Abstract
We evaluated the cardioprotective effect of Aloe vera gel isoprenaline (ISO)-administered myocardial infarction in rats. ISO administration increased lipid peroxidation and oxidative stress in rats, which were ameliorated by A. vera gel supplementation. Our study also revealed that creatine kinase-MB (CK-MB) activities were increased in ISO-administered rats, while the activities of cellular antioxidants, such as superoxide dismutase and catalase, and glutathione concentration were decreased. A. vera gel lowered CK-MB enzyme activities and the glutathione concentration in ISO-administered rats, and increased antioxidant activities. Histopathological examination also revealed increases in thickness of the left ventricle myocardium, increases in mononuclear cell infiltrations, increased degeneration of focal areas of the endocardium, and increased fibrous tissue deposition in the heart of ISO-administered rats; whereas, A. vera prevented infiltration of inflammatory cells and reduced left ventricular fibrosis. In conclusion, we show that A. vera supplementation protects against development of cardiac inflammation, fibrosis, and oxidative stress in ISO-administered rats.
Keywords: isoprenaline, cardiac hypertrophy, oxidative stress, inflammation, fibrosis
INTRODUCTION
Cardiovascular diseases (CVD) are increasing in both developed and developing countries. According to the World Health Organization, about 23.6 million people will die from CVD by 2030 (Cui et al., 2011). CVD includes several types of cardiac dysfunction, such as myocardial infarction (MI), hypertension, myocarditis, and dilated cardiomyopathy (Kehat and Molkentin, 2010). In myocardial infarction, necrosis of the myocardium occurs due to an imbalance between the coronary blood supply and the myocardial demand of oxygen. Following MI, the most significant structural changes occur to left ventricular (LV) remodeling (Hori and Nishida, 2009). Thickening of ventricular walls occurs, which ultimately results in changes to geometry and chamber size, also known as cardiac hypertrophy (Zordoky et al., 2008). These cardiac remodeling events are associated with many biological reactions where oxidative stress and inflammatory responses play a censorious role. Due to the presence of excessive reactive oxygen species (ROS), cardio depressive reactions occur, resulting in stimulation of cardiac fibroblast proliferation, matrix metalloproteinase activated collagen synthesis, and myocardial fibrosis (Tsutsui et al., 2011). Prolonged overstimulation of β-adrenergic receptors (β-ARs) is one of the primary sources of oxidative stress in cardiac myocyte and cerebral artery. Indeed, stimulation of the sympathetic nervous system attributes to cardiovascular insult (Parati and Esler, 2012). The auto-oxidized products of catecholamine, such as adrenochrome, aminochromes, and catecholamine-O-quinones, are responsible for cardiotoxicity (Dhalla et al., 2010). The β-adrenergic agonist isoprenaline (ISO) induces severe stress to the myocardium, leading to irreversible myocardial necrosis. Coronary insufficiency, calcium overload, energy depletion, hypoxia, and ischemia are responsible for ISO-induced cardiotoxicity (Upaganlawar et al., 2011). Early clinical studies suggest that isoproterenol treated patients show poor survival rates due to chronic heart failure (Lefkowitz et al., 2000). The rat model of ISO-induced myocardium necrosis is a well-defined and can be used to explore several cardiac dysfunctions, including arrhythmias, myocyte loss, and fibrosis with advancement of heart failure (Krenek et al., 2009). In this study, we subcutaneously injected rats with ISO as an experimental model to induce myocardium insult.
Natural products have been renowned as a mainstay source of medicine in pharmaceutical biology for thousands of years. Around 20% of plants in the world have been tested for biological or pharmacological properties (Sahu et al., 2013). Aloe vera, locally known as ‘Gritkumari’ and which belongs to the Asphodelaceae family, is the most widely used natural plant. A. vera is a perennial, succulent plant that possesses many pharmacological properties such as anti-inflammatory, immunostimulant, wound healing, antiulcer, antidiabetic, and antitumor activities (Joseph and Raj, 2010; Ozsoy et al., 2009). A. vera’s biological activity can be attributed to several chemical components, including anthraquinones (also known as emodin), glycoproteins or aloin (important for cathartic activity), polysaccharides, vitamins, and enzymes (Kaithwas et al., 2011). Two different parts of A. vera are usually used: leaf exudates, which are responsible for laxative effects, and mucilaginous gels (Radha and Laxmipriya, 2015). This gel contains several major components; among these are alprogen, an anti-allergic glycol protein, and c-glycosylchromone, a novel anti-inflammatory component (Anilakumar et al., 2010). The anti-oxidative properties of A. vera are due to a polysaccharide present in the pulp, which is vastly used in medicinal and nutraceutical applications (Radha and Laxmipriya, 2015). Some important chemical compounds, including β-carotene, folic acid, vitamin (A, C, E, and B12), and zinc, are found naturally in A. vera; these compounds make A. vera a formidable antioxidant plant that abets to protect against pro-oxidant-induced membrane and cellular damage. A. vera gel can modulate oxidative status through scavenging different superoxide anions, and can impact neurotransmitter process (Saada et al., 2003). Studies have suggested that the free radical scavenging activity of A. vera (72.2%) is superior than α-tocopherol (65.6%) (Anilakumar et al., 2010). Early investigations also showed beneficial effects of A. vera in thrombosis (Kishore, 2015) and diabetes (Yagi et al., 2009). A. vera gel also shows beneficial effects in age related disorders, such as cardiac thrombosis in aged rats (Ikeno et al., 2002). However, very few investigations were conducted to explore the benefit of A. vera in cardiac dysfunction. Based on previous reports of A. vera on oxidative stress, we hypothesized that A. vera may be useful as an alternative therapy in cardiac dysfunction. The aim of this work was to evaluate the cardioprotective effect of A. vera gel on ISO-induced oxidative stress and fibrosis in rats.
MATERIALS AND METHODS
Plants
A. vera was obtained from the local market (Dhaka, Bangladesh) and authenticated by Dr. Md Ashraful Alam, Associate Professor, Department of Pharmaceutical Science, North South University, Dhaka, Bangladesh. A specimen was also preserved in the Department of Pharmaceutical Science, North South University for future reference. A. vera leaves were dried and grinded into coarse powder.
Preparation of hydroalcoholic extract
5.0 g of dried powder sample and 250 mL of 70% ethanol in water (v/v) was mixed in an orbital shaker. The ethanol extract was filtered with a cloth to get a crude extract. This extract was concentrated using a rotary vacuum evaporator.
Mobile phase for high-performance liquid chromatography (HPLC)-diode-array detector (DAD) separation and analysis
Acetonitrile (solvent A), acetic acid solution, pH 3.0 (solvent B), and methanol (solvent C) was used as the mobile phase. The gradient elution program used was as follows: A : B=5:95 (0~10 min), A : B=10:90 (11~15 min), A : B : C=15:70:15 (16~25 min), A : B : C=20:60:20 (26~30 min), A : B : C=30:40:30 (31~35 min), A : B : C= 40:50:10 (36~40 min), and A : B=5:95 (41~45 min).
HPLC detection and quantification of polyphenolic compounds in the hydroalcoholic extract of A. vera
The phenolic compounds in the ethanol extract of A. vera were determined by HPLC-DAD analysis as described previously (Hossain et al., 2016). A UltiMate 3000 system (Dionex, Sunnyvale, CA, USA) equipped with quaternary rapid separation pump (LPG-3400RS, Dionex) and photodiode array detector (DAD-3000RS, Dionex) was used for HPLC-DAD. An Acclaim® C18 (5 μm) Dionex column (4.6×250 mm, Dionex) was used to separate the crude extract at 30°C. The flow rate of the mobile phase was 1 mL/min and the sample injection volume was 20 μL.
Animals
Animals were derived from the Animal House at the Department of Pharmaceutical Sciences, North South University. Long Evans male rats (12~14-month-old, 235~250 g body weight) were used for this study.
Animal housing
Individual cages were used to keep the animals in a well ventilated airconditione room where room temperature 22±3°C and humidity 55% were maintained, with a 12-h dark/light cycles. The animals were given everyday the standard laboratory chow diet and drinking water ad libitum.
Ethical approval
The Ethical Committee for animal care and experimentation of North South University, Dhaka, Bangladesh approved the experimental protocols and euthanesia process (AEC-002-2015).
Experimental groups
Eighteen Long Evan male rats were evenly divided into 3 groups: group I, the control group, was administered with saline only; group II was administered with ISO [50 mg/kg subcutaneous (S.C.) twice a week for 14 days]; group III, the ISO+A. vera group, was supplemented with A. vera gel (5% gel mixed with powder chow food, w/w) and ISO (50 mg/kg S.C. for 14 days on twice a week).
The body weight of all animals were measured using an electronic balance, and all animals were checked for any unwanted health effect on a daily basis for 14 days.
Euthanasia and sample collection
After 14 days of treatment, all rats were fasted for 12~14 h and were sacrificed through euthanasia using high dose of pentobarbitone (65 mg/kg). The blood and organs (including heart and kidneys) were collected immediately after the sacrifice. Blood was centrifuged at 6,000 g and 4°C to collect the plasma. The plasma was then stored in a refrigerator at −20°C for use in biochemical assays. All harvested organs were weighed; one part was then stored in neutral buffer formalin and the other part in a refrigerator at −20°C for further studies.
Assessment of cardiotoxicity
Enzyme [alanine amino transferase (ALT), alkaline phosphatase (ALP), and aspartate amino transferase (AST)] activity in plasma was estimated using commercial kits (Diatec Diagnostics, Macquarie Park, NSW, Australia), according to the manufacturer’s protocol. A commercials kits for the creatine kinase-MB (CK-MB) was also purchased from Diatec Diagnostics and used following the manufacturer’s standard protocol.
Preparation of tissue samples and assessment of tissue antioxidants
Phosphate buffer solution (10 mL, pH 7.4) was used to homogenize the heart and kidney tissues, and these were then centrifuged at 8,000 rpm for 15 min at 4°C. The supernatants of the tissue homogenates were collected and stored for further analysis. Measurement of protein concentration and enzymatic assays were conducted as described below. Superoxide dismutase (SOD) activity was measured according to a previously described assay (Misra and Fridovich, 1972). Catalase (CAT) activity was determined following as previously described (Chance and Maehly, 1955; Khan, 2012), and a previously described method was used to determine levels of reduced glutathione (GSH) (Jollow et al., 1974).
Estimation of oxidative stress markers
Nitric oxide (NO) was calculated from the amount of nitrate following a method described previously (Tracey et al., 1995). Malondialdehyde (MDA) was measured as thiobarbituric acid reactive substances (TBARS). Lipid peroxidation in plasma, heart, and kidney was estimated by using a previously described method (Niehaus and Samuelsson, 1968). Advanced protein oxidation products (APOP) levels were also calculated according as previously described (Tiwari et al., 2014; Witko-Sarsat et al., 1996).
Myeloperoxidase (MPO) activity assay
A di-anisidine-H2O2 based method was used to determine MPO activity (Bradley et al., 1982). The rate of absorbance change was measured at 460 nm. Results were expressed as units of MPO/mg protein.
Histopathological analysis
The neutral buffered formalin (10%) fixed heart tissues were cleaned and processed by using graded ethanol and xylene treatment. The processed tissues were then embedded in paraffin blocks, used to prepare tissue sections of about 5 μm thickness. The sectioning was carried out by employing a rotary microtome. The first set of these tissue sections were stained with hematoxylin and eosin using standard routine procedures. The second set were stained using Sirius red for fibrosis determination. Stained tissue sections were then mounted onto glass slides and photographed under a light microscope (Axioscope, ZEISS, Darmstadt, Germany) at 40× magnifications. All slides were examined for morphological changes.
Statistical analysis
The values generated in this study are expressed as mean±standard error of the mean (SEM). The results were analyzed statitically by one way ANOVA followed by Newman Keul’s test using GraphPad Prism (GraphPad, San Diego, CA, USA). Statistical significance was considered as P<0.05 in all cases.
RESULTS
Effect of A. vera gel on body weight of rats administered with ISO
The body weights of rats in the different groups are given in Fig. 1A. ISO administration decreased rat body weight significantly compared to controls. A. vera supplementation in rats administered with ISO prevented loss of body weight compared to the ISO-treated group (Fig. 1A). Furthermore, ISO-treated rats showed significantly (P<0.05) increased wet heart, LV, and right ventricle weights compared to control rats (Fig. 1B~1D). A. vera gel treatment in ISO-treated rats prevented increases in organ weight, preserving them similar to those of control rats (Fig. 1B~1D).
Fig. 1.
Effect of Aloe vera gel on (A) body weight and organ wet weight [(B) total heart weight, (C) left ventricular (LV) of heart weight, and (D) right ventricular (RV) of heart weight] of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM (n=6). Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at *P <0.05 and **P <0.01. LV, left ventricle; RV, Right ventricle.
Effect of A. vera gel on AST, ALT, and ALP activities
AST, ALT, and ALP enzymes activities were considerably (P<0.05) increased in ISO-treated rats compared to control rats (Fig. 2). A. vera treatment significantly lowers the AST and ALP enzyme activities compared to the ISO-treated rats (Fig. 2B and 2C). However, ALT activity was not significantly reduced by A. vera treatment (Fig. 2A).
Fig. 2.
Effect of Aloe vera gel on (A) alanine amino transferase (ALT), (B) aspartate amino transferase (AST), and (C) alkaline phosphatase (ALP) activities in isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM (n=6). Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at *P <0.05 and **P <0.01.
Effect of A. vera gel treatment on oxidative stress parameters and antioxidant enzymes in ISO-induced rats
We analyzed MDA, NO, and APOP levels, and in plasma and tissues. Moreover, we analyzed CAT and SOD activities, and GSH levels in plasma and tissues as antioxidant parameters.
ISO administration significantly lowered the activities of antioxidant enzymes such as SOD and CAT in plasma and tissues compared to those of control rats (P<0.05; Fig. 3). A. vera treatment significantly restored SOD and CAT activities in all samples from ISO-treated rats (Fig. 3). Moreover, ISO administration significantly depleted levels of GSH in plasma, heart, and kidney tissues compared to those of control rats (P<0.05; Fig. 4). Depleted GSH was also restored in plasma and heart through A. vera supplementation (Fig. 4). However, GSH levels in kidney was unable to be restored by A. vera.
Fig. 3.
Effect of Aloe vera gel on superoxide dismutase (SOD) [(A), (B), and (C), respectively] and catalase (CAT) [(D), (E), and (F), respectively] activities in the plasma, hearts, and kidneys of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM (n=6). Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at *P <0.05 and **P <0.01.
Fig. 4.
Effect of Aloe vera gel on glutathione (GSH) activity in (A) plasma, (B) hearts, and (C) kidneys of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM, n=6. Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at **P <0.01.
ISO treatment also increased nitric oxide in plasma and tissues compared to that of control rats (Fig. 5), and A. vera treatment normalized raised NO levels in the plasma, hearts, and kidneys of ISO-treated rats (Fig. 5). ISO treatment increased levels of MDA in both plasma and tissues above those of control rats (Fig. 5), and A. vera treatment significantly lowered these raised MDA levels from ISO-treated rats (Fig. 5).
Fig. 5.
Effect of Aloe vera gel on the oxidative stress parameters nitric oxide (NO) [(A), (B), and (C), respectively] and malondialdehyde (MDA) [(D), (E), and (F), respectively] in plasma, heart, and kidney tissue homogenates of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM (n=6). Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at *P <0.05 and **P <0.01.
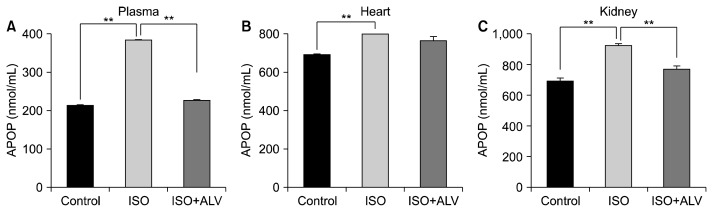
Furthermore, ISO treatment also raised the APOP concentrations in plasma and tissues compared to those of control rats (Fig. 6), which could be significantly lowered by A. vera treatment in plasma and kidney (Fig. 6). However, APOP levels in the heart were unable to be decreased A. vera supplementation in ISO-treated rats.
Fig. 6.
Effect of A. vera gel on oxidative the stress parameter, advanced protein oxidation products (APOP) in (A) plasma, (B) heart, and (C) kidney tissue homogenates of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM, n=6. Statistical analysis was carried out using one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at **P <0.01.
Effect of A. vera gel treatment on MPO and CK-MB activities in ISO-induced rats
MPO activity in tissue homogenates, such as of heart and kidneys, was significantly increased in ISO-treated rats (Fig. 7). Supplementation with A. vera prevented the rise of MPO activity in the heart and kidneys observed in ISO-treated rats (Fig. 7). Moreover, ISO-induced rats also showed significant (P<0.05) increases in plasma CK-MB activity compared to control rats (Fig. 7), and A. vera treatment significantly reduced CK-MB activity to near normal (Fig. 7).
Fig. 7.
Effect of A. vera gel on myeloperoxidase (MPO) activity in hearts and kidneys, and of creatine kinase-MB (CK-MB) in plasma of isoprenaline (ISO)-treated rats. Data are expressed as mean±SEM (n=6). Statistical analysis was done by one-way ANOVA followed by Newman-Keul’s post hoc test. Comparisons were made between the control vs. ISO group, and the ISO vs. ISO+Aloe vera (ALV) group. Statistical significance was considered at *P <0.05 and **P <0.01.
Effect of A. vera treatment on histological assessment of heart and kidney structures in ISO-induced rats
Cardiac damage in ISO-treated rats was explored by histological staining of heart sections. Infiltration of mononuclear inflammatory cells was observed in the hearts of ISO-treated rats compared to those of control rats (Fig. 8, upper panel). A. vera supplementation prevented the inflammatory cell infiltration and necrosis in the hearts of ISO-administered rats (Fig. 8, upper panel). In addition, ISO-induced rats showed cardiomyocyte hypertrophy, heart fibrosis, and inflammation (Fig. 8, lower panel). A. vera gel ameliorated fibrosis in the heart of ISO-treated rats (Fig. 8, lower panel).
Fig. 8.
Effect of Aloe vera gel on infiltration of inflammatory cells (upper panel) and fibrosis (lower panel) in the heart of isoprenaline (ISO)-treated rats. Hematoxylin and eosin staining was carried out (upper panel) to examine the normal architecture of the tissue. (A) Control rats showing normal orientation of cardiomyocytes, with regular sizes and shapes. (B) ISO-treated rats showing hypertrophied cardiomyocytes, necrosis, and infiltrating cells, which were absent in (C) ISO+A. vera gel administered rats. The lower panels show images of tissue sections stained with Sirius red to identify collagen deposition and fibrosis. (D) Images from control rats showing baseline collagen in very small amounts, or almost not present at all. (E) ISO-treated rats showing large amounts of collagen deposition in the perivascular and pericardiomycyte zones (marked in red on the yellow background) this is lowered in (F) ISO-administered rats treated with A. vera . All scale bar are 50 μm, a 40× magnification was used.
Analysis of A. vera alcoholic extracts by HPLC-DAD
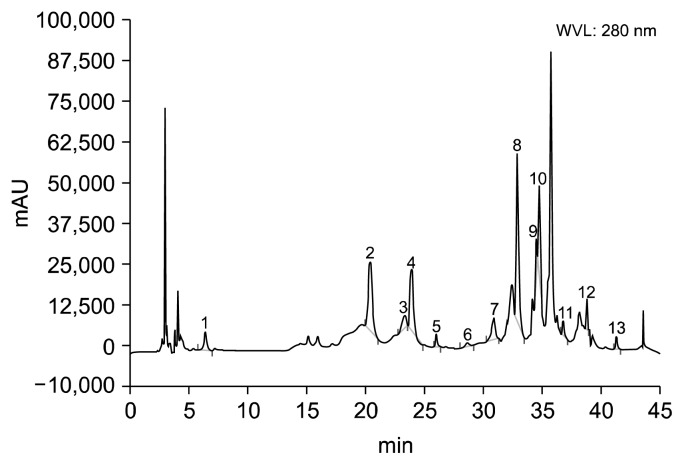
The presence of polyphenols in A. vera extracts is shown in Fig. 9. The content of each phenolic compound was calculated using the corresponding calibration curve. These values are presented as the mean of five determinations, as shown in Table 1. These results indicate that A. vera hydroalcoholic extracts contain good amounts of phenolic antioxidants, such as chatechin hydrate, caffeic acid, ferulic acid, ellagic acid, and quercetin (Table 1).
Fig. 9.
High-performance liquid chromatography chromatogram of Aloe vera hydroalcoholic extracts. Peaks: 1, gallic acid; 2, (+)-catechin hydrate; 3, vanillic acid; 4, caffeic acid; 5, (−)-epicatechin; 6, vanillin; 7, p-coumaric acid; 8, trans-ferulic acid; 9, ellagic acid; 10, rutin hydrate; 11, rosmarinic acid; 12, quercetin hydrate; 13, kaempferol.
Table 1.
Components of polyphenolic compounds in the Aloe vera hydroalcoholic extracts (n=5)
| (unit: mg/100 g of dry extract) | ||
|---|---|---|
|
| ||
| Polyphenolic compound | Hydroalcoholic extract of A. vera | % RSD |
| Gallic acid | 11.06 | 0.07 |
| Catechin hydrate | 113.89 | 0.87 |
| Vanillic acid | 15.17 | 0.09 |
| Caffeic acid | 39.64 | 0.55 |
| (−)-Epicatechin | 24.83 | 0.11 |
| Vanilli | 3.12 | 0.02 |
| p-Coumaric acid | 20.13 | 0.10 |
| Ferulic acid | 41.28 | 0.63 |
| Ellagic acid | 36.17 | 0.41 |
| Rutin hydrate | 23.01 | 0.11 |
| Rosmarinic acid | 3.38 | 0.03 |
| Quercetin | 45.04 | 0.68 |
| Kaempferol | 3.17 | 0.02 |
RSD, relative standard deviation.
DISCUSSION
There has been a major switch in the framework of disease this millennium, despite a noticeable improvement in life expectancy. This may be due to striking changes in diet and lifestyle, which may have contributed to an outbreak of non-communicable diseases (NCDs). Currently, public health services in developing countries are overstretched by a growing demand to survive long-term with NCD. CVDs are the main benefactor of the worldwide burden of NCDs. With advanced age, detrimental changes occur, which are followed by reductions in physiological activity and increase the chances of disease and death. Among these, one of the most debilitating phenomena is loss of myocardial function. Oxidative stress is the main denominator of this functional loss due to excess ROS production, resulting in alterations in the defensive mechanism of myocytes (Misra et al., 2009). In addition, altered inflammatory mediator expression, the inability of senescent cells to respond to growth factors, and peroxidation of mitochondrial phospholipids are responsible for age-related adverse cardiac remodeling (Bujak et al., 2008; Littarru and Tiano, 2007). Oxidative damage is most prevalent in cardiac tissue because oxidative phosphorylation is the highest demanding process for energy production (Ochoa et al., 2005). At normal physiological conditions, catecholamines are beneficial for heart function, and produce a positive inotropic effect. However, at higher doses of catecholamine the heart experiences rapid energy depletion, resulting in biochemical and structural changes in cardiomyocytes (Upaganlawar et al., 2011); this is demonstrated in this study. A rat model of cardiac remodeling was developed by subcutaneously injecting ISO, inducing increased LV wet weight, elevated oxidative stress, and lowered levels of antioxidants in the plasma and heart. Moreover, cellular infiltration and extracellular matrix deposition were induced in the infarct area of the heart.
A. vera has many active ingredients that assuage human life and health in a myriad ways. The vast pharmacological properties of A. vera arise from its various chemical constituents including polysaccharide, anthraquinones, salicylic acid, saponins, amino acids, enzymes, minerals, and vitamins (Nandal and Bhardwaj, 2012). The antioxidant activity of A. vera gel is due to the presence of α-tocopherol (vitamin E), carotenoids, ascorbic acid (vitamin C), tannins, and flavonoids (Radha and Laxmipriya, 2015). A. vera gel can strongly scavenge free radicals including 2,2-diphenyl-1-picrylhydrazyl, hydroxyl, and alkyl radical due to the presence of polysaccharides (Kang et al., 2014). Through analysis of A. vera alcoholic extracts by HPLC, we confirmed the presence of a good amount of phenolic antioxidants, such as chatechin hydrate, caffeic acid, ferulic acid, ellagic acid, and quercetin. These strong antioxidants may synergistically scavenge the free radicles more efficiently than single antioxidant compounds. Moreover, A. vera gel also maintains levels free radical scavenging enzymes or antioxidants, such as SOD, CAT, and GSH (Jain, 2015). A. vera contains emodin, an anthraquinone derivative that induces well-documented anti-inflammatory effect by decreasing production of the pro-inflammatory cytokines tumor necrosis factor-α and interleukin-1β (Song et al., 2012). C-glucosylchromone, a potent anti-inflammatory compound in A. vera gel, helps inhibit activity of bradykinin (an inflammatory substance) due to the presence of the peptidase bradykinase (Sahu et al., 2013). Previous studies have shown that treatment with emodin or A. vera gel significantly improves LV function by maintaining normal myofibril structures (Jain, 2015; Song et al., 2012). Our research shows that treatment with A. vera gel helps minimize oxidative stress and inflammatory cell infiltration in the hearts of ISO-treated rats.
β-AR agonists and synthetic catecholamine similar to ISO induce energy depletion in cardiac muscles and promote complex biochemical and structural changes, which lead to myocardial necrosis and cell damage. ISO administration results in elevation of AST, ALT, and ALP levels in rat plasma (Adaramoye and Lawal, 2015). In this study, treatment with A. vera gel normalized levels of ALT and AST activity in plasma. Studies have also shown that A. vera gel treatment is effective in inhibiting the rise of these enzymes in plasma (Iji et al., 2010). CK-MB is another superior and special marker of myocardial necrosis (Maynard et al., 2000). CK-MB activity was also reported to be increased in the plasma of ISO-induced aged rats (Priscilla and Prince, 2009). Our study is supported by a previous which also showed that A. vera gel can reduce CK-MB activity induced by doxorubicin-induced myocardial oxidative stress (Kaithwas et al., 2014).
Following ISO administration, oxidative stress is generated through β-AR stimulation, which influences production of highly cytotoxic free radicals by spontaneous oxidation of catecholamine, and increases the chance of calcium overload. These events lead to myocardial dysfunction (Liaudet et al., 2014). Lipid peroxidation is suggestive of elevated oxidative stress. Disintegration of membrane polyunsaturated fatty acids cause increases in TBARS production, which is a measure of lipid peroxidation usually seen after myocardial infarction. A previous study also reported that catecholamine increases MDA levels in myocardial tissues (Geng et al., 2004). In our study, the content of MDA, a product of lipid peroxidation, was increased in ISO-treated rats that were attenuated by A. vera gel supplementation; this is supported by a previous study (Jain, 2015). In addition, levels of advanced oxidation proteins were also increased in the ISO-administered group, which was subsequently lowered significantly by A. vera gel treatment. Myocyte growth, function, remodeling, and cardiovascular homeostasis are controlled by NO, a highly reactive molecule (Takimoto and Kass, 2007). Increased inducible NO synthase expression in the LV may be responsible for elevations in NO production, which is associated with increased apoptosis, and which were reported in ISO-induced cardiac hypertrophy (Krenek et al., 2009). In normal physiological conditions, NO is a vasodilator. However, uncoupled NO synthase is the main contributor of ROS due to abnormal production of NO. Peroxynitrite results from excess concentrations of NO, and can induce drastic protein oxidation and nitration, which can ultimately result in myocardial injury (Takimoto and Kass, 2007). This study revealed that levels of NO were also increased in ISO-treated rats; however, A. vera gel successfully normalized elevations of NO oxide in ISO administered rats. Phenolic antioxidants are capable of preventing lipid peroxidation and prevent conversion of peroxynitrite (Alam et al., 2016). The presence of phenolic antioxidants in A. vera may partially contribute to preventing lipid peroxidation and peroxynitrite formation in ISO-treated rats.
Antioxidants, the primary defense mechanisms for the body, can avert free radicals from initiating toxic effects. SOD, CAT, and GSH are endogenous antioxidants, together comprising the first line of defense against oxidative injury. GSH levels were significantly lowered in the blood and tissues from isoproterenol-treated rats due to increases in lipid peroxidation (Karthikeyan et al., 2007). GSH possesses high antioxidant activity; GSH directly destroys hydrogen peroxide and promotes formation of reduced forms of ascorbate. In the present study, significant reductions of CAT, SOD, and GSH levels were observed in plasma and hearts of ISO-treated rats compared to those in the control group. A. vera gel treatment significantly restored these antioxidant levels. Oxidative stress-mediated tissue damage also recruits immune cells to infiltrate into the scar site of the myocardium to scavenge dead tissues. However, immune cell recruitment further stimulate extracellular matrix deposition and ultimately cause fibrosis in the heart (Wynn and Ramalingam, 2012). ISO administration in rats induces infiltration immune cell and fibrosis of the heart and kidneys (Sagor et al., 2015). In this study, A. vera gel treatment of ISO-administered rats decreased immune cell infiltration and fibrosis of the heart.
In conclusion, in this study we suggest that A. vera gel treatment in rats administered with ISO is cardioprotective. This effect of A. vera may be attributed to restoration of antioxidant enzymes and decreased lipid peroxidation of the heart. The presence of various phenolic compounds is suggested to contribute synergistically to the beneficial cardioprotective effect in ISO-treated rats.
ACKNOWLEDGEMENTS
The research was conducted in Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh. The authors gratefully acknowledge the logistic support provided by the Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Adaramoye OA, Lawal SO. Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J Basic Clin Physiol Pharmacol. 2015;26:65–72. doi: 10.1515/jbcpp-2013-0139. [DOI] [PubMed] [Google Scholar]
- Alam MA, Subhan N, Hossain H, Hossain M, Reza HM, Rahman MM, et al. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr Metab. 2016;13:27. doi: 10.1186/s12986-016-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilakumar KR, Sudarshanakrishna KR, Chandramohan G, Ilaiyaraja N, Khanum F, Bawa AS. Effect of Aloe vera gel extract on antioxidant enzymes and azoxymethane-induced oxidative stress in rats. Indian J Exp Biol. 2010;48:837–842. [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidase. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- Cui Z, Dewey S, Gomes AV. Cardioproteomics: advancing the discovery of signaling mechanisms involved in cardiovascular diseases. Am J Cardiovasc Dis. 2011;1:274–292. [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Adameova A, Kaur M. Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol. 2010;24:539–546. doi: 10.1111/j.1472-8206.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- Hossain H, Rahman SE, Akbar PN, Khan TA, Rahman MM, Jahan IA. HPLC profiling, antioxidant and in vivo anti-inflammatory activity of the ethanol extract of Syzygium jambos available in Bangladesh. BMC Res Notes. 2016;9:191. doi: 10.1186/s13104-016-2000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iji OT, Oyagbemi AA, Azeez OI. Assessment of chronic administration of Aloe vera gel on haematology, plasma biochemistry, lipid profiles and erythrocyte osmotic resistance in Wistar rats. Niger J Physiol Sci. 2010;25:107–113. [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Yu BP, Herlihy JT. The influence of long-term Aloe vera ingestion on age-related disease in male Fischer 344 rats. Phytother Res. 2002;16:712–718. doi: 10.1002/ptr.1022. [DOI] [PubMed] [Google Scholar]
- Jain NN. Dissertation. Jamia Hamdard University; New Delhi, India: 2015. Antidiabetic antioxidant and cardioprotective activities of Clitoria ternatea and Aloe vera gel and development of chromatographic markers. [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe vera Linn–an overview. Int J Pharm Sci Rev Res. 2010;4:106–110. [Google Scholar]
- Kaithwas G, Dubey K, Pillai KK. Effect of aloe vera (Aloe barbadensis Miller) gel on doxorubicin-induced myocardial oxidative stress and calcium overload in albino rats. Indian J Exp Biol. 2011;49:260–268. [PubMed] [Google Scholar]
- Kaithwas G, Singh P, Bhatia D. Evaluation of in vitro and in vivo antioxidant potential of polysaccharides from aloe vera (Aloe barbadensis Miller) gel. Drug Chem Toxicol. 2014;37:135–143. doi: 10.3109/01480545.2013.834350. [DOI] [PubMed] [Google Scholar]
- Kang MC, Kim SY, Kim YT, Kim EA, Lee SH, Ko SC, et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr Polym. 2014;99:365–371. doi: 10.1016/j.carbpol.2013.07.091. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K, Bai BR, Devaraj SN. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int J Cardiol. 2007;115:326–333. doi: 10.1016/j.ijcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RA. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-induced oxidative stress in the thyroid tissue of rats. BMC Complement Altern Med. 2012;12:181. doi: 10.1186/1472-6882-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore K. Effect of aloe vera (Aloe barbadensis) on thrombosis in mice. Pharmacologia. 2015;6:347–354. doi: 10.5567/pharmacologia.2015.347.354. [DOI] [Google Scholar]
- Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, et al. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009;11:140–146. doi: 10.1093/eurjhf/hfn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac β-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.CIR.101.14.1634. [DOI] [PubMed] [Google Scholar]
- Liaudet L, Calderari B, Pacher P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail Rev. 2014;19:815–824. doi: 10.1007/s10741-014-9418-y. [DOI] [PubMed] [Google Scholar]
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- Maynard SJ, Menown IB, Adgey AA. Troponin T or troponin I as cardiac markers in ischaemic heart disease. Heart. 2000;83:371–373. doi: 10.1136/heart.83.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- Nandal U, Bhardwaj RL. Aloe vera: a valuable wonder plant for food, medicine and cosmetic use–a review. Int J Pharm Sci Rev Res. 2012;13:59–67. [Google Scholar]
- Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Ochoa JJ, Quiles JL, Huertas JR, Mataix J. Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J Gerontol A Biol Sci Med Sci. 2005;60:970–975. doi: 10.1093/gerona/60.8.970. [DOI] [PubMed] [Google Scholar]
- Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, β-carotene and β-tocopherol in Aloe vera. Oxid Med Cell Longev. 2009;2:99–106. doi: 10.4161/oxim.2.2.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Priscilla DH, Prince PSM. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: a systematic review. J Tradit Complement Med. 2015;5:21–26. doi: 10.1016/j.jtcme.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada HN, Ussama ZS, Mahdy AM. Effectiveness of Aloe vera on the antioxidant status of different tissues in irradiated rats. Pharmazie. 2003;58:929–931. [PubMed] [Google Scholar]
- Sagor MAT, Tabassum N, Potol MA, Alam MA. Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/478039. 478039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu PK, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, et al. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol Pharm. 2013;4:599–610. doi: 10.4236/pp.2013.48086. [DOI] [Google Scholar]
- Song ZC, Wang ZS, Bai JH, Li Z, Hu J. Emodin, a naturally occurring anthraquinone, ameliorates experimental autoimmune myocarditis in rats. Tohoku J Exp Med. 2012;227:225–230. doi: 10.1620/tjem.227.225. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacol. 2014;2014 doi: 10.1155/2014/608590. 608590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WR, Tse J, Carter G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther. 1995;272:1011–1015. [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- Upaganlawar A, Gandhi H, Balaraman R. Isoproterenol induced myocardial infarction: protective role of natural products. J Pharmacol Toxicol. 2011;6:1–17. doi: 10.3923/jpt.2011.1.17. [DOI] [Google Scholar]
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi A, Hegazy S, Kabbash A, Wahab EAE. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm J. 2009;17:209–215. doi: 10.1016/j.jsps.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordoky BN, Aboutabl ME, El-Kadi AO. Modulation of cytochrome P450 gene expression and arachidonic acid metabolism during isoproterenol-induced cardiac hypertrophy in rats. Drug Metab Dispos. 2008;36:2277–2286. doi: 10.1124/dmd.108.023077. [DOI] [PubMed] [Google Scholar]