual calibration of sound localization by barn owls. J Neurosci. 1990;10:222–232. doi: 10.1523/JNEUROSCI.10-01-00222.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981;21:467–476. [PubMed] [Google Scholar]
- 27.Boothe RG, Kiorpes L, Hendrickson A. Anisometropic amblyopia in Macaca nemestrina monkeys produced by atropinization of one eye during development. Invest Ophthalmol Vis Sci. 1982;22:228–233. [PubMed] [Google Scholar]
- 28.Abrahamsson M, Sjostrand J. Contrast sensitivity and acuity relationship in strabismic and anisometropic amblyopia. Br J Ophthalmol. 1988;72:44–49. doi: 10.1136/bjo.72.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Chen L, Liu Z, Liu C, Zhou Y. Low-level processing deficits underlying poor contrast sensitivity for moving plaids in anisometropic amblyopia. Vis Neurosci. 2012;29:315–323. doi: 10.1017/S095252381200034X. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Liu C, Liu Z, Hu X, Yu YQ, Zhou Y. Processing deficits of motion of contrast-modulated gratings in anisometropic amblyopia. PLoS One. 2014;9:e113400. doi: 10.1371/journal.pone.0113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace DK, Edwards AR, Cotter SA, et al. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113:904–912. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter SA, Edwards AR, Wallace DK, et al. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen PL, Chen JT, Tai MC, Fu JJ, Chang CC, Lu DW. Anisometropic amblyopia treated with spectacle correction alone: possible factors predicting success and time to start patching. Am J Ophthalmol. 2007;143:54–60. doi: 10.1016/j.ajo.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Freeman RD, Bradley A. Monocularly deprived humans: nondeprived eye has supernormal vernier acuity. J Neurophysiol. 1980;43:1645–1653. doi: 10.1152/jn.1980.43.6.1645. [DOI] [PubMed] [Google Scholar]
- 35.Mohindra I, Jacobson SG, Zwaan J, Held R. Psychophysical assessment of visual acuity in infants with visual disorders. Behav Brain Res. 1983;10:51–58. doi: 10.1016/0166-4328(83)90150-x. [DOI] [PubMed] [Google Scholar]
- 36.Jastrzebski G, Marg E, Hoyt CS. Superacuity in the spared eyes of monocular deprivation amblyopes: visual evoked response measurements. In: Breinen G, Siegel I, editors. Advances in Diagnostic Visual Optics Springer Series in Optical Sciences Vol. 41. Heidleburg: Springer;; 1983. pp. 180–186. [Google Scholar]
- 37.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 38.Hou C, Good WV, Norcia AM. Validation study of VEP vernier acuity in normal-vision and amblyopic adults. Invest Ophthalmol Vis Sci. 2007;48:4070–4078. doi: 10.1167/iovs.06-1368. [DOI] [PubMed] [Google Scholar]
- 39.Kubova Z, Kuba M, Juran J, Blakemore C. Is the motion system relatively spared in amblyopia? Evidence from cortical evoked responses. Vision Res. 1996;36:181–190. doi: 10.1016/0042-6989(95)00055-5. [DOI] [PubMed] [Google Scholar]
- 40.Chung ST, Bedell HE. Velocity dependence of Vernier and letter acuity for band-pass filtered moving stimuli. Vision Res. 2003;43:669–682. doi: 10.1016/s0042-6989(02)00628-4. [DOI] [PubMed] [Google Scholar]
- 41.Chung ST, Bedell HE. Vernier and letter acuities for low-pass filtered moving stimuli. Vision Res. 1998;38:1967–1982. doi: 10.1016/s0042-6989(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 42.Burton EA, Wattam-Bell J, Rubin GS, Atkinson J, Braddick O, Nardini M. The effect of blur on cortical responses to global form and motion. J Vis. 2015;15(15):12. doi: 10.1167/15.15.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orel-Bixler D, Norcia AM. Differential growth of acuity for steady-state pattern reversal and transient onset-offset VEP's. Clin Vis Sci. 1987;2:1–9. [Google Scholar]
- 44.Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003;43:729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- 45.Simmers AJ, Ledgeway T, Hess RF. The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res. 2005;45:449–460. doi: 10.1016/j.visres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Wattam-Bell J, Birtles D, Nystrom P, et al. Reorganization of global form and motion processing during human visual development. Curr Biol. 2010;20:411–415. doi: 10.1016/j.cub.2009.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.