Abstract
Background
Delay discounting, or the tendency to devalue rewards as a function of their delayed receipt, is associated with myriad negative health behaviors. Individuals from medically underserved areas are disproportionately at risk for chronic health problems. The higher rates of delay discounting and consequent adverse outcomes evidenced among low-resource and unstable environments suggest this may be an important pathway to explain health disparities among this population.
Purpose
The current study examined the effectiveness of a computerized working memory training program to decrease rates of delay discounting among residents of a traditionally underserved region.
Methods
Participants (N = 123) were recruited from a community center serving low income and homeless individuals. Subjects completed measures of delay discounting and working memory and then took part in either an active or control working memory training.
Results
Analyses indicated that participants in the active condition demonstrated significant improvement in working memory and that this improvement mediated the relation between treatment condition and reductions in delay discounting.
Conclusions
Results suggest that a computerized intervention targeting working memory may be effective in decreasing rates of delay discounting in adults from medically underserved areas (ClinicalTrials.gov number NCT03501706).
Keywords: Delay discounting, Working memory, Medically underserved, Cognitive training, mid-age
Adults from a medically-underserved area demonstrated increases in working memory and subsequent decreases in delay discounting following a computerized working memory training program.
Introduction
Individual choices underlie numerous chronic illnesses and disorders. For instance, decision-making characterized by a preference for smaller but immediately available rewards relative to larger, delayed rewards has been found to be associated with health-compromising behaviors, such as consuming more caloric and unhealthy foods while foregoing exercise [1]. In this case, the reward of eating fattening food is immediate, while the pay-off of good health and weight maintenance associated with avoiding unhealthy foods and exercising are largely delayed. Over time, the cumulative influence of these individual choices can lead to chronic health problems [2]. Indeed, individuals who steeply devalue outcomes that are in the future, known as delay discounting, are more likely to be obese and engage in substances misuse [3, 4]. Higher rates of delay discounting (i.e., greater devaluation of future outcomes), in combination with highly appetitive reinforcers (such as unhealthy foods and illicit drugs), are hypothesized to be central to the development of addictive behavior disorders [5].
Delay discounting may also help partially explain the noted disparities in health outcomes that result from factors including poverty and geographic access to care [6, 7]. Specifically, medically underserved areas characterized by lack of resources and high levels of instability may reinforce preference for immediately available rewards relative to larger, delayed rewards. For instance, a number of studies have found that lower education, income, and associated demographic factors are associated with greater rates of discounting [8–11]. The preference for the immediate option in contexts characterized by resource scarcity and environmental instability may constitute an adaptive response to contextual circumstances evolutionarily associated with a threat to survival. Following the example above, individuals who face food scarcity may overconsume high-caloric foods when it is accessible. While individual decisions to overconsume in this context may be adaptive, the cumulative effect of these repeated decisions may carry detrimental consequences in the long term. Thus, the demonstrated direct links between resource scarcity, stress, and delay discounting [11, 12] suggest that delay discounting may be a critical mechanism linking resource-poor environments to a variety of negative health behaviors and subsequent outcomes.
As delay discounting has been deemed a unifying risk factor that is common across diseases, including behavioral addictions, substance use disorders, poor health practices, neurobehavioral developmental disorders, and comorbidities [13], targeting this mechanism of transdisease processes has the potential to be effective in preventing a variety of negative health outcomes when broadly disseminated across at-risk communities. Moreover, health risk behaviors, such as eating highly caloric foods and smoking, often occur in “clusters” [14, 15], yet only a limited number of studies have demonstrated efficacy in reducing multiple health-risk behaviors, and this research has been largely limited by its reliance on affluent populations [16]. Thus, decreasing rates of delay discounting is a worthy target of intervention, specifically among adults in underserved communities.
Efficacy of Targeting Working Memory to Decrease Delay Discounting
Working memory is commonly described as the capacity to hold information while engaging in complex mental tasks, including reasoning, comprehension, and learning [17]. Working memory appears to be essential to cognitive control and guidance of decision-making [18–20] that appear to be relevant for delay discounting, and is proximally linked to rates of delay discounting. For instance, results from human [21–23] and animal models [24] suggest significant associations between rates of delay discounting and specific cognitive functions related to working memory. Damage to the brain areas associated with working memory function has also been shown to increase humans’ preference for immediate gratification [25]. This is supported by extant meta-analytic data on brain functionality, which shows that working memory and delay discounting share unique functions in the left lateral prefrontal cortex [26]. Additionally, behavioral research suggests that delay discounting and working memory are negatively correlated (see, e.g., [22]) and that imposing a working memory load during a delay discounting task heightens individuals’ preference for smaller and sooner rewards relative to larger, later rewards [27]. One reason that delay discounting and working memory are associated may stem from having to face decisions of “now” or “later” while manipulating reward values and time frames, all of which need to occur simultaneously.
Emerging research suggests the utility of targeting working memory to reduce delay discounting [25, 28–30]. Bickel et al. [28] examined the efficacy of a computer-based intervention that targeted working memory to decrease delay discounting. In this study, participants meeting criteria for a substance use disorder were administered either a computerized working memory intervention or a control training program. Results indicated that the active training condition significantly decreased delay discounting relative to the control treatment, with a post-training association between working memory and delay discounting. A modest sample size precluded an analysis of improved working memory as a mechanism of decreased delay discounting. However, the question of whether improvement in working memory results in reductions in delay discounting remains unanswered.
The Current Study
The current study attempts to address several of the limitations in the previous literature by examining whether a treatment designed to target working memory can, in turn, decrease delay discounting in adults from medically underserved areas. Participants received either a computer-based working memory training program or a matched control training program. Participants completed pretraining and post-training assessments of cognitive function and delay discounting. Hypotheses included: (a) participants in the active treatment would demonstrate increases in working memory, (b) these increases would predict decreases in delay discounting following the intervention, and (c) these changes in working memory would mediate the relation between computer training and postintervention delay discounting.
Methods and Materials
Participants and Procedures
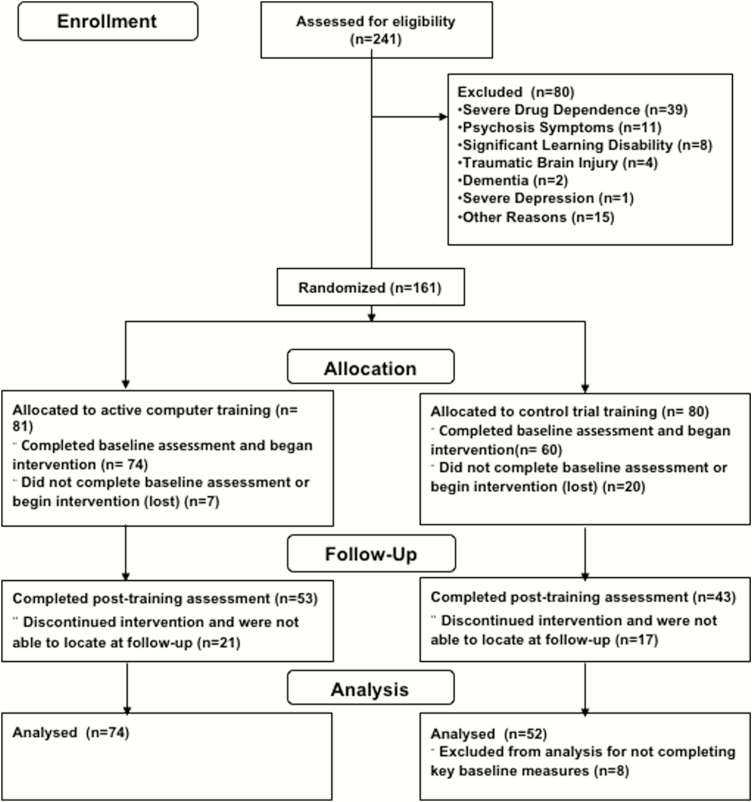
All procedures were approved by the Institutional Review Board of the University of Maryland and meet the standards set forth by relevant national and institutional guidelines (ClinicalTrials.gov number NCT03501706). Participants were recruited from a community center in a medically underserved area of Baltimore, Maryland, as defined by the Health Resources and Services Administration. The community center offers over 20 public health programs that range from crisis services to prevention services and approximately 60% of the individuals using the community outreach center are homeless. Interested individuals provided informed consent and took part in an initial screening to determine whether they met the inclusion criteria, including (a) being able to read at a 5th grade reading level and (b) not meeting criteria for current substance use disorders or having severe memory problems, psychotic symptoms, or suicidal ideations. Additionally, the study targeted participants in their midlife (40–60-years old), because this is a time in which the delayed consequences of unhealthy behaviors typically manifest and intertemporal decision-making trade-offs could have significant impact. The final sample included 126 individuals in the active (n = 74) or control (n = 52) conditions (see Fig. 1). Of those participants, 96 individuals also completed measures at postintervention into the active (n = 53) or control (n = 43) condition. Demographic information for participants is reported by treatment condition in Table 1.
Fig. 1.
Consolidated Standards of Reporting Trials diagram, depicting participant enrollment and completion of the clinical trial.
Table 1.
Demographics by treatment group
| Construct | Active condition |
Control condition |
|---|---|---|
| Sex (male) | 40.5% | 48.7% |
| Mean age (SD) | 50.0 (5.8) | 51.2 (5.7) |
| Race/ethnicity | ||
| White | 20.3% | 17.9% |
| Black/African American | 75.9% | 79.5% |
| Native American/American Indian | 2.5% | 0% |
| Other | 1.3% | 2.6% |
| Highest level of education | ||
| Less than high school degree/General Educational Development exam | 13.9% | 35.9% |
| High school degree/General Educational Development exam | 65.8% | 48.7% |
| Associates degree or trade school | 10.1% | 2.6% |
| Bachelors or advanced degree | 5.1% | 10.3% |
| Current employment status | ||
| Unemployed | 35.4% | 32.1% |
| Part-time | 10.1% | 9.0% |
| Full-time | 6.3% | 2.6% |
| Unable to work | 38.0% | 52.6% |
| Student/other | 9.0% | 3.7% |
| Current annual household income | ||
| $0–9,999 | 75.9% | 74.4% |
| $10,000–19,999 | 12.7% | 12.8% |
| $20,000–29,999 | 3.8% | 1.3% |
| $30,000+ | 5.2% | 1.3% |
| Don’t know/refused to answer | 2.5% | 6.4% |
SD standard deviation.
Participants received gift cards for completing measurements immediately before and after training. Participants were also awarded $2.50 in gift cards for attendance and $2.50 for improvement on each module (see below), at each training session. Thus, payment ranged from $2.50 (attended session but no improvements in scores) to $12.50 (attended session and improved score on all four training modules). A pseudorandomization process was used to control for incentive levels in the active and control conditions. Participants were initially assigned only to the active treatment condition in order to record incentive schedules for participants who completed training (defined as finishing 15 sessions). Subsequent participants were then randomized to either the active or control condition. Participants in the control condition were randomly matched to a participant in the active condition who had completed training and received payment based on their matched counterpart’s compensation schedule. Participants in the control condition were informed that they would receive a “surprise” amount of payment for each session that they completed.
Measures
Demographics
Participants were asked to self-report their age, biological sex, race/ethnicity, educational attainment and annual income. Income was then dummy coded: (a) $0–9,999; (b) $10,000–19,999; (c) $20,000–29,999; (d) $30,000–39,999; (e) $40,000–49,999; and (f) $50,000–59,999.
Assessments of working memory and related constructs
Three measures of cognitive function were used:
The Tower of Hanoi (TOH; [31]) assesses the working memory and planning components of cognitive function. This task involves the participant moving five circular disks between three pegs in accordance with a number of rules. Participants are asked to recreate an increasingly difficult arrangement of discs without violating any of these rules. In the current analyses, we used the total achievement score, with higher scores indicating greater cognitive function skills [32].
The Hopkins Verbal Learning Test—Revised (HVLT-R; [33]) is a standardized test of verbal learning and memory. As part of the task, the participant is read a list of 12 words, three times, and asked to recall as many words as possible. In the current study, the Total Recall Score was used, which is a scaled sum of correct words recalled across all three trials [33].
Letter Number Sequencing (LNS; [34]) is a measure of working memory. Participants are read increasingly long combinations of mixed letters and numbers and asked to reorder them. The total scaled score was used in all analyses, reflecting the overall performance on the task [34].
Delay discounting
A computerized binary choice task was used to assess delay discounting. Participants were presented with a series of trials in which they were asked to make a choice between an amount of money available immediately and larger amount of money available after a specified delay (1 day, 1 week, 1 month, 6 months, 1 year, 5 years, 25 years). A computerized algorithm [35] adjusted the immediately available reward across seven trials to determine an indifference point for each amount/delay pairing, resulting in seven indifference points (corresponding to each delay). Indifference points were then used to calculate rate of delay discounting (k) for both the $50 and $1,000 “larger later” sum. Because k values are skewed, they were transformed using a natural log function. Previous research has established the validity and reliability of discounting tasks for hypothetical rewards [36].
Intervention Conditions
The active training program consisted of 15 sessions in which participants completed four modules during each session. The order in which the modules were completed was counterbalanced across sessions. The four modules used are commercially available (PSSCogReHab, Psychological Software Services) and have been shown to improve working memory in a similar experiment [28]. The intervention included:
Sequence Recall of Digits—Auditory: Participants were read a series of increasingly long strings of numbers and asked to memorize and recall them immediately after. Five incorrect attempts ended the module.
Sequence Recall of Reversed Digits—Auditory: In this module, participants were again read a string of numbers but asked to recall the series in the reverse order. The module was terminated after five incorrect responses.
Sequenced Recall of Words—Visual: Participants were presented with an increasing longer list of four-letter words to memorize and asked to recognize these words from a larger list after a 3 s delay. The module ended after five incorrect attempts.
Verbal Memory—Categorizing: Participants were given a word bank containing 20 words, each of which fell into one of four categories listed in boxes above the word bank. Participants were instructed to properly categorize each word and then identify these words from a larger list. The module was terminated following five incorrect answers.
The control training condition was designed to utilize the same essential features of the active training, including the stimulus, response, and feedback, without engaging working memory. In this condition, participants completed the same tasks but the correct answers to items were presented visually on the screen within the task and participants were told to select the indicated item so that they would not have to engage their working memory.
Sessions were scheduled to occur 3 days per week and were completed between 5 and 7 weeks. If participants did not complete the training by Week 7, they were no longer able to receive the training. Each training session had an estimated duration of 30 min.
Data Analytic Plan
First, chi-square and t-tests were conducted to examine mean-level differences among key variables by treatment condition. Next, correlational analyses were conducted to examine relations among key study variables. In order to examine the indirect effects of training on delay discounting, via changes in executive function, a series of structural equation models was tested using Mplus 6.0 [37]. Mplus utilizes full information maximum likelihood (ML) methods to estimate missing data, which are robust to nonnormally distributed observations and appropriate for use with continuous and binary variables under missing completely at random and missing at random assumptions. ML also provides less biased parameter estimates compared to listwise or pairwise deletion [38]. Thus, we were able to conduct all analyses on the full sample of 126 participants. Four fit indices were used to determine model fit: the χ2 statistic, the comparative fit index (CFI; [39]), the root mean square error of approximation (RMSEA; [40]), and the standardized root mean square residual (SRMR). Nonsignificant chi-square values indicate good fit; however, this index is sensitive to sample size [41]. CFI values greater than .90, RMSEA values less than .08, and SRMR values under 0.10 suggest acceptable fit [42].
A latent executive function factor composed of individual scaled scores on the TOH, HVLT, and LNS at pretreatment and post-treatment was created. In line with recommendations by Cole et al. [43], cross-wave correlations were allowed between error terms for preintervention and postintervention measures. The first model examined whether treatment predicted increases in working memory. Post-treatment working memory factor was regressed onto the intervention condition and pretreatment levels of working memory. Given noted relations between performance on cognitive function measures and biological sex [44, 45], as well as previous support for dosage effects (as indexed by attendance) on delay discounting outcomes [28], participant sex and number of sessions attended were included as covariates in all models. Next, the indirect effects of treatment condition on delay discounting at both $50 and $1,000 values were examined in two separate but parallel models. Post-treatment delay discounting was regressed onto post-treatment working memory and treatment condition, controlling for pretreatment delay discounting (at either the $50 or $1,000 level). The significance of each indirect effect was determined by estimating its confidence interval (CI), using the nonparametric bootstrapping procedure recommended by Preacher and Hayes [46]. Unlike hypothesis testing based on parametric statistics (such as the Sobel test), bootstrapping procedures do not assume that estimates of the indirect effect are normally distributed [46]. An indirect effect with a confidence interval that excludes 0 is statistically significant.
Results
Preliminary Analyses
First, chi-square and t-tests were conducted to examine differences between active and control training groups on participants’ sex, age, race/ethnicity, educational attainment, and annual income. No significant differences were found between treatment conditions. Means, standard deviations, and correlations between all study variables are reported in Table 2. Skew and kurtosis of each outcome variable was also examined and found to be within an acceptable range (≤3.0).
Table 2.
Correlations, means (M), and standard deviations (SD) of key constructs by treatment condition
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex (female) | 1.00 | −.10a | −.08a | −.13a | .01a | −.10a | −.05a | −.12a | .24a | −.09a | −.09a | .25a | .03a | .20a |
| 2. Age | .20a | 1.00 | .25* | .08 | −.06 | −.01 | .09 | −.05 | −.02 | −.14 | −.13 | <.01 | −.03 | −.09 |
| 3. Income | −.06a | −.07 | 1.00 | .23 | .06 | −.01 | .10 | −.09 | −.01 | −.09 | −.07 | −.15 | −.13 | −.11 |
| 4. Attendance | −.01a | .30* | .11 | 1.00 | .11 | −.13 | .20 | .27 | −.27 | .14 | −.03 | −.20 | −.06 | −.43** |
| 5. T1 TOH | −.24* a | .07 | .13 | .18 | 1.00 | .51** | .37** | .43** | .12 | .21 | .06 | −.19 | −.01 | −.26 |
| 6. T2 TOH | −.30* a | .11 | <.01 | .11 | .61** | 1.00 | .19 | .17 | .25 | .33* | .32* | −.15 | .17 | −.23 |
| 7. T1 HVLT-R | .05a | .12 | .13 | −.12 | .24* | .22 | 1.00 | .68** | .22 | .20 | −.15 | −.34* | −.34* | −.57** |
| 8. T2 HVLT-R | .24a | .09 | .10 | .11 | .10 | .09 | .47** | 1.00 | .23 | .49** | .14 | −.09 | −.04 | −.38* |
| 9. T1 LNS | .09a | .14 | .06 | .06 | .39** | .37** | .39** | .44** | 1.00 | .55** | .14 | .30 | .11 | .08 |
| 10. T2 LNS | .06a | .03 | −.41 | .01 | .30* | .16 | .28* | .14 | .37** | 1.00 | .49** | .09 | .18 | −.26 |
| 11. T1 DD ($50) | .09a | .07 | −.20 | −.06 | −.02 | .07 | −.02 | .06 | .22 | .08 | 1.00 | .31 | .72** | .15 |
| 12. T2 DD ($50) | .25a | .01 | .36** | −.02 | .06 | −.15 | −.07 | .21 | .11 | −.14 | .27 | 1.00 | .55** | .78** |
| 13. T1 DD ($1,000) | .13a | .05 | −.14 | .08 | −.18 | .10 | −.17 | .91 | .12 | .08 | .66** | .32* | 1.00 | .49* |
| 14. T2 DD ($1,000) | .23a | .04 | .12 | −.06 | −.06 | <.01 | −.06 | .17 | .10 | −.02 | .41** | .68** | .58** | 1.00 |
| M (SD) Active Condition | 0.59 (0.49) | 50.04 (5.82) | 1.37 (0.91) | 10.00 (5.53) | 6.99 (3.55) | 9.02 (3.67) | 35.19 (12.49) | 39.77 (11.34) | 7.07 (2.64) | 7.19 (2.39) | −3.46 (2.75) | −3.65 (2.81) | −4.36 (3.79) | −4.73 (3.06) |
| M (SD) Control Condition | 0.51 (0.50) | 51.24 (5.72) | 1.21 (0.54) | 9.02 (6.09) | 7.32 (3.53) | 7.24 (3.71) | 35.31 (10.76) | 37.88 (9.56) | 6.74 (2.38) | 6.29 (2.83) | −4.19 (3.36) | −4.19 (3.16) | −4.31 (4.09) | −4.31 (3.92) |
Correlations among study variables for the control condition are listed in the top right (above the diagonal) and correlations for the active condition are listed in the bottom left (below the diagonal). Sex is coded 0 = female, 1 = male. T1 = pretraining, T2 = post-training.
DD delay discounting; HVLT-R Hopkins Verbal Learning Test—Revised; LNS Letter Number Sequencing; TOH Tower of Hanoi.
aPoint-biserial correlation coefficients; all other correlations are Pearson product-moment correlation coefficients.
*p < .05; **p < .01.
Treatment Condition Predicting Changes in Working Memory
Initial models examined whether treatment condition predicted changes in a latent working memory factor. The model fit the data well: χ2 (20) = 24.81; p = .209; CFI = .96; RMSEA = .04 (90% CI = .00 to .09); SRMR = .06. Consistent with hypothesis 1, results suggest a significant effect of treatment on changes in working memory (standardized estimate = .36; p = .006). Specifically, the active treatment condition predicted increases in working memory.
Predicting Changes in Discounting in the $50 Condition
Next, a model examining a post-treatment latent working memory factor as a mediator between treatment and follow-up delay discounting at the $50 level was evaluated. The model provided an adequate fit to the data: χ2 (34) = 53.03; p = .020; CFI = .87; RMSEA = .07 (90% CI = .03 to .10); SRMR = .08. Results indicate that treatment predicted changes in working memory (std. est. = .33; p = .007), suggesting that gains in working memory were largest for participants in the active condition. There was not a significant direct effect of treatment on changes in discounting postintervention (standardized estimate = .12; p = .326), nor did changes in working memory predict decreases in discounting (standardized estimate = −.20; p = .334). Moreover, inconsistent with hypothesis 2, the indirect effect of treatment on discounting was not significant (indirect effect = −.06; standard error (SE) = .08; 95% CI = −.21 to .08).
Predicting Changes in Discounting in the $1,000 Condition
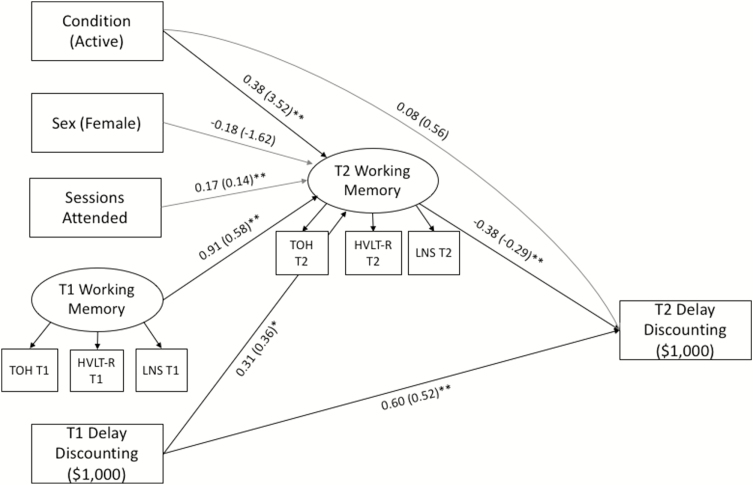
The final model (see Fig. 2) examined the indirect effect of treatment on delay discounting at the $1,000 level via changes in working memory. This model also provided an adequate fit to the data χ2 (34) = 46.54; p = .074; CFI = .93; RMSEA = .05 (90% CI = .00 to .09); SRMR = .08. Both treatment (standardized estimate = .38; p = .001) and baseline discounting (standardized estimate = .31; p = .014) predicted change in working memory, indicating that participants in the active trial and those who evidenced greater discounting at baseline demonstrated larger increases in working memory post-treatment. Further treatment did not have a direct effect on discounting (standardized estimate = .08; p = .460). Conversely, post-treatment working memory predicted changes in discounting (standardized estimate = −.38; p = .004), indicating that greater increases in working memory were associated with steeper decreases in discounting. In support of hypothesis 3, the indirect effect of treatment on discounting via changes in working memory was significant (indirect effect = −.15, SE = .07, 95% CI = −.292 to −.001).
Fig. 2.
Significant standardized (and unstandardized) path estimates for final mediation model. TOH = Tower of Hanoi; HVLT-R = Hopkins Verbal Learning Test—Revised; LNS = Letter Number Sequencing. *p < .05; **p < .01.
Discussion
Overall, the study’s main findings support our hypotheses: active treatment led to improvements in working memory, which, in turn, decreased rate of delay discounting. Additionally, attendance was found to be similar across conditions, which suggested that there were no significant differential attrition effects. The current study also demonstrates the feasibility of implementing this promising intervention in real-world community settings which provides services to individuals from medically underserved areas.
The study has several noteworthy implications. Although delay discounting shows relative stability over time [47, 48], this study suggests that changes in delay discounting are observed among individuals who show improvements in working memory after receiving a computerized training. The study findings are encouraging given that treatment research has demonstrated that individuals with lower rates of delay discounting have better treatment outcomes for weight loss and substance use problems [49–53]. The present findings suggest the potential of a computerized working memory-training intervention as a beneficial adjunct to empirically supported treatments for the variety of health behaviors. With respect to the extant literature, these findings are consistent with another published study that also failed to find a main effect of cognitive treatment on rates of discounting [54]; however, inconsistent with the current results, the other study also did not find a direct effect of cognitive training on rates of discounting. It is important to note that that study was composed of high-functioning young adults (85% some college or college graduate, mean Shipley IQ score = 110.7), which might have resulted in a functional floor effect on reductions in delay discounting. Furthermore, that study did not report on the indirect pathway via the relation between delay discounting and improved working memory.
A noteworthy finding of the present study is that improvements in working memory were directly related to decreases in rates of discounting. Given that delay discounting has been deemed a trans-disease process [13], working memory training constitutes a novel intervention strategy that could be directed at strengthening self-control through delay discounting and applied to chronic conditions such as obesity [46, 55], addiction [3], and serious mental illness [56]. As efforts to train working memory can be computer-delivered, this approach may be particularly well-suited for individuals from geographic regions with limited access to care, as computerized interventions do not require the significant resources associated with interventions that require trained therapists or private therapy spaces. Thus, computer-based approaches may hold particular promise for widespread dissemination and implementation efforts to reduce delay discounting in medically underserved communities.
Research in this area complements the established literature supporting life history perspective, which would hypothesize that children who grow up in stressful and unstable environments subsequently develop adaptive improvements on some behavioral tendencies that manifest later in life. For example, childhood adversity has been associated with an enhanced ability to effectively alternate between one task and another (i.e., set-shifting) in adulthood under specific conditions [57]. A synthesis of these overlapping literatures would suggest that adaptations in delay discounting result from demands imposed by stressful environments and that this adaptivity may prove beneficial in specific environments and maladaptive in others. As steep delay discounting conceptualized in the present study refers to generalized sooner/smaller responding, it may be detrimental in the long term and contribute to the maintenance of unhealthy behaviors [5] in this population.
The finding that changes in working memory mediated the relation between computer training and delay discounting for the $1,000 magnitude was consistent with the third hypothesis, yet the mediation effect was not significant for the $50 hypothetical value. Although the reason for this discrepancy remains unclear, accumulating evidence suggests that small magnitude reward conditions may be less able to detect within- or between-group differences [58] that are revealed in larger magnitude conditions. This has resulted in the explicit warning [58] regarding reliance on a single, small-magnitude condition. Though the mechanism for this issue remained undetermined, one possibility is that individuals tend to be more attentive to discounting in the $1,000 magnitude, as it is viewed as an important or impactful decision. Lower discounting with greater magnitudes is in accord with laboratory studies of risk taking in which individuals tend to adopt a risk-averse strategy to their decision-making in the face of larger rewards [59]. Furthermore, the significant outcomes exclusive to the $1,000 magnitude could also reflect that interventions are more likely to make a difference when individuals view that their decision-making could have impactful outcomes.
This is the first mechanistic study to assess the efficacy of a computerized training program to improve delay discounting by targeting working memory. This study replicates the positive findings from a randomized controlled trial that evaluated whether neurocognitive intervention resulted in delay discounting decreases among individuals who met criteria for stimulant dependence [28]. It also extends this research significantly by isolating working memory as an important mechanism of treatment change that should be targeted specifically in training interventions. In fact, these results support findings from neuroimaging investigations that have noted a significant overlap in patterns of brain activation when individuals perform delay discounting and executive function tasks (e.g., [26]). A future line of research inquiry would be to examine brain activation patterns among individuals preworking and postworking memory computer training with the goal of elucidating the brain areas that show change postintervention in addition to identifying useful neuromarkers that confer greater chance for a positive training outcome. Additionally, in accordance to the competing neurobehavioral decision systems theory, neuroimaging would allow the examination of how the impulsive and executive systems (e.g., working memory) operate preworking and postworking memory training [13].
A strength of the current research lies in the sample: participants from a medically underserved area. These individuals are particularly at-risk for chronic health conditions as well as high levels of delay discounting and consequent detrimental decision-making for delayed outcomes. In fact, research has found that exposure to low-income and unstable environments are associated with steeper delay discounting [60]. Additionally, investigations have also noted a positive correlation between cognitive function and socioeconomic status beginning in childhood [61] and across the lifespan [9, 62].
The study has important limitations and avenues for future research. First, the current research did not include specific health outcome measures due to the study’s focus on impacting mechanisms of disease processes. Despite the well-established relations between rates of delay discounting and chronic health conditions, it will be important to evaluate whether the improvement in working memory that led to changes in delay discounting ultimately have a positive impact on health-relevant behaviors. Second, the study was conducted in only one urban location with a relatively small sample size. Future research is needed to replicate these findings across other medically underserved communities. Third, while participants in the control condition received a training that mirrored active training in motor output and visual and auditory stimuli, it is possible that the monetary incentive participants in the active condition received for improved training performance resulted in important differences in motivation between the two groups. In other words, it may be that an unintended consequence of reinforcing participants’ motivation to engage with the working memory training resulted in differences in levels of effort between treatment groups. Future research should work to disentangle improvements in working memory from psychological factors like motivation. Lastly, it is unclear whether treatment effects will sustain after the intervention period. Longitudinal studies examining the long-term health effects of impacting working memory and consequent delay discounting are needed.
Taken together, the current findings complement the extant research on the benefits derived from implementing computerized training programs designed to strengthen cognitive functions. This is the first study to undertake the examination of the mechanism through which the observed changes in delay discounting occur. In sum, the study findings provide strong evidence that a computerized working memory training program shows promise in decreasing levels of delay discounting among those who exhibit changes in working memory and that the program is efficacious among adults from medically underserved areas. Thus, this intervention suggests particular promise to address a critical trans-disease process that has implications for impacting a range of chronic health behaviors and conditions.
Acknowledgements
Funding This project was funded by grants from the National Institutes of Health (R01 AG048904, R56 AG048904) awarded to R. Yi. NIH did not participate in the design, collection, analysis, or interpretation of the data. This content is the responsibility of the authors and does not represent the views of NIH.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Julia W. Felton, Anahi Collado, Katherine M. Ingram, Kelly Doran, and Richard Yi declare that they have no conflict of interest.
Authors’ Contributions RY conceived and designed the study and oversaw the data collection. JF analyzed the data. All authors contributed to collecting the data and drafting the manuscript.
Ethical approval All procedures, including informed consent processes, were performed in accordance to the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments.
References
- 1. Sweeney AM, Culcea I. Does a future-oriented temporal perspective relate to body mass index, eating, and exercise? A meta-analysis. Appetite. 2017;112:272–285. [DOI] [PubMed] [Google Scholar]
- 2. Cardi M, Munk N, Zanjani F, Kruger T, Schaie KW, Willis SL. Health behavior risk factors across age as predictors of cardiovascular disease diagnosis. J Aging Health. 2009;21:759–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction. 2017;112:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. [DOI] [PubMed] [Google Scholar]
- 5. Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: Reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown TM, Parmar G, Durant RW, et al. Health professional shortage areas, insurance status, and cardiovascular disease prevention in the reasons for geographic and racial differences in stroke (REGARDS) study. J Health Care Poor Underserved. 2011;22:1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rereddy SK, Jordan DR, Moore CE. Dying to be screened: Exploring the unequal burden of head and neck cancer in health provider shortage areas. J Cancer Educ. 2015;30:490–496. [DOI] [PubMed] [Google Scholar]
- 8. de Wit H, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Pers Individ Dif. 2007; 42(1):111–121. [Google Scholar]
- 9. Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: The role of age and income. Psychol Aging. 1996;11:79–84. [DOI] [PubMed] [Google Scholar]
- 10. Harrison GW, Lau MI, Williams MB. Estimating individual discount rates in Denmark: A field experiment. Am Econ Rev. 2002;92(5):1606–1617. [Google Scholar]
- 11. Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Pers and Individ Dif. 2009;47(8):973–978. [Google Scholar]
- 12. Bickel WK, Wilson AG, Chen C, Koffarnus MN, Franck CT. Stuck in time: Negative income shock constricts the temporal window of valuation spanning the future and the past. PLoS One. 2016;11:e0163051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacol Ther. 2012;134:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuit AJ, van Loon AJ, Tijhuis M, Ocké M. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35:219–224. [DOI] [PubMed] [Google Scholar]
- 15. Linardakis M, Smpokos E, Papadaki A, Komninos ID, Tzanakis N, Philalithis A. Prevalence of multiple behavioral risk factors for chronic diseases in adults aged 50+, from eleven European countries - the SHARE study (2004). Prev Med. 2013;57:168–172. [DOI] [PubMed] [Google Scholar]
- 16. King K, Meader N, Wright K, et al. Characteristics of interventions targeting multiple lifestyle risk behaviours in adult populations: A systematic scoping review. PLoS One. 2015;10:e0117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baddeley A. Working memory. Curr Biol. 2010;20:R136–R140. [DOI] [PubMed] [Google Scholar]
- 18. Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol Learn Motiv. 2004;44:145–200. [Google Scholar]
- 19. Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychol Bull. 2004;130:553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redick TS. Cognitive control in context: Working memory capacity and proactive control. Acta Psychol (Amst). 2014;145:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Basile AG, Toplak ME. Four converging measures of temporal discounting and their relationships with intelligence, executive functions, thinking dispositions, and behavioral outcomes. Front Psychol. 2015;6:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shamosh NA, Deyoung CG, Green AE, et al. Individual differences in delay discounting: Relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. [DOI] [PubMed] [Google Scholar]
- 23. Weatherly JN, Ferraro FR. Executive functioning and delay discounting of four different outcomes in university students. Pers Individ Diff. 2011;51(2):183–187. [Google Scholar]
- 24. Hernandez CM, Vetere LM, Orsini CA, et al. Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 × brown Norway hybrid rats. Neurobiol Aging. 2017;60:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. [DOI] [PubMed] [Google Scholar]
- 26. Wesley MJ, Bickel WK. Remember the future II: Meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. [DOI] [PubMed] [Google Scholar]
- 28. Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bogdanova Y, Yee MK, Ho VT, Cicerone KD. Computerized cognitive rehabilitation of attention and executive function in acquired brain injury: A systematic review. J Head Trauma Rehabil. 2016;31:419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu HL, Chu H, Tsai JC, et al. The effect of cognitive-based training for the healthy older people: A meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delis DC, Kaplan E, Kramer JH.. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 32. Delis DC, Kaplan E, Kramer JH.. Delis-Kaplan Executive Function System: Technical Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 33. Brandt J, Benedict RHB.. The Hopkins Verbal Learning Test—Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 34. Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. 3rd ed.San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 35. Du W, Green L, Myerson J. Cross-cultural comparisons of discounting delayed and probabilistic rewards. Psychol Rec. 2002;52:479–492. [Google Scholar]
- 36. Matusiewicz AK, Carter AE, Landes RD, Yi R. Statistical equivalence and test-retest reliability of delay and probability discounting using real and hypothetical rewards. Behav Processes. 2013;100:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muthén LK, Muthén BO.. Mplus: Statistical Analysis with Latent Variables: User’s Guide. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 38. Little RJA, Rubin DB.. Statistical Analysis with Missing Data. New York, NY: John Wiley; 1987. [Google Scholar]
- 39. Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. [DOI] [PubMed] [Google Scholar]
- 40. Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behav Res. 1990;25:173–180. [DOI] [PubMed] [Google Scholar]
- 41. Kline RB. Principles and Practice of Structural Equation Modelling. 2nd ed.London, UK: The Guilford Press; 2005. [Google Scholar]
- 42. Schweizer K. Some guidelines concerning the modeling of traits and abilities in test construction. Eur J Psychol Assess. 2010;26(1):1–2. [Google Scholar]
- 43. Cole DA, Ciesla JA, Steiger JH. The insidious effects of failing to include design-driven correlated residuals in latent-variable covariance structure analysis. Psychol Methods. 2007;12:381–398. [DOI] [PubMed] [Google Scholar]
- 44. Kalkut EL, Han SD, Lansing AE, Holdnack JA, Delis DC. Development of set-shifting ability from late childhood through early adulthood. Arch Clin Neuropsychol. 2009;24:565–574. [DOI] [PubMed] [Google Scholar]
- 45. Zilles D, Lewandowski M, Vieker H, et al. Gender differences in verbal and visuospatial working memory performance and networks. Neuropsychobiology. 2016;73:52–63. [DOI] [PubMed] [Google Scholar]
- 46. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 47. Kirby KN. One-year temporal stability of delay-discount rates. Psychon Bull Rev. 2009;16:457–462. [DOI] [PubMed] [Google Scholar]
- 48. Odum AL. Delay discounting: Trait variable? Behav Processes. 2011;87:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Best JR, Theim KR, Gredysa DM, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheffer C, Mackillop J, McGeary J, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Washio Y, Higgins ST, Heil SH, et al. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2011;19:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15:176–186. [DOI] [PubMed] [Google Scholar]
- 54. Kable JW, Caulfield MK, Falcone M, et al. No effect of commercial cognitive training on brain activity, choice behavior, or cognitive performance. J Neurosci. 2017;37:7390–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, Schmidt U. A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neurosci Biobehav Rev. 2016;71:506–528. [DOI] [PubMed] [Google Scholar]
- 56. Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mittal C, Griskevicius V, Simpson JA, Sung S, Young ES. Cognitive adaptations to stressful environments: When childhood adversity enhances adult executive function. J Pers Soc Psychol. 2015;109:604–621. [DOI] [PubMed] [Google Scholar]
- 58. Mellis AM, Woodford AE, Stein JS, Bickel WK. A second type of magnitude effect: Reinforcer magnitude differentiates delay discounting between substance users and controls. J Exp Anal Behav. 2017;107:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). J Exp Psychol Appl. 2002;8:75–84. [DOI] [PubMed] [Google Scholar]
- 60. Jachimowicz JM, Chafik S, Munrat S, Prabhu JC, Weber EU. Community trust reduces myopic decisions of low-income individuals. Proc Natl Acad Sci USA. 2014;114:5401–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Richards M, Wadsworth ME. Long term effects of early adversity on cognitive function. Arch Dis Child. 2004;89:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]