Abstract
Nanoemulsion drug delivery systems are advanced modes for delivering and improving the bioavailability of hydrophobic drugs and the drug which have high first pass metabolism. The nanoemulsion can be prepared by both high energy and low energy methods. High energy method includes high-pressure homogenization, microfluidization, and ultrasonication whereas low energy methods include the phase inversion emulsification method and the self-nanoemulsification method. Low energy methods should be preferred over high energy methods as these methods require less energy, so are more efficient and do not require any sophisticated instruments. However high energy methods are more favorable for food grade emulsion as they require lower quantities of surfactant than low energy methods. Techniques for formulation of nanoemulsion drug delivery system are overlapping in nature, especially in the case of low energy methods. In this review, we have classified different methods for formulation of nanoemulsion systems based on energy requirements, nature of phase inversion, and self-emulsification.
Keywords: nanoemulsion, drug delivery, high energy method, low energy method, phase inversion methods
INTRODUCTION
Nanoemulsion drug delivery systems are a promising tool for delivering and improving the bioavailability of hydrophobic drugs and bioactive food components in the blood. The majority of drugs are hydrophobic (lipophilic) in nature, thus leads to low solubility and bioavailability problems (Karthik et al., 2017; Mu et al., 2013; Qian and McClements, 2011); the bioactive food components also show low bioavailabilities in conventional doses. Such drug and food component formulations have low oral bioavailability, uncertain absorption profiles, dose variations, wide intra and inter-subject variabilities, and increased the chance of food effect. Thus, these drugs and bioactive food components express poor therapeutic efficacy (Chatterjee et al., 2016; Dokania and Joshi, 2015; Karthik et al., 2017).
Currently, lipid-based formulations are a good choice for delivering drug and bioactive food components, which have low oral bioavailability and other formulation problems (Feeney et al., 2016). Nanoemulsion drug delivery systems are lipid-based formulation system which improve the solubility and bioavailability of hydrophobic drugs and bioactive food components (Belhaj et al., 2010; Gursoy and Benita, 2004; Mohsin et al., 2009; Yuan et al., 2008). Flavonoids (flavanols, flavones, flavanones, and isoflavones), nonflavonoids (hydroxybenzoic acids, stilbenes, and curcuminoids), and carotenoids (carotenes and xanthophylls) are food bioactive compounds that have been encapsulated successfully in nanoemulsions formulations (Donsì, 2018).
These nanoemulsion systems have high interfacial areas and stabilities, protect compounds from adverse environmental conditions and improve their stability (Madene et al., 2006). Nanoemulsion systems can be used for delivering drugs via transmucosal and transdermal routes. Therefore these systems can effectively improve bioavailability (Rehman et al., 2017).
NANOEMULSION
Nanoemulsion is defined as a colloidal dispersion of two immiscible liquids that is thermodynamically unstable. In nanoemulsion, one of the liquids forms the dispersed phase and other liquid forms the dispersing medium (McClements, 2012). Nanoemulsion comprises droplets with diameters ranging from 10~200 nm and each droplet has a protective coating of emulsifier molecules (Acosta, 2009; Cerpnjak et al., 2013; Gibaud and Attivi, 2012; Rehman et al., 2017).
Self-emulsifying formulation
Self-emulsifying formulations generally comprise of self-emulsifying drug delivery systems (SEDDS) and self-nanoemulsifying drug delivery systems (SNEDDS). SEDDS give coarse emulsion whereas SNEDDS provide nano-size emulsion. These systems are isotropic mixtures of an oil, surfactant, and co-surfactant. Upon in vivo dilution by the aqueous phase, these systems form emulsions (in case of SEDDS) or fine and optical clear nanoemulsions (in case of SNEDDS) under gentle agitation, experienced due to gastrointestinal tract (GIT) motility. SEDDS and SNEDDS are generally described as emulsion or nanoemulsion pre-concentrates because the emulsion or nanoemulsion is formed from dilution in aqueous media in vivo (Pouton, 2000; Pouton and Porter, 2008).
Component of nanoemulsion
Components of nanoemulsion systems include oils, lipids, surfactants, water-soluble co-solvents, and water. In the formulation of nanoemulsions, the oil phase may include triglycerides like tri-, di-, or mono-acylglycerols, vegetable oils, mineral oils, free fatty acids etc. (Gonçalves et al., 2018). Oil selection is generally based on the drug solubility. Oil phases which have high drug loading is generally used for development of nanoemulsion (Qadir et al., 2016). Common surfactants used in the nanoemulsion systems for drug delivery and food ingredients are spans (sorbitan fatty acid esters), tweens [polyoxyethylene (POE) derivatives of sorbitan fatty acid ester], Cremophor® EL (polyoxyl-35 castor oil), lauroyl macro-golglycerides (Gelucire® 44/14), polysaccharides (gum and starch derivatives), phospholipids (egg, soy, or dairy lecithin), and amphiphilic proteins (whey protein isolate and caseinate) (Komaiko and McClements, 2016; Singh et al., 2017).
Ultra-low negative interfacial tension is required for nanoemulsion formation. For this purpose, co-surfactants or co-solvents are used along with a surfactant. Co-surfactants or co-solvents that are generally used in formulation of nanoemulsion systems are polyethylene glycol, propylene glycol, ethanol, transcutol-P (diethylene glycol monoethyl ether), ethylene glycol, glycerin, and propanol (Khan et al., 2012; Singh et al., 2017).
FORMULATION TECHNIQUES OF NANOEMULSION DRUG DELIVERY SYSTEMS
The techniques employed in formulation of nanoemulsion drug delivery systems are diverse and show a large degree of overlapping. We have classified different methods for preparation of nanoemulsion drug delivery systems by energy requirements, nature of phase inversion and self-emulsification
High energy methods
High-pressure homogenization
Microfluidization
Ultrasonication
Low energy methods
-
i) Phase inversion emulsification method
-
– Transitional phase inversion (TPI)
Phase inversion temperature (PIT)
Phase inversion composition (PIC)
-
– Catastrophic phase inversion (CPI)
Emulsion inversion point (EIP)
-
The self-nanoemulsification method
High energy methods
High energy methods are extensively used to formulate nanoemulsion (Mahdi Jafari et al., 2006). High mechanical energy is used that provide strong disruptive forces, which break up large droplets to nano-sized droplets and produce nanoemulsions with high kinetic energy. The disruptive forces are created by using mechanical devices such as ultrasonicators, microfluidizer, and high-pressure homogenizers (Gonçalves et al., 2018). By using high energy methods, we can achieve a greater control of particle size with a choice of formulation composition. High energy methods also provide controls for stability, rheology, and color of the emulsion (Graves et al., 2005; Gursoy and Benita, 2004). In case of food ingredients, high energy methods of nanoemulsion formulation have the advantage of reducing risk of spoilage and inactivation of food components without affecting food safety, and nutritional and sensory attributes (Gharibzahedi et al., 2019). High energy methods involve the following methods.
High-pressure homogenization
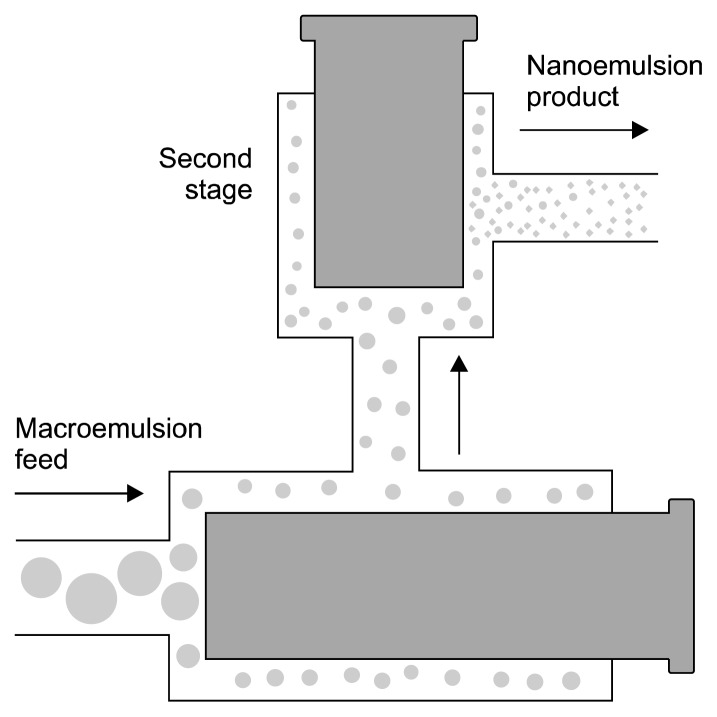
High-pressure homogenizers supply high energy and give homogeneous flow to generate smallest particle sizes. Therefore, high-pressure homogenizers are most widely used to prepare nanoemulsions. High-pressure homogenizers are used to create intensely disruptive forces that form nanoemulsions of extremely low particle size (up to 1 nm) (Rai et al., 2018). The coarse emulsion is then passed through a small orifice with high pressure (500 to 5,000 psi) (Fig. 1). Several forces, such as intense turbulence, hydraulic shear, and cavitation, are applied together during this process, to give nanoemulsions with very small droplet sizes (Floury et al., 2000; Schultz et al., 2004).
Fig. 1.
High-pressure homogenization techniques (Wang, 2014).
The particle size of nanoemulsions produced by high-pressure homogenizers depend on sample composition, homogenizer type, and homogenizer operating conditions such as energy intensity, time, and temperature (Qian and McClements, 2011). Increasing the intensity of homogenization decreases the size of droplets of the nanoemulsions. In certain cases, such as when biopolymers are used as an emulsifier, intense homogenization can lead to an increase in particle size of resulting nanoemulsion. Hence small-molecule surfactants should be used as emulsifiers in high-pressure homogenizers as they are more effective than biopolymer for producing nanoemulsions (Azeem et al., 2009a; Azeem et al., 2009b; Jafari et al., 2008; Jafari et al., 2007). High-pressure homogenization is widely used to form food, pharmaceutical, and biotechnological ingredient nanoemulsions, as shown in Table 1 (Hsieh et al., 2012; Yuan et al., 2008).
Table 1.
Various active ingredients, loaded in nanoemulsion using different techniques
| Techniques | Active ingredients |
|---|---|
| High-pressure homogenization | Quercetin (Karadag et al., 2013), peppermint oil (Liang et al., 2012), carotenoid (Salvia-Trujillo et al., 2015), peanut milk (Zaaboul et al., 2019), capsaicin (Akbas et al., 2018), primaquine (Singh and Vingkar, 2008), and paclitaxel (Yang et al., 2014). |
| Microfluidization | Essential oil (Salvia-Trujillo et al., 2015), D-limonene (Mahdi Jafari et al., 2006), fish oil (García-Márquez et al., 2017), curcumin (Kim et al., 2016), and β-carotene (Luo et al., 2017). |
| Ultrasonicator | Cinnamon oil (Sugumar et al., 2014), eucalyptus oil (Ghosh et al., 2013b), bovine serum, albumin (Tabibiazar et al., 2015), capsaicin (Akbas et al., 2018), aspirin (Tang et al., 2012), and artemether (Laxmi et al., 2015). |
| Phase inversion composition method | Hexadecane and oleic acid mixture (Maestro et al., 2008), lidocaine (Sadurní et al., 2005), and hydrogenated polyisobutene (Sonneville-Aubrun et al., 2009). |
| Phase inversion temperature method | Fisetin (Ragelle et al., 2012), isohexadecane (Izquierdo et al., 2004), minaral oil (Morales et al., 2006), cinnamon oil (Chuesiang et al., 2018), and lemon oil (Mashhadi et al., 2016). |
| Emulsion inversion point method | Curcumin (Borrin et al., 2016) and vitamin E (Mayer et al., 2013). |
| Self-nanoemulsification method | Glimepiride (Mohd et al., 2015), ibuprofen (Zhao et al., 2015), valsartan (Bandyopadhyay et al., 2015), and glibenclamide (Shakeel et al., 2013). |
Microfluidization
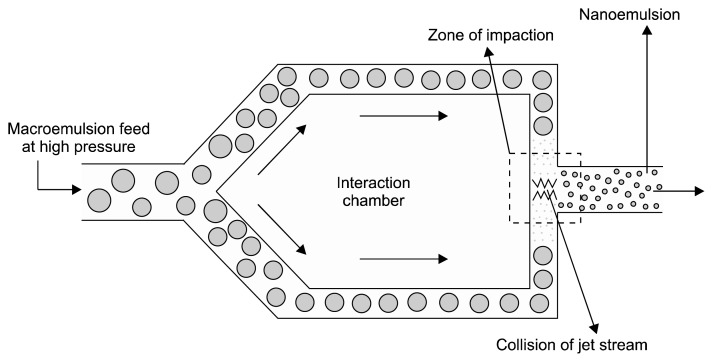
Microfluidization is a mixing technology at micro size level that uses a device called microfluidizer. In microfludization, fluids are forced to pass through the microchannels under high-pressure (500~20,000 psi). Microchannels are generally micro size channels which allow mixing at micro size level (Qadir et al., 2016; Whitesides, 2006). The phases of macroemulsion (aqueous and oil phases) are mixed together and then passed through the microfluidizer. The macroemulsion is guided through the microchannels under high pressure towards the interaction chamber. In the interaction chamber, two streams of macroemulsions strike each other at high velocity. This collision creates forces like shearing, cavitation, and impact, which produce stable nanoemulsions (Fig. 2) (Jafari et al., 2007; Lovelyn and Attama, 2011; Maa and Hsu, 1999; Mahdi Jafari et al., 2006).
Fig. 2.
Microfluidization techniques (Singh et al., 2017).
Microfluidizers produce narrower and smaller nanoemulsion particle size distributions of than homogenizers (Perrier-Cornet et al., 2005; Wooster et al., 2008). Also, microfluidizers produce stable nanoemulsions at low surfactant concentrations (Pinnamaneni et al., 2003). Microfluidization methods have been used to produce food ingredient nanoemulsions (Goh et al., 2015; Zhang et al., 2015). Microfluidization techniques produce food grade nanoemulsions with uniform droplet size distributions and greater stabilities (Villalobos-Castillejos et al., 2018).
Ultrasonication
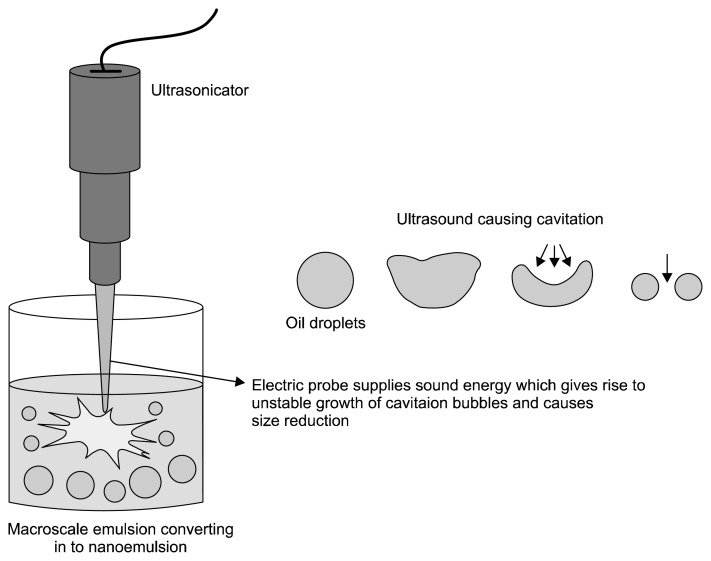
Ultrasonication is better than other high energy methods in terms of operation and cleaning (Leong et al., 2009; Mahdi Jafari et al., 2006). In ultrasonic emulsifications, ultrasonic waves provide cavitation forces that break the macroemulsion to nanoemulsion. In this method, ultrasonicators are used, which consist of a probe that emits ultrasonic waves. By varying ultrasonic energy input and time, we can achieve the desired particle size and stability of the nanoemulsion. In ultrasonic emulsification, physical shear is mainly provided by the process of acoustic cavitation (Jayasooriya et al., 2004). Cavitation is the phenomenon of formation and growth of microbubbles and then collapse of microbubbles, which are caused by the pressure fluctuations of the acoustic wave (Fig. 3). The collapse of microbubbles cause intense turbulence that causes formation of nano-sized droplets (Canselier et al., 2002; Leong et al., 2009).
Fig. 3.
Ultrasonication techniques (Singh et al., 2017).
Irradiation of an oil and water system by ultrasound causes cavitation forces and provide excess energy for new interface formations, forming nano-sized emulsion droplets. Through ultrasonication, nanoemulsions can be produced in the absence of surfactants (Gaikwad and Pandit, 2008; Jafari et al., 2007). In a recent study, it was shown that efficiency of ultrasonic emulsification is dependent on ultrasonication intensity, time, and nature of the surfactant (Landfester et al., 2004). Ultrasonication has been used extensively for producing nanoemulsions of drugs and food ingredients. Food grade ultrasonication nanoemulsion shows greater stability and smaller droplet size, and requires less energy input than other high energy method (Ghosh et al., 2013a; Salvia-Trujillo et al., 2014; Tiwari et al., 2006).
Low energy method
These methods require low energy for production of nanoemulsion systems. Low-energy emulsification methods are more energy efficient as these methods utilize internal chemical energy of the systems, and require only gentle stirring for production of the nanoemulsions (Solans and Solé, 2012). Low-energy emulsification methods generally involve phase inversion emulsification and self-emulsification. Generally low energy methods are not considered for formulation of food grade nanoemulsions as they require high concentration of surfactant, which adversely affect food formulation taste and safety (Komaiko and McClements, 2016).
Phase inversion emulsification method
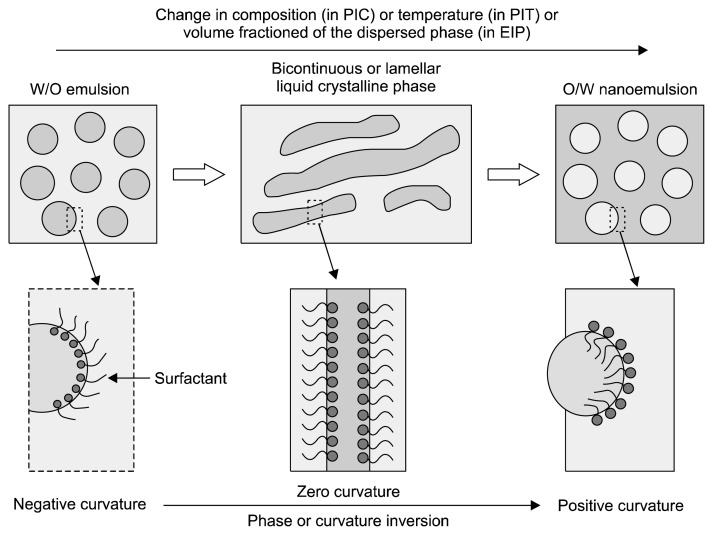
In this method, spontaneous curvature of surfactant causes phase transition during the emulsification process. Changes in spontaneous curvature of the surfactant occur by changes in parameters like temperature, composition, etc. (Fig. 4) (Solè et al., 2010). There are two types of phase inversion emulsification methods: TPI methods, which involve PIT and PIC, and CPI methods, which involves EIP (Fig. 4).
Fig. 4.
Phase inversion emulsification techniques (Solans and Solé, 2012). PIC, phase inversion composition; PIT, phase inversion temperature; EIP, emulsion inversion point; O/W, oil-in-water emulsion; W/O, water-in-oil emulsion.
Transitional phase inversion takes place due to the changes in spontaneous curvature or affinity of the surfactant due to changes in parameters like temperature and composition (Ishak and Annuar, 2016; Solans and Solé, 2012; Solè et al., 2010). However, CPI occurs when dispersed phase added continuously until the dispersed phase drops are aggregated with each other to form bi-continuous/lamellar structural phases (Ishak and Annuar, 2016). The catastrophe means a sudden change in the behavior of a system, due to changing conditions. For catastrophic phase inversion to occur, it is important that the surfactant is chiefly presented in the dispersed phase, thus the rate of coalescence is high, which leads to rapid phase inversion (Armanet and Hunkeler, 2007). During transitional phase inversion, spontaneous curvature or surfactant affinity is changed, whereas in catastrophic phase inversion spontaneous curvature or surfactant affinity does not change.
– PIT method
In the PIT method, surfactant spontaneous curvature is inversed by changing temperature. Nonionic surfactants, such as polyethoxylated surfactants, undergo dehydration of POE groups of polyethoxylated surfactant, which makes it more lipophilic and leads to changes in curvature of the surfactant. Thus, phase inversion occurs and nanoemulsion is produced (Moreira de Morais et al., 2006; Solans and Solé, 2012). In this method, oil, water, and nonionic surfactants are mixed at room temperature to form oil-in-water (O/W) emulsions. Then, as the temperature gradually increases, dehydration of surfactant POE groups occurs that makes surfactant more lipophilic and surfactant start showing a higher affinity towards the oily phase. This cause phase inversion from the initial O/W emulsion to water-in-oil (W/O) nanoemulsion through intermediate liquid crystalline or bi-continuous structures (e.g. lamellar phase). At hydrophile-lipophile balance (HLB) temperatures (an intermediate temperature) the non-ionic surfactant has zero curvature and shows a similar affinity to the aqueous and oily phases (Izquierdo et al., 2005; Izquierdo et al., 2004; Izquierdo et al., 2002). For efficient phase inversion, rapid cooling or heating of HLB (for obtaining O/W or W/O emulsions, respectively) is required. Rapid cooling or heating produces kinetically stable nanoemulsions (Solans and Solé, 2012).
– PIC method
The phase inversion composition or PIC method is similar to the PIT method; however, in PIC, phase inversion is achieved by changing the system composition rather than the system temperature (Sokolov, 2014).
In PIC, one of the components such as water is added to a mixture, and oil-surfactant or oil is added to the water-surfactant mixture. POE type nonionic surfactants are generally used in PIC method to formulate nanoemulsions, although other types can also be used. When water is added slowly to the oil phase and as the volume of the water fraction increases, surfactant POE chain hydration occurs. The surfactant hydrophilic-lipophilic properties of the water phase will become balanced and spontaneous curvature of surfactant will change to zero, similar to at the HLB temperature in the PIT method. During this transition, a bi-continuous or lamellar structure is formed. When additional water is added the transition composition is exceeded, and the structures of the surfactant layer with zero curvature change to having high positive curvature. This change in curvature leads to phase inversion and causes nano-size droplet formation. Thus, changing the composition of the system causes phase inversion (Solans and Solé, 2012; Vandamme and Anton, 2010). Similarly, other composition parameters, such as the addition of salt and pH changes, also cause nano-size emulsion droplets by transitional phase inversion (Maestro et al., 2008; McClements and Rao, 2011; Sokolov, 2014).
– EIP method
In the EIP method, phase inversion occurrs through CPI mechanisms (Sokolov, 2014). The catastrophic phase inversion is induced by changing the fractioned volume of the dispersed phase rather than the surfactant properties (Fernandez et al., 2004; Ishak and Annuar, 2016; McClements and Rao, 2011). As the water phase is added to the oil-surfactant mix, the system starts acting as a W/O nanoemulsion. When increasing amounts of water is added to above a critical water content with continuous stirring, water droplets merge with each other and the phase inversion point is reached; this causes bi-continuous or lamellar structures to be formed. Further dilution with water causes phase inversion from a W/O to an O/W system through intermediate bi-continuous microemulsion. The sizes of the nanoemulsion droplets formed depend on the process variables, such as the rate of water addition and the stirring speed. For catastrophic phase inversion to occur, the surfactant should primarily present in the dispersed phase, so the rate of coalescence is high and rapid phase inversion occurs. Small molecule surfactants can be used in catastrophic phase inversion. These surfactants are able to stabilize both W/O emulsions and O/W emulsions (Armanet and Hunkeler, 2007; Fernandez et al., 2004; Sokolov, 2014). Initially in catastrophic phase inversion, the surfactant is mainly present in the dispersed phase, thus it behaves as an abnormal emulsion (unstable emulsion) which does not obey Bancroft’s rules. According to Bancroft’s rules, for a stable emulsion (normal emulsion) emulsifier should predominantly present in the continuous phase (Perazzo et al., 2015). Therefore, catastrophic phase inversion occurs from the abnormal emulsion to form a more stable normal emulsion.
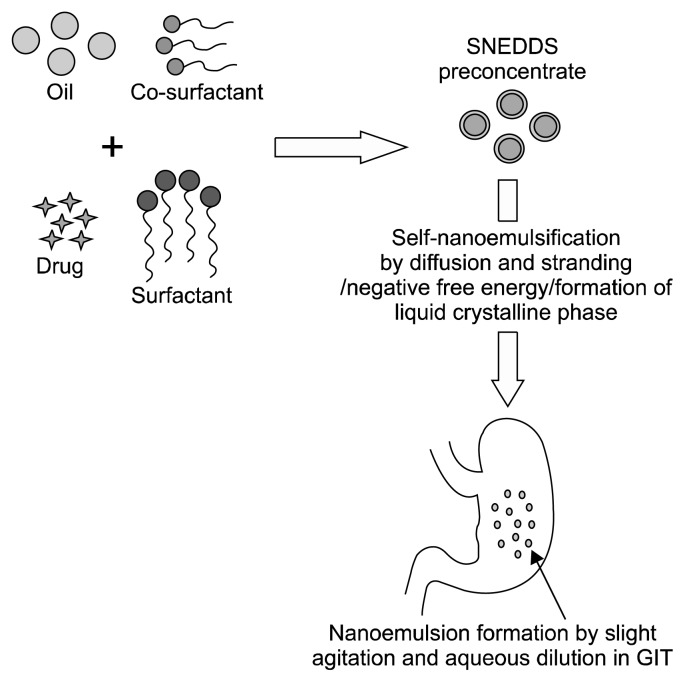
Self-nanoemulsification method
In the self-emulsification method, nanoemulsion formation is achieved without changing the spontaneous curvature of the surfactant. Surfactant and/or co-solvent molecules rapidly diffuse from the dispersed phase to the continuous phase, which causes turbulence and creates nano-sized emulsion droplets. The self-emulsification method is also referred to as the spontaneous emulsification method (Solans et al., 2016; Solans and Solé, 2012; Solè et al., 2012). SNEDDS are based on the self-emulsification phenomenon and contain more hydrophilic surfactants or co-surfactants (co-solvents), and a lower lipid content (Agrawal et al., 2012). SNEDDS can be defined as isotropic mixture of an oil, surfactant, co-surfactant, and drug. When this mixture is diluted by aqueous fluids in vivo, it form fine and optical clear O/W nanoemulsion, aided by gentle agitation provided by digestive motility of the stomach and intestine (Fig. 5) (Bandyopadhyay et al., 2014; Khan et al., 2015).
Fig. 5.
Self-nanoemulsification techniques (Alshamsan et al., 2018). SNEDDS, self-nanoemulsifying drug delivery system; GIT, gastrointestinal tract.
The two most commonly reported mechanisms of nanoemulsion formation from SNEDDS are diffusion of the hydrophilic co-solvent or co-surfactant from the organic phase into the aqueous phase (Pouton, 2000; Solans and Solé, 2012; Solè et al., 2012), and formation of nanoemulsion negative free energy at transient negative or ultra-low interfacial tensions (Agrawal et al., 2012; Gurram et al., 2015; Kohli et al., 2010). SNEDDS are also the most popular and promising tool for delivery of hydrophobic drugs with low bioavailability (Meena et al., 2012; Patel and Vavia, 2007; Patel et al., 2016; Suryawanshi and Kondawar, 2014). SNEDDS have also been used for delivery of bioactive food components (Kheawfu et al., 2018).
Stability of nanoemulsion system
During storage, nanoemulsion may become turbid or phases of nanoemulsion may separate due to mechanisms of instability such as flocculation, sedimentation, coalescence, and Ostwald ripening (Karthik et al., 2017). Nanoemulsion system destabilization kinetics are very slow (several months), therefore nanoemulsion systems are kinetically stable (Rehman et al., 2017). Nanoemulsion systems produce smaller droplets sizes than conventional macro emulsions; thus, Brownian motion effects are much more dominate then gravitational forces and have greater gravitational separation stability. Flocculation and coalescence occur due to the attractive forces between the droplets, which are generally very low in nano-sized emulsion systems. Thus, nanoemulsion also shows much better stability towards the flocculation and coalescence (Qian and McClements, 2011). Ostwald ripening is another mechanism of nanoemulsion instability, which commonly occurs in food grade nanoemulsion containing essential oil and short chain triglycerides. Dairy based nanoemulsion is relatively stable towards Ostwald ripening due to the presence of insoluble long-chain triglyceride oils. Ostwald repining can be prevented by using greater hydrophobic oil during formulation (Karthik et al., 2017).
ADVANTAGES OF NANOEMULSION DRUG DELIVERY SYSTEM
Nanoemulsion drug delivery systems are effective in solubilizing active lipophilic compounds, and therefore have for several applications (Gutiérrez et al., 2008). The very small particle sizes of nanoemulsion drug delivery systems is a promising advantage over conventional emulsions; these systems therefore have optically transparent appearances, and show greater stability against droplet flocculation and coalescence (Qian and McClements, 2011; Solans et al., 2005; Tadros et al., 2004). Nanoemulsion drug delivery system has shown potential for effective systemic delivery of active components, such as food ingredients and lipophilic drugs, via oral, parenteral, ocular, and topical routes (Hu et al., 2004; McClements et al., 2007; de Oca-Ávalos et al., 2017; Sanguansri and Augustin, 2006; Solans et al., 2005; Weiss et al., 2008). O/W vitamin nanoemulsions and nutraceuticals facilitate solubilization of these hydrophobic bioactive food components in GIT, therefore increasing bioavailability (Komaiko and McClements, 2016).
Nanoencapsulation of food ingredients in nanoemulsion systems also increase the physio-chemical stability of bioactive compound. Moreover, nanoemulsion food component systems enhance delivery the bioactive compounds to the intradermal layers through diffusion, and are therefore helpful in developing herbal cosmetics (Karthik et al., 2017). The cosmetic applications of nanoemulsion systems derive from properties of system such as nano-sized droplets, low viscosity, and transparency (Solans et al., 2005; Sonneville-Aubrun et al., 2004; Thakur et al., 2012). The physio-chemical properties of nanoemulsion systems improve bioactivity of encapsulated components, and have wide application for delivery of antibiotics, anticancer agents, disinfectants, and antiseptics (Karthik et al., 2017). Thus, nanoemulsion drug delivery systems are promising methods for formulation of drugs, food ingredients, and cosmetic agents.
CONCLUSION
Nanoemulsion drug delivery systems effectively overcome the low bioavailability drawback associated with drugs and food components which are hydrophobic, and having high first pass metabolism. Methods for nanoemulsion drug delivery system formulation can be conveniently classified in a rigid fashion based on the energy required, nature of phase inversion and self-emulsification. High energy methods for nanoemulsion drug delivery system formulation have greater control over dispersion of particle sizes and are more flexible for the choice of composition. High energy methods have been used by researchers to improve delivery of drugs and bioactive food components. For providing high energy, sophisticated instruments are required; high energy methods are therefore more costly then low energy methods as these methods require low energy and are more efficient. High energy methods are more useful for delivery of nanoemulsions containing bioactive food components, as these methods require low concentrations of surfactant. For delivery of hydrophobic drugs with low bioavailability, SNEDDS are the most popular methods used by researchers. However, further research is required to uncover the potential of phase inversion emulsification methods in effective drug loading and delivery.
ACKNOWLEDGEMENTS
The corresponding author acknowledges University Grants Commission (UGC), Government of India, for a Junior Research Fellowship [UGC-NET JRF Award, F.15-6(DEC.2013)/2014(NET)].
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface Sci. 2009;14:3–15. doi: 10.1016/j.cocis.2008.01.002. [DOI] [Google Scholar]
- Agrawal S, Giri TK, Tripathi DK, Ajazuddin Alexander A. A review on novel therapeutic strategies for the enhancement of solubility for hydrophobic drugs through lipid and surfactant based self micro emulsifying drug delivery system: a novel approach. Am J Drug Discovery Dev. 2012;2:143–183. doi: 10.3923/ajdd.2012.143.183. [DOI] [Google Scholar]
- Akbas E, Soyler B, Oztop MH. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT. 2018;96:266–273. doi: 10.1016/j.lwt.2018.05.043. [DOI] [Google Scholar]
- Alshamsan A, Kazi M, Badran MM, Alanazi FK. Role of alternative lipid excipients in the design of self-nanoemulsifying formulations for fenofibrate: characterization, in vitro dispersion, digestion and ex vivo gut permeation studies. Front Pharmacol. 2018;9:1219. doi: 10.3389/fphar.2018.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanet L, Hunkeler D. Phase inversion of polyacrylamide-based inverse-emulsions: influence of inverting-surfactant type and concentration. J Appl Polym Sci. 2007;103:3567–3584. doi: 10.1002/app.25062. [DOI] [Google Scholar]
- Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009a;10:69–76. doi: 10.1208/s12249-008-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeem A, Rizwan M, Ahmad FJ, Khar RK, Iqbal Z, Talegaonkar S. Components screening and influence of surfactant and co-surfactant on nanoemulsion formation. Curr Nanosci. 2009b;5:220–226. doi: 10.2174/157341309788185505. [DOI] [Google Scholar]
- Bandyopadhyay S, Beg S, Katare OP, Sharma G, Singh B. QbD-Oriented development of self-nanoemulsifying drug delivery systems (SNEDDS) of valsartan with improved biopharmaceutical performance. Curr Drug Deliv. 2015;2:544–563. doi: 10.2174/1567201812666150227125639. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Katare OP, Singh B. Development of optimized supersaturable self-nanoemulsifying systems of ezetimibe: effect of polymers and efflux transporters. Expert Opin Drug Deliv. 2014;11:479–492. doi: 10.1517/17425247.2014.877885. [DOI] [PubMed] [Google Scholar]
- Belhaj N, Arab-Tehrany E, Linder M. Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process Biochem. 2010;45:187–195. doi: 10.1016/j.procbio.2009.09.005. [DOI] [Google Scholar]
- Borrin TR, Georges EL, Moraes ICF, Pinho SC. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: an evaluation of process parameters and physico-chemical stability. J Food Eng. 2016;169:1–9. doi: 10.1016/j.jfoodeng.2015.08.012. [DOI] [Google Scholar]
- Canselier JP, Delmas H, Wilhelm AM, Abismaïl B. Ultrasound emulsification–an overview. J Dispersion Sci Technol. 2002;23:333–349. doi: 10.1080/01932690208984209. [DOI] [Google Scholar]
- Cerpnjak K, Zvonar A, Gašperlin M, Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63:427–445. doi: 10.2478/acph-2013-0040. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23:3639–3652. doi: 10.1080/10717544.2016.1214990. [DOI] [PubMed] [Google Scholar]
- Chuesiang P, Siripatrawan U, Sanguandeekul R, McLandsborough L, McClements DJ. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: impact of oil phase composition and surfactant concentration. J Colloid Interface Sci. 2018;514:208–216. doi: 10.1016/j.jcis.2017.11.084. [DOI] [PubMed] [Google Scholar]
- de Oca-Ávalos JMM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. Curr Opin Food Sci. 2017;16:1–6. doi: 10.1016/j.cofs.2017.06.003. [DOI] [Google Scholar]
- Dokania S, Joshi AK. Self-microemulsifying drug delivery system (SMEDDS)–challenges and road ahead. Drug Deliv. 2015;22:675–690. doi: 10.3109/10717544.2014.896058. [DOI] [PubMed] [Google Scholar]
- Donsì F. Applications of nanoemulsions in foods. In: Jafari SM, McClements DJ, editors. Nanoemulsions: Formulation, Applications, and Characterization. Academic Press; San Diego, CA, USA: 2018. pp. 349–376. [DOI] [Google Scholar]
- Feeney OM, Crum MF, McEvoy CL, Trevaskis NL, Williams HD, Pouton CW, et al. 50 years of oral lipid-based formulations: provenance, progress and future perspectives. Adv Drug Deliv Rev. 2016;101:167–194. doi: 10.1016/j.addr.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Fernandez P, André V, Rieger J, Kühnle A. Nano-emulsion formation by emulsion phase inversion. Colloids Surf A Physicochem Eng Aspects. 2004;251:53–58. doi: 10.1016/j.colsurfa.2004.09.029. [DOI] [Google Scholar]
- Floury J, Desrumaux A, Lardières J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov Food Sci Emerg Technol. 2000;1:127–134. doi: 10.1016/S1466-8564(00)00012-6. [DOI] [Google Scholar]
- Gaikwad SG, Pandit AB. Ultrasound emulsification: effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason Sonochem. 2008;15:554–563. doi: 10.1016/j.ultsonch.2007.06.011. [DOI] [PubMed] [Google Scholar]
- García-Márquez E, Higuera-Ciapara I, Espinosa-Andrews H. Design of fish oil-in-water nanoemulsion by microfluidization. Innov Food Sci Emerg Technol. 2017;40:87–91. doi: 10.1016/j.ifset.2016.11.007. [DOI] [Google Scholar]
- Gharibzahedi SMT, Hernández-Ortega C, Welti-Chanes J, Putnik P, Barba FJ, Mallikarjunan K, et al. High pressure processing of food-grade emulsion systems: antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll. 2019;87:307–320. doi: 10.1016/j.foodhyd.2018.08.012. [DOI] [Google Scholar]
- Ghosh V, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason Sonochem. 2013a;20:338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: investigation of its bactericidal activity. J Nanosci Nanotechnol. 2013b;13:114–122. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- Gibaud S, Attivi D. Microemulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv. 2012;9:937–951. doi: 10.1517/17425247.2012.694865. [DOI] [PubMed] [Google Scholar]
- Goh PS, Ng MH, Choo YM, Amru NB, Chuah CH. Production of nanoemulsions from palm-based tocotrienol rich fraction by microfluidization. Molecules. 2015;20:19936–19946. doi: 10.3390/molecules201119666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Nikmaram N, Roohinejad S, Estevinho BN, Rocha F, Greiner R, et al. Production, properties, and applications of solid self-emulsifying delivery systems (S-SEDS) in the food and pharmaceutical industries. Colloids Surf A Physicochem Eng Aspects. 2018;538:108–126. doi: 10.1016/j.colsurfa.2017.10.076. [DOI] [Google Scholar]
- Graves S, Meleson K, Wilking J, Lin MY, Mason TG. Structure of concentrated nanoemulsions. J Chem Phys. 2005;122:134703. doi: 10.1063/1.1874952. [DOI] [PubMed] [Google Scholar]
- Gurram AK, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy MS. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci. 2015;77:249–257. doi: 10.4103/0250-474X.159596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, González C, Maestro A, Solè I, Pey CM, Nolla J. Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13:245–251. doi: 10.1016/j.cocis.2008.01.005. [DOI] [Google Scholar]
- Hsieh CW, Li PH, Lu IC, Wang TH. Preparing glabridin-in-water nanoemulsions by high pressure homogenization with response surface methodology. J Oleo Sci. 2012;61:483–489. doi: 10.5650/jos.61.483. [DOI] [PubMed] [Google Scholar]
- Hu J, Johnston KP, Williams RO., III Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm. 2004;30:233–245. doi: 10.1081/DDC-120030422. [DOI] [PubMed] [Google Scholar]
- Ishak KA, Annuar MSM. Phase inversion of medium-chain-length poly-3-hydroxyalkanoates (mcl-PHA)-incorporated nanoemulsion: effects of mcl-PHA molecular weight and amount on its mechanism. Colloid Polym Sci. 2016;294:1969–1981. doi: 10.1007/s00396-016-3957-9. [DOI] [Google Scholar]
- Izquierdo P, Esquena J, Tadros TF, Dederen C, Garcia MJ, Azemar N, et al. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir. 2002;18:26–30. doi: 10.1021/la010808c. [DOI] [Google Scholar]
- Izquierdo P, Esquena J, Tadros TF, Dederen JC, Feng J, Garcia-Celma MJ, et al. Phase behavior and nano-emulsion formation by the phase inversion temperature method. Langmuir. 2004;20:6594–6598. doi: 10.1021/la049566h. [DOI] [PubMed] [Google Scholar]
- Izquierdo P, Feng J, Esquena J, Tadros TF, Dederen JC, Garcia MJ, et al. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J Colloid Interface Sci. 2005;285:388–394. doi: 10.1016/j.jcis.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Jafari SM, Assadpoor E, He Y, Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008;22:1191–1202. doi: 10.1016/j.foodhyd.2007.09.006. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Jayasooriya SD, Bhandari BR, Torley P, D’Arcy BR. Effect of high power ultrasound waves on properties of meat: a review. Int J Food Prop. 2004;7:301–319. doi: 10.1081/JFP-120030039. [DOI] [Google Scholar]
- Karadag A, Yang X, Ozcelik B, Huang Q. Optimization of preparation conditions for quercetin nanoemulsions using response surface methodology. J Agric Food Chem. 2013;61:2130–2139. doi: 10.1021/jf3040463. [DOI] [PubMed] [Google Scholar]
- Karthik P, Ezhilarasi PN, Anandharamakrishnan C. Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr. 2017;57:1435–1450. doi: 10.1080/10408398.2015.1006767. [DOI] [PubMed] [Google Scholar]
- Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin Drug Deliv. 2012;9:1305–1317. doi: 10.1517/17425247.2012.719870. [DOI] [PubMed] [Google Scholar]
- Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22:552–561. doi: 10.3109/10717544.2013.878003. [DOI] [PubMed] [Google Scholar]
- Kheawfu K, Pikulkaew S, Rades T, Müllertz A, Okonogi S. Development and characterization of clove oil nanoemulsions and self-microemulsifying drug delivery systems. J Drug Delivery Sci Technol. 2018;46:330–338. doi: 10.1016/j.jddst.2018.05.028. [DOI] [Google Scholar]
- Kim SH, Ji YS, Lee ES, Hong ST. Ostwald ripening stability of curcumin-loaded MCT nanoemulsion: influence of various emulsifiers. Prev Nutr Food Sci. 2016;21:289–295. doi: 10.3746/pnf.2016.21.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15:958–965. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Komaiko JS, McClements DJ. Formation of food-grade nanoemulsions using low-energy preparation methods: a review of available methods. Compr Rev Food Sci Food Saf. 2016;15:331–352. doi: 10.1111/1541-4337.12189. [DOI] [PubMed] [Google Scholar]
- Landfester K, Eisenblätter J, Rothe R. Preparation of polymerizable miniemulsions by ultrasonication. J Coat Technol Res. 2004;1:65–68. doi: 10.1007/s11998-004-0026-y. [DOI] [Google Scholar]
- Laxmi M, Bhardwaj A, Mehta S, Mehta A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells Nanomed Biotechnol. 2015;43:334–344. doi: 10.3109/21691401.2014.887018. [DOI] [PubMed] [Google Scholar]
- Leong TSH, Wooster TJ, Kentish SE, Ashokkumar M. Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem. 2009;16:721–727. doi: 10.1016/j.ultsonch.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Liang R, Xu S, Shoemaker CF, Li Y, Zhong F, Huang Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem. 2012;60:7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2:626–639. doi: 10.4236/jbnb.2011.225075. [DOI] [Google Scholar]
- Luo X, Zhou Y, Bai L, Liu F, Deng Y, McClements DJ. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: physical and chemical stability. J Colloid Interface Sci. 2017;490:328–335. doi: 10.1016/j.jcis.2016.11.057. [DOI] [PubMed] [Google Scholar]
- Maa YF, Hsu CC. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharm Dev Technol. 1999;4:233–240. doi: 10.1081/PDT-100101357. [DOI] [PubMed] [Google Scholar]
- Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release–a review. Int J Food Sci Technol. 2006;41:1–21. doi: 10.1111/j.1365-2621.2005.00980.x. [DOI] [Google Scholar]
- Maestro A, Solè I, González C, Solans C, Gutiérrez JM. Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method. J Colloid Interface Sci. 2008;327:433–439. doi: 10.1016/j.jcis.2008.07.059. [DOI] [PubMed] [Google Scholar]
- Mahdi Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization–a comparison. Int J Food Prop. 2006;9:475–485. doi: 10.1080/10942910600596464. [DOI] [Google Scholar]
- Mashhadi S, Javadian H, Tyagi I, Agarwal S, Gupta VK. The effect of Na2SO4 concentration in aqueous phase on the phase inversion temperature of lemon oil in water nano-emulsions. J Mol Liq. 2016;215:454–460. doi: 10.1016/j.molliq.2016.01.045. [DOI] [Google Scholar]
- Mayer S, Weiss J, McClements DJ. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: factors influencing droplet size and stability. J Colloid Interface Sci. 2013;402:122–130. doi: 10.1016/j.jcis.2013.04.016. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- Meena AK, Sharma K, Kandaswamy M, Rajagopal S, Mullangi R. Formulation development of an albendazole self-emulsifying drug delivery system (SEDDS) with enhanced systemic exposure. Acta Pharm. 2012;62:563–580. doi: 10.2478/v10007-012-0031-0. [DOI] [PubMed] [Google Scholar]
- Mohd AB, Sanka K, Bandi S, Diwan PV, Shastri N. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of glimepiride: development and antidiabetic activity in albino rabbits. Drug Deliv. 2015;22:499–508. doi: 10.3109/10717544.2013.879753. [DOI] [PubMed] [Google Scholar]
- Mohsin K, Long MA, Pouton CW. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: precipitation of drug after dispersion of formulations in aqueous solution. J Pharm Sci. 2009;98:3582–3595. doi: 10.1002/jps.21659. [DOI] [PubMed] [Google Scholar]
- Morales D, Solans C, Gutiérrez JM, Garcia-Celma MJ, Olsson U. Oil/water droplet formation by temperature change in the water/C16E6/mineral oil system. Langmuir. 2006;22:3014–3020. doi: 10.1021/la052324c. [DOI] [PubMed] [Google Scholar]
- Moreira de Morais J, dos Santos ODH, Delicato T, Gonçalves RA, da Rocha-Filho PA. Physicochemical characterization of canola oil/water nano-emulsions obtained by determination of required HLB number and emulsion phase inversion methods. J Dispersion Sci Technol. 2006;27:109–115. doi: 10.1081/DIS-200066829. [DOI] [Google Scholar]
- Mu H, Holm R, Müllertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453:215–224. doi: 10.1016/j.ijpharm.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007;9:E344–E352. doi: 10.1208/aapsj0903041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G, Shelat P, Lalwani A. Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment. Drug Deliv. 2016;23:3027–3042. doi: 10.3109/10717544.2016.1141260. [DOI] [PubMed] [Google Scholar]
- Perazzo A, Preziosi V, Guido S. Phase inversion emulsification: current understanding and applications. Adv Colloid Interface Sci. 2015;222:581–599. doi: 10.1016/j.cis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Perrier-Cornet JM, Marie P, Gervais P. Comparison of emulsification efficiency of protein-stabilized oil-in-water emulsions using jet, high pressure and colloid mill homogenization. J Food Eng. 2005;66:211–217. doi: 10.1016/j.jfoodeng.2004.03.008. [DOI] [Google Scholar]
- Pinnamaneni S, Das NG, Das SK. Comparison of oil-in-water emulsions manufactured by microfluidization and homogenization. Pharmazie. 2003;58:554–558. [PubMed] [Google Scholar]
- Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Delivery Rev. 2008;60:625–637. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11:S93–S98. doi: 10.1016/S0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Qadir A, Faiyazuddin MD, Talib Hussain MD, Alshammari TM, Shakeel F. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J Mol Liq. 2016;214:7–18. doi: 10.1016/j.molliq.2015.11.050. [DOI] [Google Scholar]
- Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25:1000–1008. doi: 10.1016/j.foodhyd.2010.09.017. [DOI] [Google Scholar]
- Ragelle H, Crauste-Manciet S, Seguin J, Brossard D, Scherman D, Arnaud P, et al. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int J Pharm. 2012;427:452–459. doi: 10.1016/j.ijpharm.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Rehman FU, Shah KU, Shah SU, Khan IU, Khan GM, Khan A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS) Expert Opin Drug Deliv. 2017;14:1325–1340. doi: 10.1080/17425247.2016.1218462. [DOI] [PubMed] [Google Scholar]
- Sadurní N, Solans C, Azemar N, García-Celma MJ. Studies on the formation of O/W nano-emulsions, by low-energy emulsification methods, suitable for pharmaceutical applications. Eur J Pharm Sci. 2005;26:438–445. doi: 10.1016/j.ejps.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L, Rojas-Graü A, Soliva-Fortuny R, Martín-Belloso O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015;43:547–556. doi: 10.1016/j.foodhyd.2014.07.012. [DOI] [Google Scholar]
- Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O. Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control. 2014;37:292–297. doi: 10.1016/j.foodcont.2013.09.015. [DOI] [Google Scholar]
- Sanguansri P, Augustin MA. Nanoscale materials developmenta food industry perspective. Trends Food Sci Technol. 2006;17:547–556. doi: 10.1016/j.tifs.2006.04.010. [DOI] [Google Scholar]
- Schultz S, Wagner G, Urban K, Ulrich J. High-pressure homogenization as a process for emulsion formation. Chem Eng Technol. 2004;27:361–368. doi: 10.1002/ceat.200406111. [DOI] [Google Scholar]
- Shakeel F, Haq N, Alanazi FK, Alsarra IA. Impact of mixed nonionic surfactants on self-nanoemulsification efficiency of Sefsol-218 in glibenclamide nanoemulsion. Curr Nanosci. 2013;9:723–729. doi: 10.2174/157341371130900100. [DOI] [Google Scholar]
- Singh KK, Vingkar SK. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int J Pharm. 2008;347:136–143. doi: 10.1016/j.ijpharm.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Sokolov YV. Nanoemulsion formation by low-energy methods: a review. Vìsn farm. 2014;3:16–19. doi: 10.24959/nphj.14.1981. [DOI] [Google Scholar]
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nanoemulsions. Curr Opin Colloid Interface Sci. 2005;10:102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- Solans C, Morales D, Homs M. Spontaneous emulsification. Curr Opin Colloid Interface Sci. 2016;22:88–93. doi: 10.1016/j.cocis.2016.03.002. [DOI] [Google Scholar]
- Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci. 2012;17:246–254. doi: 10.1016/j.cocis.2012.07.003. [DOI] [Google Scholar]
- Solè I, Pey CM, Maestro Al, González C, Porras M, Solans C, et al. Nano-emulsions prepared by the phase inversion composition method: preparation variables and scale up. J Colloid Interface Sci. 2010;344:417–423. doi: 10.1016/j.jcis.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Solè I, Solans C, Maestro A, González C, Gutiérrez JM. Study of nano-emulsion formation by dilution of microemulsions. J Colloid Interface Sci. 2012;376:133–139. doi: 10.1016/j.jcis.2012.02.063. [DOI] [PubMed] [Google Scholar]
- Sonneville-Aubrun O, Babayan D, Bordeaux D, Lindner P, Rata G, Cabane B. Phase transition pathways for the production of 100 nm oil-in-water emulsions. Phys Chem Chem Phys. 2009;11:101–110. doi: 10.1039/B813502A. [DOI] [PubMed] [Google Scholar]
- Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108–109:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Sugumar S, Ghosh V, Nirmala MJ, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason Sonochem. 2014;21:1044–1049. doi: 10.1016/j.ultsonch.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Suryawanshi MR, Kondawar MS. Formulation and evaluation of solid self micro emulsifying drug delivery system for Clarithromycin. Pharm Sin. 2014;5:27–35. [Google Scholar]
- Tabibiazar M, Davaran S, Hashemi M, Homayonirad A, Rasoulzadeh F, Hamishehkar H, et al. Design and fabrication of a food-grade albumin-stabilized nanoemulsion. Food Hydrocoll. 2015;44:220–228. doi: 10.1016/j.foodhyd.2014.09.005. [DOI] [Google Scholar]
- Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Tang SY, Sivakumar M, Ng AMH, Shridharan P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int J Pharm. 2012;430:299–306. doi: 10.1016/j.ijpharm.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Thakur N, Garg G, Sharma PK, Kumar N. Nanoemulsions: a review on various pharmaceutical application. Global J Pharmacol. 2012;6:222–225. [Google Scholar]
- Tiwari SB, Shenoy DB, Amiji MM. Nanoemulsion formulations for improved oral delivery of poorly soluble drugs. NSTI-Nanotech. 2006;1:475–478. [Google Scholar]
- Vandamme TF, Anton N. Low-energy nanoemulsification to design veterinary controlled drug delivery devices. Int J Nano-medicine. 2010;5:867–873. doi: 10.2147/IJN.S13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Castillejos F, Granillo-Guerrero VG, Leyva-Daniel DE, Alamilla-Beltrán L, Gutiérrez-López GF, Monroy-Villagrana A, et al. Fabrication of nanoemulsions by microfluidization. In: Jafari SM, McClements DJ, editors. Nanoemulsions: Formulation, Applications, and Characterization. Academic Press; San Diego, CA, USA: 2018. pp. 207–232. [DOI] [Google Scholar]
- Wang Y. Master’s thesis. Massey University; Auckland, New Zealand: 2014. Preparation of nano- and microemulsions using phase inversion and emulsion titration methods. [Google Scholar]
- Weiss J, Decker EA, McClements DJ, Kristbergsson K, Helgason T, Awad T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophysics. 2008;3:146–154. doi: 10.1007/s11483-008-9065-8. [DOI] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24:12758–12765. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang D, Ma Y, Zhao Q, Fallon JK, Liu D, et al. Theranostic nanoemulsions: codelivery of hydrophobic drug and hydrophilic imaging probe for cancer therapy and imaging. Nano-medicine. 2014;9:2773–2785. doi: 10.2217/nnm.14.50. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Gao Y, Zhao J, Mao L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int. 2008;41:61–68. doi: 10.1016/j.foodres.2007.09.006. [DOI] [Google Scholar]
- Zaaboul F, Raza H, Cao C, Yuanfa L. The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil bodies. Food Chem. 2019;280:270–277. doi: 10.1016/j.foodchem.2018.12.047. [DOI] [PubMed] [Google Scholar]
- Zhang J, Peppard TL, Reineccius GA. Preparation and characterization of nanoemulsions stabilized by food biopolymers using microfluidization. Flavour Fragrance J. 2015;30:288–294. doi: 10.1002/ffj.3244. [DOI] [Google Scholar]
- Zhao T, Maniglio D, Chen J, Chen B, Motta A, Migliaresi C. Design and optimization of self-nanoemulsifying formulations for lipophilic drugs. Nanotechnology. 2015;26:125102. doi: 10.1088/0957-4484/26/12/125102. [DOI] [PubMed] [Google Scholar]