Abstract
Prenatal testosterone (T)-treated female sheep display reproductive deficits similar to women with polycystic ovarian syndrome (PCOS), including an increase in LH pulse frequency due to actions of the central GnRH pulse generator. In this study, we used multiple-label immunocytochemistry to investigate the possibility of changes in the γ-aminobutyric acid (GABA) neurotransmitter system at two key components of the GnRH pulse generator in prenatal T-treated sheep: kisspeptin/neurokinin B/dynorphin (KNDy) neurons of the arcuate nucleus, and GnRH neurons in the preoptic area (POA) and mediobasal hypothalamus (MBH). We observed a significant decrease and increase, respectively, in the number of GABAergic synapses onto POA and MBH GnRH neurons in prenatal T-treated ewes; additionally, there was a significant increase in the number of GABAergic inputs onto KNDy neurons. To determine the actions of GABA on GnRH and KNDy neurons, we examined colocalization with the chloride transporters NKCC1 and KCC2, which indicate stimulatory or inhibitory activation of neurons by GABA, respectively. Most GnRH neurons in both POA and MBH colocalized NKCC1 cotransporter whereas none contained the KCC2 cotransporter. Most KNDy neurons colocalized either NKCC1 or KCC2, and 28% of the KNDy population contained NKCC1 alone. Therefore, we suggest that, as in the mouse, GABA in the sheep is stimulatory to GnRH neurons, as well as to a subset of KNDy neurons. Increased numbers of stimulatory GABAergic inputs to both MBH GnRH and KNDy neurons in prenatal T-treated animals may contribute to alterations in steroid feedback control and increased GnRH/LH pulse frequency seen in this animal model of PCOS.
Polycystic ovarian syndrome (PCOS) is the leading cause of infertility in women of reproductive age (1). PCOS is characterized by polycystic ovaries, anovulation, hyperandrogenism, hyperinsulemia, and central obesity (2). Reproductive deficits associated with PCOS include impaired steroid hormone feedback to the hypothalamic–pituitary–gonadal axis, with an increase in LH frequency, indicative of abnormal steroid hormone feedback to GnRH neurons (3), resulting in the downstream consequences of the syndrome. Although the symptoms of PCOS manifest in adolescence, the neuroendocrine deficits may originate in utero. Prenatal testosterone (T)-treated female sheep that are treated on days 30 to 90 of gestation show both reproductive and metabolic symptoms (4) that mimic the deficits observed in human women, including anovulation, polycystic ovaries, and increased pulsatile release of LH due to impaired steroid hormone feedback (5, 6). Studies done using the sheep model of PCOS have shown impaired progesterone and 17β-estradiol (E2) negative and positive feedback (6, 7). In addition to the sheep model, other animal models, including monkeys (4), rats (8), and mice (9, 10), have shown altered sensitivity to steroid hormone feedback and reproductive abnormalities when exposed to prenatal T or DHT.
GnRH neurons represent the final output in the neuronal network controlling fertility, as they control the secretion of the gonadotropins LH and FSH from the anterior pituitary in mammals (11). GnRH release influences the pulse amplitude and frequency of LH and FSH secretion, resulting in follicular development, gonadal steroid synthesis, and ovulation (12). Defects in the GnRH neurons or in the GnRH neuronal network result in defective puberty and fertility (11). Interestingly, GnRH neurons do not contain the subtypes of steroid hormone receptors required for feedback control of GnRH/LH secretion, and, therefore, this control is likely dependent on an afferent neuronal network leading to GnRH neurons (13–16).
Currently, compelling evidence suggests that kisspeptin neurons play a pivotal role in conveying the feedback effects of gonadal steroid hormones on GnRH secretion during puberty and the estrous cycle. Previous work done in the sheep has focused on a subset of kisspeptin neurons in the arcuate nucleus (ARC) that coexpress kisspeptin (stimulatory), neurokinin B (NKB; stimulatory), and dynorphin (inhibitory) peptides, termed “KNDy” neurons, which play a critical role in the steroid feedback regulation of GnRH neurons (17–19). A very high percentage of KNDy neurons contain estrogen receptor α, progesterone receptors, and androgen receptors (17, 20–22), consistent with the hypothesis that these neurons are a key target for gonadal steroid actions in the brain. Additionally, KNDy neurons also form reciprocal connections (KNDy–KNDy) that are thought to be responsible for the synchronous activity corresponding to GnRH/LH pulses (18, 19). These reciprocal connections and the presence of the synchronous activity of this arcuate neuronal population have led to the hypothesis that KNDy neurons constitute the “pulse generator” driving the pulsatile secretion of GnRH and LH (23, 24). Recent work, directly manipulating these cells by optogenetic stimulation and inhibition in mice, has provided strong confirmation of this hypothesis (25). KNDy neurons appear to also be prime targets of the effects of prenatal T; long-term effects of the prenatal T treatment in sheep include changes in KNDy peptide expression (decreases in NKB and dynorphin) (17), morphological changes (hypertrophy), and changes in synaptic connections within the KNDy–GnRH circuitry, including that of glutamatergic inputs onto both KNDy and GnRH neurons (26).
Recent work in a prenatally androgenized (PNA) mouse model of PCOS has also implicated changes in γ-aminobutyric acid (GABA)ergic signaling to GnRH neurons in the etiology of this disorder (10, 27). Specifically, PNA female mice showed an increase in the density of GABAergic contacts onto GnRH neurons in the preoptic area (POA) arising from progesterone receptor–containing cells in the ARC (27). Studies have shown that GABA excitation of GnRH neurons via the GABAA receptor is due to high internal chloride stores in GnRH neurons as a result of the presence of NKCC1 transporters, which accumulate chloride. Thus, upon exposure to GABA, the cell’s membrane potential will move toward the reversal potential as chloride ions leave the cell, resulting in depolarization. The opposite happens for neurons that contain KCC2 transporters that extrude chloride, resulting in a lower internal chloride store in the neurons: GABA hyperpolarizes them as the chloride enters the cell when the GABAA receptor opens (28). As GABA is stimulatory to GnRH neurons in the mouse (29), this suggests a mechanism by which enhanced GABA signaling at the level of GnRH neurons may drive the increased LH pulse frequency characteristic of PCOS.
Although the reproductive and metabolic consequences of prenatal T exposure in sheep have been well described, our understanding of the neural mechanisms responsible for neuroendocrine defects in this model is still largely incomplete. The prenatal T-treated (prenatal T) sheep has several advantages as a model of PCOS compared with prenatal T exposure models in other species. These include attributes that very closely mimic those seen in women with PCOS, including alterations in both reproductive and metabolic endpoints (6). Additionally, sheep have a similar developmental trajectory to humans with precocial development of the reproductive neuroendocrine system (6). Furthermore, there are significant differences among rodents, sheep, and primates in the functional organization of KNDy cells (30), making a comparative approach important for validating eventual translation to humans. Therefore, the goals of the current study were (i) to investigate whether GABAergic inputs to GnRH and KNDy neurons are altered in a prenatal T sheep model of PCOS, and (ii) to determine whether GnRH and arcuate kisspeptin neurons in the sheep express NKCC1 or KCC2 cotransporter as an indication of whether GABA has potentially stimulatory or inhibitory effects on those populations.
Materials and Methods
Animals
Prenatal T-treated females
Suffolk sheep were maintained outdoors at the Sheep Research Facility of the University of Michigan (Ann Arbor, MI) as described in our previous publication (26). Animals were maintained under natural photoperiods with a daily maintenance feeding and free access to water. To generate prenatal T sheep, pregnant Suffolk sheep were injected with 100 mg per injection of T propionate (catalog no. T1875; Sigma-Aldrich, St. Louis, MO) suspended in cottonseed oil (catalog no. C7767; Sigma-Aldrich) twice weekly from 30 to 90 days of gestation of a 147-day pregnancy. Controls were untreated, as previous work showed no effect of vehicle treatment on reproductive or metabolic attributes (31). Lambs were born in March and April and weaned at 10 weeks. Endocrine deficits were confirmed in these animals in previous publications (32) and included disruptions in the timing and magnitude of primary gonadotropin surges, luteal defects, and reduced responsiveness to progesterone negative feedback. As adults (21 months of age), and 3 to 4 weeks prior to tissue collection, animals (control, n = 8; prenatal T, n = 7) were ovariectomized and were treated subcutaneously continuously with a 1-cm-long E2 implant and for 11 to 12 days with two controlled internal drug release progesterone implants (CIDR; InterAG, Hamilton, Waikato, New Zealand) and then for 1 day with four 3-cm-long E2 implants to simulate ovarian steroid levels during the late follicular phase of the cycle. Eighteen hours after the E2 implants were inserted, animals were perfused.
Tissue collection
At the time of tissue collection, all ewes received two IV injections (at 10-minute intervals) of 25,000 U of heparin (catalog no. 504505; Fresenius Kabi USA, Lake Zurich, IL). Ewes were anesthetized IV with sodium pentobarbital (390 mg/mL/kg; Fatal Plus; Vortech, Dearborn, MI) and rapidly decapitated. The heads were immediately perfused through the internal carotid artery with 6 L of 4% paraformaldehyde (Sigma-Aldrich) dissolved in 0.1 M phosphate buffer (Sigma-Aldrich) containing 0.1% sodium nitrate (Sigma-Aldrich) and 10 U/mL heparin (Fresenius Kabi). The brain was removed and tissue blocks containing POA and hypothalamus were dissected and postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 18 hours and transferred to 30% sucrose in 0.1 M phosphate buffer for cryoprotection. Coronal sections were cut (45 μm) using a freezing microtome (Microm HM400R) and stored at 20°C in a cryopreservative solution (30% ethylene glycol, 1% polyvinylpyrrolidone, 30% sucrose in sodium phosphate buffer) until further processing.
Immunohistochemistry and analyses overview
First, the effects of prenatal T treatment on GABAergic contacts on GnRH and kisspeptin neurons were examined, using tissues from control and prenatal T ewes. Second, potential colocalization of NKCC1 and KCC2 in GnRH and kisspeptin neurons and the potential effects of prenatal T treatment were examined using tissues from control and prenatal T ewes. Finally, colocalization of NKCC1 and KCC2 in KNDy cells was explored using tissue from control animals. Sections from animals in all groups were processed simultaneously for each of the different staining combinations. The experimenter was blinded to the treatment group of the animal during tissue processing and analysis.
Immunohistochemistry and general methods
All steps were performed at room temperature, with gentle agitation, and sections were free floating. Tissue sections were washed with PBS (pH 7.35, 0.1 M phosphate buffer containing 0.9% NaCl) between steps. Sections were treated with 10% (kisspeptin triple label) or 1% (all other protocols) hydrogen peroxide (10 minutes; catalog no. H325; Fisher Scientific, Hampton, NH) and washed with antibody incubation solution [1 hour; consisting of 4% normal goat serum (catalog no. 005-000-121; Jackson ImmunoResearch Laboratories, West Grove, PA)] in PBS with 0.4% Triton X-100 (catalog no. BP151-500; Sigma-Aldrich) to prevent nonspecific labeling. All primary and secondary antibodies were diluted in incubation solution. Once tissue was incubated in the proper fluoroprobe, tissue was protected from light from that step forward. After staining, sections were mounted onto Superfrost slides (Fisher Scientific), coverslipped with an aqueous mounting medium (Gelvatol) containing an antifading agent (1,4-diazabicyclo[2,2]octane, 50 mg/mL, catalog no. D2522; Sigma-Aldrich), and stored at 4°C until analysis. Sections from all animals in groups that were statistically compared were processed simultaneously for each of the different staining combinations detailed below.
GnRH, vesicular GABA transporter, and synapsin-1
To examine synaptic inputs onto GnRH neurons in the POA and mediobasal hypothalamus (MBH), four sections from each control (n = 8) and prenatal T (n = 7) animals containing POA or six sections containing MBH were processed for GnRH, vesicular GABA transporter (VGAT), and synapsin-1. Sections were incubated with rabbit anti-VGAT (1:15,000; 17 hours; catalog no. 131-003; Synaptic Systems; RRID: AB_887869) (33), followed by biotinylated goat anti-rabbit IgG (1:500; 1 hour; catalog no. BA-9200; Vector Laboratories, Burlingame, CA), avidin–biotin complex (ABC) reagent (1:500; 1 hour; catalog no. pk-6100; Vector Laboratories), biotinylated tyramine [1:250; 10 minutes; tissue sample amplification (TSA); catalog no. NEL700A; PerkinElmer Life Sciences, Waltham, MA], and Cy3-conjugated streptavidin (1:200; 30 minutes; catalog no. 016-160-084; Jackson ImmunoResearch Laboratories). Sections were then simultaneously incubated with rabbit anti-synapsin-1 (1:300; 17 hours; catalog no. 5297S; Cell Signaling Technology; RRID: AB_2616578) (34) and mouse anti-GnRH (1:800; 17 hours; catalog no. SMI-41R; Covance; RRID: AB_10123893) (35), followed by DyLight 488 goat anti-mouse (1:200; 30 minutes; catalog no. 35503; Thermo Fisher Scientific, Waltham, MA) and, next, Alexa Fluor 647 goat anti-rabbit (1:200; 30 minutes; catalog no. 111-605-144; Jackson ImmunoResearch Laboratories).
Kisspeptin, VGAT, and synaptophysin
To examine synaptic inputs onto kisspeptin neurons in the ARC, four hemisections containing ARC from each control (n = 8) and prenatal T (n = 7) animal were processed for kisspeptin, VGAT and synaptophysin. Sections were coincubated with rabbit anti-VGAT (1:15,000; 17 hours; catalog no. 131-003; Synaptic Systems; RRID: AB_887869) (33) and mouse anti-synaptophysin (1:200; 17 hours; catalog no. S5768; Sigma-Aldrich; RRID: AB_477523) (36), followed by Alexa Fluor 647 goat anti-mouse (1:100; 30 minutes; catalog no. 115-605-166; Jackson ImmunoResearch Laboratories), biotinylated goat anti-rabbit IgG (1:500; 1 hour), followed by ABC reagent (1:500; 1 hour), TSA (1:250; 10 minutes; catalog no. NEL700A), and Alexa Fluor 555 streptavidin (1:100; 30 minutes; red). Sections were next incubated with rabbit anti-kisspeptin (1:5000; 17 hours; ab566 gifted by A. Caraty, French National Institute for Agricultural Research, Paris, France; RRID: AB_2622231) (37), followed by DyLight 488 goat anti-rabbit (1:100; 30 minutes; catalog no. 35553; Thermo Scientific).
GnRH/kisspeptin and NKCC1
To examine expression of NKCC1 in GnRH neurons in the POA and MBH and kisspeptin neurons in the ARC, five sections from each control (n = 6) and prenatal T (n = 4) animal for each brain area were processed for GnRH/kisspeptin and NKCC1. Sections were incubated with mouse anti-NKCC1 (1:500; 17 hours; Developmental Studies Hybridoma Bank; RRID: AB_528406) (38). Next, sections were washed and incubated with biotinylated goat anti-mouse IgM (1:500; 1 hour; catalog no. BA-2020; Vector Laboratories), followed by ABC (1:500; 1 hour), TSA (1:250; 10 minutes), and Cy3-streptavidin (1:200; 30 minutes). The tissue was then washed and incubated with either rabbit anti-GnRH (1:400; 17 hours; catalog no. 20075; Immunostar; RRID: AB_572248) (39) or rabbit anti-kisspeptin (1:2000; 17 hours; catalog no. AB9754; Millipore; RRID: AB_2296529) (40). Sections were then washed and incubated with DyLight 488 goat anti-rabbit (1:200; 30 minutes).
GnRH and KCC2
To examine expression of KCC2 in GnRH neurons in the POA and MBH, control (n = 6) and prenatal T (n = 4) ewes were used to examine transporter expression. Five sections from each animal for each brain area were processed for GnRH and KCC2. Sections were incubated with mouse anti-GnRH (1:800; 17 hours). Next, sections were washed and incubated with biotinylated goat anti-mouse IgM (1:500; 1 hours), then incubated in Alexa Fluor 555 goat anti-mouse (1:200; 30 minutes; catalog no. A21422; Molecular Probes, Eugene, OR). The tissue was then washed and incubated with rabbit anti-KCC2 (1:250; 17 hours; catalog no. 07-432; Millipore; RRID: AB_310611) (41). Sections were then washed and incubated with DyLight 488 goat anti-rabbit (1:200; 30 minutes).
Kisspeptin and KCC2
To examine expression of KCC2 in kisspeptin neurons in the ARC, control (n = 6) and prenatal T (n = 4) ewes were used to observe cotransporter expression. Three to four sections from three animals were processed for kisspeptin and KCC2. Sections were incubated with rabbit anti-kisspeptin (1:40,000; 17 hours; Millipore). Next, sections were washed and incubated with biotinylated goat anti-rabbit IgM (1:500; 1 hour; catalog no. BA-9200; Vector Laboratories), followed by ABC (1:500; 1 hour; catalog no. pk-6100; Vector Laboratories), TSA (1:250; 10 minutes), and Cy3 streptavidin (1:200; 30 minutes; catalog no. 016-160-08; Jackson ImmunoResearch Laboratories). The tissue was then washed and incubated with rabbit anti-KCC2 (1:250; 17 hours). Sections were then washed and incubated with DyLight 488 goat anti-rabbit (1:200; 30 minutes).
Triple-label immunolabeling for NKB/NKCC1/KCC2
To examine the expression of NKCC1 and KCC2 in the same neurons in the ARC, three to four hemisections per animals from prenatal vehicle-treated control ewes (n = 6) were processed for NKB, NKCC1, and KCC2. Sections were incubated with mouse anti-NKCC1 (1:500; 17 hours). Next, sections were washed and incubated with goat anti-mouse with biotinylated goat anti-mouse IgM (1:500; 1 hour), followed by ABC reagent (1:500; 1 hour; catalog no. pk-6100; Vector Laboratories), biotinylated tyramine (1:250; 10 minutes; catalog no. NEL700A, diluted; PerkinElmer Life Sciences), and Cy3-streptavidin (1:200; 30 minutes; red). Sections were coincubated with the primary antibody guinea pig anti-NKB (1:1000; 17 hours; catalog no. 210493; gifted by Ciofi; RRID: AB_2732894) (42) and rabbit anti-KCC2 (1:250; 17 hours). The tissue was then washed and incubated with goat anti-guinea pig DyLight 488 (1:200; 30 minutes; green) and Alexa Fluor 647 goat anti-rabbit (1:200; 30 minutes; blue). Sections were mounted onto slides, coverslipped with Gelvatol mounting medium, covered, and stored at 4°C until analyzed.
Peptide blocking controls
Specificity of the VGAT antibody was tested using peptide-blocking controls. Preabsorption (overnight at 4°C) of the VGAT antibody (1:15,000; catalog no. 131-003; Synaptic Systems ; RRID: AB_887869) (33) with the synthesizing peptide (20 μg/mL; catalog no. 131-0P; lot no. 131-0P/6; Synaptic Systems) prevented all staining. GnRH, VGAT, synaptophysin, synapsin-1, and kisspeptin have previously been validated in sheep (26, 43, 44). Omission of primary antibodies, while applying the secondary antibodies in all of the above-detailed combinations and with NKCC1 and KCC2, completely eliminated labeling for the first antigen. Purified antigen for NKCC1 is not available, and thus a preabsorption control was not possible. However, previous publications have validated this antibody using the western blot analysis, which yielded a single band between 145 and 205 kDa in 23 cell types (45), and demonstrated a lack of NKCC1 labeling in brain tissue of NKCC1-knockout mice (46, 47). Finally, this antibody has been used by a number of investigative groups (48–50). Preabsorption (overnight at 4°C) of the KCC2 antibody (rabbit × KCC2) with the synthesizing peptide [25 μg/mL; amino acids 932 to 1043 of rat K+/Cl− cotransporter (KCC2), custom-made by Phoenix Pharmaceuticals] prevented all staining for KCC2 in the hypothalamus. For KCC2, immunolabeling was compared from two different antibodies (catalog no. 07-432, Millipore; catalog no. KCC2-Rb-Af720, Frontier Institute; RRID: AB_2571782) (51) using confocal microscopy and yielded immunolabeling that was indistinguishable for both antibodies.
Image capture and analysis of GABAergic inputs
Confocal Z-stacks comprised of 1.0-μm optical sections through immunostained sections were captured at ×60 magnification on a Nikon D-Eclipse C1 laser-scanning confocal system attached to a Nikon Eclipse E800 microscope; fluorophores were detected by three lasers at wavelengths of 488, 543, and 633 nm. Per animal, a total of 10 kisspeptin-positive neurons in the ARC and 10 GnRH-positive neurons in the POA and 4 to 6 GnRH-positive neurons in the MBH were imaged and analyzed for putative contacts. Images were imported into ImageJ software (National Institutes of Health, Bethesda, MD), sharpened, and adjusted for contrast and brightness, identically for all images. A putative contact was defined as an immunolabeled bouton that was positive for the presynaptic marker, synaptophysin/synapsin-1, and in direct apposition to the neuron or proximal dendrite. No single-labeled VGAT or kisspeptin contacts were observed. For each kisspeptin cell, for each optical section, the number of double-labeled VGAT/synaptophysin and kisspeptin/synaptophysin inputs, as well as single-labeled synaptophysin-positive inputs, were counted. For each GnRH cell in the POA and MBH, for each optical section, the number of double-labeled VGAT/synapsin-1 and single-labeled synapsin-1–positive inputs were counted for both the cell body and the proximal dendrite.
The perimeter of the soma of each kisspeptin or GnRH neuron was measured for each optical section of the neuron and averaged per number of optical sections. The numbers of inputs per kisspeptin neuron and GnRH neuron soma and dendrite were then calculated per 10 μm of analyzed perimeter to control for any difference in cell and dendrite perimeter between the control and prenatal T groups. There were no differences in the length of the proximal dendrite measured for POA and MBH GnRH neurons between control and prenatal T animals. Additionally, there was no difference in the number of optical sections analyzed between control and prenatal T animals. The values for the synaptic inputs, as well as neuron and dendrite perimeters, were averaged per cell and then per animal.
NKCC1 and KCC2 transporter analysis
Colocalizations of NKCC1 in GnRH neurons in the POA and MBH were analyzed in five sections per brain area per ewe at ×20 magnification using a digital camera (Microfire A/R; Optronics) attached to a microscope (Leica DM500B; Leica Microsystems, Wetzlar, Germany). The total numbers of single- and double-labeled neurons were analyzed for each section, averaged per animal, and reported as the percentages of the following: GnRH immunoreactive (ir) neurons containing NKCC1 and single-labeled GnRH-ir neurons. All data are expressed as the mean ± SEM.
Colocalization of NKCC1 in kisspeptin neurons in the ARC was analyzed in four sections per ewe at ×20 magnification using a digital camera (Microfire A/R; Optronics, Goshen, IN) attached to a microscope (Leica DM500B; Leica Microsystems). The total numbers of single- or double-labeled neurons were analyzed for each section, averaged per animal, and reported as the percentages of kisspeptin-ir neurons containing NKCC1 or kisspeptin-ir only.
Owing to the localization of KCC2 to the membrane of cell bodies and dendrites, confocal microscopy was used to determine colocalization of kisspeptin neurons with KCC2, and colocalization of NKB neurons with NKCC1 and KCC2. Z-stacks of 1-μm-thick optical sections were captured through the ARC using an Olympus FV3000 confocal laser scanning system attached to a motorized inverted microscope (1X83; Olympus Corporation, Center Valley, PA) using a 60× objective with ×1 zoom. Three to four sections were scanned per ewe. In sections labeled for kisspeptin and KCC2, the total number of single-labeled kisspeptin-ir neurons or dual-labeled kisspeptin-ir neurons containing KCC2 were analyzed, averaged per animal, and reported as the percentage of kisspeptin cells. In sections labeled for NKB, NKCC1, and KCC2, the number of NKB-ir cells containing either NKCC1, KCC2, or both and NKB-ir only were analyzed, averaged per animal, and reported as a percentage of NKB cells. All data are expressed as the mean ± SEM.
Data analyses
Statistical comparisons between control and prenatal T sheep were performed using a Student t test, and a P value <0.05 was considered a significant difference.
Results
Inputs to GnRH neurons in the POA and MBH
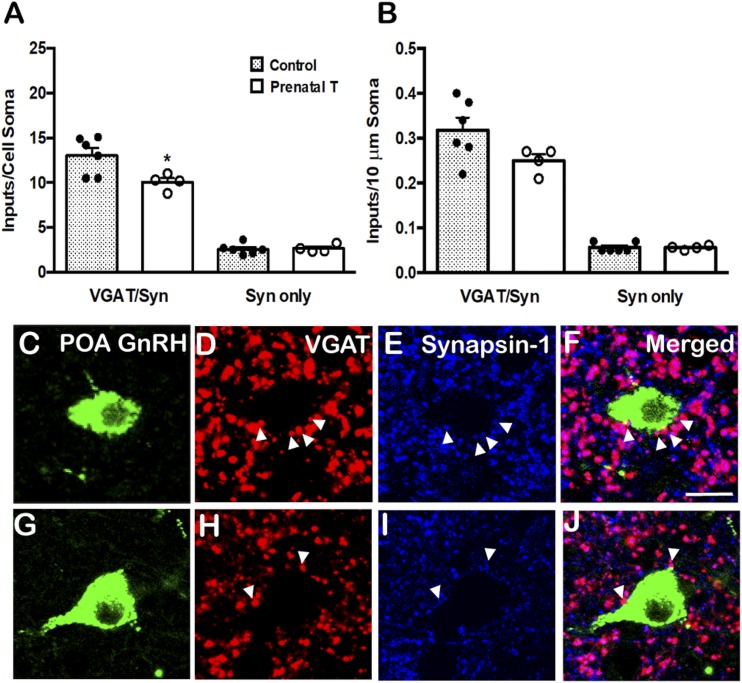
The number of VGAT/synapsin-1 inputs to GnRH neurons in tissues triple-labeled for GnRH, VGAT, and synapsin-1, in both the POA and MBH, are shown in Figs. 1 and 2. In the POA, there was a significant decrease in the number of VGAT/synapsin-1 inputs (P = 0.031) to the soma of GnRH neurons in prenatal T animals compared with controls (Fig. 1A). However, when the VGAT/synapsin-1 inputs were analyzed with respect to their density per 10-μm somal cell surface, this difference did not reach statistical significance (P = 0.095) (Fig. 1B), even though there were no significant differences in GnRH soma size between the groups (Table 1). In contrast to the changes in VGAT inputs to GnRH somas in prenatal T animals, no differences were seen in number or density of VGAT/synapsin-1 inputs to the proximal dendrite of GnRH neurons (Table 2). Finally, there were no differences between prenatal T and control animals in the number or density of single-labeled synapsin-1 inputs onto either the cell soma (Fig. 1B) or proximal dendrites (data not shown) of POA GnRH neurons.
Figure 1.
Prenatal T decreased VGAT inputs onto POA GnRH neurons. (A) Mean (±SEM) numbers of VGAT/synapsin (VGAT/Syn) double-labeled inputs and synapsin (Syn) single-labeled inputs onto the somas of POA GnRH neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. Individual data points representing each animal are shown superimposed above each bar in (A) and (B). (B) Mean (±SEM) numbers of VGAT/Syn and Syn inputs per 10-μm somal cell surface of POA GnRH neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. *P < 0.05. (C–J) Representative confocal images of 1-μm optical sections triple-labeled for (C and G) GnRH (green), (D and H) VGAT (red), (E and I) synapsin-1 (blue), and (F and J) the merged images from a (C–F) control and (G–J) prenatal T animal. Arrowheads indicate double-labeled inputs on somas. Scale bar, 10 μm.
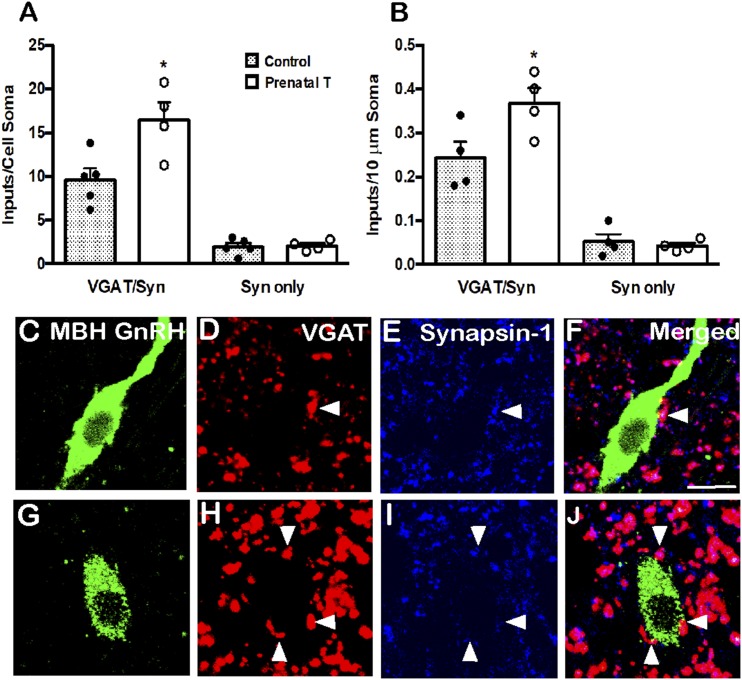
Figure 2.
Prenatal T increased VGAT inputs onto MBH GnRH neurons. (A) Mean (±SEM) numbers of VGAT/synapsin (VGAT/Syn) double-labeled inputs and synapsin (Syn) single-labeled inputs onto the somas of MBH GnRH neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. Individual data points representing each animal are shown superimposed above each bar in (A) and (B). (B) Mean (±SEM) numbers of VGAT/Syn and Syn inputs per 10-μm somal cell surface of MBH GnRH neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. *P < 0.05. (C–J) Representative confocal images of 1-μm optical sections triple-labeled for (C and G) GnRH (green), (D and H) VGAT (red), (E and I) synapsin-1 (blue), and (F and J) the merged images from a (C–F) control and (G–J) prenatal T animal. Arrowheads indicate double-labeled inputs on somas. Scale bar, 10 μm.
Table 1.
Effect of Prenatal T on Soma Perimeters for GnRH and ARC Kisspeptin
| Cell Type | Mean Soma Perimeter, µm | |
|---|---|---|
| Control | Prenatal T | |
| POA GnRH | 49.57 ± 1.81 | 48.19 ± 0.70 |
| MBH GnRH | 48.90 ± 1.38 | 47.09 ± 1.40 |
| ARC kisspeptin | 38.75 ± 1.07 | 42.47 ± 2.37 |
Data are expressed as means ±SEM.
Table 2.
Effect of Prenatal T on the Density of GABAergic Inputs to GnRH Proximal Dendrites
| Cell Type | Density of VGAT/synaptophysin Inputs to Proximal Dendrites, Inputs per 10 µm | |
|---|---|---|
| Control | Prenatal T | |
| POA GnRH | 0.02 ± 0.003 | 0.02 ± 0.002 |
| MBH GnRH | 0.19 ± 0.12 | 0.65 ± 0.14a |
Data are expressed as means ±SEM.
P < 0.05, prenatal T vs control.
In contrast to the POA, there was a significant increase in both the number and density of VGAT/synapsin-1 inputs to the cell soma of GnRH neurons in the MBH of prenatal T animals compared with controls (P = 0.02 and P = 0.048; Fig. 2A and 2B). As in the POA, there was no significant difference in MBH GnRH soma size between the groups (data not shown). Furthermore, prenatal T animals showed significantly increased density of VGAT/synapsin-1 inputs to the proximal dendrite of MBH GnRH neurons (P = 0.017; Table 2). Again, no differences between groups were seen in the number or density of single-labeled synapsin-1 inputs onto either the cell soma (Fig. 2B) or proximal dendrites (data not shown) of MBH GnRH neurons.
Inputs to ARC kisspeptin neurons
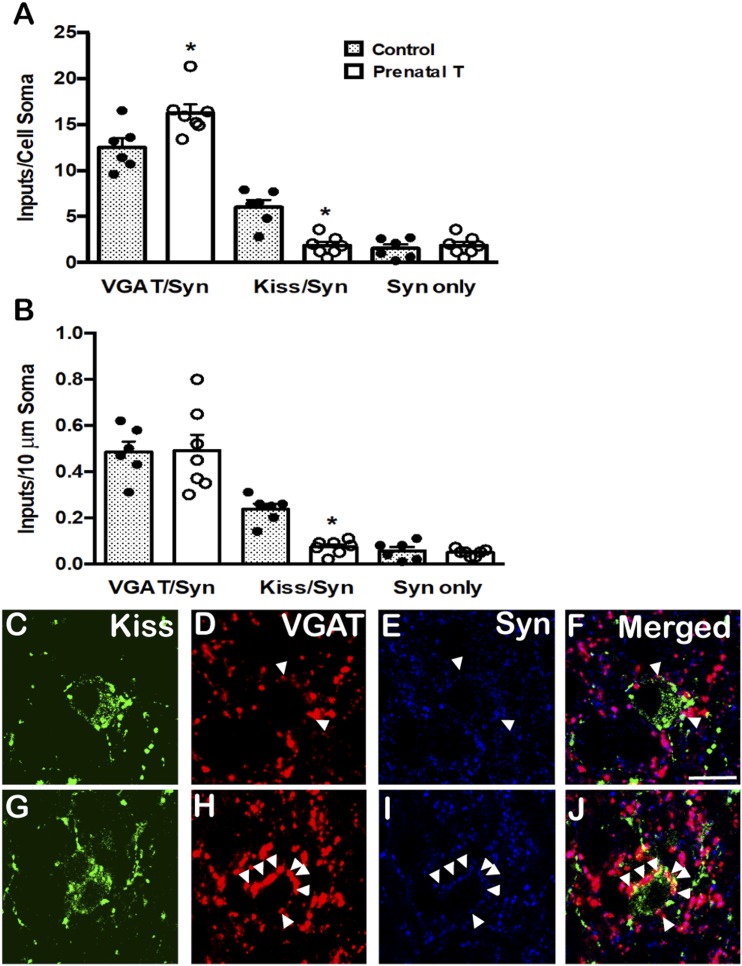
Analyses of the numbers of VGAT- and kisspeptin-positive synaptic inputs onto ARC kisspeptin neurons found a significant increase in prenatal T ewes in the number of VGAT/synaptophysin inputs (P = 0.02) to the soma of ARC kisspeptin neurons but not in the density of VGAT/synaptophysin inputs per 10-μm somal cell surface (Fig. 3A and 3B). In contrast, there was a significant decrease in the same animals in the number (P < 0.001) and density (P < 0.001) of kisspeptin/synaptophysin inputs onto ARC kisspeptin cells (Fig. 3A and 3B), consistent with that reported in an earlier study (7). There were no differences in the number or density of single-labeled, synaptophysin only contacts between control and prenatal T animals (Fig. 3A and 3B), nor were there significant differences in ARC kisspeptin soma size between the groups (Table 1).
Figure 3.
Prenatal T increased VGAT inputs onto KNDy neurons. (A) Mean (±SEM) numbers of VGAT/synaptophysin (VGAT/Syn) and kisspeptin/synaptophysin (Kiss/Syn) double-labeled inputs and synaptophysin (Syn) single-labeled inputs onto the somas of KNDy neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. Individual data points representing each animal are shown superimposed above each bar in (A) and (B). (B) Mean (±SEM) numbers of VGAT/Syn and Kiss/Syn inputs per 10-μm somal cell surface of KNDy neurons in control (filled bars; n = 6) and prenatal T (open bars; n = 4) ewes. *P < 0.05. (C–J) Representative confocal images of 1-μm optical sections triple-labeled for (C and G) Kisspeptin (green), (D and H) VGAT (red), (E and I) synaptophysin (blue), and (F and J) the merged images from a (C–F) control and (G–J) prenatal T animal. Arrowheads indicate double-labeled inputs on somas. Scale bars, 10 μm.
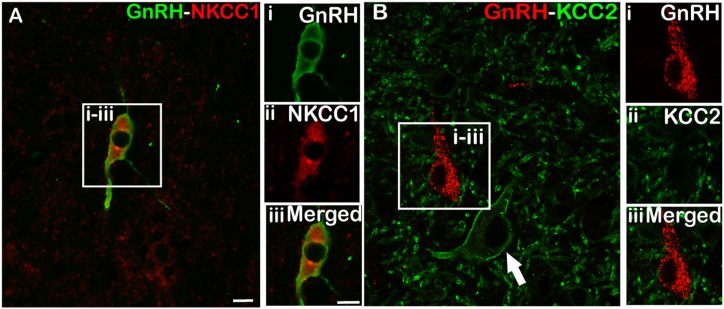
GnRH neurons colocalize NKCC1 but not KCC2
Analysis of sections dual-labeled for GnRH and NKCC1 showed that most POA GnRH neurons in both control (72% ± 3.95%, mean ± SEM) and prenatal T (78% ± 7.64%, mean ± SEM) ewes colocalized NKCC1 (Fig. 4A). Similarly, most MBH GnRH neurons in both control (67% ± 7.75%, mean ± SEM) and prenatal T (72% ± 7.29%, mean ± SEM) animals colocalized NKCC1. There were no significant differences between control and prenatal T animals in the percentage of NKCC1 colocalization in GnRH neurons in either the POA (P = 0.467) or MBH (P = 0.496). In both POA and MBH GnRH neurons, NKCC1 labeling was associated with both the cell membrane and cytoplasm of GnRH neurons (Fig. 4A), with no qualitative differences in subcellular distribution noted between control and prenatal T animals. In contrast to the colocalization of NKCC1, none of the total of GnRH neurons analyzed in the POA (628 neurons) and MBH (108 neurons) in either control or prenatal T animals showed colocalization with KCC2, despite the presence of KCC2 in adjacent neurons (Fig. 4B).
Figure 4.
Representative images of dual-labeled GnRH neurons used for NKCC1 and KCC2 transporter analysis. (A) A POA GnRH cell that colocalizes (i) GnRH (green), (ii) NKCC1 (red) and (iii) merged image. (B) A section immunostained for (i) GnRH (red) and (ii) KCC2 (green), and (iii) the merged image depicting a POA GnRH neuron that does not contain KCC2 despite the presence of adjacent KCC2-labeled cells (arrow). Scale bars, 10 μm.
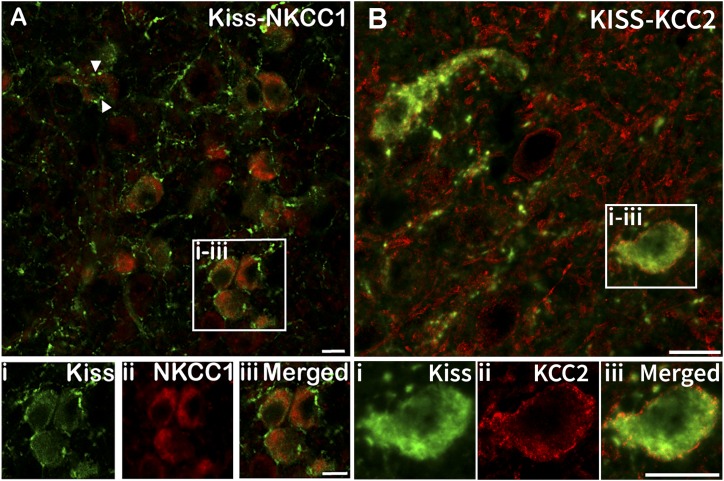
ARC kisspeptin neurons colocalize NKCC1 and KCC2
Analysis of sections through the ARC dual-labeled for kisspeptin and NKCC1 revealed that a large majority of kisspeptin neurons colocalized NKCC1 in both control (90% ± 3.79%, mean ± SEM) and prenatal T (94% ± 2.52%, mean ± SEM) animals. No significant differences were detected between groups (P = 0.35). ARC kisspeptin neurons displayed NKCC1 labeling associated with both the membrane and cytoplasm of the neuron (Fig. 5A). Additionally, we observed kisspeptin fibers in contact with NKCC1-positive neurons that were not dual-labeled for kisspeptin (Fig. 6). Analysis of sections through the ARC dual-labeled for kisspeptin and KCC2 (Fig. 5B) revealed colocalization in both control (59.5% ± 6.65%, mean ± SEM) and prenatal T (72.7% ± 4.52%, mean ± SEM) ewes, with no significant differences between the groups (P = 0.18).
Figure 5.
(A) Representative images obtained using a ×20 objective of dual-labeled neurons in the ARC colocalizing (i) kisspeptin (Kiss; green) and (ii) NKCC1 (red) and (iii) a merged image. Most ARC kisspeptin neurons colocalized NKCC1 [examples shown in (i–iii)]. Additionally, kisspeptin-positive fibers were seen in close contact with non-kisspeptin NKCC1-positive cells [e.g., arrowheads in (A)]. (B) Representative confocal 1-μm-thick optical sections obtained using a ×60 objective of neurons in the ARC colocalizing (i) kisspeptin (green) and (ii) KCC2 (red) and (iii) a merged image. Scale bars, 10 μm.
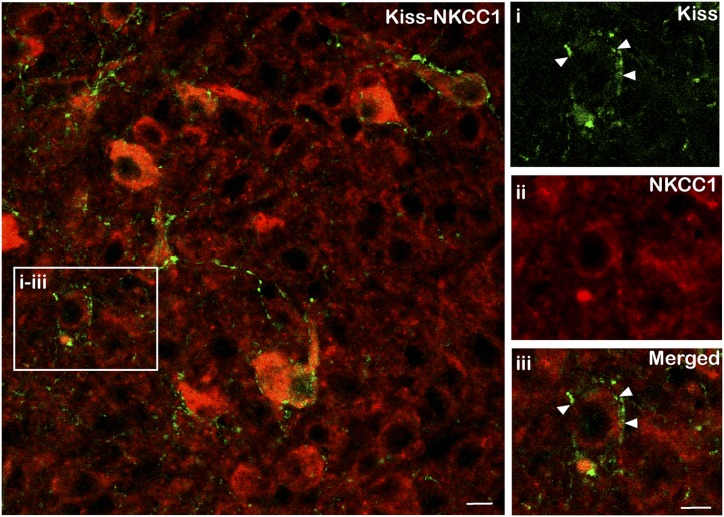
Figure 6.
Representative images of dual-labeled neurons where (i) kisspeptin-positive fibers (green) were seen in close contact with (ii and iii) non-kisspeptin NKCC1-positive cells (red, arrowheads). Scale bars, 10 μm.
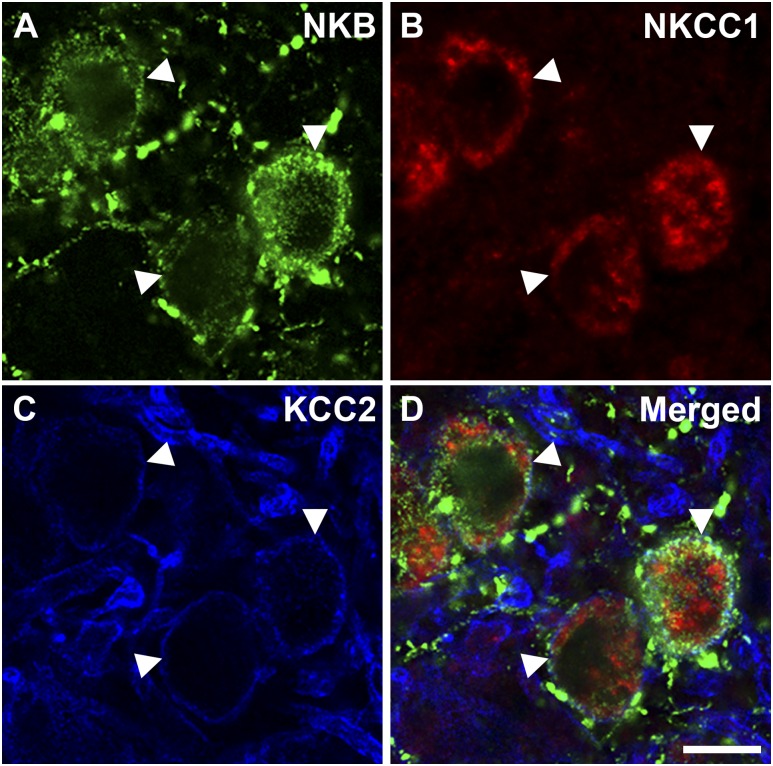
Triple-label detection of NKB (another KNDy cell marker), NKCC1, and KCC2 revealed that of NKB neurons analyzed, 72.0% ± 8.7% (mean ± SEM) contained NKCC1 and 60.5% ± 4.2% (mean ± SEM) contained KCC2. A subset of NKB cells contained either NKCC1 alone (28.2% ± 4.2%, mean ± SEM) or KCC2 alone (16.6% ± 7.3%, mean ± SEM), and 43.8% ± 8.1% (mean ± SEM) of NKB cells contained both NKCC1 and KCC2 (Fig. 7).
Figure 7.
Representative confocal 1-μm-thick optical section of triple-labeled neurons in the ARC colocalizing (A) NKB, (B) NKCC1, and (C) KCC2 and (D) a merged image of the three. Arrowheads indicate triple-labeled NKB/NKCC1/KCC2 cells. Scale bar, 10 μm.
Discussion
Results of the current study build on previous observations regarding the role of GABAergic inputs in animal models of PCOS, as well as provide new insights into potential sites of action of GABA in regulating the GnRH system. There are several novel findings. First, in contrast to the increase in GABAergic inputs reported for preoptic GnRH neurons in PNA mice (27), we found decreased inputs to preoptic GnRH neurons in the sheep, as well as an increase only in the subset of GnRH neurons found in the MBH, a population of GnRH neurons present in sheep and humans but not in rodents. The latter observation is particularly noteworthy because the MBH subpopulation of GnRH neurons in sheep has been specifically implicated in pulsatile GnRH/LH secretion (41). Second, we report, to our knowledge for the first time, increased GABAergic inputs to KNDy neurons in prenatal T sheep as well as the presence of the cotransporters NKCC1 and KCC2 in this population; 28% of KNDy (NKB) neurons had NKCC1 alone, suggesting that GABA may play an excitatory role in a subset of adult KNDy cells. Finally, although colocalization of NKCC1 in GnRH cells has previously been reported in mice (42), to our knowledge the current report is the first demonstration of this in another mammal and supports the idea that excitatory GABA signaling is a common feature among mammalian GnRH neurons in the adult brain.
There are several possible explanations for the difference in the direction of change of GABAergic inputs to GnRH neurons between the PNA mouse and prenatal T sheep. As suggested above, one possibility is a functional difference between POA and MBH GnRH neurons in sheep, with evidence suggesting that MBH but not POA GnRH neurons mediate pulsatile secretion (44, 52, 53), although both MBH and POA GnRH neurons are activated during the preovulatory GnRH surge (54). Because the MBH GnRH neuron population is smaller in rodents compared with the ewe, it is possible that the subset of GnRH neurons responsible for pulsatile secretion exists in rodents as part of the preoptic population, and that neurons in that subset differ from other GnRH cells in their degree of GABAergic input. Another possible explanation for the difference in the direction of changes in GABAergic inputs between sheep and mice may arise from the model itself: in the sheep model, animals are treated in utero with T whereas PNA mice are treated prenatally with the nonaromatizable androgen DHT. Although T may act upon either androgen or estrogen receptors (the latter via aromatization of T to E2), DHT binds predominantly to the androgen receptor; therefore, different developing neuronal populations may be targeted by each treatment. Finally, it is possible that the anatomical difference between sheep and mice is reflected by differences in the pharmacological effects of GABA antagonists. Electrophysiological recordings in the mouse have shown that GABAA receptor antagonists increased the firing rate of 80% of active GnRH neurons (55); however, when ionotropic glutamate receptors were blocked, GABAA receptor antagonist blockade decreased firing consistent with an excitatory role for GABA (56). In the ewe, studies of the effects of antagonists to GABAA have yielded conflicting results. GABA levels measured by microdialysis in the POA of sheep are lower during the LH surge (57), and injections of the GABAA receptor agonist muscimol into the POA of OVX+E sheep (58) have been shown to suppress pulsatile LH release. However, antagonism of GABAA receptors with bicuculline in the POA also decreased pulsatile LH secretion in OVX+E sheep (58). Note that some (or all) of these effects could reflect actions on interneurons, rather than direct effects on GnRH neurons. Therefore, further studies are needed to determine the direction of effects of GABA antagonists on GnRH neurons in the ewe.
Previous work in the sheep model has shown a variety of long-term changes in the connections and morphology of KNDy neurons in the female sheep brain as a consequence of prenatal T exposure. These include changes in peptide expression (decreases in NKB and dynorphin) (17), hypertrophy of KNDy cell bodies (26), as well as decreases in the density of synaptic inputs from KNDy cells to GnRH neurons, and inputs to KNDy neurons from other KNDy cells as well as other, nonidentified cells (26). We now add to this evidence of changes in the number of GABAergic inputs to KNDy as well as GnRH neurons as a result of prenatal T treatment. What might be the functional consequences of these changes? Key neuroendocrine alterations seen in prenatal T sheep include a decrease in the inhibitory influence of progesterone upon pulsatile GnRH secretion (59), similar to that seen in women with PCOS (3), as well as changes in the ability of estradiol to induce the GnRH/LH surge responsible for ovulation (60). Studies in the ewe have provided strong evidence that dynorphin secreted from KNDy neurons, acting via κ receptors on MBH GnRH cells, is responsible for mediating progesterone negative feedback on pulsatile GnRH secretion (53, 61, 62). This includes data showing decreased numbers of dynorphin but not kisspeptin cells in the ARC of prenatal T sheep (17); we would expect that dynorphin inputs to GnRH arising from KNDy neurons would also be decreased in prenatal T sheep, but this has yet to be demonstrated. Nonetheless, we previously hypothesized that decreased numbers of KNDy inputs onto GnRH neurons might underlie reduced responsiveness to progesterone negative feedback mediated via dynorphin. It is unclear where the source of GABAergic inputs are and how increased GABA inputs to KNDy and MBH GnRH neurons may fit into this functional consequence of prenatal T exposure, but it may be that the actions of these inputs act in a complementary way to decrease inhibitory dynorphin (KNDy) release, leading to increased pulse frequency. We note that although changes were seen in numbers of GABAergic input to KNDy cells in prenatal T animals, the density of inputs remained unchanged (Fig. 3). It is possible that the change in number but not density of inputs is due to the slightly increased cell soma size for KNDy neurons in prenatal T sheep, even though this did not reach statistical significance in the current study (Table 1). Finally, although we cannot unequivocally conclude that the changes reported in this study for prenatal T sheep would be expected to have a physiological effect on GnRH/LH pulse frequency, the increase we observed in GABA inputs onto KNDy cells in this study (∼33%) is similar in magnitude, albeit opposite in direction, to that decrease in glutamatergic inputs to KNDy cells (∼20%) previously shown in the same animal model (26).
Alternatively, prenatal T-induced changes in GABAergic inputs to GnRH and/or KNDy neurons could contribute to alterations in the timing and amplitude of the preovulatory GnRH/LH surge seen in this animal model (32). As noted above, in sheep, GnRH neurons in both the POA and MBH are activated at the time of the surge (54). Thus, it may be that decreased numbers of stimulatory GABA inputs onto POA GnRH neurons are in part responsible for the decreased amplitude of the surge reported in prenatal T sheep. Note that both kisspeptin and NKB have also been implicated in modulation of surge amplitude in the ewe (63, 64), and it is possible that decreased NKB reported in KNDy cells (17) also contributes to this deficit. Additionally, the rostral population of kisspeptin cells located in the POA in sheep is also activated during the surge in sheep (44, 64). We did not analyze ovine kisspeptin cells in the POA, as the current study was focused on KNDy and GnRH neurons as components of the pulse generator. Nevertheless, changes in GABAergic inputs onto POA kisspeptin neurons could also potentially affect the generation of the surge, and represent an area for future study.
As noted, our findings of NKCC1 colocalization suggest the actions of GABA in the sheep brain upon GnRH cells and a subset of KNDy neurons may be predominantly excitatory rather than inhibitory. Electrophysiological data have demonstrated GABA to be excitatory to preoptic GnRH and KNDy neurons in the mouse (29, 65, 66). The presence of NKCC1 in GnRH neurons suggests that GABA may likewise have an excitatory influence on GnRH neurons in sheep, but this needs to be confirmed with electrophysiological studies. However, a technical obstacle to this approach is the current lack of transgenic GnRH-GFP models in this species, needed to enable in vitro identification of the scattered population of GnRH neurons in sheep as in other mammals. However, recent innovations in in vitro fertilization may allow for more rapid and easier generation of transgenic sheep models (67), and in coming years it may be feasible to generate these models more easily. Additionally, previous studies in the sheep have shown the presence of GABAB receptor 1 and 2 subunits in most sheep GnRH neurons (68), but to date colocalization of the GABAA receptor, which mediates stimulatory actions of GABA in murine GnRH cells, has not been examined in the ewe. Finally, it is possible that the expression of the cotransporters NKCC1 and KCC2 is influenced by gonadal status; the E2 replacement protocol we used in this experiment for both control and prenatal T sheep was designed to closely mimic late follicular phase levels as seen in ovary-intact ewes. Nonetheless, future work could determine whether changing levels of progesterone and E2 across the estrous cycle influence expression of the transporters and their levels of colocalization in GnRH and/or KNDy cells.
In summary, changes in GABAergic synaptic inputs to GnRH and KNDy neurons were seen in adult female sheep exposed to T during prenatal life, an animal model that closely mimics the features of PCOS. Furthermore, changes in GABA inputs, together with other morphological alterations in prenatal T sheep, may underlie defects in both pulsatile and surge secretion of GnRH seen in this model. Additionally, our anatomical data suggest that GABA in the sheep brain is stimulatory to GnRH neurons similar to its actions in the mouse and raise the possibility that GABA may also be stimulatory to a subset of ARC kisspeptin (KNDy) neurons. Future work is needed to determine the regions and nuclei where GABAergic inputs to GnRH and KNDy neurons in the sheep arise from, as well as pharmacological and electrophysiological studies to provide further evidence for the role of GABA in the regulation of GnRH secretion in the normal and prenatal T sheep brain.
Acknowledgments
We thank Kathryn Lucas and Dayanara Moore for excellent technical support.
Financial Support: This work was supported by National Institutes of Health Grant P01 HD44232 to V.P., L.M.C., and M.N.L.
Author Contributions: Designed experiments: D.T.P., L.M.C., M.N.L., A.M.M., and V.P. Performed experiments, collected and analyzed data: D.T.P., J.A.C., L.M.C., and A.M.M. Wrote and edited the article: all authors. Provided funding, equipment, reagents, and supplies: V.P., L.M.C., R.L.G., and M.N.L. All authors had full access to the data, take responsibility for the integrity of the data and accuracy of the data analysis, and approved the final manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- ABC
avidin–biotin complex
- ARC
arcuate nucleus
- E2
17β-estradiol
- GABA
γ-aminobutyric acid
- -ir
immunoreactive
- KNDy
kisspeptin/neurokinin B/dynorphin
- MBH
mediobasal hypothalamus
- NKB
neurokinin B
- PCOS
polycystic ovarian syndrome
- PNA
prenatally androgenized
- POA
preoptic area
- prenatal T (animal)
prenatal T-treated (animal)
- T
testosterone
- TSA
tissue sample amplification
- VGAT
vesicular γ-aminobutyric acid transporter
References and Notes
- 1. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650–4658. [DOI] [PubMed] [Google Scholar]
- 2. Stein I, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–191. [Google Scholar]
- 3. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 4. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 5. Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1–2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson J. Prenatal programming of the female reproductive neuroendocrine system by androgens. Reproduction. 2006;132(4):539–547. [DOI] [PubMed] [Google Scholar]
- 8. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 9. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452–466. [DOI] [PubMed] [Google Scholar]
- 12. Herbison A. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, ed. Knobil and Neill’s Physiology of Reproduction. 3rd ed. New York, NY: Raven Press; 2006:1415–1482. [Google Scholar]
- 13. Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133(2):876–886. [DOI] [PubMed] [Google Scholar]
- 14. Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology. 1993;57(4):751–759. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 1995;685(1–2):198–200. [DOI] [PubMed] [Google Scholar]
- 16. Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–579. [DOI] [PubMed] [Google Scholar]
- 17. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. [DOI] [PubMed] [Google Scholar]
- 21. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 22. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]
- 23. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miles R. Neurobiology. A homeostatic switch. Nature. 1999;397(6716):215–216. [DOI] [PubMed] [Google Scholar]
- 29. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153(11):5105–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147(10):4843–4851. [DOI] [PubMed] [Google Scholar]
- 32. Jackson LM, Mytinger A, Roberts EK, Lee TM, Foster DL, Padmanabhan V, Jansen HT. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154(4):1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID:AB_887869, https://scicrunch.org/resolver/AB_887869.
- 34. RRID:AB_2616578, https://scicrunch.org/resolver/AB_2616578.
- 35. RRID:AB_10123893, https://scicrunch.org/resolver/AB_10123893.
- 36. RRID:AB_477523, https://scicrunch.org/resolver/AB_477523.
- 37. RRID:AB_2622231, https://scicrunch.org/resolver/AB_2622231.
- 38. RRID:AB_528406, https://scicrunch.org/resolver/AB_528406.
- 39. RRID:AB_572248, https://scicrunch.org/resolver/AB_572248.
- 40. RRID:AB_2296529, https://scicrunch.org/resolver/AB_2296529.
- 41. RRID:AB_310611, https://scicrunch.org/resolver/AB_310611.
- 42. RRID:AB_2732894, https://scicrunch.org/resolver/AB_2732894.
- 43. Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144(8):3663–3676. [DOI] [PubMed] [Google Scholar]
- 44. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lytle C, Xu JC, Biemesderfer D, Forbush B III. Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol. 1995;269(6 Pt 1):C1496–C1505. [DOI] [PubMed] [Google Scholar]
- 46. Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25(1):54–66. [DOI] [PubMed] [Google Scholar]
- 47. Liu Q, Wong-Riley MT. Postnatal development of Na+–K+–2Cl− co-transporter 1 and K+–Cl− co-transporter 2 immunoreactivity in multiple brain stem respiratory nuclei of the rat. Neuroscience. 2012;210:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarez-Leefmans FJ, León-Olea M, Mendoza-Sotelo J, Alvarez FJ, Antón B, Garduño R. Immunolocalization of the Na+–K+–2Cl− cotransporter in peripheral nervous tissue of vertebrates. Neuroscience. 2001;104(2):569–582. [DOI] [PubMed] [Google Scholar]
- 49. Gilbert D, Franjic-Würtz C, Funk K, Gensch T, Frings S, Möhrlen F. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int J Dev Neurosci. 2007;25(7):479–489. [DOI] [PubMed] [Google Scholar]
- 50. Mykoniatis A, Shen L, Fedor-Chaiken M, Tang J, Tang X, Worrell RT, Delpire E, Turner JR, Matlin KS, Bouyer P, Matthews JB. Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am J Physiol Cell Physiol. 2010;298(1):C85–C97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. RRID:AB_2571782, https://scicrunch.org/resolver/AB_2571782.
- 52. Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929–5936. [DOI] [PubMed] [Google Scholar]
- 53. Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, Lehman MN. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology. 2018;159(9):3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. [DOI] [PubMed] [Google Scholar]
- 55. Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145(2):495–499. [DOI] [PubMed] [Google Scholar]
- 56. Moenter SM, DeFazio RA. Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146(12):5374–5379. [DOI] [PubMed] [Google Scholar]
- 57. Robinson JE, Kendrick KM, Lambart CE. Changes in the release of gamma-aminobutyric acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. J Neuroendocrinol. 1991;3(4):393–399. [DOI] [PubMed] [Google Scholar]
- 58. Scott CJ, Clarke IJ. Evidence that changes in the function of the subtypes of the receptors for gamma-amino butyric acid may be involved in the seasonal changes in the negative-feedback effects of estrogen on gonadotropin-releasing hormone secretion and plasma luteinizing hormone levels in the ewe. Endocrinology. 1993;133(6):2904–2912. [DOI] [PubMed] [Google Scholar]
- 59. Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the GnRH neuronal network to inhibition by progesterone. Endocrinology. 1999;140(12):5797–5805. [DOI] [PubMed] [Google Scholar]
- 60. Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66(4):924–933. [DOI] [PubMed] [Google Scholar]
- 61. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 62. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 63. Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30(1):94–102. [DOI] [PubMed] [Google Scholar]
- 65. Taylor-Burds C, Cheng P, Wray S. Chloride accumulators NKCC1 and AE2 in mouse GnRH neurons: implications for GABAA mediated excitation. PLoS One. 2015;10(6):e0131076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 2014;34(49):16296–16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walker SK, Heard TM, Seamark RF. In vitro culture of sheep embryos without co-culture: successes and perspectives. Theriogenology. 1992;37(1):111–126. [Google Scholar]
- 68. Sliwowska JH, Billings HJ, Goodman RL, Lehman MN. Immunocytochemical colocalization of GABA-B receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience. 2006;141(1):311–319. [DOI] [PubMed] [Google Scholar]